Despite increased incidence of anal squamous cell carcinoma (ASCC), treatment recommendations have remained unchanged for the past 35 years. This article profiles the tumor microenvironment of patients with localized ASCC, examining CD8, PD‐1, PD‐L1, IDO1 and HLA class I expression and, specifically, characterizes expression of IDO1 in the context of several key components of the immune microenvironment.
Keywords: Anal cancer; Immune microenvironment; Indoleamine 2,3 dioxygenase 1; Chemoradiation; Outcomes
Abstract
Background.
This study characterizes the tumor‐immune microenvironment in pretreatment, localized anal squamous cell carcinoma (ASCC), including two markers that have not previously been studied in ASCC: indoleamine 2,3 dioxygenase 1 (IDO1) and human leukocyte antigen (HLA) class I.
Materials and Methods.
Retrospective review identified 63 patients with ASCC receiving definitive chemoradiation between 2005 and 2016 with pretreatment tissue available. Immunohistochemistry was used to quantify cluster of differentiation 8 (CD8), programmed cell death protein 1, programmed death‐ligand 1, HLA class I, and IDO1. Cox proportional hazards models evaluated associations between outcomes and immune markers, controlling for clinical characteristics.
Results.
With a median follow‐up of 35 months, 3‐year overall survival was 78%. The only marker found to have a robust association with outcome was tumor IDO1. In general, the percentage of tumor cells expressing IDO1 was low (median 1%, interquartile range 0%–20%); however, patients with >50% of tumor cells expressing IDO1 had significantly worse overall survival (hazard ratio [HR] 4.7, p = .007) as well as higher local recurrence (HR 8.6, p = .0005) and distant metastasis (HR 12.7, p = .0002). Tumors with >50% IDO1 were also more likely to have the lowest quartile of CD8 infiltrate (<40 per high‐power field, p = .024).
Conclusion.
ASCC has a diverse immune milieu. Although patients generally do well with standard therapy, IDO1 may serve as a prognostic indicator of poor outcome and could help identify a patient population that might benefit from IDO‐targeted therapies.
Implications for Practice.
After definitive chemoradiation, patients with locally advanced anal cancer may experience significant treatment morbidity and high risk of recurrence. The goal of the current study is to identify novel prognostic factors in the tumor‐immune microenvironment that predict for poor outcomes after definitive chemoradiation. This study characterizes the tumor‐immune microenvironment in pre‐treatment, localized anal squamous cell carcinoma (ASCC), including two markers which have not previously been studied in ASCC: indoleamine 2,3 dioxygenase 1 (IDO1) and HLA class I. With a median follow‐up of 3 years, this study demonstrated that high IDO1 expression is correlated with significantly worse 3‐year overall survival (88% vs. 25%). Whereas recent studies of IDO1 inhibitors have shown mixed results, this study suggests that patients with anal cancer with high IDO1 expression have dismal prognosis and may represent a patient population primed for response to targeted IDO1 inhibition.
摘要
背景。本研究描述了局部肛门鳞状细胞癌 (ASCC)治疗前的肿瘤免疫微环境,包括以前未在 ASCC 中研究的两种标志物:吲哚胺 2,3 双加氧酶 1 (IDO1) 和人白细胞抗原 (HLA) I 类的特性。
材料和方法。回顾性调查确定了在 2005 年至 2016 年期间接受根治性放化疗的 63 例 ASCC 患者,并可获取治疗前组织。免疫组织化学用于量化分化簇 8 (CD8),程序性细胞死亡蛋白 1,程序性死亡‐配体 1,HLA I 类和 IDO1。Cox 比例风险模型,在控制临床特征的同时,评估预后和免疫标记物之间的关联。
结果。中位随访时间为 35 个月,3 年总生存率为 78%。发现与预后具有强烈关联的唯一标记物是肿瘤 IDO1。通常,表达 IDO1 的肿瘤细胞的百分比较低(中位数 1%,四分位数范围 0%‐20%);然而,表达 IDO1 的肿瘤细胞 > 50% 的患者的总生存率 [风险比 (HR)4.7, p = 0.007]显著降低,并且局部复发率(HR 8.6, p = 0.000 5)和远处转移发生率(HR 12.7, p = 0.000 2)较高。具有 > 50% IDO1 的肿瘤也更可能具有最低四分位数的 CD8 浸润(每个高倍视野 <40, p = 0.024)。
结论。ASCC 具有多样化的免疫环境。尽管患者通常在标准治疗方面表现良好,但 IDO1 可作为预后不良的一个指标,可帮助确定可能受益于 IDO 靶向治疗的患者群体。
实践意义:在根治性放化疗后,患有局部晚期肛门癌的患者可能会有显著的治疗病损率和高复发风险。本研究的目的是确定肿瘤免疫微环境中的新型预后因素,以预测根治性放化疗的不良预后。本研究描述了局部肛门鳞状细胞癌 (ASCC) 治疗前的肿瘤免疫微环境,包括以前未在 ASCC 中研究过的两种标志物:吲哚胺 2,3 双加氧酶 1 (IDO1) 和 HLA I 类的特性。中位随访时间为 3 年,该研究表明,高 IDO1 表达与显著较低的 3 年总生存率相关(88% vs. 25%)。尽管最近对 IDO1 抑制剂的研究显示出的结果好坏不一,但本研究表明具有高 IDO1 表达的肛门癌患者具有并不乐观的预后并且可能代表了对靶向 IDO1 抑制发生反应的患者群体。
Introduction
Anal squamous cell carcinoma (ASCC) is a relatively rare malignancy with about 27,000 new cases per year worldwide [1]. The incidence has steadily increased over the last few decades, secondary to exposure to oncogenic subtypes of the human papilloma virus (HPV), the major driver of ASCC in >90% of patients [2], [3], [4], [5]. Despite this increase in incidence, the treatment paradigm for locally advanced ASCC has been essentially unchanged for the past 35 years, when definitive chemoradiation was first found to be an effective alternative to abdominoperineal resection [6]. This standard regimen of 5‐fluorouracil (5FU) and mitomycin‐C (MMC) with concurrent radiation results in good outcomes for many patients, but despite best practices, about 25% of patients with advanced T‐ or N‐stage cancer will not be cured of their disease [7], [8], [9].
Identifying novel biomarkers that are associated with poor prognosis will help define the cohort of patients who merit treatment escalation. Several studies have found that HPV‐negative tumors have worse outcomes, but the role of other immunohistochemical or genomic markers is less well established [10], [11], [12], [13]. Given the viral etiology of this disease, it is perhaps not surprising that prior studies have shown that the immune microenvironment in ASCC plays an important role in disease prognosis. Tumor‐infiltrating lymphocytes (TILs) are the most frequently studied component of the immune microenvironment, with most studies finding higher TIL to be associated with improved outcomes [14], [15], [16], [17]. Other immunologic factors are less well studied in ASCC. Several groups have examined the effect of programmed cell death protein 1 (PD‐1)/programmed death‐ligand 1 (PD‐L1) expression on prognosis but have come to different conclusions regarding whether higher expression of these immune checkpoints is negatively or positively prognostic [18], [19]. However, despite these differing results, there is an emerging consensus that an immunosuppressive tumor microenvironment is likely to be associated with poor outcomes.
A variety of novel immunomodulatory therapies have shown that inhibiting immunosuppressive signaling can improve clinical outcomes. The most clinically developed of these agents are immune checkpoint inhibitors, such as anti‐PD‐1/PD‐L1 drugs, which have led to exciting new treatment options for a wide variety of metastatic or recurrent malignancies, including HPV‐related malignancies such as head and neck cancer, cervical cancer, and ASCC [20], [21], [22]. Although a subset of patients will have dramatic and often durable responses, fewer than 20% of patients in these studies typically respond to treatment. Predicting which patients will respond to immune checkpoint therapy is challenging. Although PD‐L1 expression has been associated with anti‐PD‐1/PD‐L1 response in a variety of settings, several studies have shown a lack of association between PD‐L1 expression and response to PD‐1 inhibition [23]. A variety of other markers are under investigation.
Indoleamine‐2,3 dioxygenase 1 (IDO1), a component of the kynurenine pathway that initiates the first and rate‐limiting step of tryptophan degradation, depletes tryptophan and thus promotes activated T‐cell anergy while enhancing regulatory T‐cell activity [24], [25], [26], [27]. IDO1 can be expressed by tumor cells as well as myeloid and lymphoid cells, with expression being associated with poor outcome in several cancers including endometrial cancer, Hodgkin lymphoma, colorectal cancer, cervical cancer, gastric cancer, laryngeal cancer, and breast cancer [28], [29], [30], [31], [32], [33], [34], [35], [36]. IDO1 inhibitors are currently in clinical trials in a variety of malignancies in combination with several anti‐PD‐1 agents.
The current study aims to profile the tumor microenvironment of patients with localized ASCC by examining cluster of differentiation 8 (CD8), PD‐1, PD‐L1, IDO1, and human leukocyte antigen (HLA) class I expression. This is the first study, to our knowledge, to specifically examine IDO1 in ASCC and demonstrate that patients with tumors expressing high IDO1 levels have significantly worse outcomes. More broadly, this study also characterizes expression of IDO1 in the context of several key components of the immune microenvironment and suggests the existence of several ASCC‐microenvironment subtypes, each of which have unique drivers of immune evasion.
Materials and Methods
Patient Cohort
With Institutional Review Board approval, we reviewed the medical records of all patients with nonmetastatic ASCC treated at Massachusetts General Hospital (MGH) Cancer Center and Brigham & Women's Hospital/Dana‐Farber Cancer Institute (BWH/DFCI) who met the following criteria: (a) treatment with definitive chemoradiation between 2005 and 2016, with (b) ≥3 months follow‐up from start of radiation (if alive at last follow‐up), and (c) accessible formalin‐fixed paraffin‐embedded (FFPE) material with sufficient tumor for further analysis.
Pathologic Assessment
All FFPE blocks for each patient were retrieved and the corresponding hematoxylin and eosin‐stained slides were reviewed to identify the block with the highest inflammatory infiltrate as well as high tumor cellularity. Five micron sections were cut from the selected paraffin block and stained by immunohistochemistry (IHC). The specific antibodies and conditions used for each immune marker were as follows: (a) CD8—clone 4B11 (Leica Biosystems, Wetzlar, Germany); (b) PD‐1—clone EH33 (Cell Signaling Technology, Danvers, MA); (c) PD‐L1—clone E1L3N (Cell Signaling Technology); (d) HLA class I—clone HC10 (which recognizes an epitope expressed on β2m‐free HLA‐A3, ‐A10, ‐A28, ‐A29, ‐A30, ‐A31, ‐A32, and ‐A33, and all β2m‐free HLA‐B excluding ‐B5702, ‐B5804, and ‐B73, as well as HLA‐C heavy chains, provided courtesy of Dr. Saldano Ferrone [37], [38]); and (e) IDO1—clone D5J4E (Cell Signaling Technology). Each antibody was used on individual tissue sections except CD8 and PD‐L1, which were costained with 3‐amino‐9‐ethylcarbazole (AEC) and 3,3’ diaminobenzidine (DAB). IHC was quantified by two pathologists blinded to clinical outcome (V.D. and D.B.). CD8‐positive (CD8+) and PD‐1‐positive (PD‐1+) TILs were quantified as the number of cells per high‐power field (cells/hpf) in the area of tumor with the most positive cells. IDO1, PD‐L1, and HLA class I were quantified as percentage of tumor cells positive. The number of IDO1‐positive (IDO1+) macrophages/hpf was also quantified. HPV status was determined by in situ hybridization (ISH) using a probe set designed for detection of high‐risk subtypes (subtypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66; catalog number 725‐4713; Ventana Medical Systems, Oro Valley, AZ).
Clinical Parameters Collected and Treatment Details
The medical record was retrospectively reviewed for each patient to collect pretreatment clinical characteristics (including age at diagnosis, sex, performance status, smoking history, and immunosuppression), disease characteristics (including stage and grade), and treatment characteristics (including surgery, chemotherapy, and radiation). Patients were followed for clinical complete response, and development of local‐regional recurrence (defined as recurrence within the pelvis) and distant metastases (defined as recurrence outside the pelvis). Date of death or last follow‐up alive was also recorded.
Statistical Analysis
To determine the immunologic subgrouping, unsupervised hierarchical clustering analysis was performed using a variance filter of 0.45 (σ/σmax) in Qlucore Omics Explorer, v.3.2 (Lund, Sweden).
Kaplan‐Meier analyses were used to evaluate overall survival (OS) and time to recurrence (any, local, and distant). Patients without an event were censored at the date of last follow‐up. To look for associations between immune marker expression and outcomes, we set expression cutoff values to dichotomize the data at the 25th, 50th, 75th, and 90th percentile of each marker's distribution. Each cutoff was tested for association with risk of death, any recurrence, locoregional recurrence (LRR), or distant metastases (DM). Because of multiple testing, a Bonferroni correction was applied such that p < .0125 was considered a statistically significant association with outcome. The cutoff with the lowest p value was selected for further analysis.
The log‐rank test was used to compare time from diagnosis to event or censoring. Cox proportional hazards models were used to evaluate associations between outcomes and immune markers, controlling for patient and tumor characteristics. With 63 patients, this study had 80% power to detect hazard ratios for survival ranging from 3.8 (using the 50th percentile as the marker's cutoff) to 6.3 (using the 90th percentile), corresponding to decreases in 3‐year survival from 85% (the background rate in this patient population) to levels ranging from 54% to 36%, respectively, based on a two‐sided log‐rank test at the .05 significance level.
Results
Patient, Disease, and Treatment Characteristics
Sixty‐three patients treated for nonmetastatic ASCC between 2005 and 2016 with FFPE tissue available constituted the cohort for analysis. This included 48 patients from MGH and 15 patients from BWH/DFCI. The patient, disease, and treatment characteristics of the cohort are shown in Table 1. The median age of patients in the study was 61 years (range 33–92), with 59% being female and 92% having Eastern Cooperative Oncology Group performance status 0–1. Fifty‐two percent of patients were prior or current smokers, and 30% had a potential source of immunosuppression including human immunodeficiency virus diagnosis or being on immunosuppressive medication (most commonly after organ transplant). The distribution of disease stages was I—11% (7 patients), II—38% (24 patients), IIIA—14% (9 patients), IIIB—33% (21 patients), and unknown—3% (2 patients). HPV status was determined by ISH for 38 patients, with 37 and 1 patients having HPV‐positive and ‐negative ASCC, respectively.
Table 1. Patient, disease, and treatment characteristics.

Abbreviations: 5FU/MMC, 5‐fluorouracil/mitomycin‐C; APR, abdominoperineal resection; Cape, capecitabine; Cis, Cisplatin; ECOG, Eastern Cooperative Oncology Group; dys, days; Gy, Gray; HIV, human immunodeficiency virus; Med., median; n, number of patients; RT, radiation; x, cycles; yrs, years.
Because of obstructive symptoms, 14% of patients had upfront surgical diversion with colostomy followed by chemoradiation. The remaining patients received definitive chemoradiation. Eighty‐three percent of patients were treated as per Radiation Therapy Oncology Group (RTOG) 0529 with dose‐painted intensity‐modulated radiation therapy to 50.4 Gy/42 Gy for T2N0 disease and 54 Gy/50.4 Gy/45 Gy for T3‐4N0‐3 disease [39]. Seventeen percent of patients were treated with a 3D‐conformal successive cone down technique, but all patients received 45–60 Gy. Fifty‐three patients received two cycles of concurrent 5FU and MMC, five patients received only one cycle because of persistent leukopenia, three patients received single‐agent 5FU or capecitabine because of comorbidity, and two patients received concurrent 5FU and cisplatin because of physician preference.
Disease Outcomes
Median follow‐up from initiation of radiation was 35 months (range excluding two patients who died on treatment: 5–145 months). Overall outcomes are shown in supplemental online Table 1. Because 3 patients died prior to follow‐up assessment and 1 patient had an upfront abdominoperineal resection (apr), 59 patients were evaluable for clinical response to chemoradiation. Ninety percent of evaluable patients had a complete clinical response. Of the six patients who had residual disease, four had subsequent abdominoperineal resection and the remaining two developed metastatic disease and did not have local surgery.
Of 61 patients who were alive at the end of treatment, 16 patients developed disease recurrence, including 7 patients with isolated LRR. However, despite having no documentation of DM, four of these seven patients with isolated LRR died of ASCC (secondary to bleeding or failure to thrive) and three of these patients were salvaged with surgery and alive without disease at last follow‐up. Twenty‐nine percent of patients died during follow‐up, including 2 patients who died on treatment, 5 patients who died during follow‐up of causes thought to be unrelated to ASCC, and 11 patients who died of ASCC.
Immune Marker Expression
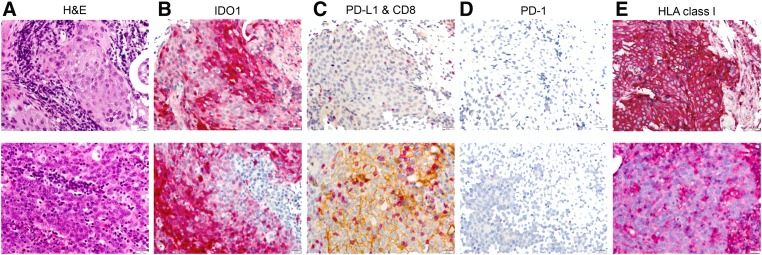
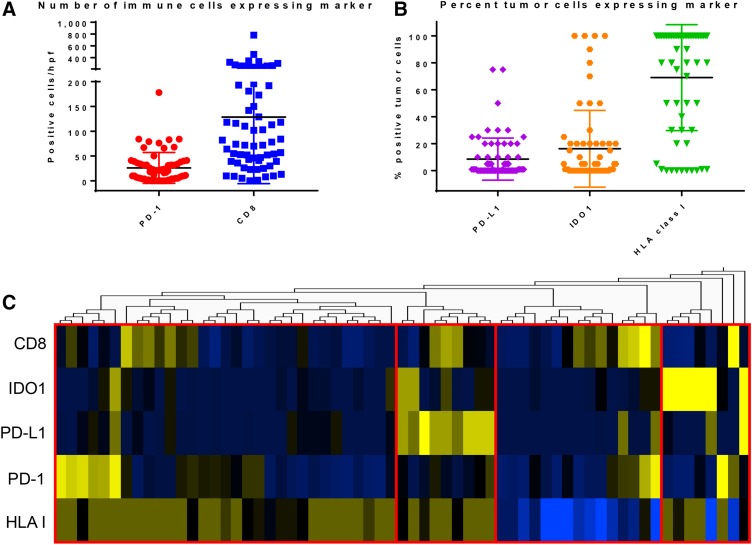
Examples of immune marker staining in two patients with high IDO1 are shown in Figure 1. Expression of the five immune markers examined varied substantially across the 63 patients in the studied cohort (Fig. 2A, 2B). Each marker had the following median (med) and interquartile range (IQR): (1) tumor IDO1—med 1% (IQR 0%–20%), (2) tumor PD‐L1—med 6% (IQR 0%–21%), (3) HLA class I—med 100% (IQR 50%–100%), (4) CD8—med 78/hpf (IQR 40–193/hpf), and (5) PD‐1—med 17/hpf (IQR 4–35/hpf).
Figure 1.
Immune marker staining for two patients with high IDO1 expression. (A): H&E. (B): IDO1 with 3‐amino‐9‐ethylcarbazole (AEC) substrate. (C): PD‐L1 with 3,3' diaminobenzidine tetrahydrochloride (DAB) substrate and CD8 with AEC substrate. (D): PD‐1 with AEC substrate. (E): HC10 for HLA class I with AEC substrate. ×20 magnification shown.
Abbreviations: H&E, hematoxylin and eosin; HLA, human leukocyte antigen; IDO1, indoleamine 2,3 dioxygenase 1; PD‐1, programmed cell death protein 1; PD‐L1, programmed death‐ligand 1.
Figure 2.
Distribution of immune marker expression across anal squamous cell carcinoma tumors. (A): The number of immune cells expressing PD‐1 or CD8 per high‐power field. (B): The percentage of tumor cells expressing PD‐L1, IDO1, or HLA class I. For both A and B, the bar represents the mean with the graphical range representing the standard deviation. (C): Heatmap showing hierarchical clustering of the five examined immune markers with the four broad microenvironment subgroups demarcated in red.
Abbreviations: CD8, cluster of differentiation 8; HLA, human leukocyte antigen; hpf, high‐power field; IDO1, indoleamine 2,3 dioxygenase 1; PD‐1, programmed cell death protein 1; PD‐L1, programmed death‐ligand 1.
Fifty‐seven percent of patients (n = 36) had at least 1% IDO1+ tumor cells and 10% (n = 6) had a high level of staining, defined as >50% tumor cells being positive. Macrophages were identified by morphology and found to be IDO1+ in 52% of patients (n = 33, median IDO+ macrophages/hpf: 5.8 with IQR 0–21). Forty‐one percent of patients had >1% tumor cells that were PD‐L1 positive, and one patient had >50% PD‐L1‐positive tumor cells. Nineteen percent of patients had <50% of tumor cells stain positive for HLA class I, with five patients (8%) having no staining for class I HLA.
Hierarchical clustering of immune marker expression suggested four broad subgroups (Fig. 2C). One subgroup largely consisted of patients with high IDO1 expression and tended to have lower CD8 infiltrate. Correspondingly, the tumors with >50% IDO1 expression were significantly more likely to have the lowest quartile of CD8+ TIL (<40/hpf), with 67% (4/6) of IDO1‐high tumors having low CD8+ TIL compared with 21% (12/57) of IDO1‐low tumors having low CD8+ TIL (p = .024). A second subgroup consisted of patients with low HLA class I expression. Although CD8+ TIL levels in this subgroup varied, there was significant positive correlation between CD8+ TIL and PD‐1+ immune cells (R2 = 0.40, p = .0083). A third subgroup consisted of tumors with high PD‐L1 levels, with a subset of these tumors having high CD8+ TILs. Several patients in this subgroup also had IDO1+ tumors (although <50% positive). The fourth subgroup had a heterogeneous immune microenvironment including a subset with high immune cell PD‐1 and a subset with high CD8+ TIL. Overall, patients with higher CD8+ TIL tended to have higher PD‐1 (R2 = 0.47, p = .0006) as well as tumor PD‐L1 (R2 = 0.36, p = .04).
Association Between IHC Features and Clinical Outcomes
As shown in Table 2, among various patient and disease characteristics, only advanced T stage and lymph node positivity were associated with increased risk of death or recurrence on univariate analysis, consistent with prior published results [9]. T3–T4 (vs. T1–T2) disease was specifically associated with a significantly increased risk of any recurrence or LRR (hazard ratio [HR] 3.41, p = .036 and HR 5.56, p = .029, respectively) and showed a trend for association with worse OS (HR 2.58, p = .060). The presence of clinically positive lymph nodes was associated with worse OS (HR 3.35, p = .022) and showed a trend for increased risk of DM (HR 4.39, p = .065). These results are consistent with previously published prospective studies of ASCC. Sex, age, smoking status, and immunosuppression were not significantly associated with outcome.
Table 2. Univariate predictors of outcome.
Bold indicates p < .05 for clinical variables and p < .0125 for immune marker variables.
Abbreviations: CD8, cluster of differentiation 8; CI, confidence interval; DM, distant metastasis; HLA, human leukocyte antigen; hpf, high‐power field; HR, hazard ratio, ID01, indoleamine 2,3 dioxygenase 1; LN, lymph node; LRR, local‐regional recurrence; PD‐1, programmed cell death protein 1; PD‐L1, programmed death‐ligand 1.
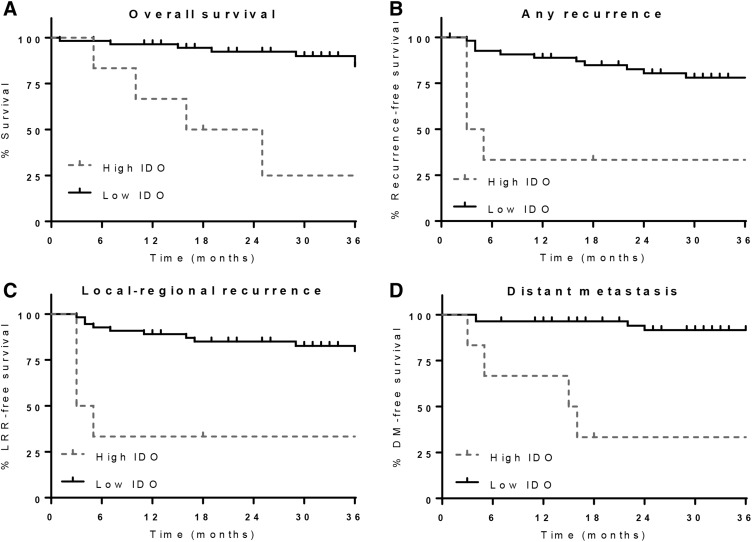
Associations between immune marker expression and outcomes are shown in Table 2. Patients with >50% IDO+ tumor cells had a significantly higher likelihood of poor outcomes, including increased risk of death (HR 4.7, 95% confidence interval [CI] 1.52–14.56, p = .0074), any recurrence (HR 6.38, 95% CI 2.02–20.09, p = .0016), LRR (HR 8.66, 95% CI 2.58–29.13, p = .0005), and DM (HR 12.66, 95% CI 3.26–49.2, p = .002). The 3‐year OS for patients with IDO1 ≤ 50% was 88% (95% CI 76%–95%) compared with a 3‐year OS of 25% (95% CI 1%–65%) for patients with IDO1 > 50% (Fig. 3). The association between IDO1 levels and clinical outcomes remained significant in a multivariate model that included all clinical parameters that either were significant or showed a trend toward significance on univariate examination (Table 3). Of note, no other immune marker was found to be significantly associated with any outcome, including the presence of IDO1+ macrophages. Similarly, when clinical outcomes across the four subgroups defined by hierarchical clustering were compared, the subgroup defined by high IDO1 continued to have significantly worse prognosis without a significant difference among the other subgroups (supplemental online Fig. 1).
Figure 3.
Outcomes for patients with ASCC by tumor cell IDO expression. Overall survival (A), recurrence‐free survival (B), local‐regional recurrence‐free survival (C), and distant metastasis‐free survival (D) for patients with ASCC with either high IDO1 expression (>50% tumor cells positive) or low IDO1 expression (≤50% tumor cells positive).
Abbreviations: DM, distant metastasis; IDO, indoleamine 2,3 dioxygenase; LRR, locoregional recurrence.
Table 3. Multivariate predictors of outcome.
Abbreviations: —, non‐significant; CI, confidence interval; DM, distant metastasis; HR, hazard ratio; IDO1, indoleamine 2,3 dioxygenase 1; LN, lymph node; LRR, local‐regional recurrence.
Discussion
The current study is the first, to our knowledge, to show that high IDO1 tumor cell expression is associated with poor outcomes for patients with ASCC. IDO1 expression in tumor cells was detectable by IHC in 57% of cases, and IDO1 expression in macrophages was present in 52% of cases; however, only tumor cell IDO1 expression was associated with outcomes. Among the 10% of patients with high (≥50%) tumor IDO1 expression, there was a 4.7‐fold increased risk of death and associated decrease in 3‐year OS from 88% to 25% (p = .0074). Correspondingly, there was a 6.4‐fold increased risk of any recurrence including an 8.7‐fold increased risk of LRR and 12.7‐fold increased risk of DM.
IDO1 is commonly expressed in tumors and has been shown to play a role in resisting immune rejection [24]. More recently, data from The Cancer Genome Atlas have revealed that IDO1 is expressed in a wide variety of malignancies [40]. Like ASCC, cervical cancer and head and neck squamous cell carcinoma are commonly associated with HPV infection and have a subset of cases with high IDO1 expression [40]. Several retrospective studies specifically examining cervical cancer outcomes have confirmed that patients with high tumor IDO1 expression by IHC or high kynurenine levels, indicating a high degree of tryptophan metabolism, are associated with poor outcomes including worse OS in one study and worse disease‐specific survival in a second study [32], [33].
The mechanism by which IDO1 produces poor clinical outcomes is incompletely understood but may be driven by the depletion of tryptophan and accumulation of kynurenine‐pathway metabolites in the tumor microenvironment. Several groups have shown that tryptophan depletion causes an accumulation of uncharged transfer RNA in T cells, which signals amino acid insufficiency and acts on downstream signaling pathways including activation of the stress response kinase GCN2. This kinase inhibits the translation initiation factor eIF2α and suppresses the mammalian target of rapamycin complex 1/PKCθ growth pathway [41], [42]. Ultimately, these signaling pathways prevent T‐cell activation and promote de novo regulatory T‐cell (Treg) differentiation while also enhancing existing Treg activity. Together, these effects act to create a profoundly immunosuppressive environment.
Several groups have found an association between TIL and outcome in ASCC that was not seen in the current study. Reasons for this discrepancy include differences in the underlying patient population, the methodology used to quantify T cells, and the cutoffs used to define patients with high versus low expression. Specifically, Gilbert et al. quantified lymphocytes on hematoxylin and eosin‐stained slides using low‐power magnification with a pathologist assessing the degree of infiltrate as low, medium, or high; Hu et al. quantified intratumoral CD8+ cells by IHC examining three high‐power fields and setting an average of >10 cells as high; and Grabenbauer et al. performed IHC for CD8 (as well as CD3 and CD4) and used image analysis software to quantify the number of tumor‐infiltrating cells per 100 tumor cells with >2.1 intratumoral CD8+ cells per 100 tumor cells considered high. These varying methods of TIL detection, quantification, and dichotomization into high‐ versus low‐expression groups differ as well from the approach used in the current study of quantifying intratumoral CD8+ hot spots. It may be that more comprehensive tumor evaluation rather than evaluating the area of maximum signal is a better proxy for total tumor TIL. However, it is not clear whether total tumor TIL versus area of maximal tumor TIL is a better marker of overall tumor‐immune recognition.
Several studies have examined the association between immune markers, such as PD‐1 and PD‐L1, and outcomes in ASCC. One study showed that tumors with high PD‐1 (in addition to high CD8+ TIL) had better local control and disease‐free survival, with high PD‐L1 specifically correlated with better local control [18]. However, a different group has shown presence of PD‐L1 on tumor cells to be inversely correlated with CD8+ TIL such that high PD‐L1 is negatively prognostic in terms of response to standard therapy [19]. Although our results did not show these markers to be individually prognostic, it is interesting that IDO1 was robustly associated with poor outcome and was associated with the lowest quartile of CD8+ TIL, which fits in the general paradigm of low TIL being negatively prognostic. Similarly, the current study also showed a statistically significant association between the number of CD8+ TIL and the number of PD‐1+ cells as well as the percentage of tumor cells that are PD‐L1+, which may suggest that a subset of ASCC tumors in our study represents the exhausted phenotype described by Balermpas et al., which can be activated by chemoradiation to help contribute to good outcomes [18].
Ultimately, in describing the tumor microenvironment, we hoped to gain insight into potential avenues for therapeutic intervention that may improve outcomes for the subset of patients who are not cured by upfront chemoradiation. A phase II study of nivolumab in metastatic ASCC showed a 24% response rate to anti‐PD‐1‐directed therapy, which serves as proof of principle that immunotherapy can have an important role in this disease [22]. Responders were found to have higher CD8, PD‐1, and PD‐L1 levels.
Given that our study has shown the dismal prognosis of patients with high tumor IDO1 levels, as well as the association of high IDO1 level with the lowest quartile of CD8+ TIL, it seems likely that anti‐PD‐1 therapy may not be sufficient in treating patients with ASCC with high IDO1. Rather, this study suggests that IDO1‐targeted therapies may have an important role in IDO1‐high ASCC, either in the metastatic setting or, given the very short time to disease recurrence in patients with IDO1‐high primary tumors, in the adjuvant setting after definitive chemoradiation. Future clinical studies will be needed to examine this question with prospective collection of tissue and/or blood before and after standard therapy and any potential immune targeted therapy.
Conclusion
ASCC, although near‐universally HPV related, is a disease with a diverse immune milieu. Although patients generally do well with standard therapy, those who have tumors with high IDO1 expression have significantly worse survival and increased risk of disease recurrence. Tumors with high IDO1 expression also tend to have the lowest quartile of CD8+ TIL, suggesting that high IDO1 expression can drive an immunosuppressive microenvironment, which may be responsible for the resulting poor prognosis. Collectively, this study suggests that IDO1 may serve as a prognostic indicator while also helping identify a patient population who could benefit from IDO‐targeted therapies.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
Funding for this study was provided by the American Cancer Society Institutional Review Grant (ACS‐IRG, awarded to J.Y.W.) and Rare Cancer Genetics Registry Grant (NIH 4R01CA160233‐05, Principal Investigator: Dianne Finkelstein).
Contributed equally.
Author Contributions
Conception/design: Devarati Mitra, Vikram Deshpande, Jennifer Y. Wo
Provision of study material or patients: Kent W. Mouw, Jason L. Hornick, Soldano Ferrone, Theodore S. Hong, Harvey Mamon, Jeffrey W. Clark, Aparna R. Parikh, Jill N. Allen, David P. Ryan, David T. Ting, Vikram Deshpande, Jennifer Y. Wo
Collection and/or assembly of data: Devarati Mitra, Nora Horick, Diane G. Brackett, Kent W. Mouw, Jason L. Hornick, Vikram Deshpande, Jennifer Y. Wo
Data analysis and interpretation: Devarati Mitra, Nora Horick, Vikram Deshpande, Jennifer Y. Wo
Manuscript writing: Devarati Mitra, Vikram Deshpande, Jennifer Y. Wo
Final approval of manuscript: Devarati Mitra, Nora Horick, Diane G. Brackett, Kent W. Mouw, Jason L. Hornick, Soldano Ferrone, Theodore S. Hong, Harvey Mamon, Jeffrey W. Clark, Aparna R. Parikh, Jill N. Allen, David P. Ryan, David T. Ting, Vikram Deshpande, Jennifer Y. Wo
Disclosures
Aparna R. Parikh: Puretech, Eisai (C/A); David T. Ting: Merrimack Pharmaceuticals, Ventana Roche, EMD Millipore Sigma (C/A), ACD Biotechne (RF), PanTher Therapeutics (OI); Vikram Deshpande: Agios (C/A), Agios, ACD Biotechne (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.de Martel C, Ferlay J, Franceschi S et al. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol 2012;13:607–615. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute . Surveillance, Epidemiology, and End Results Program U.S. Population Data – 1969–2016. Available at www.seer.cancer.gov/popdata. Accessed 2017.
- 3.Soeberg MJ, Rogers K, Currow DC et al. Trends in incidence and survival for anal cancer in New South Wales, Australia, 1972‐2009. Cancer Epidemiol 2015;39:842–847. [DOI] [PubMed] [Google Scholar]
- 4.Brewster DH, Bhatti LA. Increasing incidence of squamous cell carcinoma of the anus in Scotland, 1975‐2002. Br J Cancer 2006;95:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frisch M, Glimelius B, van den Brule AJ et al. Sexually transmitted infection as a cause of anal cancer. N Engl J Med 1997;337:1350–1358. [DOI] [PubMed] [Google Scholar]
- 6.Nigro ND, Seydel HG, Considine B et al. Combined preoperative radiation and chemotherapy for squamous cell carcinoma of the anal canal. Cancer 1983;51:1826–1829. [DOI] [PubMed] [Google Scholar]
- 7.James RD, Glynne‐Jones R, Meadows HM et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous‐cell carcinoma of the anus (ACT II): A randomised, phase 3, open‐label, 2 × 2 factorial trial. Lancet Oncol 2013;14:516–524. [DOI] [PubMed] [Google Scholar]
- 8.Gunderson LL, Winter KA, Ajani JA et al. Long‐term update of US GI intergroup RTOG 98‐11 phase III trial for anal carcinoma: Survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol 2012;30:4344–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunderson LL, Moughan J, Ajani JA et al. Anal carcinoma: Impact of TN category of disease on survival, disease relapse, and colostomy failure in US Gastrointestinal Intergroup RTOG 98‐11 phase 3 trial. Int J Radiat Oncol Biol Phys 2013;87:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meulendijks D, Tomasoa NB, Dewit L et al. HPV‐negative squamous cell carcinoma of the anal canal is unresponsive to standard treatment and frequently carries disruptive mutations in TP53. Br J Cancer 2015;112:1358–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koerber SA, Schoneweg C, Slynko A et al. Influence of human papillomavirus and p16(INK4a) on treatment outcome of patients with anal cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol 2014;113:331–336. [DOI] [PubMed] [Google Scholar]
- 12.Serup‐Hansen E, Linnemann D, Skovrider‐Ruminski W et al. Human papillomavirus genotyping and p16 expression as prognostic factors for patients with American Joint Committee on Cancer stages I to III carcinoma of the anal canal. J Clin Oncol 2014;32:1812–1817. [DOI] [PubMed] [Google Scholar]
- 13.Mouw KW, Cleary JM, Reardon B et al. Genomic evolution after chemoradiotherapy in anal squamous cell carcinoma. Clin Cancer Res 2017;23:3214–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grabenbauer GG, Lahmer G, Distel L et al. Tumor‐infiltrating cytotoxic T cells but not regulatory T cells predict outcome in anal squamous cell carcinoma. Clin Cancer Res 2006;12:3355–3360. [DOI] [PubMed] [Google Scholar]
- 15.Hu WH, Miyai K, Cajas‐Monson LC et al. Tumor‐infiltrating CD8(+) T lymphocytes associated with clinical outcome in anal squamous cell carcinoma. J Surg Oncol 2015;112:421–426. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert DC, Serup‐Hansen E, Linnemann D et al. Tumour‐infiltrating lymphocyte scores effectively stratify outcomes over and above p16 post chemo‐radiotherapy in anal cancer. Br J Cancer 2016;114:134–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gooden MJ, de Bock GH, Leffers N et al. The prognostic influence of tumour‐infiltrating lymphocytes in cancer: A systematic review with meta‐analysis. Br J Cancer 2011;105:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balermpas P, Martin D, Wieland U et al. Human papilloma virus load and PD‐1/PD‐L1, CD8+ and FOXP3 in anal cancer patients treated with chemoradiotherapy: Rationale for immunotherapy. Oncoimmunology 2017;6:e1288331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao YJ, Sun WP, Peng JH, et al. Programmed death‐ligand 1 expression correlates with diminished CD8+ T cell infiltration and predicts poor prognosis in anal squamous cell carcinoma patients. Cancer Manag Res 2018;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferris RL, Blumenschein G, Fayette J et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frenel JS, Le Tourneau C, O'Neil B et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1–positive cervical cancer: Results from the phase Ib KEYNOTE‐028 trial. J Clin Oncol 2017;35:4035–4041. [DOI] [PubMed] [Google Scholar]
- 22.Morris VK, Salem ME, Nimeiri H et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): A multicentre, single‐arm, phase 2 study. Lancet Oncol 2017;18:446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maleki Vareki S, Garrigós C, Duran I. Biomarkers of response to PD‐1/PD‐L1 inhibition. Crit Rev Oncol Hematol 2017;116:116–124. [DOI] [PubMed] [Google Scholar]
- 24.Uyttenhove C, Pilotte L, Théate I et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3‐dioxygenase. Nat Med 2003;9:1269–1274. [DOI] [PubMed] [Google Scholar]
- 25.Prendergast GC. Immune escape as a fundamental trait of cancer: Focus on IDO. Oncogene 2008;27:3889–3900. [DOI] [PubMed] [Google Scholar]
- 26.Chung DJ, Rossi M, Romano E et al. Indoleamine 2,3‐dioxygenase‐expressing mature human monocyte‐derived dendritic cells expand potent autologous regulatory T cells. Blood 2009;114:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon YW, Hajjar J, Hwu P et al. Targeting the indoleamine 2,3‐dioxygenase pathway in cancer. J Immunother Cancer 2015;3:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ino K, Yoshida N, Kajiyama H et al. Indoleamine 2,3‐dioxygenase is a novel prognostic indicator for endometrial cancer. Br J Cancer 2006;95:1555–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ino K, Yamamoto E, Shibata K et al. Inverse correlation between tumoral indoleamine 2,3‐dioxygenase expression and tumor‐infiltrating lymphocytes in endometrial cancer: Its association with disease progression and survival. Clin Cancer Res 2008;14:2310–2317. [DOI] [PubMed] [Google Scholar]
- 30.Choe JY, Yun JY, Jeon YK et al. Indoleamine 2,3‐dioxygenase (IDO) is frequently expressed in stromal cells of Hodgkin lymphoma and is associated with adverse clinical features: A retrospective cohort study. BMC Cancer 2014;14:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandacher G, Perathoner A, Ladurner R et al. Prognostic value of indoleamine 2,3‐dioxygenase expression in colorectal cancer: Effect on tumor‐infiltrating T cells. Clin Cancer Res 2006;12:1144–1151. [DOI] [PubMed] [Google Scholar]
- 32.Inaba T, Ino K, Kajiyama H et al. Indoleamine 2,3‐dioxygenase expression predicts impaired survival of invasive cervical cancer patients treated with radical hysterectomy. Gynecol Oncol 2010;117:423–428. [DOI] [PubMed] [Google Scholar]
- 33.Ferns DM, Kema IP, Buist MR et al. Indoleamine‐2,3‐dioxygenase (IDO) metabolic activity is detrimental for cervical cancer patient survival. Oncoimmunology 2015;4:e981457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu H, Shen Z, Wang Z et al. Increased expression of IDO associates with poor postoperative clinical outcome of patients with gastric adenocarcinoma. Sci Rep 2016;6:21319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye J, Liu H, Hu Y et al. Tumoral indoleamine 2,3‐dioxygenase expression predicts poor outcome in laryngeal squamous cell carcinoma. Virchows Arch 2013;462:73–81. [DOI] [PubMed] [Google Scholar]
- 36.Yu J, Du W, Yan F et al. Myeloid‐derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol 2013;190:3783–3797. [DOI] [PubMed] [Google Scholar]
- 37.Sernee MF, Ploegh HL, Schust DJ. Why certain antibodies cross‐react with HLA‐A and HLA‐G: Epitope mapping of two common MHC class I reagents. Mol Immunol 1998;35:177–188. [DOI] [PubMed] [Google Scholar]
- 38.Perosa F, Luccarelli G, Prete M et al. Beta 2‐microglobulin‐free HLA class I heavy chain epitope mimicry by monoclonal antibody HC‐10‐specific peptide. J Immunol 2003;171 :1918–1926. [DOI] [PubMed] [Google Scholar]
- 39.Kachnic LA, Winter KA, Myerson RJ et al. Two‐year outcomes of RTOG 0529: A phase II evaluation of dose‐painted IMRT in combination with 5‐fluorouracil and mitomycin‐C for the reduction of acute morbidity in carcinoma of the anal canal. J Clin Oncol 2011;29(suppl 4):368a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Baren N, Wan den Eynde BJ. Tryptophan‐degrading enzymes in tumoral immune resistance. Front Immunol 2015;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metz R, Rust S, Duhadaway JB et al. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: A novel IDO effector pathway targeted by D‐1‐methyl‐tryptophan. Oncoimmunology 2012;1:1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munn DH, Sharma MD, Baban B et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3‐dioxygenase. Immunity 2005;22:633–642. [DOI] [PubMed] [Google Scholar]