Abstract
Lessons Learned.
The triple combination chemotherapy of SOXIRI (S‐1/oxaliplatin/irinotecan) in patients with unresectable pancreatic ductal adenocarcinoma was an effective treatment that appeared to be better tolerated than the widely used FOLFIRINOX regimen.
SOXIRI regimen may provide an alternative approach for advanced pancreatic cancer.
Background.
In our previous phase I study, we determined the recommended dose of a biweekly S‐1, oxaliplatin, and irinotecan (SOXIRI) regimen in patients with unresectable pancreatic ductal adenocarcinoma (PDAC). This phase II study was conducted to assess the safety and clinical efficacy in patients with unresectable PDAC.
Methods.
Patients with previously untreated metastatic and locally advanced PDAC were enrolled. The primary endpoint was response rate (RR). Secondary endpoints were adverse events (AEs), progression‐free survival (PFS), and overall survival (OS). Patients received 80 mg/m2 of S‐1 twice a day for 2 weeks in alternate‐day administration, 150 mg/m2 of irinotecan on day 1, and 85 mg/m2 of oxaliplatin on day 1 of a 2‐week cycle.
Results.
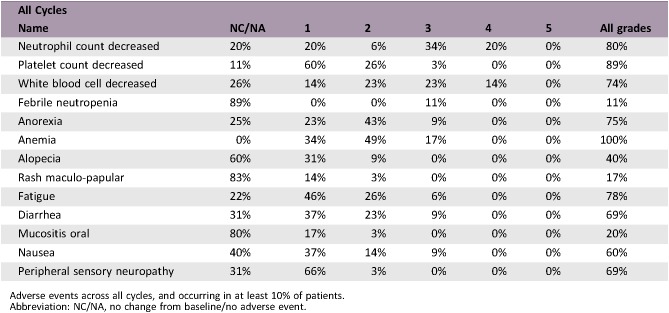
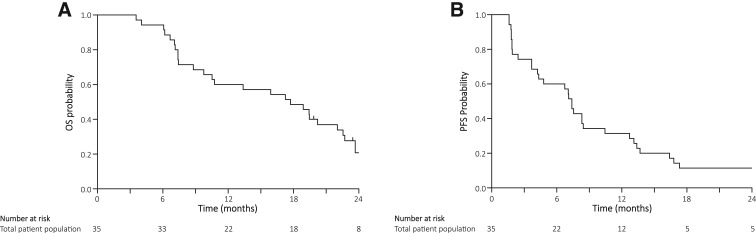
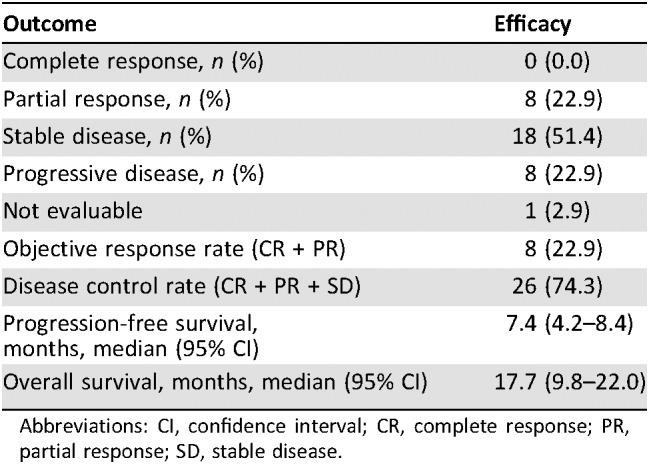
Thirty‐five enrolled patients received a median of six (range: 2–15) treatment cycles. The RR was 22.8% (95% confidence interval [CI]: 10.4–40.1); median OS, 17.7 months (95% CI: 9.8–22.0); and median PFS, 7.4 months (95% CI: 4.2–8.4). Furthermore, the median OS in patients with distant metastasis was 10.1 months, whereas that in patients with locally advanced PDAC was 22.6 months. Major grade 3 or 4 toxicity included neutropenia (54%), anemia (17%), febrile neutropenia (11%), anorexia (9%), diarrhea (9%), and nausea (9%). There were no treatment‐related deaths.
Conclusion.
SOXIRI is considered a promising and well‐tolerated regimen in patients with unresectable PDAC.
Abstract
经验获取
•SOXIRI(S‐1/奥沙利铂/伊立替康)三联化疗治疗无法切除的胰腺导管腺癌患者,似乎比目前广泛使用的 FOLFIRINOX 方案要更耐受,是一种有效的治疗方案。
•SOXIRI 方案可能为晚期胰腺癌提供另一种治疗方法。
摘要
背景。在我们之前的 I 期研究中,我们确定了两周一次的 S‐1,奥沙利铂和伊立替康 (SOXIRI) 方案的推荐剂量用于治疗患有无法切除的胰腺导管腺癌 (PDAC) 的患者。进行这项 II 期研究旨在评估无法切除的 PDAC 患者的安全性和临床疗效。
方法。招募未经治的转移性和局部晚期 PDAC 的患者。主要终点为缓解率 (RR)。次要终点为不良事件 (AE),无进展生存期 (PFS) 和总生存期 (OS)。患者接受 2 周S‐1 80 mg/m2,隔日给药,每天两次, 在第 1 天接受伊立替康 150 mg/m2 和奥沙利铂 85mg/m2,2 周为一周期。
结果。35 名入组患者接受中位数为 6(范围:2‐15 )的治疗周期。RR 为 22.8% [95% 置信区间 (CI):10.4‐40.1];中位OS,17.7 个月(95% CI:9.8‐22.0);中位PFS,7.4 个月(95% CI:4.2‐8.4)。此外,发生远处转移的患者的中位 OS 为 10.1 个月,而局部晚期 PDAC 患者的中位 OS 为 22.6 个月。主要的 3 级或 4 级毒性包括中性粒细胞减少症(54%)、贫血症(17%)、发热性中性粒细胞减少症(11%)、厌食(9%)、腹泻(9%)和恶心(9%)。无治疗相关的死亡。
结论。对于无法切除的 PDAC 患者,SOXIRI 被认为是一种颇具前景且耐受良好的治疗方案。
Discussion
Since Conroy et al. reported the significant efficacy in OS and quality of life with FOLFIRINOX compared with gemcitabine (GEM) in patients with metastatic pancreatic cancers, this regimen has been one of the most effective current standard treatments for unresectable PDAC [1]. However, this treatment carries significantly more adverse events and cannot be used in all patients with advanced pancreatic cancer [2], [3]. We previously performed a phase I study to determine the recommended dose of a biweekly S‐1, oxaliplatin, and irinotecan (SOXIRI) regimen, using S‐1 in alternate‐day administration instead of 5‐fluorouracil (5‐FU), potentially more feasible than FOLFIRINOX in patients with unresectable PDAC [4]. The recommended dose of S‐1, oxaliplatin, and irinotecan is 80, 85, and 150 mg/m2, respectively.
S‐1 is a drug combination comprising three agents at a 1:0.4:1 molar ratio: tegafur, a prodrug of 5‐FU; 5‐chloro‐2,4‐dihydroxypyridine, which blocks dihydropyrimidine dehydrogenase, the first step in 5‐FU metabolism; and potassium oxonate, which blocks the enzyme orotate phosphoribosyltransferase, thereby reducing the gastrointestinal toxicity of 5‐FU. S‐1 has regulatory approval in Japan and in Europe.
This phase II study was carried out to investigate the efficacy and safety of a SOXIRI regimen in chemotherapy‐naïve patients with unresectable PDAC. The RR, which was the primary endpoint of this study, was 22.8% (95% CI: 10.4–40.1) with the lower limit of the 95% CI being above the threshold RR of 10%. The disease control rate (74.0%) was similar to the findings of the former FOLFIRINOX phase II/III study (70.2%) [1] and the FOLFIRINOX phase II study in Japanese patients (69.4%) [3]. In addition, the median OS (17.7 months) and the median PFS (7.4 months) were also favorable (Fig. 2). Accordingly, we consider the SOXIRI regimen to be effective in patients with unresectable PDAC.
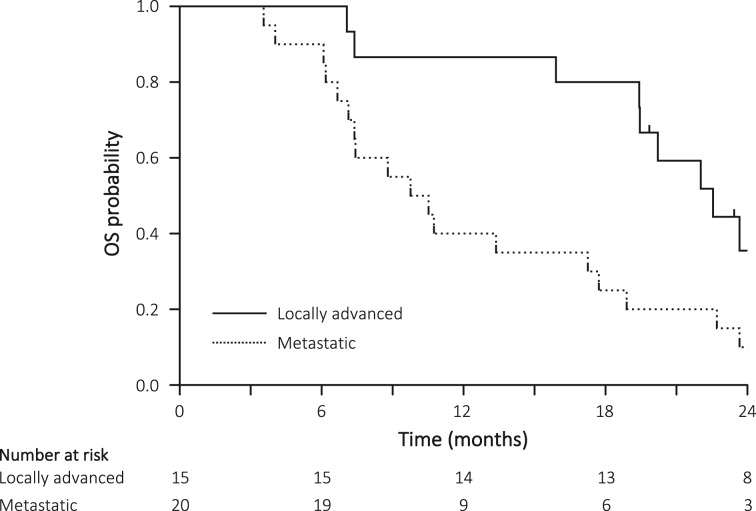
Figure 2.
OS in the locally advanced cohort (solid line) and in the metastatic cohort (dotted line).
Abbreviation: OS, overall survival.
The incidences of grade 3–4 neutropenia and febrile neutropenia in this study were lower (54% and 11%) than those in the FOLFIRINOX phase II study for Japanese patients (vs. 78% and 22%, respectively) [3] and similar to the FOLFIRINOX phase II/III study (vs. 46% and 5%, respectively) [1]. With regard to nonhematologic events, the incidence of grade 3 or 4 fatigue, nausea, neuropathy (6%, 9%, 0%) was similar to that in the FOLFIRINOX phase II study for Japanese patients (vs. 0%, 0%, 5.6%, respectively) [3] and lower than in the Conroy et al. FOLFIRINOX phase II/III study (vs. 5.6%, 23.6%, 9%, respectively) [1]. Taken together, the toxicities including hematological and nonhematological events are relatively mild compared with previous studies.
In summary, our present findings suggest that the SOXIRI regimen appears comparable efficacy with acceptable AEs in patients with unresectable PDAC. Thus, the SOXIRI regimen could be an optional regimen for the FOLFIRINOX in patients with unresectable PDAC.
Trial Information
- Disease
Pancreatic cancer
- Disease
Advanced cancer
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
None
- Type of Study ‐ 1
Phase II
- Type of Study ‐ 2
Single arm
- Primary Endpoint
Overall response rate
- Secondary Endpoint
Safety
- Secondary Endpoint
Progression‐free survival
- Secondary Endpoint
Overall survival
- Additional Details of Endpoints or Study Design
- Study Design: The study was an open‐label, single‐arm, phase II study that was carried out at Nara Medical University and Kansai Medical University in Japan. Patients received 80 mg/m2 of S‐1 twice a day for 2 weeks in alternate‐day administration, 150 mg/m2 of irinotecan on day 1, and 85 mg/m2 of oxaliplatin on day 1 of a 2‐week cycle. The RR was the primary endpoint of this study and was determined using RECIST version 1.1. The response status was evaluated at least every 6 weeks. For the confirmation of complete response or partial response, computed tomography was carried out after 4 weeks when the tumor response was measured. OS and PFS were assessed by determining the length of time from the day on which SOXIRI was started to the day of assessment.
- Patients: Eligibility criteria were as follows: locally advanced or metastatic PDAC with at least one measurable lesion; a histologically or cytologically proven diagnosis of adenocarcinoma; no prior chemotherapy or radiotherapy for PDAC; age between 20 and 75 years; an Eastern Cooperative Oncology Group performance status of 1 or less; adequate hematological, hepatic, and renal functions defined by hemoglobin ≥8.0 g/dL, absolute neutrophil count ≥1,500/mm3, platelet count ≥100,000/mm3, total bilirubin ≤1.5 × the upper normal limit (UNL) of the institution, serum transaminases (aspartate aminotransferase, alanine aminotransferase), and alkaline phosphatase ≤2.5 × UNL (or in case of biliary stent, bilirubin ≤3.0 × UNL); and serum creatinine level ≤1.2 mg/dL. Patients were excluded if they had uridine diphosphate glucuronosyltransferase (UGT) genetic polymorphisms of homozygous UGT1A1*28 or UGT1A1*6 or heterozygous UGT1A1*6 and UGT1A1*28.
- Statistical analysis: The expected and threshold RRs for the SOXIRI regimen were set as 30% and 10%, respectively. If an exact binomial test was carried out at a one‐sided significance level of 2.5%, according to the binomial distribution for the null hypothesis that the threshold RR was 10%, a sample size of 29 subjects would result in a power of 80%. Accordingly, the target sample size was set at 35 subjects, to account for exclusion of patients. The median OS and corresponding 95% confidence intervals for OS were estimated using the Kaplan‐Meier method. We used the JMP software program (version 11.0; SAS Institute, Inc., Cary, NC) for the statistical analyses.
- Investigator's Analysis
Active and should be pursued further
Drug Information
- Drug 1
- Generic/Working Name
S‐1
- Drug Type
Small molecule
- Drug Class
Antimetabolite
- Dose
80 mg/m2
- Route
p.o.
- Schedule of Administration
Twice a day every 2 weeks
- Drug 2
- Generic/Working Name
Oxaliplatin
- Drug Type
Small molecule
- Drug Class
Platinum compound
- Dose
85 mg/m2
- Route
IV
- Schedule of Administration
Day 1 of a 2‐week cycle
- Drug 3
- Generic/Working Name
Irinotecan
- Drug Type
Small molecule
- Drug Class
Topoisomerase I
- Dose
150 mg/m2
- Route
IV
- Schedule of Administration
Day 1 of a 2‐week cycle
Patient Characteristics
- Number of Patients, Male
19
- Number of Patients, Female
16
- Stage
Locally advanced only: 15; metastatic disease: 20
- Age
Median (range): 65 (42–75)
- Number of Prior Systemic Therapies
Median (range): none
- Performance Status: ECOG
-
0 — 28
1 — 7
2 —
3 —
Unknown —
- Other
Additional details for patient and treatment characteristics can be found in Table 1 and 2.
Table 1. Tumor response (n = 35).
Abbreviations: CI, confidence interval; CR, complete response; PR, partial response; SD, stable disease.
Table 2. Patient characteristics.
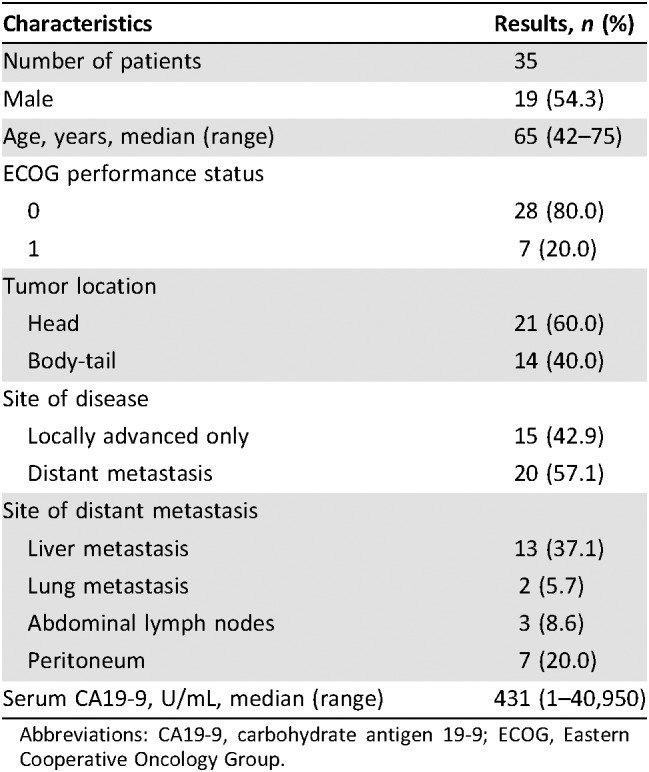
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; ECOG, Eastern Cooperative Oncology Group.
Primary Assessment Method
- Title
- Number of Patients Screened
35
- Number of Patients Enrolled
35
- Number of Patients Evaluable for Toxicity
35
- Number of Patients Evaluated for Efficacy
34
- Evaluation Method
RECIST 1.1
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 8 (23%)
- Response Assessment SD
n = 18 (51%)
- Response Assessment PD
n = 8 (23%)
- Response Assessment OTHER
n = 1 (3%)
- (Median) Duration Assessments PFS
7.4 months, CI: 4.2–8.4
- (Median) Duration Assessments OS
17.7 months, CI: 9.8–22.0
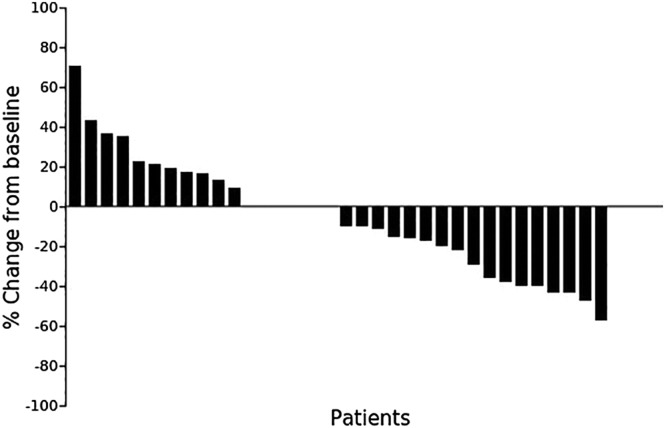
Maximum percentage changes from baseline in size of target lesions according to the RECIST criteria (n = 34).
Adverse Events
Adverse events across all cycles, and occurring in at least 10% of patients.
Abbreviation: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Active and should be pursued further
In 2011, FOLFIRINOX was compared with GEM in a phase II/III study involving patients with metastatic pancreatic ductal adenocarcinoma (PDAC) [1]. FOLFIRINOX exhibited a significant improvement in overall survival (OS) and quality of life versus GEM. The FOLFIRINOX regimen requires close monitoring and must be limited to patients with good performance status because of significant toxicity [2]. In a phase II study conducted in Japan, febrile neutropenia occurred during the first cycle in 22.2% of patients [3]. Because of the high incidence of severe neutropenia and febrile neutropenia, the relative dose intensity of bolus 5‐fluorouracil (5‐FU) was only 15.9% [3]. These significant toxicities in Japan, as well as in Western countries, resulted in modification of the FOLFIRINOX regimen; several studies using reduced irinotecan or omitting the bolus fluorouracil have been reported [5], [6], [7].
S‐1 (TS‐1; Taiho Pharmaceutical Co., Ltd., Tokyo, Japan) is an oral fluoropyrimidine derivative in which tegafur is combined with two 5‐chloro‐2, 4‐dihydroxypyridine modulators and oteracil potassium, a potentiator of 5‐FU's antitumor activity that also decreases gastrointestinal toxicity [8]. In Japan, clinical studies of S‐1 have reported promising results in various cancer [9], [10]. With respect to pancreatic cancer, combination chemotherapy with GEM plus S‐1 is reportedly well tolerated and active against advanced PDAC [11], [12]. In addition, S‐1 is often used as a substitute of 5‐FU in various combination chemotherapies such as 5‐FU and folinic acid with either oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) for metastatic colorectal cancer [13], [14]. Although S‐1 is associated with various nonhematologic toxicities, including anorexia, nausea, vomiting, stomatitis, and diarrhea [10], [15], [16], we adopted the alternate‐day dosage of S‐1 as a substitute for 5‐FU in this study because previous studies about gastric cancer reported that this method of administration reduced adverse effects without compromising efficacy [17], [18]. Although a multicenter, randomized, phase II study comparing S‐1 alternate‐day therapy with the standard daily regimen in patients with unresectable advanced PDAC failed to demonstrate noninferiority to the daily treatment (9.4 months, 95% confidence interval 7.6–11.1 vs. 10.4 months, 7.9–12.8) in OS rate. On the other hand, the incidence of anorexia, fatigue, neutropenia, pigmentation, and pneumonitis was significantly lower in alternate‐day treatment compared with daily treatment and comparable median OS (9.4 months) [19]. Considering the comparable effectiveness and the improved safety of SOXIRI in this study, the S‐1 alternate‐day treatment instead of 5‐FU in the FOLFIRINOX regimen may be of value in the treatment of pancreatic cancer.
There are several limitations in this study. First, the current study included a relatively high population of patients with locally advanced pancreatic cancer, which might lead to the favorable OS when we compared all cohorts with previous studies using FOLFIRINOX. However, the median survival time of patients with metastatic PDAC (10.1 months) in this study was comparable to previous studies [1], [3], [7] (Figure 2). Therefore, we thought the SOXIRI regimen was not inferior to the original regimen. Second, although this study was conducted at dual institutions in Japan, almost the entire study population comprised an only Asian population, that is, Japanese patients. With respect to the applicability of this regimen in white patients, it should be noted that there are some pharmacogenomic differences regarding S‐1 metabolism between Asians and whites. The most important component of S‐1, tegafur, is converted to cytotoxic fluorouracil by cytochrome P450 (CYP) enzyme [20]. One of the CYP family, CYP2A6, plays a central role in this conversion process [21]. Because of the different polymorphisms in the CYP2A6 gene among Asians and whites [22], [23], the efficacy of CYP2A6 in whites is higher than that in Asians. Therefore, the area under the curve of fluorouracil in whites is higher than that in Asians [24] and the tolerability against the same dose of S‐1 in whites is lower. However, the alternate‐day administration of S‐1, which is milder than the usual administration, may reduce AEs in white populations. Furthermore, the study population is relatively small. Further larger studies are needed to confirm our findings.
In conclusion, the substitution of S‐1 for infusional 5‐fluorouracil, in combination with irinotecan and oxaliplatin, retained the efficacy with good tolerability in the first‐line treatment of unresectable PDAC. Our findings may provide an alternative approach for reducing patient burden with comparable efficacy to the original FOLFIRINOX for advanced PDAC.
Figures and Table
Figure 1.
Kaplan‐Meier curves for overall survival and progression‐free survival. (A): Overall survival. (B): Progression‐free survival.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
We acknowledge Dr. Takahashi Inoue for the statistical analysis. This study was not funded by any foundation, company, industry, or other external sources.
Footnotes
ClinicalTrials.gov Identifier: UMIN000014339
Sponsor(s): None
Principal Investigator: Takahiro Akahori
IRB Approved: Yes
Disclosures
The authors indicated no financial relationships.
References
- 1.Conroy T, Desseigne F, Ychou M et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 2.Saif MW, Chabot J. Chemotherapy: Metastatic pancreatic cancer—Is FOLFIRINOX the new standard? Nat Rev Clin Oncol 2011;8:452–453. [DOI] [PubMed] [Google Scholar]
- 3.Okusaka T, Ikeda M, Fukutomi A et al. Phase II study of FOLFIRINOX for chemotherapy‐naive Japanese patients with metastatic pancreatic cancer. Cancer Sci 2014;105:1321–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yanagimoto H, Satoi S, Sho M et al. Phase I study assessing the feasibility of the triple combination chemotherapy of SOXIRI (S‐1/oxaliplatin/irinotecan) in patients with unresectable pancreatic ductal adenocarcinoma. Cancer Chemother Pharmacol 2016;77:35–41. [DOI] [PubMed] [Google Scholar]
- 5.Mahaseth H, Brutcher E, Kauh J et al. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas 2013;42:1311–1315. [DOI] [PubMed] [Google Scholar]
- 6.Oikonomopoulos GM, Syrigos KN, Skoura E et al. FOLFIRINOX: From the ACCORD study to 2014. JOP 2014;15:103–105. [DOI] [PubMed] [Google Scholar]
- 7.Stein SM, James ES, Deng Y et al. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer 2016;114:737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirasaka T, Shimamato Y, Ohshimo H et al. Development of a novel form of an oral 5‐fluorouracil derivative (S‐1) directed to the potentiation of the tumor selective cytotoxicity of 5‐fluorouracil by two biochemical modulators. Anticancer Drugs 1996;7:548–557. [DOI] [PubMed] [Google Scholar]
- 9.Koizumi W, Kurihara M, Nakano S et al. Phase II study of S‐1, a novel oral derivative of 5‐fluorouracil, in advanced gastric cancer. For the S‐1 Cooperative Gastric Cancer Study Group. Oncology 2000;58:191–197. [DOI] [PubMed] [Google Scholar]
- 10.Ohtsu A, Baba H, Sakata Y et al. Phase II study of S‐1, a novel oral fluorophyrimidine derivative, in patients with metastatic colorectal carcinoma. S‐1 Cooperative Colorectal Carcinoma Study Group. Br J Cancer 2000;83:141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura K, Yamaguchi T, Ishihara T et al. Phase II trial of oral S‐1 combined with gemcitabine in metastatic pancreatic cancer. Br J Cancer 2006;94:1575–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueno H, Okusaka T, Ikeda M et al. A phase I study of combination chemotherapy with gemcitabine and oral S‐1 for advanced pancreatic cancer. Oncology 2005;69:421–427. [DOI] [PubMed] [Google Scholar]
- 13.Muro K, Boku N, Shimada Y et al. Irinotecan plus S‐1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second‐line chemotherapy for metastatic colorectal cancer: A randomised phase 2/3 non‐inferiority study (FIRIS study). Lancet Oncol 2010;11:853–860. [DOI] [PubMed] [Google Scholar]
- 14.Yamada Y, Tahara M, Miya T et al. Phase I/II study of oxaliplatin with oral S‐1 as first‐line therapy for patients with metastatic colorectal cancer. Br J Cancer 2008;98:1034–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawahara M, Furuse K, Segawa Y et al. Phase II study of S‐1, a novel oral fluorouracil, in advanced non‐small‐cell lung cancer. Br J Cancer 2001;85:939–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakata Y, Ohtsu A, Horikoshi N et al. Late phase II study of novel oral fluoropyrimidine anticancer drug S‐1 (1 M tegafur‐0.4 M gimestat‐1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer 1998;34:1715–1720. [DOI] [PubMed] [Google Scholar]
- 17.Arai W, Hosoya Y, Hyodo M et al. Alternate‐day oral therapy with TS‐1 for advanced gastric cancer. Int J Clin Oncol 2004;9:143–148. [DOI] [PubMed] [Google Scholar]
- 18.Arai W, Hosoya Y, Haruta H et al. Comparison of alternate‐day versus consecutive‐day treatment with S‐1: Assessment of tumor growth inhibition and toxicity reduction in gastric cancer cell lines in vitro and in vivo. Int J Clin Oncol 2008;13:515–520. [DOI] [PubMed] [Google Scholar]
- 19.Yamaue H, Shimizu A, Hagiwara Y et al. Multicenter, randomized, open‐label phase II study comparing S‐1 alternate‐day oral therapy with the standard daily regimen as a first‐line treatment in patients with unresectable advanced pancreatic cancer. Cancer Chemother Pharmacol 2017;79:813–823. [DOI] [PubMed] [Google Scholar]
- 20.El Sayed YM, Sadee W. Metabolic activation of ftorafur [R,S‐1‐(tetrahydro‐2‐furanyl)‐5‐fluorouracil]: The microsomal oxidative pathway. Biochem Pharmacol 1982;31:3006–3008. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda K, Yoshisue K, Matsushima E et al. Bioactivation of tegafur to 5‐fluorouracil is catalyzed by cytochrome P‐450 2A6 in human liver microsomes in vitro. Clin Cancer Res 2000;6:4409–4415. [PubMed] [Google Scholar]
- 22.van der Weide J, Steijns LS. Cytochrome P450 enzyme system: Genetic polymorphisms and impact on clinical pharmacology. Ann Clin Biochem 1999;36:722–729. [DOI] [PubMed] [Google Scholar]
- 23.Daigo S, Takahashi Y, Fujieda M et al. A novel mutant allele of the CYP2A6 gene (CYP2A6*11) found in a cancer patient who showed poor metabolic phenotype towards tegafur. Pharmacogenetics 2002;12:299–306. [DOI] [PubMed] [Google Scholar]
- 24.Ajani JA, Faust J, Ikeda K et al. Phase I pharmacokinetic study of S‐1 plus cisplatin in patients with advanced gastric carcinoma. J Clin Oncol 2005;23:6957–6965. [DOI] [PubMed] [Google Scholar]