This article reports on treatment of advanced cancer with anti‐PD1 antibodies, focusing on patients with pre‐existing autoimmune disorders.
Keywords: Anti‐programmed death‐1, Sex, Autoimmune disease, Immunotherapy, Performance status, Immune checkpoint inhibitors
Abstract
Background.
Patients with a history of autoimmune diseases (AIDs) have not usually been included in clinical trials with immune checkpoint inhibitors.
Materials and Methods.
Consecutive patients with advanced cancer, treated with anti‐programmed death‐1 (PD‐1) agents, were evaluated according to the presence of pre‐existing AIDs. The incidence of immune‐related adverse events (irAEs) and clinical outcomes were compared among subgroups.
Results.
A total of 751 patients were enrolled; median age was 69 years. Primary tumors were as follows: non‐small cell lung cancer, 492 (65.5%); melanoma, 159 (21.2%); kidney cancer, 94 (12.5%); and others, 6 (0.8%). Male/female ratio was 499/252. Eighty‐five patients (11.3%) had pre‐existing AIDs, further differentiated in clinically active (17.6%) and inactive (82.4%). Among patients with pre‐existing AIDs, incidence of irAEs of any grade was significantly higher when compared with patients without AIDs (65.9% vs. 39.9%). At multivariate analysis, both inactive (p = .0005) and active pre‐existing AIDs (p = .0162), female sex (p = .0004), and Eastern Cooperative Oncology Group Performance Status <2 (p = .0030) were significantly related to a higher incidence of irAEs of any grade. No significant differences were observed regarding grade 3/4 irAEs and objective response rate among subgroups. Pre‐existing AIDs were not significantly related with progression‐free survival and overall survival.
Conclusion.
This study quantifies the increased risk of developing irAEs in patients with pre‐existing AIDs who had to be treated with anti‐PD‐1 immunotherapy. Nevertheless, the incidence of grade 3/4 irAEs is not significantly higher when compared with control population. The finding of a greater incidence of irAEs among female patients ranks among the “hot topics” in gender‐related differences in immuno‐oncology.
Implications for Practice.
Patients with a history of autoimmune diseases (AIDs) have not usually been included in clinical trials with immune checkpoint inhibitors but are frequent in clinical practice. This study quantifies the increased risk of developing immune‐related adverse events (irAEs) in patients with pre‐existing AIDs who had to be treated with anti‐programmed death‐1 immunotherapy. Nevertheless, their toxicities are mild and the incidence of grade 3/4 irAEs is not significantly higher compared with those of controls. These results will help clinicians in everyday practice, improving their ability to offer a proper counselling to patients, in order to offer an immunotherapy treatment even to patients with pre‐existing autoimmune disease.
Introduction
Patients with a history of autoimmune diseases have not usually been enrolled in clinical trials with immune checkpoint inhibitors (ICIs). The advent of these treatments radically changed the cure of the majority of patients with advanced cancer. That implies that an increasing number of patients with a history of immune disorders might need to be treated with these agents, and oncologists will be called to manage the risks related to treatment choice. Furthermore, is it well known that autoimmune diseases themselves can increase the risk of developing several types of malignancies [1].
The presence of a previous (or concomitant) autoimmune disease (AID) is considered the most important risk factor for developing immune‐related adverse events (irAEs), something that oncologists must identify before starting the treatment [2]. In this case, proper management, early diagnosis, and careful pre‐ and post‐treatment monitoring are strongly recommended. Nevertheless, there is no literature evidence that helps quantify the risks of worsening/recurrence of the pre‐existing AID and of developing irAEs overall.
Many case reports have been published, but very few studies have investigated the topic with a large sample size. The first study enrolled 30 patients with advanced melanoma with AIDs treated with ipilimumab; 50% of them experienced no toxicity, whereas 33% experienced grade (G) 3 to G5 irAEs [3]. Menzies et al. investigated the safety of anti‐programmed death‐1 (PD‐1) therapy in patients with advanced melanoma and pre‐existing AIDs or major toxicity with ipilimumab; in the cohort with pre‐existing AIDs (52 patients), 71% experienced no toxicity, 10% experienced G3 irAEs, and no G4/G5 events were observed [4]. A systematic literature review collected data of 49 publications: 75% of the 123 patients analyzed experienced an exacerbation of underlying AIDs or irAEs, but prevalently managed without discontinuing therapy [5]. A retrospective study by Leonardi et al. reported 38% of irAEs (74% G1 and G2) and 23% of flares in a cohort of patients with non‐small cell lung cancer (NSCLC) with autoimmune disease treated with anti‐PD‐1/programmed death‐1 ligand (PD‐L1) agents, concluding that the incidence of irAEs was similar to what had been reported in clinical trials [6].
A recent article by Danlos et al. reported the subgroup analysis of 45 patients with advanced cancer with pre‐existing AIDs, previously enrolled in a prospective study with anti‐PD‐1 agents [7]; 44.4% of the patients with pre‐existing AIDs experienced irAEs, with a median grade 2, even though they did not perform a differentiated analysis for G3/G4 irAEs. They also reported a “flare rate” of 24.4% and a discontinuation rate due to irAEs of 11.1%. They found a greater incidence of irAEs among patients with pre‐existing AIDs when compared with patients without (44.4% vs. 29%), a shorter irAE‐free interval time (5.4 months vs. 13 months), and no significant differences in clinical outcomes between the two populations.
We planned a “real‐world” retrospective, multicenter observational study of patients with advanced cancer treated with anti‐PD‐1 agents, performing comparative safety and efficacy analyses according to the history of pre‐existing AIDs.
Materials and Methods
Patient Eligibility
This study evaluated patients with advanced cancer with histologically confirmed diagnosis of measurable stage IV cancer, who consecutively underwent treatment with single‐agent anti‐PD‐1, regardless of the treatment line, at the medical oncology departments of 15 Italian centers (supplemental online Table 1), from September 2013 to May 2018.
All patients provided written, informed consent to treatment with immunotherapy. All patients alive at the moment of the data collection provided an informed consent to participate. The procedures followed were in accordance with the precepts of Good Clinical Practice and the declaration of Helsinki. The study was approved by the local responsible committees on human experimentation (University of L'Aquila, Internal Review Board protocol number 32865, approved on July 24, 2018).
Study Design
A multicenter, retrospective observational study of consecutive patients with advanced cancer treated with anti‐PD‐1 (standard doses and schedules) agents, regardless of treatment line, was performed. The aims of the study were to evaluate the incidence of AIDs among patients with cancer undergoing immunotherapy with anti‐PD‐1, and to compare the incidence of irAEs between patients with and without pre‐existing AIDs (inactive and active). The secondary objective of the study was to evaluate the correlation between pre‐existing AIDs and clinical outcomes: objective response rate (ORR), median progression free survival (PFS), and median overall survival (OS).
The following covariates were analyzed: primary tumor (NSCLC, melanoma, kidney, and others), age (<70 vs. ≥70 years) [8], [9], [10], [11], sex (male vs. female), Eastern Cooperative Oncology Group Performance Status (ECOG‐PS; 0–1 vs. ≥2), burden of disease (number of metastatic sites ≤2 vs. >2), and treatment line (first vs. nonfirst).
Responses were evaluated with RECIST criteria (version 1.1), according to the local clinical practice of the participating centers and to the respective investigators’ evaluation [12]. χ2 and Fisher's exact test were used to evaluate ORRs, the incidence of irAEs and the discontinuation rates due to adverse events among subgroups [13], [14], using the appropriate test according to the sample size in contingency tables for each comparison. Odds ratios (ORs) with 95% confidence intervals (95% CIs) were used to estimate the association between the incidence of irAEs and covariates [15]. In the multivariate analysis, logistic regression was used to evaluate the parameters that were significant at the univariate safety analysis [16] (given its role, primary tumor was included in multivariate analysis regardless of its significance at univariate analysis). Median PFS, median OS, and median time to G3/G4 irAEs were evaluated using the Kaplan‐Meier method [17]. Median follow‐up was calculated according to the reverse Kaplan‐Meier method [18]. Cox proportional hazards model [19] was used to evaluate predictor variables in univariate and multivariate analysis for median PFS and median OS. The data cutoff period was August 2018. All statistical analyses were performed using MedCalc Statistical Software version 18.6 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2018).
Pre‐Existing Autoimmune Diseases and Immune‐Related Adverse Events Assessment
Personal history of pre‐existing AIDs was investigated in every patient. Any condition arising from an abnormal pathologic immune self‐response was considered [20] and categorized on the basis of the organ/system involved as follows: thyroid disorders, dermatologic disorders, rheumatologic disorders, gastrointestinal and hepatic disorders, neurologic disorders, and nephrologic disorders. Patients with a history of multiple pre‐existing AIDs were considered separately. In order to distinguish between patients with an active aberrant immune response at the moment of immunotherapy with anti‐PD‐1, we also categorized pre‐existing AIDs into clinically active and inactive, on the basis of the use of a concomitant disease‐oriented treatment (corticosteroids or other immunosuppressants) at the time of commencement of anti‐PD‐1 treatment. Patients were clinically monitored for safety evaluation at every preadministration visit (according to the technical files of the drugs), and as clinically indicated by the investigators subsequently.
Immune‐related AEs were defined as those AEs having an immunological basis. They were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE; version 4.0) and cumulatively reported as crude incidence. Immune‐related AEs were categorized on the basis of the organ/system involved as follows: endocrine irAEs (including thyroid disorders), skin irAEs, hepatic irAEs, gastrointestinal irAEs (excluding pancreatitis), pneumological irAEs, rheumatologic irAEs, and other irAEs (including neuromuscular, pancreatitis, fever, asthenia, and anorexia). Immune‐related AEs were defined as “single‐site” if the patient experienced just one category of irAEs as mentioned above; we defined “multiple‐site” irAEs as those occurring in patients who experienced irAEs of different categories. Time to G3/G4 irAE was defined as the length of time from the commencement of the immunotherapy to the development of the G3/G4 irAE; median time to G3/G4 irAE was computed only for patients who developed a G3/G4 irAE. In the case of worsening of underlying AIDs, the irAE constituted a flare; flares were graded in absolute terms according to CTCAE, regardless of previous clinical expression.
Results
Patients’ Characteristics
Seven hundred fifty‐one consecutive patients with stage IV cancer were enrolled. The initiation dates of the anti‐PD‐1 treatment ranged from September 2013 to April 2018. The patients’ features are summarized in Table 1. The median age was 69 years (range: 24–92), and the male/female ratio was 499/252. Primary tumors were as follows: NSCLC, 492 patients 65.5%); melanoma, 159 patients (21.2%); renal cell carcinoma, 94 patients (12.5%); and others, 6 patients (0.8%). One hundred eighty‐two (24.2%) patients were treated with pembrolizumab, 569 (75.8%) with nivolumab. Anti‐PD‐1 were administered as first‐line treatment in 175 patients (23.3%). Of 159 patients with melanoma, 31 received prior ipilimumab; just 1 of them developed a significant toxicity (hypopituitarism), which was not considered among pre‐existing AIDs. Eighty‐five patients (11.3%) had a personal history of pre‐existing AIDs: 48 (56.5%) were male (9.6% of the males overall) and 37 (43.5%) were female (14.7% of females overall). Among patients with pre‐existing AIDs, 70 (82.4%) had an inactive AID, whereas 15 (17.6%) had an active AID. All the AIDs with the relative medications are reported in Table 2.
Table 1. Patients’ characteristics.
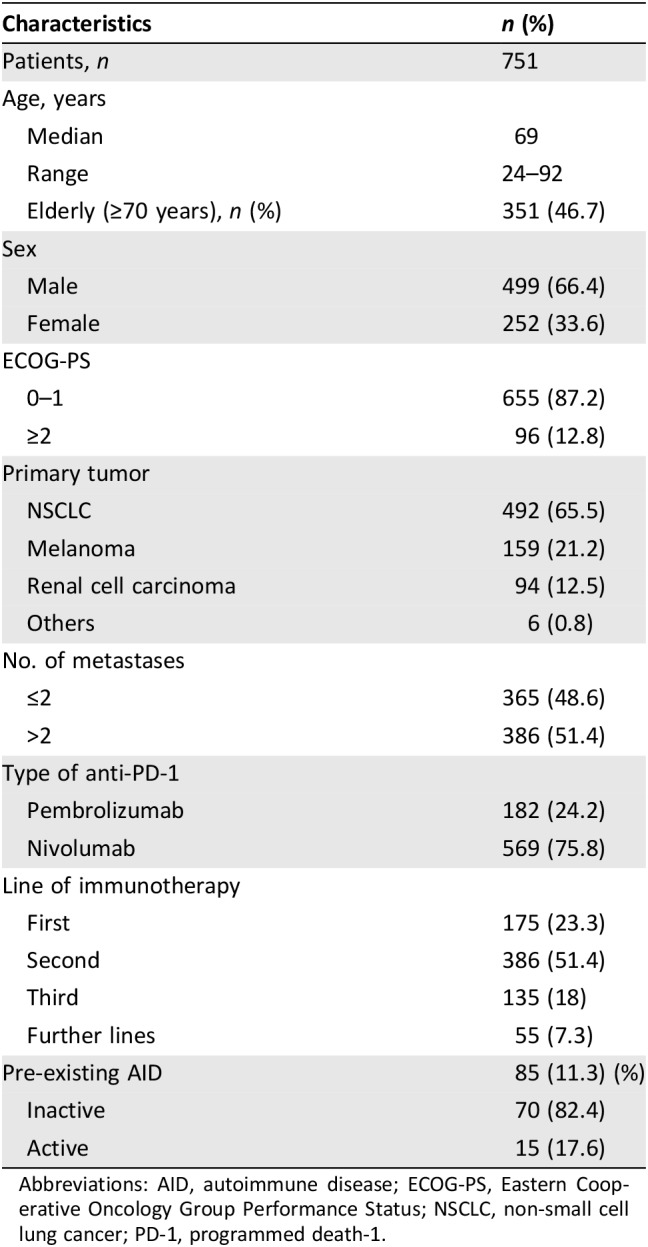
Abbreviations: AID, autoimmune disease; ECOG‐PS, Eastern Cooperative Oncology Group Performance Status; NSCLC, non‐small cell lung cancer; PD‐1, programmed death‐1.
Table 2. List of pre‐existing autoimmune disease and immunosuppressant treatments.
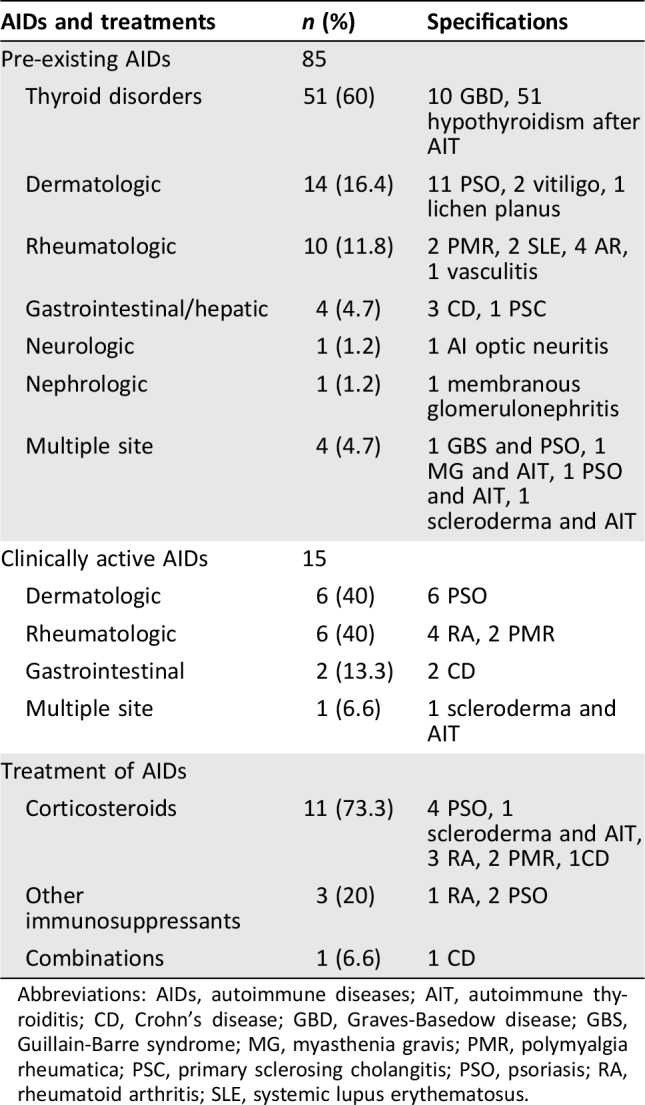
Abbreviations: AIDs, autoimmune diseases; AIT, autoimmune thyroiditis; CD, Crohn's disease; GBD, Graves‐Basedow disease; GBS, Guillain‐Barre syndrome; MG, myasthenia gravis; PMR, polymyalgia rheumatica; PSC, primary sclerosing cholangitis; PSO, psoriasis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
Safety Analysis
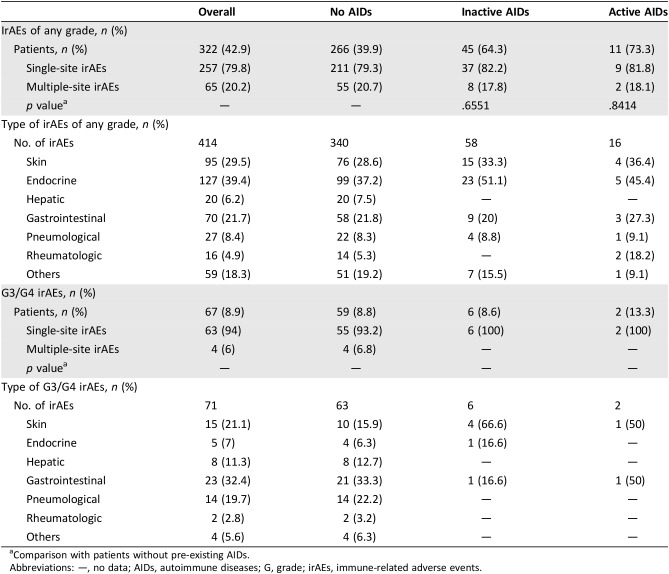
Table 3 reports the rates with confidence intervals of irAEs of any grade, G3/G4 irAEs, and flares of pre‐existing AIDs, among all the patients’ categories. The incidences of irAEs of any grade and G3/G4 irAEs in the overall population were 42.9% and 8.9%, respectively. Interestingly, the incidences of G3/G4 irAEs correspond to the flare rates in patients with both pre‐existing inactive and active AIDs.
Table 3. Immune‐related adverse events of any grade and G3/G4 immune‐related adverse events by subgroups.
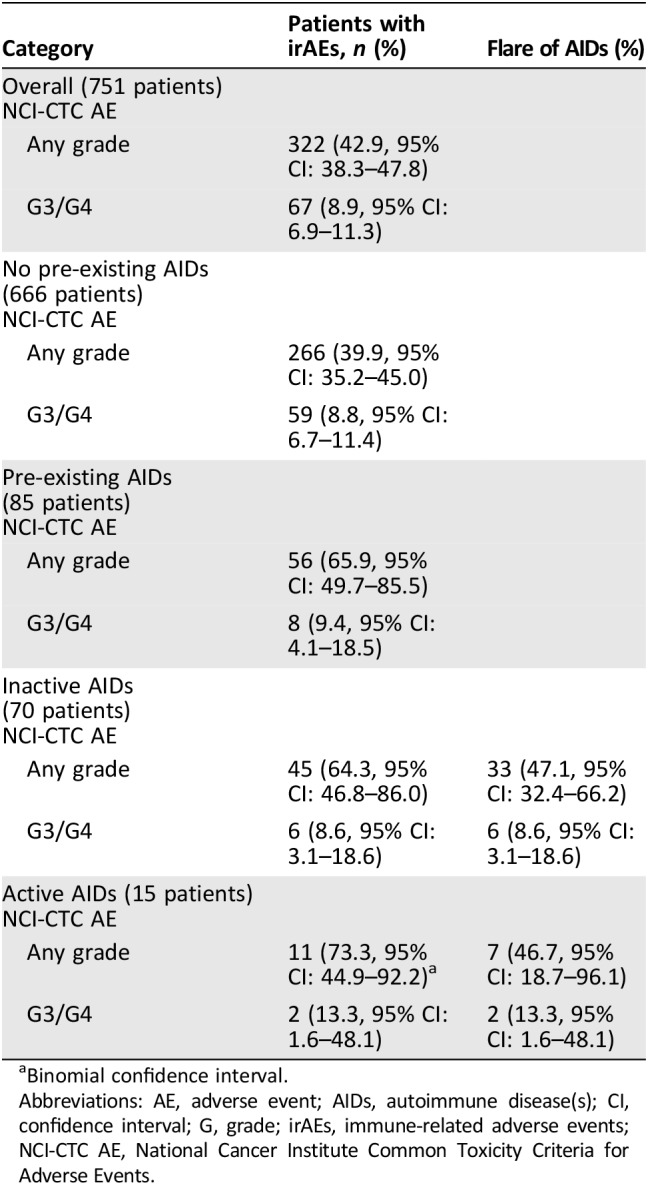
Binomial confidence interval.
Abbreviations: AE, adverse event; AIDs, autoimmune disease(s); CI, confidence interval; G, grade; irAEs, immune‐related adverse events; NCI‐CTC AE, National Cancer Institute Common Toxicity Criteria for Adverse Events.
The complete “per category” list of adverse events by subgroup is provided in Table 4; the majority of patients experienced single‐site instead of multiple‐site irAEs in all the subgroups, without significant differences in patient with pre‐existing AIDs (either active and inactive) when compared with patients without pre‐existing AIDs.
Table 4. “Per category” analysis of immune‐related adverse events of any grade and G3/G4 immune‐related adverse events among the subgroups.
Comparison with patients without pre‐existing AIDs.
Abbreviations: —, no data; AIDs, autoimmune diseases; G, grade; irAEs, immune‐related adverse events.
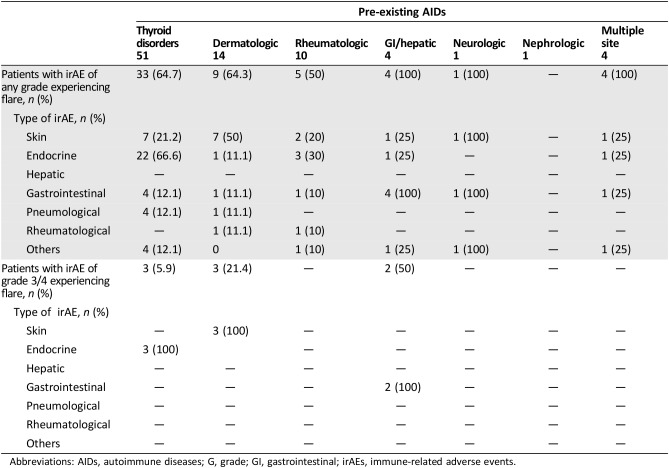
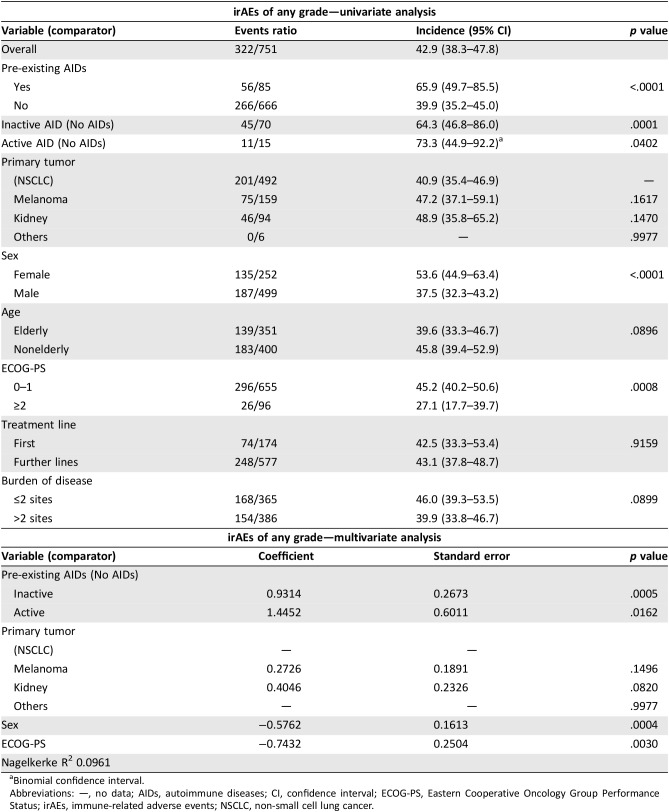
Among the 85 patients with pre‐existing AIDs, 56 (65.9%) and 8 (9.4%) experienced irAEs of any grade and G3/G4 irAEs, respectively, and 40 out of 56 (47.1%) developed a flare of the underlying AIDs. Table 5 outlines in detail the irAEs of any grade and G3/G4 irAEs among these patients; interestingly, the flare (of any grade) rates were 66.6% in patients with pre‐existing thyroid disorders, 50% in patients with pre‐existing dermatologic disorders, 10% in patients with pre‐existing rheumatologic disorders, and 100% in patients with pre‐existing gastrointestinal/hepatic disorders. As previously stated, all the G3/G4 flares in this population correspond exactly to G3/G4 irAEs events. Table 6 reports the univariate and multivariate analyses of irAEs of any grade; considering together patients with a personal history of active and inactive pre‐existing AIDs, the incidence of irAEs of any grade was significantly higher when compared with patients without AIDs (65.9% vs. 39.9%, p < .0001). Similarly, irAEs of any grade are more frequent in both subgroups of patients with pre‐existing inactive and active AIDs when separately compared with patients without AIDs. Female gender and ECOG‐PS 0–1 were also shown to be significantly related to a greater incidence of irAEs at univariate analysis. At multivariate analysis, pre‐existing AIDs (both inactive and active), female sex, and ECOG‐PS 0‐1 were all significantly related to greater incidence of irAEs of any grade.
Table 5. “Per category” analysis of immune‐related adverse events of any grade and G3/G4 immune‐related adverse events according to the pre‐existing AIDs.
Abbreviations: AIDs, autoimmune diseases; G, grade; GI, gastrointestinal; irAEs, immune‐related adverse events.
Table 6. Univariate and multivariate analyses of immune‐related adverse events of any grade.
Binomial confidence interval.
Abbreviations: —, no data; AIDs, autoimmune diseases; CI, confidence interval; ECOG‐PS, Eastern Cooperative Oncology Group Performance Status; irAEs, immune‐related adverse events; NSCLC, non‐small cell lung cancer.
Among patients with pre‐existing AIDs, the incidence of G3/G4 irAEs was 9.4%, whereas it was 8.8% in those without (p = .8663). Similarly, no statistically significant differences were observed between patients with previous inactive (p = .9638) and active (p = .5487) pre‐existing AIDs when separately compared with patients without.
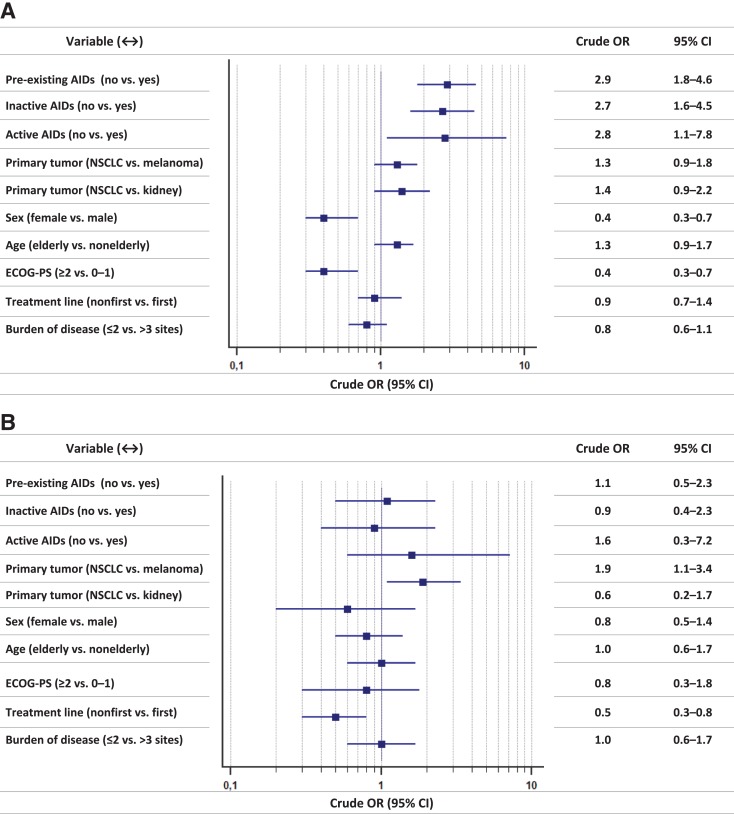
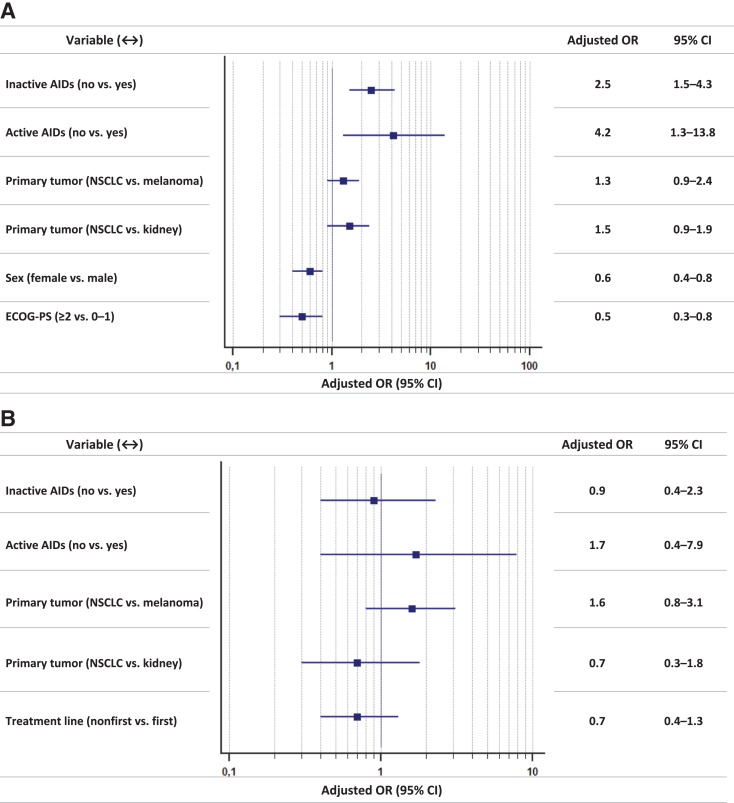
Figure 1 reports the forest plot graph, with crude ORs, of univariate analysis of irAEs of any grade and G3/G4 irAEs, whereas Figure 2 reports the forest plot graph, with adjusted ORs, of multivariate analysis of irAEs of any grade and G3/G4 irAEs.
Figure 1.
Univariate analyses. (A): Immune‐related adverse events of any grade: forest‐plot graph with crude odds ratios. (B): Grade 3/4 immune‐related adverse events: forest‐plot graph with crude odds ratios.
Abbreviations: AIDs, autoimmune diseases; CI, confidence interval; ECOG‐PS, Eastern Cooperative Oncology Group Performance Status; NSCLC, non‐small cell lung cancer; OR, odds ratio.
Figure 2.
Multivariate analyses. (A): Immune‐related adverse events of any grade: forest‐plot graph with adjusted odds ratios. (B): Grade 3/4 immune‐related adverse events: forest‐plot graph with adjusted odds ratios.
Abbreviations: AIDs, autoimmune diseases; CI, confidence interval; ECOG‐PS, Eastern Cooperative Oncology Group Performance Status; NSCLC, non‐small cell lung cancer; OR, odds ratio.
Median times to G3/G4 irAE were 3.6 months (95% CI: 2.2–4.8) in both overall population and patients without pre‐existing AIDs. Among patients with pre‐existing inactive AIDs, median time to G3/G4 irAEs was 1.9 months (95% CI: 0.7–12.2), whereas that among patients with pre‐existing active AIDs was 3.5 months (95% CI: 3.5–3.7). No statistically significant differences were found (data not shown).
Overall, 54 (7.1%) patients discontinued the treatment because of irAEs. The discontinuation rate among patients with pre‐existing AIDs was 7% (6 out of 85 patients), whereas it was 7.2% among patients without (48 out of 666 patients). Among patients with pre‐existing inactive and active AIDs, the discontinuation rates were 5.7% (four patients) and 13.3% (two patients), respectively, without statistically significant differences when compared with patients without (p = .8086 and p = .3028).
Activity and Efficacy
Among the 701 patients evaluable for activity, 251 responses of disease were observed, and ORR was 38.5% (95% CI: 31.5–40.5). Among the 624 evaluable patients without pre‐existing AIDs, ORR was 35.3% (95% CI: 30.7–40.2; 220 responses of disease). Among the 63 evaluable patients with pre‐existing inactive AIDs, ORR was 38.1% (95% CI: 24.4–56.6; 24 responses of disease), whereas among the 14 evaluable patients with pre‐existing active AIDs, ORR was 50% (95% CI: 23.0–76.9; 7 responses of disease). No significant differences were observed in ORR between patients with and without pre‐existing AIDs (p = .3170), or between patients without pre‐existing AIDs and those with inactive (p = .6539) and active ones (p = 0.2548), when separately compared.
All patients were evaluable for efficacy; after a median follow up of 14.7 months, median PFS was 8.2 months (95% CI: 6.6–9.3; 432 events) and median OS was 16.2 months (95% CI: 14.1–20.0; 329 events).
Median PFS of patients without pre‐existing AIDs was 8.0 months (95% CI: 6.6–9.1; 382 events), whereas median PFS of patients with pre‐existing inactive and active AIDs were 14.4 months (95% CI: 5.3–17.1; 41 events) and 6.8 months (95% CI: 5.1–9.4; 9 events), respectively. Pre‐existing AIDs (neither inactive nor active) were not significantly related to PFS, whereas primary tumor (melanoma), first‐line treatment, low burden of disease, and ECOG‐PS 0–1 were confirmed as independent predictors for a longer PFS at multivariate analysis (supplemental online Table 2).
With the limit of immature data, the median OS of patients without pre‐existing AIDs was 16.5 months (95% CI: 14.1–20.9; 379 censored), whereas the median OS of patients with pre‐existing inactive and active AIDs were 15.7 months (95% CI: 10.3–24.3; 37 censored) and 9.8 months (95% CI: 5.8–24.6; 6 censored), respectively. At univariate analysis, primary tumor (melanoma and kidney), female gender, first‐line treatment, low burden of disease, and ECOG‐PS 0–1 were significantly related to a longer OS. All but treatment line were confirmed as independent predictors for OS at multivariate analysis (supplemental online Table 3). Supplemental online Figure 1 reports the Kaplan‐Meier survival curves of PFS and OS.
Discussion
As previously mentioned, auto‐immunity and cancer are closely linked; there is surely a direct proportionality between chronic inflammation and cancer [1], but it is a “two‐way” relationship. Indeed, although AIDs lead to an increased risk of developing cancer, cancer itself is a condition that increases the risk of developing AIDs [21]. We focused our attention on patients with pre‐existing AIDs, finding an overall incidence of 11.3% among our “real‐life” population. These results are even lower than those observed before, in a cohort of patients with NSCLC [22], but are not negligible in the era of ICIs. In our cohort of patients with cancer, AIDs were more frequent among female patients (14.7%) than they were among male patients (9.6%).
The overall incidence of irAEs of any grade and G3/G4 irAEs were 42.9% and 8.9%, respectively; these rates are halfway between those reported in clinical trials [23], [24] and in real‐life studies with anti‐PD‐1 [25], [26].
Looking at Table 4, it is noticeable that overall, the number of patients who experienced irAEs was less than the crude number of irAEs (322 vs. 414), and the same trend was found among all the categories of irAEs. Moreover, single‐site irAEs were more frequent than multiple‐site irAEs among all the subgroups, without differences according to pre‐existing AIDs. Immune‐related AEs typically involve single organs/system, as the underlying pathological mechanism tends to be tissue specific; despite that, approximately 20% of patients experienced irAEs in multiple systems/organs.
In our study, the incidence of irAEs of any grade and G3/G4 irAEs among patients with pre‐existing AIDs were 65.9% and 9.4%, respectively. The rates of irAEs of any grade seem worse than those observed by Menzies et al. (29%), Leonardi et al. (38%), and Danlos et al. (44.4%), although G3/G4 events are quite comparable (10% of G3, and 10.7%, respectively—not reported by Danlos et al.) [4], [6], [7]. Forty patients (47.1%) among those with pre‐existing AIDs developed a flare of the underlying disease; similarly, in the studies of Leonardi et al. (23%) and Danlos et al. (24.4%), the flare rates were lower than the incidence of irAEs overall, whereas in the study of Menzies et al., it was higher (38%) than the “conventional” irAEs rate.
Danlos et al. have already clarified that patients with pre‐existing AIDs had an increased risk of developing irAEs of any grade [7]. In their experience, the median CTCAE grade was 2; they also reported a shorter irAE‐free interval in this population when compared with the control group. In our analysis, the adjusted ORs testify that the risk of developing irAEs of any grade was 2.5‐ and 4.2‐fold higher for patients with inactive and active AIDs, respectively, when compared with the control group. On the other hand, even if patients with pre‐existing AIDs have such a significant increased risk of developing irAEs overall, they are not exposed to an increased significant risk of developing serious adverse events (G3/4); furthermore, we found no significant differences among subgroups regarding discontinuation rates due to irAEs and time to G3/G4 irAEs. Nevertheless, our sample size of only 15 patients with pre‐existing active AIDs reminds us to interpret with caution the results.
The systems/organs of the underlying pre‐existing AIDs surely have the highest risk to be involved in developing irAEs. Regardless of statistical significance, looking at Table 5, we noticed that 66.6% of patients with pre‐existing thyroid disorders, 50% of patients with pre‐existing dermatologic disorders, and 100% of patients with pre‐existing gastrointestinal/hepatic disorders, respectively, experienced flares of any grade. It is noteworthy that G3/G4 irAEs in patients with both inactive and active pre‐existing AIDs were all flares of the underlying AIDs.
We can confirm that in patients with pre‐existing AIDs, particular attention must be focused on the spectrum of the underlying AIDs, which was involved in all the serious irAEs in our study. This clarifies that, before starting the treatment, a multidisciplinary assessment with organ‐specific consultants is fundamental. In the case of a history of pre‐existing AIDs, it is critical to understand what kind of autoimmune disease the patient had: is it completely resolved? How much time has passed since the last recurrence? Does it require an active immunosuppressant therapy? This information will be crucial in determining proper treatment choice and in correctly evaluating the risk/benefit ratio of the immunotherapy.
As in the study of Danlos et al. [7], in our study population, pre‐existing AIDs do not affect response rate, and at multivariate analyses, there were no correlations between AIDs, PFS, and OS. When looking at the crude values, we cannot help but notice that patients with active AIDs seemed to have a higher ORR (50%) when compared with patients with inactive AIDs and those without AIDs (38.1% and 35.3%, respectively). Similarly, median PFS tended to be longer among patients with pre‐existing inactive AIDs (14.4 months) compared with patients with active AIDs and those without AIDs (6.8 and 8.0 months, respectively). A curious trend was also observed in median OS, which was shorter among patients with pre‐existing active AIDs (9.8 months) compared with patients with inactive AIDs and those without AIDs (15.7 and 16.5 months, respectively). These findings have the limit of immature survival data but together with the safety analysis lay the foundations for some speculative reflections. It is known that irAEs could be related to a greater benefit with anti‐PD‐1/PD‐L1 treatments [27], [28], [29], and also that treatment discontinuation due to irAEs does not have an impact on treatment efficacy [30]. With that in mind, we could suppose that the aberrant immune self‐response, which underlies the AIDs, while sustaining a higher rate of irAEs, would boost the activity of the immunomodulatory treatment. This would explain the higher ORR among patients with pre‐existing active AIDs, even though this assumption seems to clash with the trend of a shorter OS in patients with pre‐existing active AIDs. The point is that we really do not know the immunological balance of these patients, and PD‐1 pathway is certainly not the only factor involved; lastly, we cannot fail to also take into consideration the impact of the AID, which itself could be a non‐negligible cause of morbidity and mortality.
Interestingly, we found a significant greater incidence of irAEs of any grade among female patients, with a risk of developing toxicities 1.4‐fold higher when compared with male patients. The greater incidence of autoimmune disease among women might be not enough to explain it; furthermore, this difference comes out from the multivariate analysis, which was performed together with pre‐existing AIDs. Sex could surely affect immune responses [31], but to date, “gender medicine” has not yet investigated the sexual dimorphism in ICIs response, and only speculative reflections have been made on the topic [32]. As previously stated [27], [28], [29], the greater incidence of irAEs among females could be related to the longer OS, which at the same time is aligned with sex‐related difference in survival among patients with cancer overall [33], and does not contradict the recent evidences of a greater benefit from immunotherapy with ICIs in male patients [34], [35].
Our findings of shorter PFS and OS in patients with ECOG‐PS ≥2 are not unexpected but deserve consideration, even more so considering that patients with a poor PS were usually not enrolled in clinical trials with ICIs. Obviously, patients with the highest PS have shorter life expectancy, but it does not imply that they cannot benefit from anti‐PD‐1 treatments. Indeed, a recent meta‐analysis showed that the pooled hazard ratios for OS were not significantly different among patients with ECOG‐PS 0 and those with ECOG‐PS 1–2 [36]. Our toxicity analysis also demonstrates that patients with a poor PS could be safely treated with these agents. Interestingly, ECOG‐PS ≥2 was significantly related to a lower incidence of irAEs of any grade at multivariate analysis. If irAEs result from pharmacodynamic activity of ICIs and are surrogates of clinical benefit, a poor PS could implies a kind of “repressed immune‐reactivity,” and thus a lower incidence of irAEs, but with shorter survivals.
Among the limits of our study, we must recognize the retrospective nature, the lack of centralized data review (imaging and toxicity), and the data availability. Indeed, we do not have the details about the pre‐existing AIDs, such as previous recurrences/treatments and the diagnosis date, so we are not able to calculate the time from the diagnosis of pre‐existing AIDs and the anti‐PD‐1 treatment commencement. We also do not have detailed data about treatments used in managing irAEs, and the time to develop irAEs of any grade. It would be interesting to collect these data for future studies.
Conclusion
Our study allows us to confirm that patients with pre‐existing AIDs who underwent anti‐PD‐1 treatments, beside the risk of worsening/recurrence of the underlying disorder, are exposed to a statistically significant increased risk of developing irAEs of any grade, regardless of primary tumor, sex, and ECOG‐PS. However, that does not mean that these patients should not be treated with anti‐PD‐1 immunotherapy in absolute terms; indeed, they are not exposed to a significant increased risk of developing G3/G4 irAEs. Surely, patients with active autoimmune disease are not the best candidates for ICIs treatments; however, clinicians are called to evaluated this option, especially if the available treatments are limited. In such a case, a multidisciplinary evaluation is strongly recommended.
Finally, our finding of a statistically significant greater incidence of irAEs among female patients remains partially unexplained and requires further investigation.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This work was supported by the Consorzio Interuniversitario Nazionale per la Bio‐Oncologia (CINBO).
Author Contributions
Conception/design: Alessio Cortellini, Sebastiano Buti, Melissa Bersanelli, Antonino Grassadonia, Giampiero Porzio, Katia Cannita, Valeria Ciciarelli, Maria Concetta Fargnoli, Paolo Antonio Ascierto, Corrado Ficorella
Provision of study material or patients: Daniele Santini, Fabiana Perrone, Raffaele Giusti, Marcello Tiseo, Melissa Bersanelli, Maria Michiara, Antonino Grassadonia, Davide Brocco, Nicola Tinari, Michele De Tursi, Federica Zoratto, Enzo Veltri, Riccardo Marconcini, Francesco Malorgio, Carlo Garufi, Marco Russano, Cecilia Anesi, Tea Zeppola, Marco Filetti, Paolo Marchetti, Andrea Botticelli, Gian Carlo Antonini Cappellini, Federica De Galitiis, Maria Giuseppa Vitale, Roberto Sabbatini, Sergio Bracarda, Rossana Berardi, Silvia Rinaldi, Marianna Tudini, Rosa Rita Silva, Annagrazia Pireddu, Francesco Atzori, Rita Chiari, Biagio Ricciuti, Daniela Iacono, Maria Rita Migliorino
Collection and/or assembly of data: Daniele Santini, Fabiana Perrone, Raffaele Giusti, Marcello Tiseo, Melissa Bersanelli, Maria Michiara, Antonino Grassadonia, Davide Brocco, Nicola Tinari, Michele De Tursi, Federica Zoratto, Enzo Veltri, Riccardo Marconcini, Francesco Malorgio, Carlo Garufi, Marco Russano, Cecilia Anesi, Tea Zeppola, Marco Filetti, Paolo Marchetti, Andrea Botticelli, Gian Carlo Antonini Cappellini, Federica De Galitiis, Maria Giuseppa Vitale, Roberto Sabbatini, Sergio Bracarda, Rossana Berardi, Silvia Rinaldi, Marianna Tudini, Rosa Rita Silva, Annagrazia Pireddu, Francesco Atzori, Rita Chiari, Biagio Ricciuti, Daniela Iacono, Maria Rita Migliorino
Data analysis and interpretation: Alessio Cortellini, Sebastiano Buti, Melissa Bersanelli, Paolo Antonio Ascierto
Manuscript writing: Alessio Cortellini, Sergio Bracarda, Antonio Rossi, Paolo Antonio Ascierto, Corrado Ficorella
Final approval of manuscript: Alessio Cortellini, Sebastiano Buti, Daniele Santini, Fabiana Perrone, Raffaele Giusti, Marcello Tiseo, Melissa Bersanelli, Maria Michiara, Antonino Grassadonia, Davide Brocco, Nicola Tinari, Michele De Tursi, Federica Zoratto, Enzo Veltri, Riccardo Marconcini, Francesco Malorgio, Carlo Garufi, Marco Russano, Cecilia Anesi, Tea Zeppola, Marco Filetti, Paolo Marchetti, Andrea Botticelli, Gian Carlo Antonini Cappellini, Federica De Galitiis, Maria Giuseppa Vitale, Roberto Sabbatini, Sergio Bracarda, Rossana Berardi, Silvia Rinaldi, Marianna Tudini, Rosa Rita Silva, Annagrazia Pireddu, Francesco Atzori, Rita Chiari, Biagio Ricciuti, Daniela Iacono, Maria Rita Migliorino, Antonio Rossi, Giampiero Porzio, Katia Cannita, Valeria Ciciarelli, Maria Concetta Fargnoli, Paolo Antonio Ascierto, Corrado Ficorella
Disclosures
Alessio Cortellini: Merck Sharp & Dohme (RF); Melissa Bersanelli: Bristol‐Myers Squibb (SAB), Pfizer, Bristol‐Myers Squibb (H), AstraZeneca (RF); Paolo Marchetti: AstraZeneca, Merck Sharp & Dohme, Novartis, Bristol‐Myers Squibb, Roche, Foundation Medicine (H), AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Incyte, Merck Sharp & Dohme, Novartis, Roche, Foundation Medicine (RF), Bristol‐Myers Squibb, Novartis, Merck Sharp & Dohme, Roche, Foundation Medicine (other). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Franks AL, Slansky JE. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res 2012;32:1119–1136. [PMC free article] [PubMed] [Google Scholar]
- 2.Champiat S, Lambotte O, Barreau E et al. Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann Oncol 2016;27:559–574. [DOI] [PubMed] [Google Scholar]
- 3.Johnson DB, Sullivan RJ, Ott PA et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2016;2:234–240. [DOI] [PubMed] [Google Scholar]
- 4.Menzies AM, Johnson DB, Ramanujam S et al. Anti‐PD‐1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017;28:368–376. [DOI] [PubMed] [Google Scholar]
- 5.Abdel‐Wahab N, Shah M, Lopez‐Olivo MA et al. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: A systematic review. Ann Intern Med 2018;168:121–130. [DOI] [PubMed] [Google Scholar]
- 6.Leonardi GC, Gainor JF, Altan M et al. Safety of programmed death‐1 pathway inhibitors among patients with non‐small‐cell lung cancer and preexisting autoimmune disorders. J Clin Oncol 2018;36:1905–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danlos FX, Voisin AL, Dyevre V et al. Safety and efficacy of anti‐programmed death 1 antibodies in patients with cancer and pre‐existing autoimmune or inflammatory disease. Eur J Cancer 2018;91:21–29. [DOI] [PubMed] [Google Scholar]
- 8.Minana B, Cozar JM, Palou J et al. Bladder cancer in Spain 2011: Population‐based study. J Urol 2014;191:323–328. [DOI] [PubMed] [Google Scholar]
- 9.Ciocan D, Barbe C, Aubin F et al. Distinctive features of melanoma and its management in elderly patients: A population‐based study in France. JAMA Dermatol 2013;149:1150–1157. [DOI] [PubMed] [Google Scholar]
- 10.Gridelli C, Balducci L, Ciardiello F et al. Treatment of elderly patients with non‐small‐cell lung cancer: Results of an international expert panel meeting of the Italian Association of Thoracic Oncology. Clin Lung Cancer 2015;16:325–333. [DOI] [PubMed] [Google Scholar]
- 11.Azawi NH, Joergensen SM, Jensen NV et al. Trends in kidney cancer among the elderly in Denmark, 1980‐2012. Acta Oncol 2016;55(suppl 1):79–84. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 13.Mantel N. Chi‐square tests with one degree of freedom: Extensions of the Mantel‐Haenszel procedure. J Am Stat Assoc 1963;58:690–700. [Google Scholar]
- 14.Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. J Royal Stat Soc 1992;85:87–94. [Google Scholar]
- 15.Mosteller F. Association and estimation in contingency tables. J Am Stat Assoc 1968;63:1–28. [Google Scholar]
- 16.Hosmer DW Jr, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd ed Hoboken, NJ: John Wiley & Sons, 2013. [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation of incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 18.Schemper M, Smith TL. A note on quantifying follow‐up in studies of failure time. Control Clin Trials 1996;17:343–346. [DOI] [PubMed] [Google Scholar]
- 19.Cox DR. Regression models and life tables (with discussion). J Royal Stat Soc Series B 1972;74:187–200. [Google Scholar]
- 20.Rose NR, Bona C. Defining criteria for autoimmune diseases (Witebsky's postulates revisited). Immunol Today 1993;14:426–430. [DOI] [PubMed] [Google Scholar]
- 21.Giat E, Ehrenfeld M, Shoenfeld Y. Cancer and autoimmune diseases. Autoimmun Rev 2017;16:1049–1057. [DOI] [PubMed] [Google Scholar]
- 22.Khan SA, Pruitt SL, Xuan L et al. Prevalence of autoimmune disease among patients with lung cancer: Implications for immunotherapy treatment options. JAMA Oncol 2016;2:1507–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Osta B, Hu F, Sadek R et al. A meta‐analysis of immune‐related adverse events (irAE) of immune checkpoint inhibitors (ICI) from cancer clinical trials. Ann Oncol 2016;27(suppl 6):1077P. [Google Scholar]
- 24.El Osta B, Hu F, Sadek R et al. Not all immune‐checkpoint inhibitors are created equal: Meta‐analysis and systematic review of immune‐related adverse events in cancer trials. Crit Rev Oncol Hematol 2017;119:1–12. [DOI] [PubMed] [Google Scholar]
- 25.Aguiar JP, Cardoso Borges F, Murteira R et al. Using a cancer registry to capture signals of adverse events following immune and targeted therapy for melanoma. Int J Clin Pharm 2018;40:852–861. [DOI] [PubMed] [Google Scholar]
- 26.So AC, Board RE. Real‐world experience with pembrolizumab toxicities in advanced melanoma patients: A single‐center experience in the UK. Melanoma Manag 2018;5:MMT05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato K, Akamatsu H, Murakami E et al. Correlation between immune‐related adverse events and efficacy in non‐small cell lung cancer treated with nivolumab. Lung Cancer 2018;115:71–74. [DOI] [PubMed] [Google Scholar]
- 28.Toi Y, Sugawara S, Kawashima Y et al. Association of immune‐related adverse events with clinical benefit in patients with advanced non‐small‐cell lung cancer treated with nivolumab. The Oncologist 2018;23:1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riudavets M, Barba A, Maroto P et al. Correlation between immune‐related adverse events (irAEs) and efficacy in patients with solid tumors treated with immune‐checkpoints inhibitors (ICIs). J Clin Oncol 2018;36(suppl 15):3064.30188784 [Google Scholar]
- 30.Schadendorf D, Wolchok JD, Hodi FS et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: A pooled analysis of randomized phase II and III trials. J Clin Oncol 2017;35:3807–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016;16:626–638. [DOI] [PubMed] [Google Scholar]
- 32.Özdemir BC, Coukos G, Wagner AD. Immune‐related adverse events of immune checkpoint inhibitors and the impact of sex‐what we know and what we need to learn. Ann Oncol 2018;29:1067. [DOI] [PubMed] [Google Scholar]
- 33.Radkiewicz C, Johansson ALV, Dickman PW et al. Sex differences in cancer risk and survival: A Swedish cohort study. Eur J Cancer 2017;84:130–140. [DOI] [PubMed] [Google Scholar]
- 34.Conforti F, Pala L, Bagnardi V et al. Cancer immunotherapy efficacy and patients' sex: A systematic review and meta‐analysis. Lancet Oncol 2018;19:737–746. [DOI] [PubMed] [Google Scholar]
- 35.Botticelli A, Onesti CE, Zizzari I et al. The sexist behaviour of immune checkpoint inhibitors in cancer therapy? Oncotarget 2017;8:99336–99346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bersanelli M, Brighenti M, Buti S et al. Patient performance status and cancer immunotherapy efficacy: A meta‐analysis. Med Oncol 2018;35:132. [DOI] [PubMed] [Google Scholar]