Few older cancer patients are enrolled in cancer clinical trials. This article assesses whether the development and conduct of older‐patient‐specific trials is justified, comparing outcomes of patients enrolled in trials purposely designed for older cancer patients versus age‐unspecified trials.
Keywords: Older patients, Clinical trials, Accrual, Breast cancer, Adjuvant
Abstract
Background.
Less than 3% of older patients with cancer are enrolled in clinical trials. To reverse this underrepresentation, we compared older patients enrolled with older‐patient‐specific trials, defined as those designed for older patients with cancer, with those enrolled in age‐unspecified trials.
Materials and Methods.
We focused on individual patient data from those ≥65 years (younger patients excluded) and included all Alliance phase III adjuvant breast cancer trials from 1985–2012.
Results.
Among 2,277 patients, 1,014 had been enrolled to older‐patient‐specific and 1,263 to age‐unspecified trials. The median age (range) in the older‐patient‐specific trials was 72 (65–89) years compared with 68 (65–84) years in the cohort of older patients in age‐unspecified trials; p < .0001. A greater percentage of patients 75 years or older had enrolled in older‐patient‐specific trials compared with the cohort of age‐unspecified trials: 26% versus 6% (p < .0001). Median overall survival (OS) was 12.8 years (95% confidence interval [CI], 11.9–13.7) and 13.5 years (95% CI, 12.9–14.1) for older‐patient‐specific and age‐unspecified trials, respectively. OS was comparable (hazard ratio [HR], 1.08; 95% CI, 0.92–1.28; p = .34; referent: age‐unspecified trials), after adjusting for age, estrogen receptor status, tumor size, and lymph node status. Similar findings were reached for recurrence‐free survival. A lower rate of grade 3–5 adverse events (hematologic and nonhematologic) was reported in older‐patient‐specific trials (43% vs. 58%; p < .0001). Sensitivity analysis with chemotherapy only trials and subset analysis, adjusted for performance score, yielded similar OS results.
Conclusion.
Older‐patient‐specific trials appear to address this underrepresentation of older patients with ostensibly comparable outcomes. Clinical trial identification numbers. NCT00003088 (CALGB 9741); NCT00024102 (CALGB 49907); NCT00068601 (CALGB 40401); NCT00005970 (NCCTG N9831)
Implications for Practice.
This work underscores the importance of clinical trials that focus on the recruitment of older patients with cancer.
Introduction
Less than 3% of older patients with cancer are enrolled in cancer clinical trials, and, for drug registration trials, less than 10% of patients are 75 years of age or older [1]. Moreover, trends in recruitment of older patients to large national cancer trials show, at best, only modest improvements [2], [3], [4], [5]. This predicament of poor accrual of older patients with cancer to cancer clinical trials creates unfilled gaps in our knowledge of how best to provide cancer care to an increasingly growing population. For breast cancer, close to half of all malignant diagnoses occur in older women, and close to half of cancer deaths also occur in older women [6]. Indeed, the incidence of breast cancer, as well as other malignancies, is expected to increase further as the population ages and as life expectancy continues to lengthen.
A variety of efforts—such as high‐level policy statements, health care provider education, legislative changes to cover reimbursement, and others—are underway to improve rates of accrual of older patients to cancer clinical trials [7], [8], [9]. One such effort consists of the purposeful design of clinical trials for older patients, herein referred to as older‐patient‐specific trials, with the goal of tailoring therapeutic interventions to address functional, biological, or other factors germane to older patients with cancer [10]. At times, however, such efforts have been met with controversy. In a commentary entitled “Do we protect or discriminate? Representation of senior adults in clinical trials,” Kázmierska points out that less aggressive treatment of older patients with cancer can yields inferior outcomes but also acknowledges heterogeneity among older patients and the fact that broad‐based enrollment of older patients provides an essential source of knowledge that enables oncology health care providers to render the best possible cancer care [11].
In this context, the current study was undertaken to assess whether the development and conduct of older‐patient‐specific trials is justified. The objective was to compare characteristics and outcomes of patients enrolled on older‐patient‐specific trials, defined as those purposely designed for older patients with cancer, with older patients enrolled on age‐unspecified trials. Importantly, to achieve this objective, the current study relied on individual patient data from prospective trials conducted within a nationally‐funded, multi‐institutional organization.
Materials and Methods
Overview
The Mayo Clinic institutional review board (IRB) approved this pooled analysis, which used individual patient data from all phase III adjuvant breast cancer trials conducted from 1985 to 2012 within Cancer and Leukemia Group B and North Central Cancer Treatment Group (both now part of the Alliance for Clinical Trials in Oncology) [12], [13], [14], [15], [16], [17], [18], [19]. A deliberate decision was made to focus only on postoperative, adjuvant clinical trials to circumvent the confounding effects of more advanced disease on overall survival (OS). Each patient had signed an IRB‐approved, protocol‐specific informed consent document, in accordance with federal and institutional guidelines.
Description of Trials and Patients
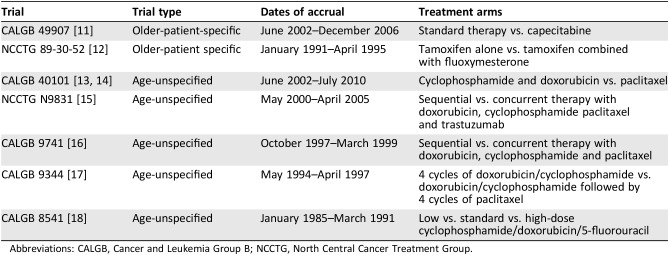
Older‐patient‐specific trials were those that restricted enrollment to older patients (Table 1). Age‐unspecified trials were those that enrolled patients 18 years of age or older (Table 1). The decision to focus exclusively on older patients appeared advantageous for answering the primary question of whether older‐patient‐specific trials are able to address this underrepresentation of older patients on cancer clinical trials. Furthermore, the choice of the age cutoff point of 65 years (patients younger than 65 years from age‐unspecified trials were excluded from all analyses) is based on precedent from previous studies and on Medicare eligibility criteria.
Table 1. Trials included in the pooled analysis.
Abbreviations: CALGB, Cancer and Leukemia Group B; NCCTG, North Central Cancer Treatment Group.
Selection of Patient Characteristics and Endpoints
We sought to compare the following demographic variables based on trial types: patient age at trial entry, patient race and ethnicity, tumor size, number of positive lymph nodes, and estrogen receptor (ER) status and progesterone receptor (PR) status. Because only two trials reported on Eastern Cooperative Oncology Group (ECOG) performance score, a subgroup analysis was used to compare this variable‐based on trial type [12], [14], [15].
For OS, the event was death from any cause, and the time to event was defined as the interval from randomization within each trial until death or last follow‐up, whichever occurred first. The event for recurrence‐free survival (RFS) was cancer recurrence or death, whichever occurred first. The time to RFS was defined as the interval from randomization to cancer recurrence, death, or last follow‐up. For both OS and RFS, patients lost to follow‐up were censored at the time of their last visit. Finally, we compared the frequency of adverse events by grade.
Statistical Methods
Baseline characteristics were summarized for each type by mean (SD) and median (range) for continuous variables and by frequency (percentage) for categorical variables. Patient characteristics were compared between trial type with the Kruskal‐Wallis test for continuous variables and Pearson's chi‐square test for categorical variables.
OS and RFS were summarized by trial type with the Kaplan‐Meier estimator. OS and RFS were compared between trial type with multivariable Cox proportional hazard regression models. The Cox models included trial type (older‐patient‐specific vs. age‐unspecified) as the main effect. Other covariates considered in the Cox models included age (<70 vs. 70–75 vs. >75 years), ER status (negative vs. positive), tumor size (diameter <3 cm vs. ≥3 cm), positive lymph nodes (0 vs. 1–3 vs. 4–9 vs. 10+), ethnicity, and ECOG performance status (0 vs. 1–2). A stepwise procedure was used to select the covariates in the final models with trial type (the main effect) included in all steps of the model selection. Variables that retained statistical significance at the two‐sided type I error of 0.05 were included in the final models. The proportional hazards assumption was tested for all variables in the Cox model. In a subgroup analysis that focused only on trials that captured performance score, adjusted survival curves based on the final Cox model were created. Adverse events were summarized by maximum grade level separately by trial type with frequencies and percentages and were compared between trial types with the Pearson's chi‐square test. A deliberate decision was made to not characterize nonhematologic adverse events based on trial type in detail, as adverse event data from trial to trial were difficult to harmonize with accuracy.
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. All analyses were performed with SAS Version 9.4 software (SAS Institute Inc., Cary, NC). Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies.
Results
Baseline Characteristics
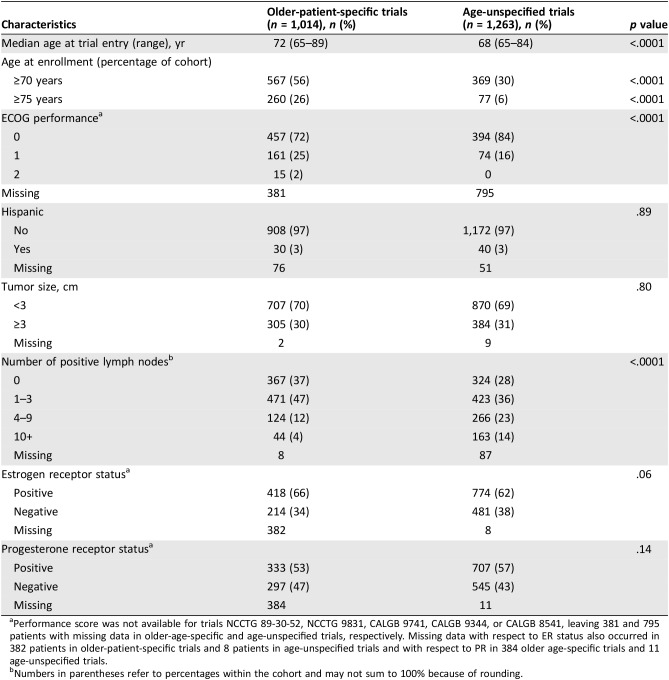
A total of 2,277 patients were included in this pooled analysis of individual patient data; 1,014 patients were from older‐patient‐specific trials, and 1,263 were from age‐unspecified trials (Table 2). The median age of older‐patient‐specific trials was 72 (range, 65–89) years versus 68 (range, 65–84) years for the older cohort in the age‐unspecified trials (p < .0001). Among older‐patient‐specific trials, 26% (260/1014) of patients were older than 75 years of age at trial entry, whereas only 6% (77/1263) in age‐unspecified trials were within this older age group (p < .0001).
Table 2. Characteristics of cohorts in older‐patient‐specific and age‐unspecified trials.
Performance score was not available for trials NCCTG 89‐30‐52, NCCTG 9831, CALGB 9741, CALGB 9344, or CALGB 8541, leaving 381 and 795 patients with missing data in older‐age‐specific and age‐unspecified trials, respectively. Missing data with respect to ER status also occurred in 382 patients in older‐patient‐specific trials and 8 patients in age‐unspecified trials and with respect to PR in 384 older age‐specific trials and 11 age‐unspecified trials.
Numbers in parentheses refer to percentages within the cohort and may not sum to 100% because of rounding.
As noted, performance score data were available for only one older‐patient‐specific trial (CALGB 49907; n = 633) and one age‐unspecified trial (CALGB 40401; n = 468). Among patients with reported ECOG performance score, 72% (457/633) of patients in older‐patient‐specific trials had a performance score of zero compared with 84% (394/468) in age‐unspecified trials (p < .0001). Other baseline characteristics appear in Table 2.
Survival
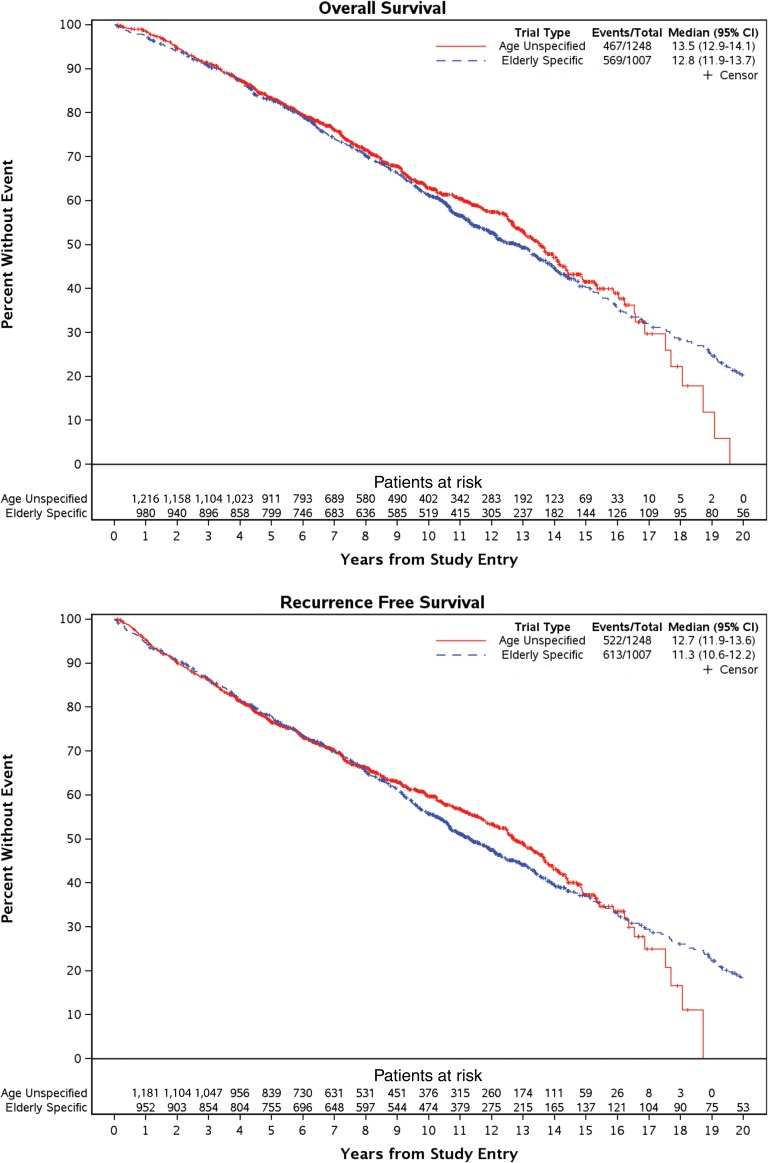
Median OS was 12.8 years (95% confidence interval [CI], 11.9–13.7) and 13.5 years (95% CI, 12.9–14.1) in older‐patient‐specific trials and age‐unspecified trials, respectively (Fig. 1). OS was comparable (hazard ratio [HR], 1.08; 95% CI, 0. 92–1. 28; p = .34; referent: age‐unspecified trials), after adjusting for age, estrogen receptor status, tumor size, and lymph node status. (Table 3, Fig. 1).
Figure 1.
Survival outcomes based on trial type. Median overall survival was comparable based on trial type: 12.8 years (95% CI, 11.9–13.7) and 13.5 years (95% CI, 12.9–14.1) in older‐patient‐specific trials and age‐unspecified trials, respectively. Similarly, median recurrence‐free survival was comparable based on trial type: 11.3 years (95% CI, 10.6–12.2) versus 12.7 years (95% CI, 11.9–13.6).
Abbreviation: CI, confidence interval.
Table 3. Multivariate analysis of overall survival (OS) and recurrence‐free survival (RFS).
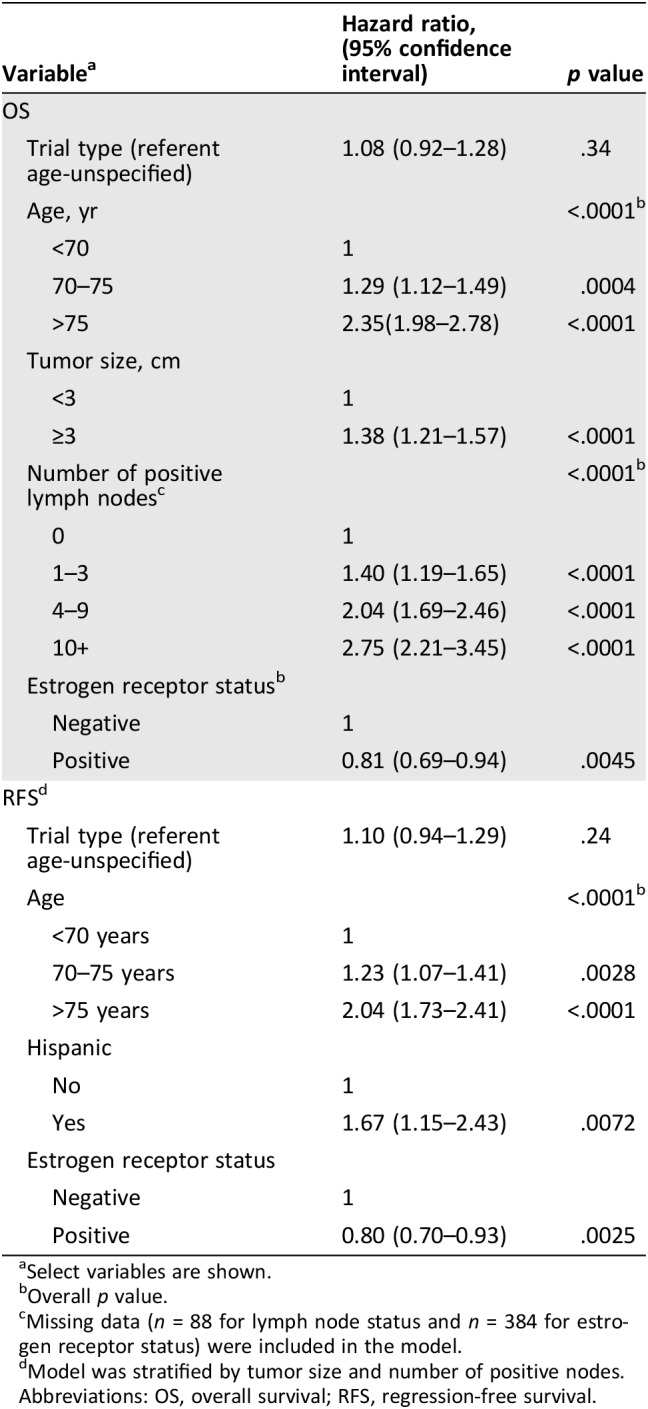
Select variables are shown.
Overall p value.
Missing data (n = 88 for lymph node status and n = 384 for estrogen receptor status) were included in the model.
Model was stratified by tumor size and number of positive nodes.
Abbreviations: OS, overall survival; RFS, regression‐free survival.
Median RFS was 11.3 years (95% CI, 10.6–12.2 years) in older‐patient‐specific trials versus 12.7 years (95% CI, 11.9–13.6 years) in age‐unspecified trials. RFS was comparable between trial type (HR, 1.10; 95% CI, 0.94–1.28; p = .24; referent: older‐patient‐specific trials), after adjusting for patient age, ethnicity, and ER status. (Table 3, Fig. 1).
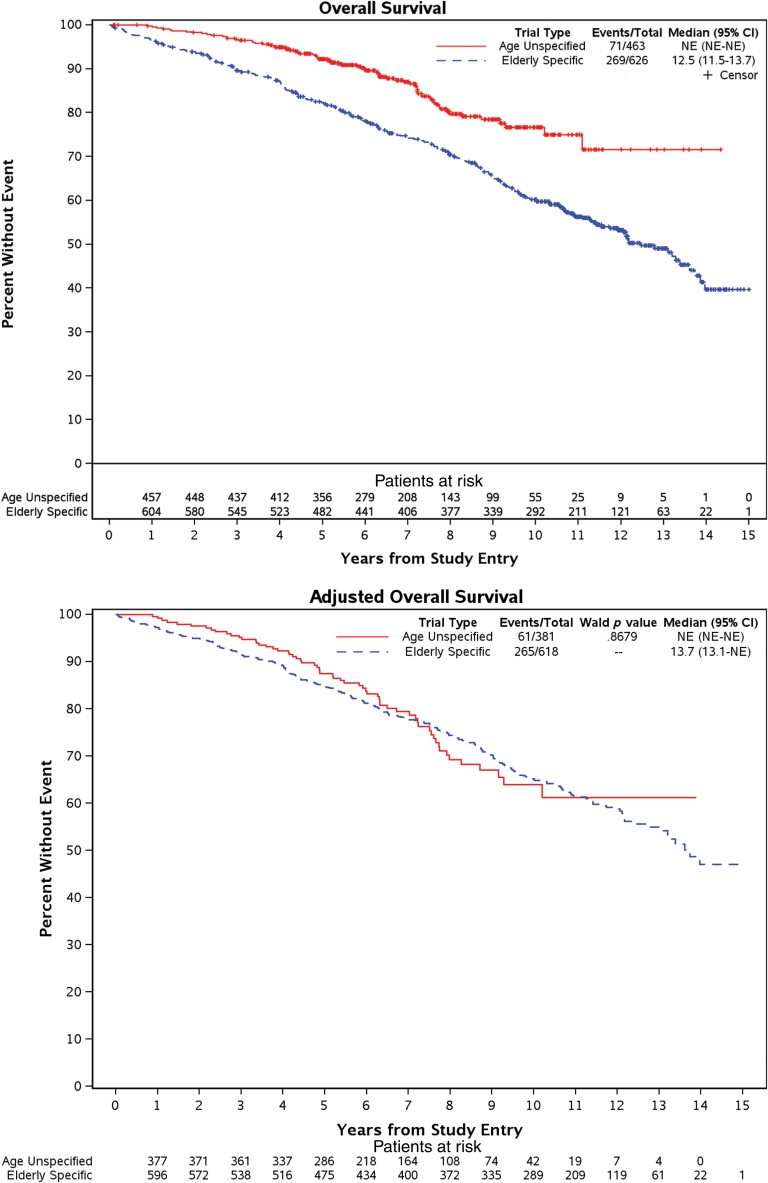
Although only two trials captured patient performance scores, and although older patients had worse scores, a two‐trial subgroup that included performance score into the model showed comparable OS based on trial type after adjusting for performance scores (Fig. 2) [11], [13], [14]. Another subgroup analysis which excluded the older‐patient‐specific trial that tested hormonal therapy yielded similar findings (data not shown).
Figure 2.
Subgroup analysis of overall survival based on trial type but with adjustment for performance score. In a subgroup analysis that included only trials that captured baseline performance score, older‐patient specific trials appeared initially to inferior survival (top set of curves): 12.5 years (95% CI, 11.5–13.7 years) in older‐patient‐specific trials and median not reached for age‐unspecified trials (the longest follow‐up was 14 years), but after adjustment for performance score, overall survival curves show comparable outcomes (bottom set of curves): 13.7 years (95% CI, 13.1 years–not reached) in older‐patient‐specific trials and median not reached for age‐unspecified trials (again, the longest follow‐up was 14 years).
Abbreviations: CI, confidence interval; NE, not estimable.
Adverse Events
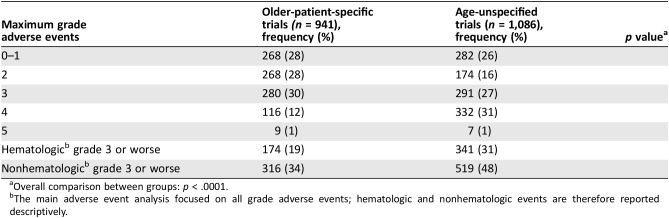
Maximum grade 3–5 adverse events (which included both hematologic and nonhematologic adverse events) were reported in 43% (405/941) of patients older‐patient‐specific trials compared with 58% (630/1086) in age‐unspecified trial. Specifically, a higher rate of grade 4 was reported in older‐patient‐specific trial (p < .0001; Table 4). A lower rate of grade 3–5 nonhematologic adverse events were reported in older‐patient‐specific trials (34% vs. 48%) and a lower rate of grade 3–5 hematologic adverse events (19% versus 31%; Table 4).
Table 4. Adverse events.
Overall comparison between groups: p < .0001.
The main adverse event analysis focused on all grade adverse events; hematologic and nonhematologic events are therefore reported descriptively.
Discussion and Conclusion
This pooled analysis of 2,277 older patients with breast cancer shows that older‐patient‐specific trials capture a much older group of patients with cancer, many of whom otherwise might never have entered a clinical trial. Importantly, we relied on individual patient data, thus making this study particularly robust. To recapitulate findings, 26% of patients enrolled in older‐patient‐specific trials were 75 years of age or older in contrast to less than 10% in the cohort of age‐unspecified trials and in cancer registration trials in general [1]. By testing gentler cancer treatment, older‐patient‐specific trials appear to render care to patients with ostensibly little compromise in outcomes. The current study suggests that well‐designed older‐patient‐specific trials serve an important role in accruing older patients—and potentially also those with poor performance scores—to clinical trials and in defining the standard of cancer care for older patients with cancer. Ostensibly, neither overly protective nor discriminatory, older‐patient‐specific trials appear to accomplish the goals of any other well‐designed clinical trial.
The findings from the current study are in keeping with a previous pooled analysis, which drew similar conclusions in patients with metastatic non‐small cell lung cancer [20]. That earlier study also revealed no statistical differences in survival outcomes between older patients enrolled in older‐patient‐specific trials and those enrolled in age‐unspecified trials; adverse event profiles across trial types were also relatively comparable. Although this pooled analysis of adjuvant breast cancer trials is far more robust, these earlier findings add credence and underscore a consistent message that older‐patient‐specific trials appear to serve an important role in helping us understand how best to render cancer treatment to older patients with cancer.
With respect to trial design, it should be emphasized that older‐patient‐specific trials merit the same careful thought and planning with respect to drug doses and other treatment conditions as any other cancer clinical trial. Previous studies have shown that undertreating older patients with cancer can, in fact, give rise to suboptimal outcomes. For example, Bouchard and others examined over 30 studies on the undertreatment of breast cancer in older patients and concluded that “undertreatment is a well‐documented phenomenon responsible for preventable cancer deaths” [21]. In concert with such observations, the study from Muss and others, as integrated into the current pooled analysis, demonstrated that adjuvant capecitabine—a gentler, oral chemotherapy regimen that patients often prefer and that is effective in metastatic breast cancer—was clearly inferior when compared with standard adjuvant chemotherapy (either cyclophosphamide, methotrexate, and fluorouracil or cyclophosphamide plus doxorubicin) [12]. Adjuvant capecitabine resulted in higher rates of cancer recurrence and death. Thus, older‐patient‐specific trials should not only be testing a hypothesis that favors a less aggressive therapeutic approach. Rather, building on the concept of equipoise, such trials can be aimed at defining an optimal treatment—whether more aggressive or less aggressive than the current standard—and contribute to an otherwise sparse evidence base for older patients with cancer.
This pooled analysis has limitations. First, as is often the case in such analyses, we were forced to contend with trial heterogeneity and missing data. To address heterogeneity, we adjusted for multiple variables in our Cox models. Performance scores were available from only two trials—one older‐patient‐specific trial and one age‐unspecified trial. To overcome these shortcomings, we conducted a subgroup analyses with our limited data on performance score only to confirm our main OS findings, as stated above, in multivariate analyses. The findings from this subgroup analysis also underscore the importance of including broader eligibility criteria in age‐unspecified trials. Second, with respect to adverse event data, we were unable to provide the desired granularity on what types of nonhematological adverse events were more commonly observed in older‐patient specific trials. The limitations entailed in harmonizing adverse event data across trials made it impossible to acquire the desired degree of detail with accuracy. Future studies might focus on understanding age‐based differences in specific adverse events. Third, an important caveat is that we focused only on clinical trial data. It is well accepted that clinical trial enrollment entails a selection process that has the potential to bias any and all trial results toward conclusions perhaps relevant to only the fittest of older patients with cancer. Importantly, in view of the types of information gathered during the conduct of these trials, we are unable to comment on baseline morbidity and differences based on trial type. Nonetheless, the fact that older‐patient‐specific trials appear to be successful in enrolling a much older patient cohort with poorer performance status suggests that these trials may capture patients more representative of the general population of older patients with cancer. Hence, these trials should be viewed as an important first step in filling knowledge gaps for how best to provide cancer care to an increasingly growing population of patients with cancer.
Finally, when examining the dates of enrollment of older‐patient‐specific trials and age‐unspecified trials, it appears that older‐patient‐specific and age‐unspecified trials heavily overlap. Yet all the studies in this pooled analysis were able to complete their accrual and provide important findings on the role of adjuvant therapy in patients with breast cancer. In essence, the data presented here also suggest that these two different trial types can successfully coexist. In summary, our results suggest the value of dedicating clinical trials to older patients, not only to answer therapeutic questions but also to ascertain the power to assess other endpoints relevant to older patients with cancer.
Acknowledgments
This work was supported by the National Cancer Institute of the National Institutes of Health [UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), P30CA033572, U10CA180790, U10CA180838, U10CA180857, U10CA180867, and UG1CA189850]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Deceased.
Author Contributions
Concept/design: Dyda Dao, Aminah Jatoi, Jennifer G. Le‐Rademacher
Provision of study material or patients: Arti Hurria, Hyman Muss, Lawrence N. Shulman, Marc Citron, Daniel Budman, Ann Partridge, Lisa Carey
Collection and/or assembly of data: Tyler Zemla, Ryan McMurray, Dyda Dao, Aminah Jatoi, Jennifer G. Le‐Rademacher
Data analysis and interpretation: Tyler Zemla, Ryan McMurray, Dyda Dao, Aminah Jatoi, Jennifer G. Le‐Rademacher
Manuscript writing: Dyda Dao, Tyler Zemla, Aminah Jatoi, Rachel A. Freedman, Arti Hurria, Hyman Muss, Harvey Jay Cohen, Lawrence N. Shulman, Marc Citron, Daniel Budman, Ryan McMurray, Ann Partridge, Lisa Carey, Mina S. Sedrak, Jacqueline M. Lafky, Jennifer G. Le‐Rademacher
Final approval of manuscript: Dyda Dao, Tyler Zemla, Aminah Jatoi, Rachel A. Freedman, Arti Hurria, Hyman Muss, Harvey Jay Cohen, Lawrence N. Shulman, Marc Citron, Daniel Budman, Ryan McMurray, Ann Partridge, Lisa Carey, Mina S. Sedrak, Jacqueline M. Lafky, Jennifer G. Le‐Rademacher
Disclosures
Mina Sedrak: Novartis, The Hope Foundation, National Institutes of Health (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Harnaker ME, Stauder R, van Munster BC. Exclusion of older patients from ongoing clinical trials for hematological malignancies: An evaluation of the National Institutes of Health Clinical Trial Registry. The Oncologist 2014;19:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis JH, Kilgore ML, Goldman DP et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol 2003;21:1383–1389. [DOI] [PubMed] [Google Scholar]
- 3.Hutchins LF, Unger JM, Goldman DP et al. Participation of patients 65 years of age or older in cancer clinical trials. N Engl J Med 1999;341:2061–2067. [DOI] [PubMed] [Google Scholar]
- 4.Freedman RA, Foster JC, Seisler DK et al. Accrual of older patients with breast cancer to alliance systemic therapy trials over time: Protocol A151527. J Clin Oncol 2017;35:421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith BD, Jiang J, McLaughlin SS et al. Improvement in breast cancer outcomes over time: Are older women missing out? J Clin Oncol 2011;29:4647–4653. [DOI] [PubMed] [Google Scholar]
- 6.American Cancer Society . Available at http://www.cancer.org/. Accessed July 6, 2018.
- 7.Kemeny MM, Peterson BL, Kornblith AB et al. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol 2003;21:2268–2275. [DOI] [PubMed] [Google Scholar]
- 8.Kornblith AB, Kemeny M, Peterson BL et al. Survey of oncologists’ perceptions of barriers to accrual of older patients with breast carcinoma to clinical trials. Cancer 2002;95:989–996. [DOI] [PubMed] [Google Scholar]
- 9.Kimmick G, Kornblith A, Mandelblatt et al. A randomized controlled trial of an educational program to improve accrual of older persons to cancer treatment protocols: CALGB 360001. J Clin Oncol 2004;22(suppl):8040a. [DOI] [PubMed] [Google Scholar]
- 10.Hurria A, Dale W, Mooney M et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol 2014;32:2587–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kázmierska J. Do we protect or discriminate? Representation of senior adults in clinical trials. Rep Pract Oncol Radiother 2012;18:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muss HB, Berry DA, Cirrincione CT et al. Adjuvant chemotherapy in older women with early‐stage breast cancer. N Engl J Med 2009;360:2055–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingle JN, Suman VJ, Mailliard JA et al. Randomized trial of tamoxifen alone or combined with fluoxymesterone as adjuvant therapy in postmenopausal women with resected estrogen receptor positive breast cancer. North Central Cancer Treatment Group Trial 89‐30‐52. Breast Cancer Res Treat 2006;98:217–222. [DOI] [PubMed] [Google Scholar]
- 14.Shulman LN, Berry DA, Cirrincione et al. Comparison of doxorubicin and cyclophosphamide versus single‐agent paclitaxel as adjuvant therapy for breast cancer in women with 0 to 3 positive axillary nodes: CALGB 40101 (Alliance). J Clin Oncol 2014;32:2311–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shulman LN, Cirrincione CT, Berry DA et al. Six cycles of doxorubicin and cyclophosphamide or Paclitaxel are not superior to four cycles as adjuvant chemotherapy for breast cancer in women with zero to three positive axillary nodes: Cancer and Leukemia Group B 40101. J Clin Oncol 2012;30:4071–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez EA, Romond EH, Suman VJ et al. Four‐year follow‐up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2‐positive breast cancer: Joint analysis of data from NCCTG N9831 and NSABP B‐31. J Clin Oncol 2011;29:3366–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Citron ML, Berry DA, Cirrincione C et al. Randomized trial of dose‐dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node‐positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 2003;21:1431–1439. [DOI] [PubMed] [Google Scholar]
- 18.Henderson IC, Berry DA, Demetri GD et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node‐positive primary breast cancer. J Clin Oncol 2003;21:976–983. [DOI] [PubMed] [Google Scholar]
- 19.Wood WC, Budman DR, Korzun AH et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node‐positive breast carcinoma. N Engl J Med 1994;330:1253–1259. [DOI] [PubMed] [Google Scholar]
- 20.Jatoi A, Hillman S, Stella P et al. Should elderly non–small‐cell lung cancer patients be offered older‐aged‐specific trials? Results of a pooled analysis from the North Central Cancer Treatment Group. J Clin Oncol 2005;23:9113–9119. [DOI] [PubMed] [Google Scholar]
- 21.Bouchardy C, Rapiti E, Blagojevis S et al. Older female cancer patients: Importance, causes, and consequences of undertreatment. J Clin Oncol 2007;25:1858–1869. [DOI] [PubMed] [Google Scholar]