A single‐center retrospective study of 40 patients with epidermal growth factor receptor mutant lung cancer with brain metastases treated with osimertinib was conducted, with a focus on whether patients who initially responded to osimertinib may be able to defer or delay radiation without reducing overall survival.
Keywords: Osimertinib, Brain metastases, EGFR‐mutant lung cancer, Stereotactic radiosurgery
Abstract
Introduction.
Osimertinib is a third‐generation tyrosine kinase inhibitor, initially approved for epidermal growth factor receptor (EGFR) mutant non‐small cell lung cancer (NSCLC) with T790M acquired resistance, and now approved in the first‐line setting. However, data supporting the use of osimertinib in untreated brain metastases are limited, although it has established central nervous system (CNS) activity. Our study compares the clinical outcomes of patients experiencing progressing brain metastases treated with cranial irradiation and osimertinib with those treated with osimertinib alone.
Methods.
Forty patients who were treated with osimertinib at the Stanford Cancer Center from November 2015 to December 2016 were identified by searching an electronic medical record database. Eleven patients had progressing brain metastases and did not receive radiation (group A), 9 patients had progressing brain metastases and received radiation when starting osimertinib (group B), and 20 patients had stable brain metastases at the time of initiating osimertinib (group C). Patient and disease characteristics, radiographic responses, and survival outcomes were evaluated retrospectively for the three groups.
Results.
The CNS response rate was 32.3%. Median time to treatment failure (TTF), overall progression‐free survival (PFS), and overall survival (OS) were 10.0 months (95% confidence interval [CI], 4.5–11.8), 8.8 months (95% CI, 6.2–12.1), and 16.2 months, respectively. Median TTF was 15.1 months for group A (95% CI, 1.7–28.5), 7.7 months for group B (95% CI, 0–15.5), and 10.7 months for group C (95% CI, 9.0–12.5). The median PFS was 8.8 months for group A (95% CI, 4.3–13.4), not reached for group B, and 8.4 months for group C (95% CI, 5.6–11.1). The median OS was not reached for group A and C, and was 16.2 months for group B. There was no apparent difference in TTF, PFS, or OS between the three groups.
Conclusion.
Receiving radiation prior to starting osimertinib for patients with progressing brain metastases did not prolong TTF, PFS, or OS in our series. To minimize the risks of radiation‐related toxicity, delaying radiation could be considered for some patients with EGFR‐mutant NSCLC with brain metastases who initially respond to osimertinib in the second‐line setting.
Implications for Practice.
Osimertinib is a third‐generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor recently approved for the first‐line treatment of EGFR‐mutant non‐small cell lung cancer. Although it appears to have central nervous system (CNS) activity, most clinical trials have excluded patients with untreated, progressing brain metastases. This study included patients with stable and progressing CNS metastases treated with osimertinib and found no apparent differences in median time to treatment failure, time to progression, and overall survival in patients who received osimertinib alone compared with those who received osimertinib and radiosurgery. This may support a clinician's decision to defer radiation for selected patients with untreated brain metastases who are candidates for osimertinib therapy.
摘要
简介。奥希替尼是第三代酪氨酸激酶抑制剂,最初被批准用于治疗具有 T790M 获得性耐药性的表皮生长因子受体 (EGFR) 突变非小细胞肺癌 (NSCLC),现已被批准用于一线用药。虽然奥希替尼被公认具有中枢神经系统 (CNS) 活性,但支持在未经治疗的脑转移中使用奥希替尼的数据非常有限。我们的研究对比了为出现进展性脑转移患者提供颅脑照射和奥希替尼治疗与奥希替尼单药治疗的临床结果。
方法。通过搜索电子病历数据库,我们确定了 40 名在 2015 年 11 月至 2016 年 12 月期间在斯坦福癌症中心接受奥希替尼治疗的患者。11 名患者出现进展性脑转移且没有接受放射治疗(A 组),9 名患者出现进展性脑转移且在开始服用奥希替尼的同时接受放射治疗(B 组),20 名患者在开始服用奥希替尼时处于稳定性脑转移状态(C 组)。我们针对 3 组的患者和疾病特征、影照学反应以及生存结果进行回顾性评估。
结果。CNS 反应率为 32.3%。中位至治疗失败时间 (TTF)、总无进展生存期 (PFS) 和总生存期 (OS) 分别为 10.0 个月 [95% 置信区间 (CI),4.5–11.8]、8.8 个月(95%CI,6.2–12.1)和 16.2 个月。A 组、B 组和 C 组的中位 TTF 分别为 15.1 个月(95% CI,1.7–28.5)、7.7 个月(95% CI,0–15.5)和 10.7 个月(95% CI,9.0–12.5)。A 组 和 C 组的中位 PFS 分别为 8.8 个月(95% CI,4.3–13.4)和 8.4 个月(95% CI,5.6–11.1),而 B 组未达到中位 PFS。A 组和 C 组未达到中位 OS,B 组的中位 OS 为 16.2 个月。3 组之间的 TTF、PFS 或 OS 没有明显差异。
结论。在我们的系列研究中,让出现进展性脑转移的患者在开始服用奥希替尼之前接受放射治疗不会延长 TTF、PFS 或 OS。为了最大限度地降低放射治疗相关毒性风险,可以考虑为部分在二线用药中对奥希替尼最初出现反应的伴有脑转移的 EGFR 突变 NSCLC 患者延迟放射治疗。
对临床实践的提示:奥希替尼是第三代表皮生长因子受体 (EGFR) 酪氨酸激酶抑制剂,最近被批准用于 EGFR 突变非小细胞肺癌的一线治疗。尽管奥希替尼似乎具有中枢神经系统 (CNS) 活性,但是,大部分临床试验已将出现未经治疗的进展性脑转移的患者排除在外。本研究包含具有稳定性和进展性脑转移的患者以接受奥希替尼治疗,研究结果表明,接受奥希替尼单药治疗的患者与接受奥希替尼和放射手术治疗的患者之间的中位至治疗失败时间、至进展时间和总生存期没有明显差异。这可以支持临床医生为经选的出现未经治疗的脑转移且申请奥希替尼治疗的患者延迟放射治疗的决定
Introduction
The management of central nervous system (CNS) metastases in EGFR‐mutant non‐small cell lung cancer (NSCLC) presents a unique challenge, particularly related to the long median overall survival from diagnosis of CNS metastases of approximately 14.5 months for patients with EGFR‐mutant NSCLC, leading to the potential for adverse effects from radiation [1]. In general, systemic chemotherapy has a limited effect on CNS disease because of poor penetration of the blood‐brain barrier. Although first‐generation tyrosine kinase inhibitors (TKIs) have shown CNS responses, therapies are limited for patients who have progressed on them. Currently, whole‐brain radiation (WBRT), stereotactic radiosurgery (SRS), or surgical resection of CNS metastases are the best options for patients whose disease is refractory to systemic therapies with CNS activity. However, a third‐generation epidermal growth factor receptor (EGFR) TKI that has efficacy against the common EGFR driver mutations as well as the T790M mutation, osimertinib (Tagrisso; AstraZeneca, London, UK) may have better efficacy than previous‐generation TKIs in the management of NSCLC with brain metastases. Osimertinib has evidence of clinically relevant penetration of the blood‐brain barrier and tumor regression in mouse models as well as responses reported in patients with stable brain and leptomeningeal metastases with no prior radiotherapy [2], [3], [4], [5], [6]. Osimertinib was initially approved by the U.S. Food and Drug Administration as second‐line therapy for patients who have progressed on first‐generation TKIs and whose tumors have the T790M acquired resistance mutation [7]. However, based on the data presented from the phase III FLAURA trial, osimertinib was approved in April 2018 as first‐line therapy for EGFR‐mutant NSCLC [8]. In the FLAURA trial, the median progression‐free survival (PFS) for osimertinib was 18.9 months compared with 10.2 months for the first‐generation drugs gefitinib or erlotinib. However, the FLAURA trial only included patients with stable or treated brain metastases at baseline.
Because these clinical trials excluded patients with progressing brain metastases, there are limited data on the effect of osimertinib in this patient population. We conducted a single‐center retrospective study of 40 patients with brain metastases treated with osimertinib at our institution to analyze outcomes. Our hypothesis is that some patients with EGFR‐mutant lung cancer with brain metastases that initially respond to osimertinib may be able to defer or delay radiation without reducing overall survival.
Materials and Methods
Study Design and Patients
We retrospectively reviewed the medical records of all patients who were treated with commercial osimertinib from November 2015 to December 2016 at our institution, using a cohort identified by the Stanford Cancer Institute Research Database. Institutional review board approval was obtained at Stanford University. The cutoff date for data collection was October 15, 2017. Sixty patients were identified who had been started on osimertinib during the study period, and of these patients, 40 patients had brain metastases at baseline. The baseline characteristics of these patients, including age, sex, stage, smoking history, T790M status, status of brain metastases, size of brain metastases, presence of neurologic symptoms, and prior treatment regimens, were noted. The dates of initial cancer diagnosis, brain metastases diagnosis, initiation and completion of osimertinib, systemic and CNS progression, most recent follow‐up, and death were noted, if known.
Patients with brain metastases were stratified into three groups: 11 patients with progressing brain lesions who did not receive radiation (group A), 9 patients with progressing brain lesions who did receive radiation (group B), and 20 patients with stable, untreated brain lesions at the time of initiating osimertinib (group C). All patients received standard 80 mg dosing of osimertinib with the exception of three patients in group A, two of whom received 160 mg daily and one of whom received 80 mg every other day. The patients in group B received Cyberknife (Accuray, Sunnyvale, CA) as the only modality of CNS irradiation, and no patients received WBRT prior to starting osimertinib. Tumor response was determined for CNS and systemic disease. Systemic tumor response was calculated based on computed tomography scans, generally performed every 2–4 months for all patients, based on RECIST version 1.1. CNS progression, based on MRI with contrast performed every 6 weeks to 4 months, was defined as the development of new brain lesions or an interpretation of growth of existing lesions by the local radiologist. Complete remission (CR) of CNS disease was defined as the disappearance of all brain lesions. Partial response (PR) of CNS disease was defined as decrease of greater than 30% of all brain lesions larger than 10 mm or an interpretation of significant reduction of brain lesions by a radiologist for patients with unmeasurable lesions at baseline. Confirmation of PR was not required. Stability of CNS disease was defined as disease activity not meeting criteria for progression, PR, or CR of CNS disease. Time to treatment failure (TTF) was measured from the date of initiation of osimertinib to the date of the event of stopping therapy or death, with censorship at the last follow‐up visit for patients lost to follow‐up, regardless of radiographic changes in disease status. PFS was measured from the date of initiation of osimertinib to the date of the event of overall progression or death, with censorship at the last follow‐up visit for patients lost to follow‐up. Time to CNS progression was measured from the date of initiation of osimertinib to the date of the event of progression of CNS disease or death, with censorship at the last follow‐up visit for patients lost to follow‐up.
Statistical Analysis
Clinical outcomes were calculated, including TTF, PFS, and overall survival (OS). Survival curves were estimated using the Kaplan‐Meier method with analysis performed using SPSS (IBM, Armonk, NY). Survival curves were compared using the log‐rank test without adjustment for variables. A swimmer chart was created using Apple Numbers (Apple, Cupertino, CA).
Results
Patient Characteristics
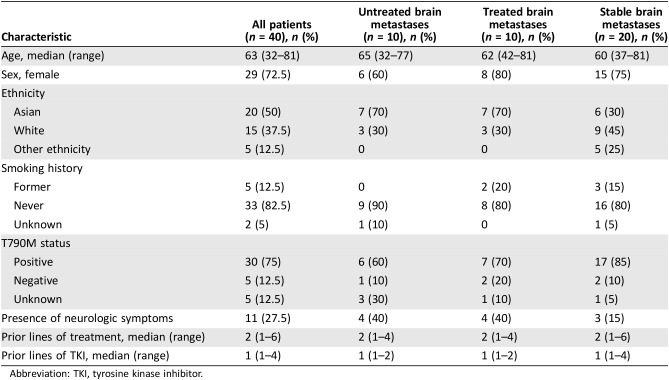
Forty patients with EGFR‐mutant adenocarcinoma with brain metastases began treatment with osimertinib during a period from November 2015 to December 2016. The patient characteristics for the three groups are summarized in Table 1. The characteristics of age, sex, smoking history, stage at diagnosis, T790M status, and prior treatment history were similar between the three groups. The median age was 63 years. Regarding sex, 72.5% of patients were female. Groups A and B each included 70% of patients with Asian ethnicity and 30% of patients with white ethnicity, whereas group C included 30% Asian patients, 45% white patients, and 25% patients of another ethnicity (Hispanic or African American). Regarding smoking history, 82.5% of patients were lifetime nonsmokers. The T790M status negativity was 12.5%. The five patients who were T790M negative were only tested with liquid biopsy, which may have led to false negative results. Groups A and B each included 40% of patients experiencing neurologic symptoms, whereas group C had 15% of patients with neurologic symptoms. All groups had median two prior lines of treatment and one prior line of TKI; 96.7% of patients had received erlotinib previously. Group A tended to have nonsignificantly smaller sizes of brain metastases compared with the patients who received radiation, with a mean size of 9 mm (range of 3–25 mm) compared with 11 mm (range of 4–30 mm; p = .468).
Table 1. Baseline characteristics of patients with brain metastases.
Abbreviation: TKI, tyrosine kinase inhibitor.
Clinical Outcomes
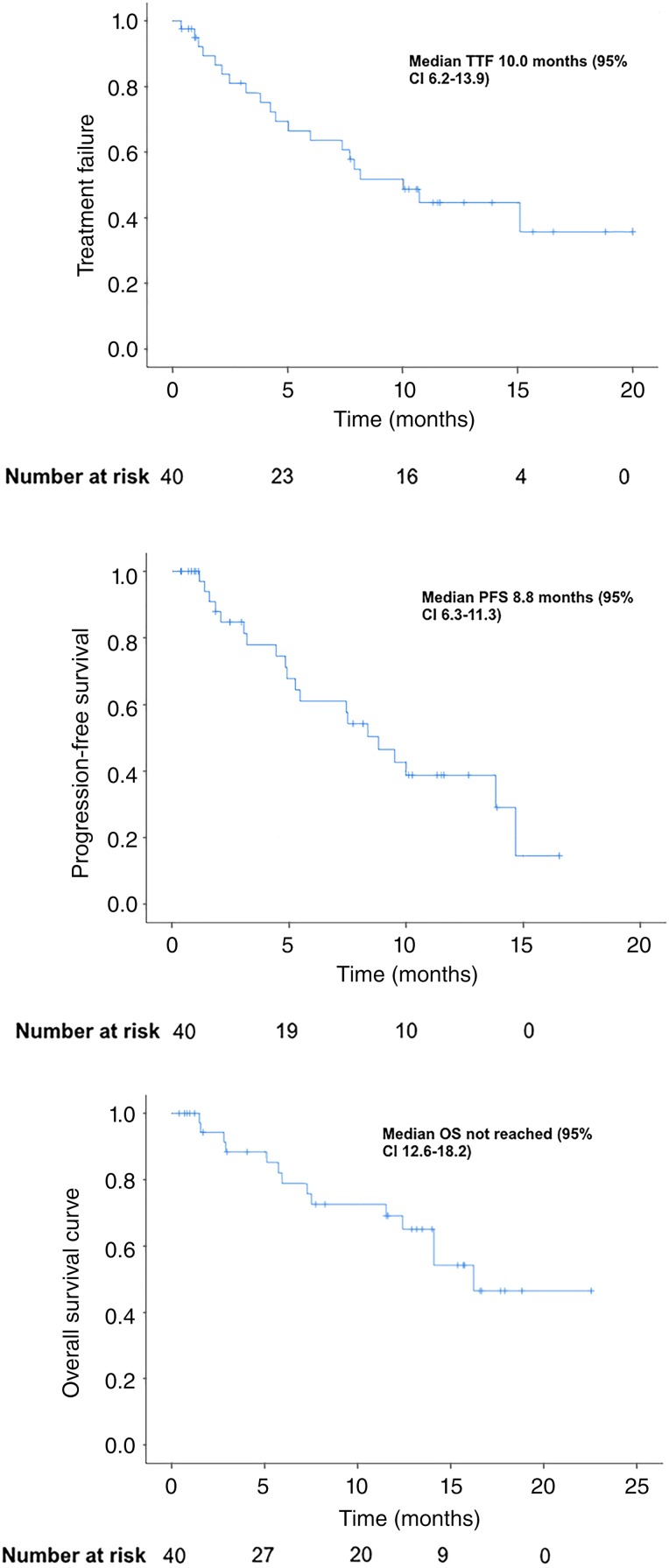
The CNS response rate to osimertinib was 32.3% for the 31 patients who had evaluable disease at 3 months, with three CRs (9.7%) noted. Of the nine nonevaluable patients, survival information was available, but their disease status was nonevaluable because five died within 3 months of starting osimertinib, and the other patients did not have accessible imaging. Of the 40 patients with brain metastases, the median TTF was 10.0 months (95% confidence interval [CI] 6.2–13.9), PFS was 8.8 months (95% CI, 6.3–11.3), and OS was 16.2 months (95% CI, 12.6–18.2), shown in Figure 1. At the data cutoff, 12 patients were still receiving osimertinib. One of the 40 patients stopped osimertinib because of toxicity resulting in fatal pneumonitis.
Figure 1.
TTF, PFS, and OS curves for all patients.
Abbreviations: CI, confidence interval; OS, overall survival; PFS, progression‐free survival; TTF, time to treatment failure.
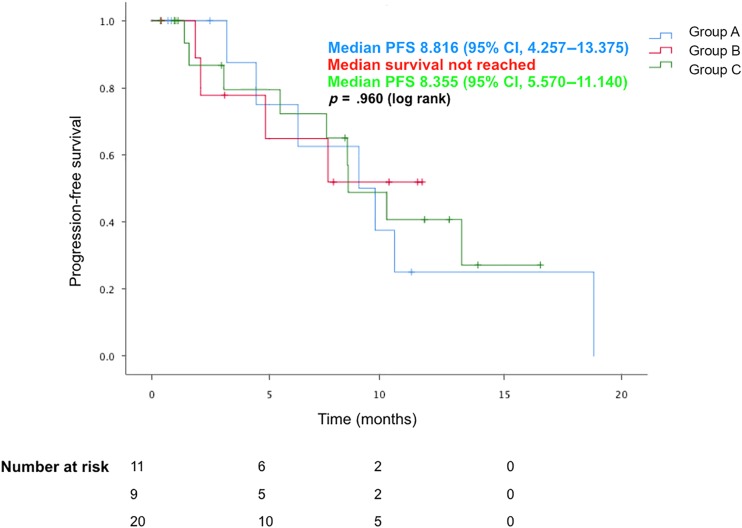
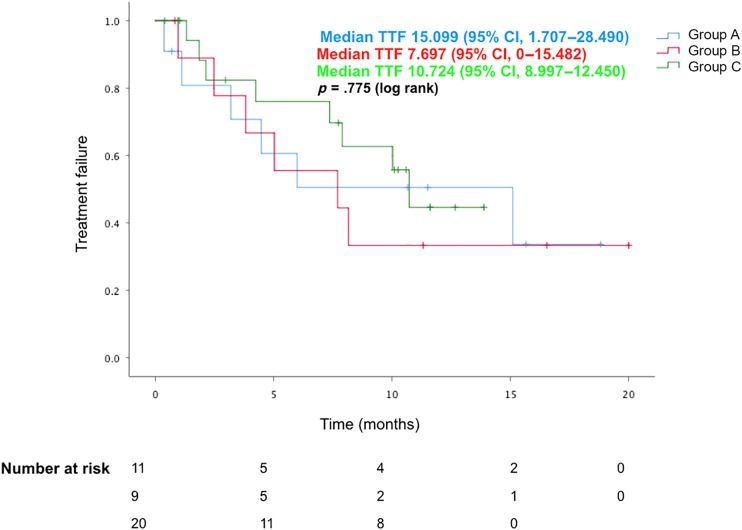
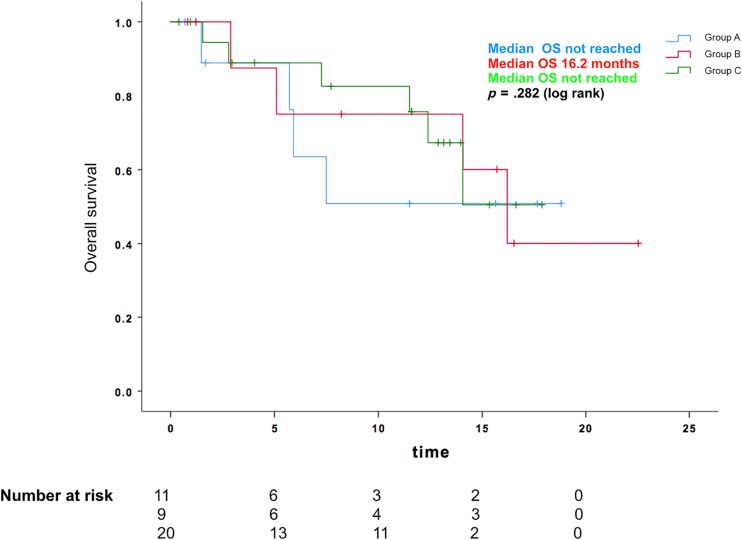
The median TTF was 15.1 months for group A (95% CI, 1.7–28.5), 7.7 months for group B (95% CI, 0–15.5), and 10.7 months for group C (95% CI, 9.0–12.5). The median PFS was 8.8 months for group A (95% CI, 4.3–13.4), not reached for group B, and 8.4 months for group C (95% CI, 5.6–11.1). The median OS was not reached for group A and C and was 16.2 months for group B.
There was no apparent difference with regard to TTF (p = .775), PFS (p = .960), or OS (p = .282) noted between the three groups, as shown in Figures 2, 3, 4. Of the patients in group A, one patient was known to later receive brain irradiation about 13 months after starting osimertinib, which was still continued at the last date of data collection. Of the patients in group B, no patients received any additional brain irradiation, and one patient received craniotomy for resection of a brain metastasis about 15 months after starting osimertinib, which was still continued at the last date of data collection.
Figure 2.
PFS for patients with progressing brain metastases without radiation, progressing brain metastases with radiation, and stable brain disease at the time of starting osimertinib.
Abbreviations: CI, confidence interval; PFS, progression‐free survival.
Figure 3.
TTF for patients with progressing brain metastases without radiation, progressing brain metastases with radiation, and stable brain disease at the time of starting osimertinib.
Abbreviations: CI, confidence interval; TTF, time to treatment failure.
Figure 4.
OS for patients with progressing brain metastases without radiation, progressing brain metastases with radiation, and stable brain disease at the time of starting osimertinib. Abbreviation: OS, overall survival.
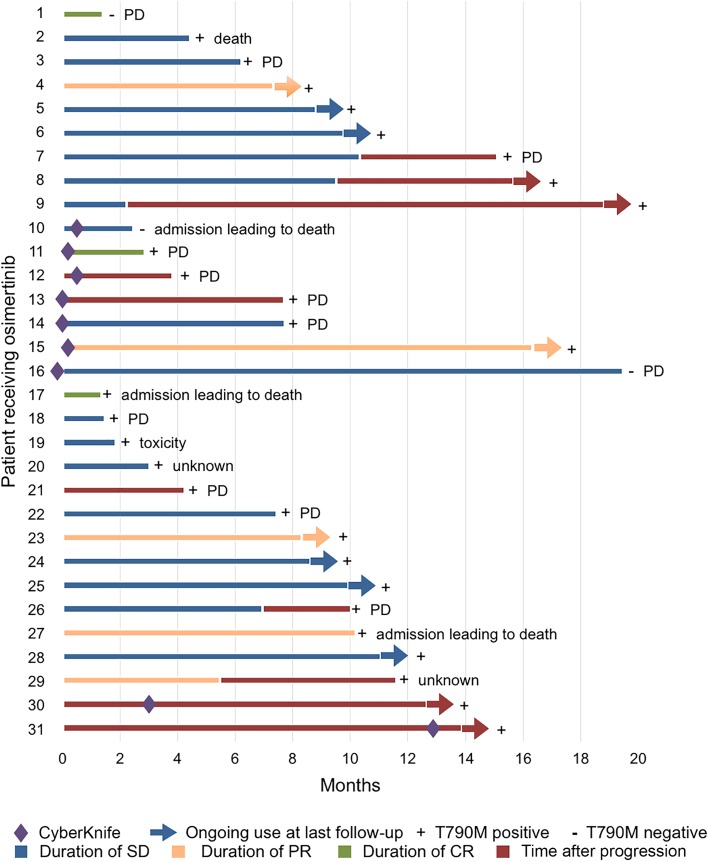
Figure 5 shows the best CNS response and duration of therapy for 31 patients with brain metastases who had follow‐up imaging. Nine patients had progressing brain metastases and did not receive local radiation. Of these nine patients, two patients had radiographic evidence of CNS response, including one patient with complete resolution of CNS lesions, and seven patients had initially stable disease, of whom two were later continued on osimertinib after progression of brain metastases. The interval MRI imaging of one of the patients who experienced a partial response on osimertinib alone is shown here (Fig. 6). Seven patients received radiation for their progressing brain metastases within 1 month of initiating osimertinib. Of these seven patients, two patients had radiographic evidence of CNS response, including one patient with complete response, two patients with progressive CNS disease, and three patients with stable disease. Fifteen patients had stable brain metastases at the time of starting osimertinib. Of these 15 patients, 4 patients had radiographic evidence of CNS response, including 1 patient with complete response, 3 patients with progressive CNS disease, and 8 patients with stable CNS disease. All groups of patients (progressing brain metastases with and without concomitant radiation, and stable brain metastases) had a similar distribution of CNS disease outcomes, including complete resolution, disease stability, and progression.
Figure 5.
Swimmer's plot of 31 patients who had evaluable brain metastases showing duration of best brain response and duration of osimertinib use after central nervous system progression. Patients with lines that terminate with arrows were still alive and receiving osimertinib at last follow‐up. Patients with lines that terminate without arrows were known to be no longer receiving osimertinib because of death, progression of disease, toxicity, or unknown reason, as stated.
Abbreviations: CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 6.
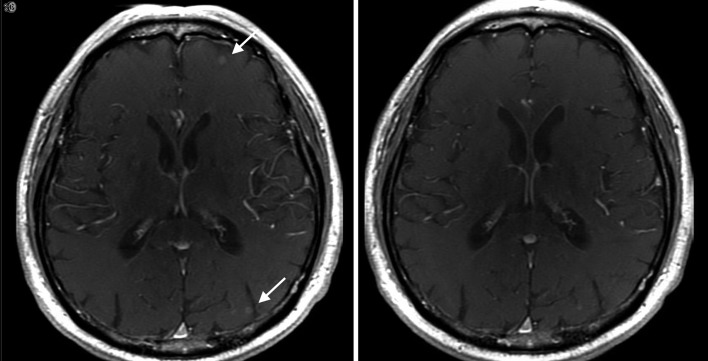
Magnetic resonance imaging of patient who demonstrated partial response at 2 months after initiating osimertinib alone. The image on the left was obtained prior to starting osimertinib, and the image on the right was after 2 months of treatment with osimertinib.
Discussion
We conducted a retrospective analysis of patients with EGFR‐mutant NSCLC with progressing brain metastases who were treated with cranial irradiation and osimertinib compared with those treated with osimertinib alone. There are currently limited data on the effectiveness of osimertinib alone for patients with progressing brain metastases. Some case reports have demonstrated that the clinical responses achieved on osimertinib may obviate the need for whole‐brain radiation in some patients [9], [10]. The phase I BLOOM study studied the effects of osimertinib 160 mg daily on 20 patients with advanced NSCLC who had progressed on prior EGFR TKIs and had confirmed leptomeningeal disease by cerebrospinal fluid cytology, but the study did not focus on parenchymal metastases [6]. The subset of 75 patients in the AURA3 trial with stable and progressing CNS metastases treated with osimertinib in the second‐line setting were noted to have a median CNS PFS of 11.7 months, whereas the overall population had a PFS of 10.1 months for osimertinib in the second‐line setting [11]. Our single‐institution data reveal a shorter median PFS of 8.8 months in NSCLC patients with brain metastases. However, this compares favorably, considering that our “real world” population may not have met the inclusion criteria for a trial such as AURA3 because of borderline functional status, higher number of prior lines of therapy, and lack of availability of tissue for T790M testing.
A recent pooled, multicenter retrospective analysis suggested that patients receiving SRS prior to first‐line erlotinib had superior overall survival (46 months) compared with those receiving WBRT (30 months) or those receiving erlotinib with deferral of radiotherapy (25 months) [12]. It is unclear whether the same survival benefit may be true for receiving SRS prior to starting osimertinib. Our study indicates that there is no apparent difference in TTF, PFS, or OS for patients with brain metastases regardless of whether they received local radiation therapy prior to starting osimertinib. It also suggests that initiating osimertinib alone may a reasonable choice even for patients with progressing brain metastases.
Our study has several limitations. Most notably it is a small retrospective analysis at a single center, which may limit the detection of statistically meaningful differences between the three groups. Furthermore, the criteria used to determine CNS and systemic response and progression were based on data available to a treating physician, including imaging and reports. Only three patients had untreated CNS lesions that were 10 mm or greater that would have allowed application of RECIST 1.1 criteria to these lesions. We believe that this methodology would be consistent with a treating physician's assessment of response and progression, but it is possible that our conclusions could differ from those obtained using central radiological review and other methods. The survival outcomes calculated are grossly comparable to those from clinical trials, but larger prospective studies are needed to further evaluate for differences in outcomes for patients receiving osimertinib alone compared with those receiving CNS radiation at the time of initiating osimertinib. In our study, there may be differences between the radiation‐receiving and non‐radiation‐receiving groups for patients with progressing brain metastases in terms of initial size of CNS lesions and performance status, leading to selection bias. The median size of the largest lesions measured was lower for the patients with progressing brain metastases who did not receive radiation compared with the patients with progressing brain metastases who did receive radiation, but this difference was not statistically significant, and otherwise the two groups had similar baseline characteristics. Additionally, differences in specific neurologic symptoms and severity may have led to a clinical decision to defer radiation in less symptomatic patients, but this effect is hard to discern from retrospective chart review. Most patients in this study were not documented to have neurologic symptoms or deficits, which may limit the generalizability of our results to a more symptomatic patient population.
Given the emergence of osimertinib as a first‐line drug for NSCLC after the FLAURA results, one remaining question is the optimal therapy for patients with brain metastases who have progressed on osimertinib in the first‐line setting. It has been hypothesized that EGFR‐mutant NSCLC may be particularly radiosensitive, as shown in one study in which 54% of patients with EGFR mutations received a response from WBRT, which was double the response in the EGFR wild‐type group [13]. In another study, EGFR‐mutant patients who received radiation had an increased time to CNS progression compared with EGFR wild‐type patients [1]. Therefore, radiotherapy may have some extended benefits for patients with EGFR‐mutant NSCLC beyond progression, but this should be weighed against the potential of long‐term toxicity from radiation.
Conclusion
This study found an initial CNS response rate to second‐line osimertinib of 32.3% and a median TTF of 10.0 months, PFS of 8.8 months, and OS of 16.2 months. There was no apparent difference in PFS, TTF, or OS for patients who did not receive cranial irradiation at the time of initiating osimertinib compared with patients treated with cranial irradiation for progressing brain metastases at the time of starting osimertinib. When selecting patients who may be appropriate for deferral of initial CNS irradiation, we recommend doing so judiciously for patients who have small lesions (<10 mm) with minimal or no vasogenic edema and are asymptomatic or minimally symptomatic. In general, our group defers WBRT when an alternative systemic therapy with CNS penetration, such as osimertinib, is available. We generally recommend follow‐up imaging every 2–3 months when using systemic therapy alone. Deferral of cranial irradiation may be considered for select patients who initially respond to second‐line osimertinib, but further prospective studies are needed to elucidate the longer‐term risks and benefits of this approach.
Author Contributions
Conception/design: Lijia Xie, Joel W. Neal
Provision of study material or patients: Lijia Xie, Seema Nagpal, Heather A. Wakelee, Gordon Li, Scott G. Soltys, Joel W. Neal
Data analysis and interpretation: Lijia Xie
Manuscript writing: Lijia Xie, Seema Nagpal, Heather A. Wakelee, Gordon Li, Scott G. Soltys, Joel W. Neal
Final approval of manuscript: Lijia Xie, Seema Nagpal, Heather A. Wakelee, Gordon Li, Scott G. Soltys, Joel W. Neal
Disclosures
Seema Nagpal: Nektar Therapeutics (RF); Heather A. Wakelee: AstraZeneca (RF, H), Genentech/Roche (RF, H), Novartis (RF, H), ACEA (H), Clovis, Exelixis, BMS, Gilead, Xcovery, Pfizer, Celgene, Eli Lilly, Pharmacyclics (RF); Scott G. Soltys: Inovio Pharmaceuticals (H); Joel W. Neal: ARIAD/Takeda (RF, H), Boehringer Ingelheim (RF, H), AstraZeneca, Clovis, Eli Lilly (H), Genentech/Roche, Novartis, Exelixis, Merck Sharpe & Dohme, Nektar Therapeutics (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Eichler AF, Kahle KT, Wang DL et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol 2010;12:1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballard P, Yates JW, Yang Z et al. Preclinical comparison of osimertinib with other EGFR‐TKIs in EGFR‐mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 2016;22:5130–5140. [DOI] [PubMed] [Google Scholar]
- 3.Koba T, Kijima T, Takimoto T et al. Rapid intracranial response to osimertinib, without radiotherapy, in nonsmall cell lung cancer patients harboring the EGFR T790M mutation: Two case reports. Medicine (Baltimore) 2017;96:e6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goss G, Tsai CM, Shepherd FA et al. CNS response to osimertinib in patients with T790M‐positive advanced NSCLC: Pooled data from two phase II trials. Ann Oncol 2018;29:687–693. [DOI] [PubMed] [Google Scholar]
- 5.Mok TS, Wu YL, Ahn MJ et al. Osimertinib or platinum‐pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med 2017;376:629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang JCH, Kim DW, Kim SW et al. Osimertinib activity in patients (pts) with leptomeningeal (LM) disease from non‐small cell lung cancer (NSCLC): Updated results from BLOOM, a phase I study. J Clin Oncol 2016;34(suppl 15):9002A. [Google Scholar]
- 7.Yang J, Ramalingam SS, Jänne PA et al. LBA2_PR: Osimertinib (AZD9291) in pre‐treated pts with T790M‐positive advanced NSCLC: Updated phase 1 (P1) and pooled phase 2 (P2) results. J Thorac Oncol 2016;11(suppl 4):S152–S153. [Google Scholar]
- 8.Soria JC, Ohe Y, Vansteenkiste J et al. Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med 2018;378:113–125. [DOI] [PubMed] [Google Scholar]
- 9.Ricciuti B, Chiari R, Chiarini P et al. Osimertinib (AZD9291) and CNS response in two radiotherapy‐naïve patients with EGFR‐mutant and T790M‐positive advanced non‐small cell lung cancer. Clin Drug Investig 2016;36:683–686. [DOI] [PubMed] [Google Scholar]
- 10.Reichegger H, Jochumb W, Förbs D et al. Rapid intracranial response to osimertinib in a patient with epidermal growth factor receptor T790M‐positive adenocarcinoma of the lung. Oncol Res Treat 2016;39:461–463. [DOI] [PubMed] [Google Scholar]
- 11.Mok T, Ahn MJ, Han JY et al. CNS response to osimertinib in patients (pts) with T790M‐positive advanced NSCLC: Data from a randomized phase III trial (AURA3). J Clin Oncol 2017;35(suppl 15):9005A. [Google Scholar]
- 12.Magnuson WJ, Lester‐Coll NH, Wu AJ et al. Management of brain metastases in tyrosine kinase inhibitor‐naïve epidermal growth factor receptor‐mutant non‐small‐cell lung cancer: A retrospective multi‐institutional analysis. J Clin Oncol 2017;35:1070–1077. [DOI] [PubMed] [Google Scholar]
- 13.Gow CH, Chien CR, Chang YL et al. Radiotherapy in lung adenocarcinoma with brain metastases: Effects of activating epidermal growth factor receptor mutations on clinical response. Clin Cancer Res 2008;14:162–168. [DOI] [PubMed] [Google Scholar]