Abstract
Lessons Learned.
First trial to report safety and activity of the microtubule inhibitor vinflunine plus the tyrosine kinase inhibitor sorafenib in post‐platinum metastatic urothelial cancer (mUC) patients.
A recommended phase II dose was identified for the treatment combination of vinflunine plus sorafenib, with main adverse events including fatigue, febrile neutropenia, neutropenia, hypertension, and hyponatremia.
An overall response rate of 41% to second‐line vinflunine plus sorafenib treatment in patients with platinum‐resistant mUC was confirmed.
Background.
Platinum‐progressive metastatic urothelial carcinoma (mUC) is a clinical challenge. The tyrosine kinase inhibitor sorafenib has demonstrated varied activity in mUC. This trial was designed to examine safety and activity of vinflunine plus sorafenib in mUC.
Methods.
In addition to standard dose of vinflunine (320 or 280 mg/m2), patients received sorafenib (400, 600, or 800 mg/day), in a 3 + 3 dose‐escalation phase I design.
Results.
Twenty‐two patients (median age 62.5 years) were included. Five patients received vinflunine 320 mg/m2 and 17 received 280 mg/m2. The maximum tolerated dose (MTD) of sorafenib with vinflunine 280 mg/m2 was 600 mg, and with vinflunine 320 mg/m2 it was not determined, owing to toxicity. Adverse events (AEs) grades 3 + 4 consisted of neutropenia (6 patients), febrile neutropenia (5), and hyponatremia (5). The overall response rate (ORR) in the efficacy‐evaluable patients was 41% (7 of 17), all partial responses evaluated by RECIST version 1.1. Median overall survival (OS) was 7.0 months (1.8–41.7).
Conclusion.
The defined recommended phase II dose (RPTD) was vinflunine 280 mg/m2 plus sorafenib 400 mg. Sorafenib was too toxic in combination with vinflunine 320 mg/m2. The ORR of 41% to this second‐line combination treatment of mUC is noteworthy and supports further trials.
Abstract
经验获取
• 第一个报告微管抑制剂长春氟宁加用酪氨酸激酶抑制剂索拉非尼治疗经铂类药物治疗后转移性尿路上皮癌 (mUC) 患者的安全性和活性的试验。
• 确定了长春氟宁和索拉非尼联合用药的推荐 II 期剂量,主要不良反应包括疲劳、发热性嗜中性白血球减少症、嗜中性白血球减少症、高血压和低钠血症。
• 确认了二线长春氟宁加用索拉非尼治疗铂类耐药 mUC 患者的总缓解率为 41%。
摘要
背景。铂类进展性转移性尿路上皮癌 (mUC) 是一项临床挑战。酪氨酸激酶抑制剂索拉非尼已在 mUC 中显示出不同的活性。本试验旨在检查长春氟宁加用索拉非尼治疗 mUC 的安全性和活性。
方法。按照 3 + 3 剂量递增 I 期试验设计,除了标准剂量的长春氟宁(320 或 280 mg/m2)之外, 患者还要服用索拉非尼(400、600 或 800 mg/天)。
结果。22 名患者(中位年龄为 62.5 岁)入组。5 名患者的长春氟宁用药剂量为 320 mg/m2, 17 名患者的用药剂量为 280 mg/m2。在加用长春氟宁280 mg/m2时,索拉非尼的最大耐受剂量 (MTD) 为 600 mg;在加用长春氟宁320 mg/m2时,由于毒性的缘故而无法确定索拉非尼的MTD。3 + 4 级不良反应 (AE) 包括中性粒细胞减少症(6 名患者)、发热性中性粒细胞减少症(5 名患者)以及低钠血症(5 名患者)。在有效性可评估的患者中,总缓解率(ORR)为 41%(17 名患者中的 7 名患者),所有的部分缓解均以 1.1 版实体瘤疗效评价标准 (RECIST) 进行评估。中位总生存期 (OS) 为 7.0 个月 (1.8–41.7)。
结论。确定的推荐 II 期剂量 (RPTD) 为长春氟宁 280 mg/m2 加用索拉非尼 400 mg。索拉非尼与长春氟宁 320 mg/m2 联合用药的毒性太大。本次 mUC 二线联合治疗的 ORR 为41%,值得注意且支持进一步试验。
Discussion
For mUC patients with rapidly progressive platinum‐resistant disease, development of effective treatment options remains a challenge and an unmet medical need. The combination of vinflunine in standard dose plus dose‐escalated sorafenib was evaluated for safety and toxicity in this phase I trial in second‐line treatment of mUC. All subjects had disease progression or relapse ≤6 months following previous platinum‐based chemotherapy, reflecting a cohort of patients with platinum‐resistant disease.
The observed high rate of toxicity for patients treated with vinflunine of 320 mg/m2 is in line with previously reported phase I vinflunine combination studies, including doublets with pazopanib and pemetrexed [1], [2]. It appears as if full‐dose vinflunine doublets for patients with platinum‐progressive disease require combination with compounds with low bone marrow toxicity. Hence, the combination with sorafenib in this trial proved only to be safe and tolerable with a vinflunine starting dose of 280 mg/m2 [3], [4], [5], [6]. The most frequent AE grades 3 + 4 in this study included neutropenia, hypertension, and hyponatremia. These side effects are potentially caused by both vinflunine and sorafenib, but more frequently reported in the former except for hypertension [7], [8].
The addition of sorafenib to vinflunine did not significantly improve median OS as compared with the vinflunine registration trial (7.0 vs. 6.9 months). For patients administered vinflunine 280 mg/m2 (e.g., Eastern Cooperative Oncology Group performance status [ECOG PS] 1, creatinine clearance 40–60 mL/minute) in this study, the prognosis was most likely even worse than for the average second‐line patient treated within the vinflunine registration trial. Considering this fact, the observed ORR of 41% and disease control rate (DCR) of 71% in this study is notable, especially compared with the ORR and DCR of 8.6% and 55.1% in the vinflunine registration trial [3]. The higher response rates are plausibly attributable to the addition of sorafenib, possibly resulting in an additive effect of this specific drug combination. Further, the results of the present study are in line with the RANGE study, reporting an advantage of combining docetaxel with another vascular endothelial growth factor receptor 2 active compound, ramucirumab, thus adding evidence that selected platinum‐refractory mUC patients may benefit from concomitant standard chemotherapy plus antiangiogenic targeted therapy [9].
Within the context of this phase I trial, an RPTD for the treatment combination of vinflunine and sorafenib was identified for mUC patients with platinum‐resistant and progressive disease. The observed side effects were expected but with a higher incidence of grade 3 + 4 hyponatremia than previously reported for vinflunine and sorafenib each administered as monotherapy. Clinically meaningful disease stabilization and objective responses were observed but with large differences in OS (Figs. 1 and 3). Future trials should aim to evaluate this treatment combination in a randomized setting, define biomarkers for treatment benefit, and explore the effects in patients with both platinum‐ and immunotherapy‐resistant disease.
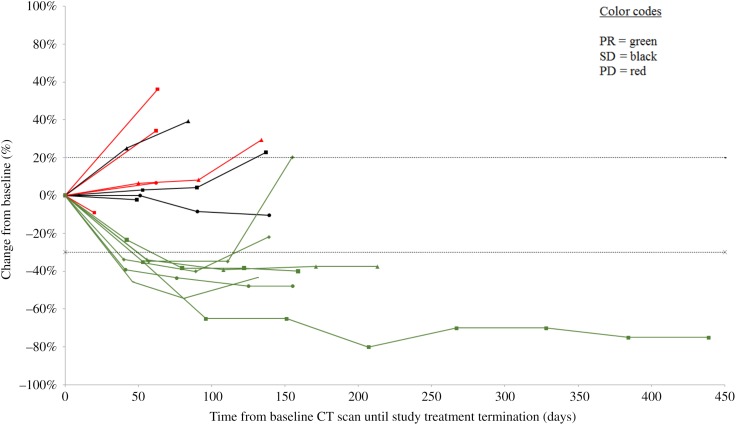
Figure 1.
Tumor response by RECIST version 1.1. Percentage change in sum of the diameters of tumor lesions from baseline.
Abbreviations: CT, computed tomography; PD, progressive disease; PR, partial response; SD, stable disease.
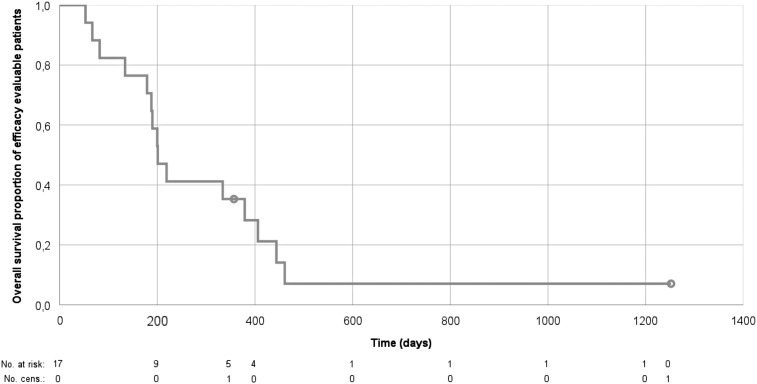
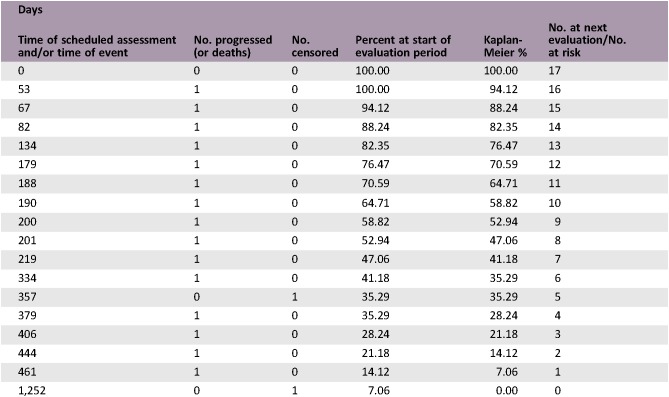
Figure 3.
Kaplan‐Meier plot of overall survival outcome. Overall survival in days from date of study assignment until recorded death among the efficacy‐evaluable patients (n = 17).
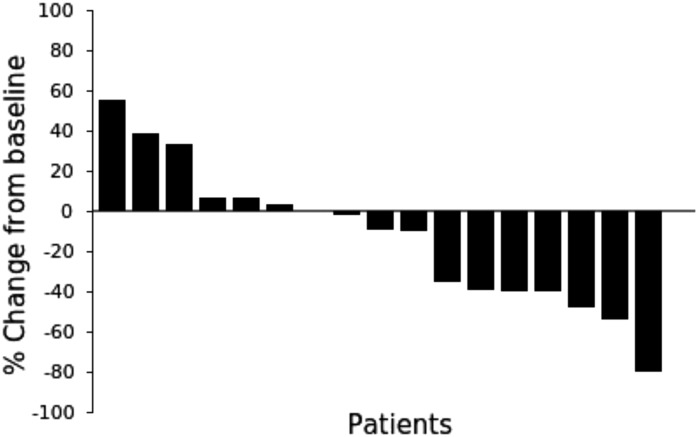
Figure 2.
Waterfall plot of individual patient's tumor response by RECIST version 1.1. Best percentage change in the sum of size (diameters) of tumor lesions from baseline until the end of study treatment. One patient (number 17) developed brain metastases (new nontarget tumor lesions) during cycle 2. No formal treatment evaluation of this patient's target lesions was performed.
Trial Information
- Disease
Bladder cancer
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
One prior regimen
- Type of Study – 1
Phase I
- Type of Study – 2
3 + 3
- Primary Endpoint
Maximum tolerated dose
- Primary Endpoint
Recommended phase II dose
- Secondary Endpoints
Efficacy; response and survival outcomes
- Additional Details of Endpoints or Study Design
- The Vinsor study was a phase I trial evaluating the treatment combination of standard‐dose vinflunine and sorafenib, in dose escalation. Patients with progressive locally advanced mUC following platinum combination‐chemotherapy were screened for inclusion at three cancer centers within the Nordic Urothelial Cancer Oncology Group collaboration, one center in Stockholm, Sweden, and two centers in Denmark, Copenhagen and Aarhus. The study was investigator‐initiated and conducted as an academic clinical trial. The trial was monitored independently at the respective sites in Sweden and Denmark. The study was approved by the regional Ethical Review Board in Stockholm (October 12, 2011, ref 2011/1398‐31/1) and by the Scientific Research Council Komité E, Region Hovedstaden, Hillerød, Denmark (August 28, 2012, ref H‐1‐2012‐079). The study was approved by the Swedish Medical Products Agency (151:2012/12127) and by the Danish Health and Medicines Authority (2012053960). Study participation was compliant with the Declaration of Helsinki. Prior to screening procedures and treatment, signed informed consent was obtained from all patients. This trial is registered with ClinicalTrials.gov, number NCT01844947.
- Eligibility Criteria: Eligible patients, aged 18–80 years, with ECOG PS 0 or 1, had a histologically confirmed diagnosis of UC (pure or mixed) of the urothelial tract. The major inclusion criteria stipulated that UC patients had a confirmed disease relapse or progression no later than at the 6‐month follow‐up visit after completion of previous platinum‐based chemotherapy. Patients were not allowed to have received more than one previous line of chemotherapy treatment for locally advanced or mUC. Alternatively, patients with mUC and contraindication to platinum‐containing chemotherapy were eligible. Any prior systemic chemotherapy needed to be permanently terminated 14 days prior to inclusion and recovery from treatment‐related toxicity (grade 1 or less). Patients needed to have measurable or nonmeasurable disease using RECIST version 1.1. Patients with predominantly adenocarcinoma, squamous cell carcinoma, or any other nontransitional cell carcinoma were excluded. Prior treatment with vinflunine was not allowed. Patients with brain metastasis or leptomeningeal involvement were not eligible. Patients with congestive heart failure (New York Heart Association class ≥III), angina pectoris, poorly controlled hypertension >150/90 mmHg, hypercalcemia, hypokalemia, or QTc time >450 ms at baseline were excluded. ECG controls were performed to exclude patients at high risk for developing arrhythmias. Included patients were required to have an acceptable hematologic function: hemoglobin ≥10 g/dL, absolute neutrophil count ≥1.0× lower limit of normal, and platelets ≥100 000 per μL; adequate hepatic function: bilirubin <1.5× upper limit of normal (ULN), and transaminases <2.5× ULN; renal function: creatinine clearance ≥40 mL/minute (measured by iohexol or 51Cr‐EDTA techniques).
- Procedures: The study subjects were treated with standard doses of vinflunine, 320 or 280 mg/m2, depending on their condition (see details below). In addition, patients were prescribed a fixed start dose of sorafenib (400, 600, or 800 mg). Patients with ECOG PS 0, age ≤74 years, presenting adequate hepatic and renal function (defined as creatinine clearance >60 mL/minute) were treated with vinflunine 320 mg/m2 intravenous (IV) on day 1, and sorafenib in 200 mg dose steps from 200 to 400 mg b.i.d. on days 2–21 every 3 weeks (Q3W). For patients with PS 1, or age 75–80 years, or previous exposure to radiation of the lower pelvic region, or with impaired renal function (creatinine clearance 40–60 mL/minute) but adequate hepatic function, the dose of vinflunine was 280 mg/m2 IV on day 1 and sorafenib as per above days 2–21, repeated every 21 days. Treatment continued until progression, unacceptable toxicity, or any other medical event requiring a stop. Pause in treatment was allowed for up to 14 days. Dose reductions were permitted as described in the protocol. Toxicity was graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Adverse events were evaluated during all treatment cycles for all patients. Study drug‐related adverse events recorded during cycle 1 and 2 served to define dose‐limiting toxicity (DLT) as hematological toxicity: grade ≥4 neutropenia (absolute neutrophil count <0.5 × 109 for ≥7 days or <0.1 × 109 for ≥3 days), or febrile neutropenia of grade ≥3 (absolute neutrophil count <1.0 × 109 and temperature ≥38.5°C), or platelet count <25 × 109/L or thrombocytopenia with bleeding or requiring platelet transfusion; and nonhematological toxicity: liver toxicity (alanine aminotransferase or aspartate aminotransferase) of grade ≥3 for >7 days, or any other grade ≥3 major organ toxicity according to CTCAE v 4.0. Any AE had to resolve to grade ≤2 within 14 days to continue study treatment. Tumor response was radiologically evaluated by computer tomography after completing every two treatment cycles. Evaluation of response to treatment was performed on measurable and/or nonmeasurable tumor lesions using RECIST v 1.1.
- Safety Assessment: Examination by physician (including performance status [World Health Organization ECOG scale] and body weight) weekly during cycle one and thereafter prior to every new treatment cycle (i.e., every 3rd week). Complete blood counts, electrolytes, and renal and hepatic function prior to and at day 8 in every treatment cycle. All patients were followed‐up by a oncology nurse at day 8 and 15 during all treatment cycles. Blood pressure was monitored weekly during the first two cycles and thereafter every 3rd week as long as the active treatment remained.
- Investigator's Analysis
Active and should be pursued further
Drug Information
- Drug 1
- Generic/Working Name
Vinflunine
- Trade Name
Javlor
- Company Name
Pierre Fabre
- Drug Type
Small molecule
- Drug Class
Tubulin/Microtubules targeting agent
- Dose
280 and 320 mg/m2
- Route
IV
- Schedule of Administration
Day 1, Q3W
- Drug 2
- Generic/Working Name
Sorafenib
- Trade Name
Nexavar
- Company Name
Bayer Healthcare AG
- Drug Type
Small molecule
- Drug Class
Raf ‐ BRAF
- Dose
400, 600, or 800 per day (200 + 200, 200 + 400, or 400 + 400) mg per flat dose
- Route
p.o.
- Schedule of Administration
Morning and evening (b.i.d.) on days 2–21, Q3W
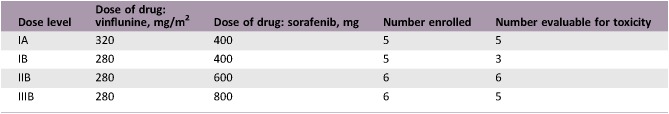
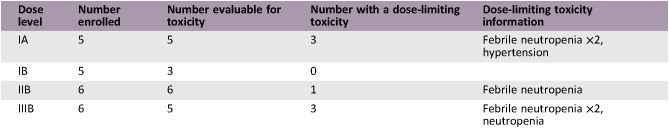
Dose Escalation Table
Patient Characteristics
- Number of Patients, Male
13
- Number of Patients, Female
9
- Stage
Metastatic urothelial cancer (all patients)
- Age
Median (range): 62.5 years, range 44–71 years
- Number of Prior Systemic Therapies
Median (range): 1, range 1–1
- Performance Status: ECOG
-
0 — 9
1 — 13
2 — 0
3 — 0
Unknown — 0
- Other
- Previous treatments included cystectomy (10 patients) and nephrectomy/ureterectomy (3). No previous surgery (9). No patient had received radiation to the pelvic region prior to study inclusion. All patients had received one line of platinum (cisplatin or carboplatin)‐containing systemic chemotherapy treatment (median 5 cycles [range 1–11]).
- Cancer Types or Histologic Subtypes
- Urothelial tract cancer (urothelial histology) ICD‐10: C66.9‐C68.9
Primary Assessment Method
- Title
Efficacy assessment
- Number of Patients Screened
69
- Number of Patients Enrolled
22
- Number of Patients Evaluable for Toxicity
19
- Number of Patients Evaluated for Efficacy
17
- Evaluation Method
RECIST 1.1
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 7 (41%)
- Response Assessment SD
n = 5 (29%)
- Response Assessment PD
n = 5 (29%)
- Response Assessment OTHER
n = 0 (0%)
- (Median) Duration Assessments PFS
4.5 months
- (Median) Duration Assessments TTP
4.2 months
- (Median) Duration assessments OS
7.0 months
- (Median) Duration Assessments Duration of Treatment
2.3 months
Kaplan‐Meier Time Units
- Outcome Notes:
- Sixty‐nine patients were screened for inclusion between April 2012 and September 2017. Screening failures included no progression on platinum within 6 months, comorbidity or insufficient PS, second‐line treatment previously administered, history of other malignancy, and prolonged QTc interval. Twenty‐two patients at three sites in two countries were included. The primary tumor was located in the bladder (18 patients), renal pelvis (2 patients), and ureter (2 patients). All included patients had confirmed histology of pure urothelial carcinoma at inclusion and disease progression within 6 months following treatment with platinum and gemcitabine. Previous surgical treatment included cystectomy in 10 patients and nephrectomy/ureterectomy in 3 patients. The median number of cycles administered was 4 (range 1–16). Median duration of study treatment was 4.1 months (0.1–14.5) among the DLT‐evaluable patients. The MTD of vinflunine 320 mg/m2 day 1 with sorafenib days 2–21 Q3W was not defined (<400 mg) because three out of five patients had a DLT in the first dose cohort. For patients treated with vinflunine 320 mg/m2, adding sorafenib, even at a dose of 400 mg daily, resulted in unacceptable toxicity. The MTD of vinflunine 280 mg/m2 day 1 with sorafenib was 600 mg (200 + 400 mg) days 2–21 Q3W. Five of eight patients with treatment‐induced hypertension received at least six cycles of treatment, and three partial responses were reported. Two of the six efficacy‐evaluable patients with skin rash had a partial response and three had stable disease on study treatment. Two patients continued with vinflunine monotherapy after study completion. Three patients received experimental treatment within clinical trial protocols and two patients received immunotherapy. At data cutoff date, October 1, 2018, two patients remained alive.
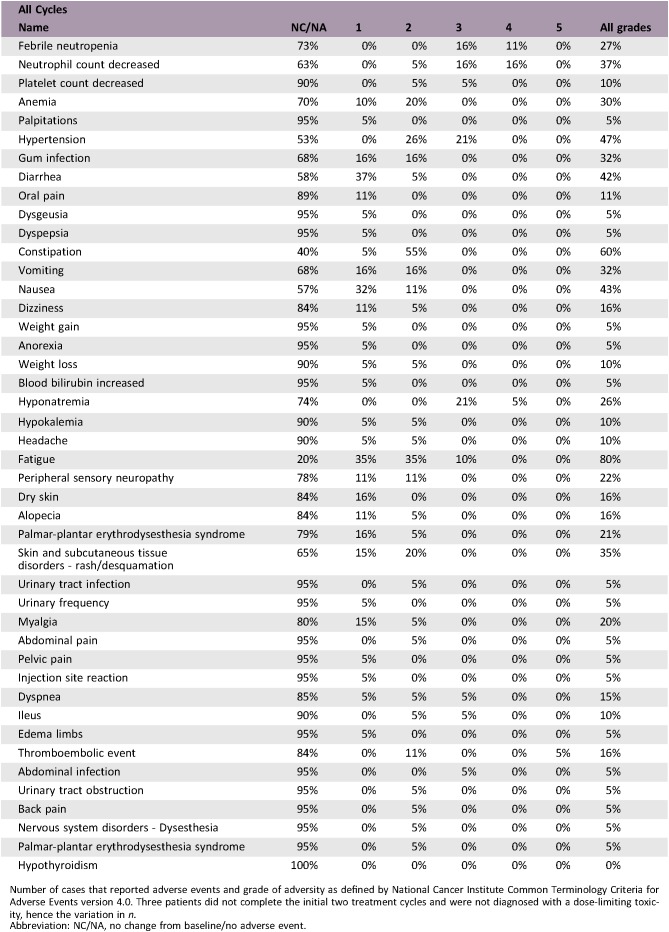
Adverse Events
Number of cases that reported adverse events and grade of adversity as defined by National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Three patients did not complete the initial two treatment cycles and were not diagnosed with a dose‐limiting toxicity, hence the variation in n.
Abbreviation: NC/NA, no change from baseline/no adverse event.
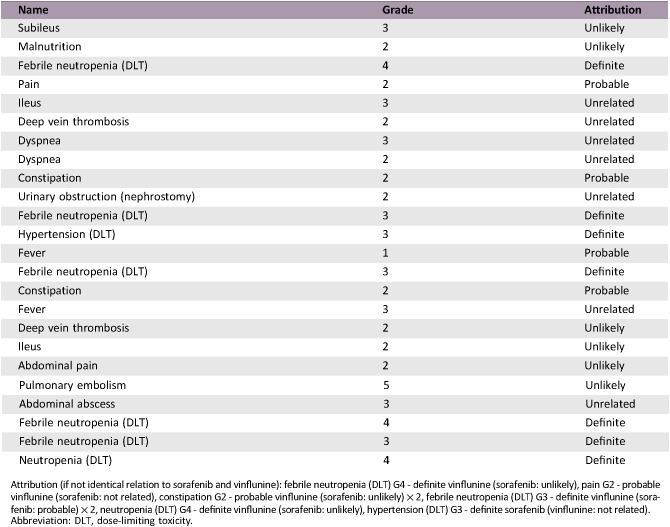
Serious Adverse Events
Attribution (if not identical relation to sorafenib and vinflunine): febrile neutropenia (DLT) G4 ‐ definite vinflunine (sorafenib: unlikely), pain G2 ‐ probable vinflunine (sorafenib: not related), constipation G2 ‐ probable vinflunine (sorafenib: unlikely) × 2, febrile neutropenia (DLT) G3 ‐ definite vinflunine (sorafenib: probable) × 2, neutropenia (DLT) G4 ‐ definite vinflunine (sorafenib: unlikely), hypertension (DLT) G3 ‐ definite sorafenib (vinflunine: not related).
Abbreviation: DLT, dose‐limiting toxicity.
Dose‐Limiting Toxicities
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Active and should be pursued further
Although the recently approved immunotherapies can induce impressive and durable responses, the majority of patients with metastatic urothelial cancer (mUC) do not have major responses. In the randomized second‐line phase III trial of pembrolizumab, the overall response rate (ORR) was limited to 21.1%, and the response rates reported for atezolizumab, durvalumab, avelumab, and nivolumab are in the same range [10], [11], [12], [13], [14]. For these postimmunotherapy patients, as well as for patients with rapidly progressive platinum‐resistant disease, further development of effective treatment options remains a challenge and an unmet medical need.
The combination of vinflunine in standard dose with dose‐escalated sorafenib was evaluated for safety and toxicity in this phase I trial for patients with mUC. All included patients in this study had disease progression or relapse within 6 months following previous platinum‐based chemotherapy, reflecting a cohort of typically platinum‐resistant patients with aggressive disease.
The observed high rate of toxicity for patients treated with a start dose of vinflunine of 320 mg/m2 is in line with previously reported phase I vinflunine combination studies, including doublets with pazopanib and pemetrexed [1], [2]. It appears as if full‐dose vinflunine doublets for patients with progressive disease after cisplatin requires combination compounds with low bone marrow toxicity. Hence, the combination with sorafenib proved only to be safe and tolerable with a vinflunine start dose of 280 mg/m2. Side effects of grade 1 and 2 were mainly gastrointestinal (constipation and diarrhea), fatigue, nausea/vomiting, and skin rash. Constipation and pain have more commonly been reported with vinflunine, whereas diarrhea is frequently seen with tyrosine kinase inhibitor therapies and fatigue with either [3], [4], [5], [6]. The top three reported adverse events (AEs) of grade ≥3 in this study included neutropenia (31.6%), hyponatremia (26.3%), and febrile neutropenia (26.3%). These side effects are potentially caused by both vinflunine and sorafenib, but are more frequent in the former except for hypertension [7], [8]. No definite treatment‐related death was recorded, but one patient had fatal pulmonary embolism (unlikely relation to study treatment). Overall, the AEs are in line with previous studies combining vinflunine and other cytotoxic agents [1], [2], [15].
The addition of sorafenib to vinflunine did not improve median overall survival (OS) as compared with the registration trial by Bellmunt et al. evaluating monotherapy with vinflunine with best supportive care (BSC) versus BSC alone (7.0 vs. 6.9 months). For the cohort of patients receiving the vinflunine start dose of 280 mg/m2 (e.g., Eastern Cooperative Oncology Group performance status 1, creatinine clearance 40–60 mL/minute), and in which the recommended phase II dose (RPTD) was defined, the prognosis is, however, most likely even worse than for the average second‐line patient treated within the vinflunine registration trial [3]. In this view, the observed ORR of 41% and DCR of 71% in this study of platinum‐refractory mUC is notable, especially compared with the ORR and DCR of 8.6% and 55.1% in the vinflunine phase III trial, and 16%–18% and 57%–67%, respectively, in previous phase II trials [3], [16], [17]. It can be speculated that the reduction of metastatic tumor burden and stabilization of disease could translate into clinical meaningful palliation and increased quality of life for some mUC patients with otherwise aggressive disease and few treatment options. Interestingly, both rash and hypertension, two side effects correlated with treatment benefit in renal cell carcinoma, were common in patients with partial response or disease stabilization. The favorable overall response rates in this study are plausibly attributable to the addition of sorafenib to vinflunine, possibly resulting in an additive effect of this specific drug combination. Further, the results of the present study are in line with the recently reported outcome of the RANGE study, reporting an advantage of combining docetaxel with another vascular endothelial growth factor receptor 2 active compound, ramucirumab, thus adding evidence that patients with platinum‐refractory mUC may benefit from combined treatment with standard chemotherapy and antiangiogenic targeted therapy [9]. Nevertheless, the overall positive response rate of this trial could still be at random considering the limited size and phase I design. In comparison, a phase II randomized study of gemcitabine and cisplatin plus sorafenib or placebo in first‐line mUC resulted in an ORR of 52.5% and DCR of 75% versus 50% and 79%, showing no additional response effect of sorafenib [18]. If this trial could be repeated, eligible patients would include those with relapse within 12 months of platinum‐containing systemic treatment, thus increasing inclusion rate. Future trials should aim to evaluate this treatment combination in a randomized setting, define biomarkers for treatment benefit, and explore the effects in patients with both platinum‐ and immunotherapy‐resistant disease.
Within the context of this phase I trial, a RPTD for the treatment combination of vinflunine and sorafenib was identified for mUC patients with platinum‐resistant and progressive disease. The observed side effects were as expected and reversible but with a higher incidence of grade 3 and 4 hyponatremia than previously reported for vinflunine and sorafenib administered as monotherapy. Clinically meaningful disease stabilization and objective responses were observed in a number of patients with poor prognosis along with interindividual variation in OS.
Table
Table 1. Baseline characteristics, including previous treatment and prognostic factors.
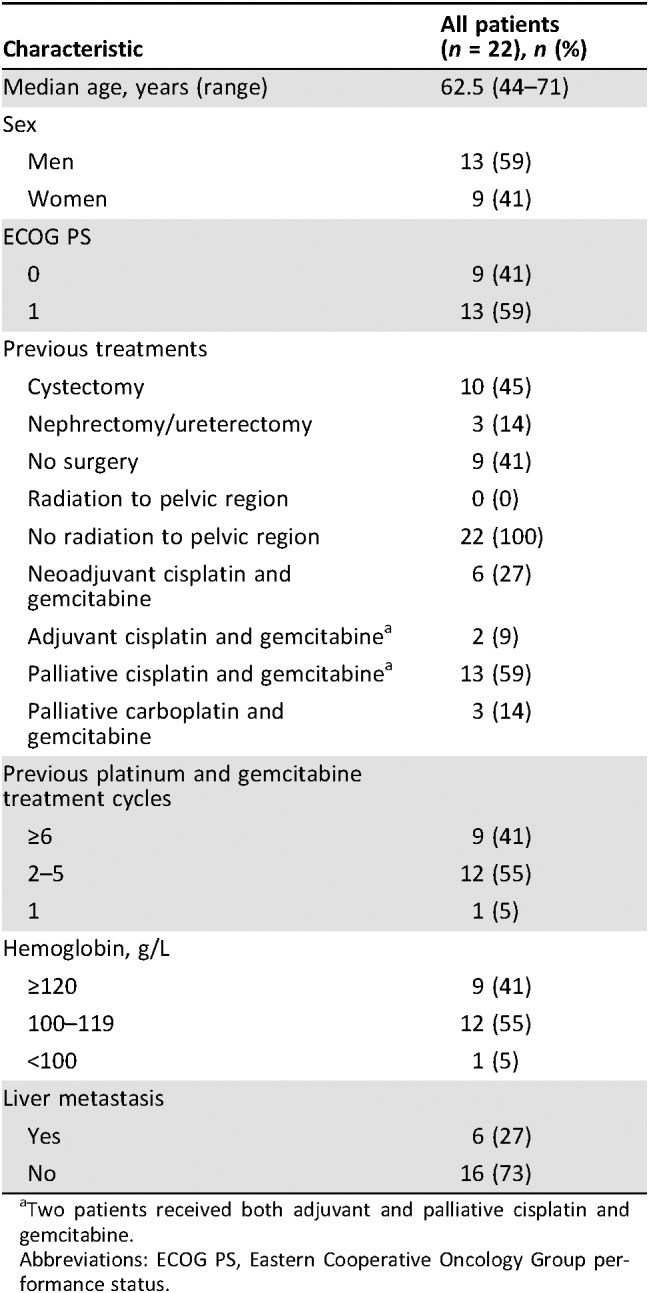
Two patients received both adjuvant and palliative cisplatin and gemcitabine.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status.
Acknowledgments
We are grateful for the administrative help of the clinical trial support staff at all three sites. We are thankful for the financial support provided by the Swedish Cancer Society, the Stockholm Cancer Society, Stockholm County Council, Pierre Fabre Pharma Norden AB, and Bayer Healthcare AG, who also provided sorafenib free of charge.
Footnotes
ClinicalTrials.gov Identifier: NCT01844947
Sponsor(s): Anders Ullén
Principal Investigator: Anders Ullén
IRB Approved: Yes
Disclosures
Carl‐Henrik Shah: Pierre Fabre Pharma Norden AB, Bayer Healthcare AG (RF); Helle Pappot: Merck Sharpe & Dohme, Roche (RF); Karin Holmsten: Pierre Fabre Pharma (RF, study/meeting expenses); Anders Ullén: Pierre Fabre Pharma (C/A), Pierre Fabre Pharma, Bayer (RF, study/meeting expenses). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Gerullis H, Eimer C, Ecke TH et al. Combined treatment with pazopanib and vinflunine in patients with advanced urothelial carcinoma refractory after first‐line therapy. Anticancer Drugs 2013;24:422–425. [DOI] [PubMed] [Google Scholar]
- 2.Pappot H, von der Maase H, Ullen A et al. Combined treatment with pemetrexed and vinflunine in patients with metastatic urothelial cell carcinoma after prior platinum‐containing chemotherapy ‐ results of an exploratory phase I study. Invest New Drugs 2018;36:615–618. [DOI] [PubMed] [Google Scholar]
- 3.Bellmunt J, Theodore C, Demkov T et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum‐containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009;27:4454–4461. [DOI] [PubMed] [Google Scholar]
- 4.Escudier B, Szczylik C, Hutson TE et al. Randomized phase II trial of first‐line treatment with sorafenib versus interferon Alfa‐2a in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:1280–1289. [DOI] [PubMed] [Google Scholar]
- 5.Brose MS, Nutting CM, Jarzab B et al. Sorafenib in radioactive iodine‐refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double‐blind, phase 3 trial. Lancet 2014;384:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–390. [DOI] [PubMed] [Google Scholar]
- 7.Summary of Product Characteristics ‐ Javlor dated 30/07/2014. Available at ema.europa.eu. Accessed October 2, 2018.
- 8.Summary of Product Characteristics ‐ Nexavar dated 30/09/2016. Available at ema.europa.eu. Accessed October 2, 2018.
- 9.Petrylak DP, de Wit R, Chi KN et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum‐based therapy (RANGE): A randomised, double‐blind, phase 3 trial. Lancet 2017;390:2266–2277. [DOI] [PubMed] [Google Scholar]
- 10.Bellmunt J, de Wit R, Vaughn DJ et al. Pembrolizumab as second‐line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel MR, Ellerton J, Infante JR et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): Pooled results from two expansion cohorts of an open‐label, phase 1 trial. Lancet Oncol 2018;19:51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powles T, O'Donnell PH, Massard C et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open‐label study. JAMA Oncol 2017;3:e172411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg JE, Hoffman‐Censits J, Powles T et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum‐based chemotherapy: A single‐arm, multicentre, phase 2 trial. Lancet 2016;387:1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma P, Retz M, Siefker‐Radtke A et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single‐arm, phase 2 trial. Lancet Oncol 2017;18:312–322. [DOI] [PubMed] [Google Scholar]
- 15.De Santis M, Wiechno PJ, Bellmunt J et al. Vinflunine‐gemcitabine versus vinflunine‐carboplatin as first‐line chemotherapy in cisplatin‐unfit patients with advanced urothelial carcinoma: results of an international randomized phase II trial (JASINT1). Ann Oncol 2016;27:449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Culine S, Theodore C, De Santis M et al. A phase II study of vinflunine in bladder cancer patients progressing after first‐line platinum‐containing regimen. Br J Cancer 2006;94:1395–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaughn DJ, Srinivas S, Stadler WM et al. Vinflunine in platinum‐pretreated patients with locally advanced or metastatic urothelial carcinoma: Results of a large phase 2 study. Cancer 2009;115:4110–4117. [DOI] [PubMed] [Google Scholar]
- 18.Krege S, Rexer H, vom Dorp F et al. Prospective randomized double‐blind multicentre phase II study comparing gemcitabine and cisplatin plus sorafenib chemotherapy with gemcitabine and cisplatin plus placebo in locally advanced and/or metastasized urothelial cancer: SUSE (AUO‐AB 31/05). BJU Int 2014;113:429–436. [DOI] [PubMed] [Google Scholar]