Immune‐related adverse events are challenging to treat, with one of the most serious consequences being pneumonitis. This article reports an illustrative case of a patient with metastatic lung cancer.
Abstract
Immunotherapy has changed the field of oncology around the world with the approval of immune checkpoint inhibitors for a number of tumor types over the last 5 years. However, immune‐mediated adverse events can be challenging and difficult to treat, with one of the most dire consequences being immune‐mediated pneumonitis.
Key Points.
Rapid intervention and aggressive management for grade 3 or greater pneumonitis
Slow taper of steroids and also recommend pneumocystis carinii pneumonia prophylaxis
Monitor carefully for a pneumonitis flare with steroid taper, which can occur in the absence of resuming anti‐programmed cell death protein 1 (PD‐1) [1], and do not resume anti‐PD‐1 therapy until completely off steroids and no clinical or radiologic evidence of recurrence
Consider observation without anti‐PD‐1 resumption—in this case, durable response was maintained even without resuming anti‐PD‐1 therapy.
Patient Story
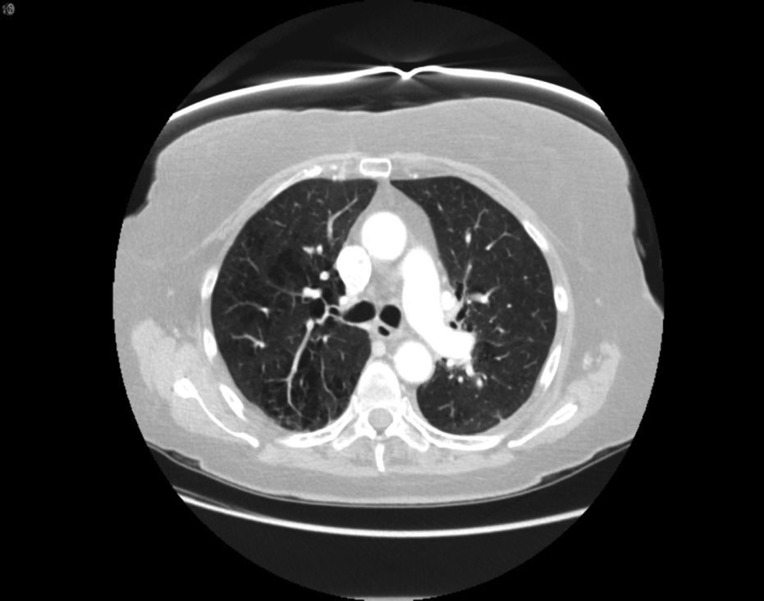
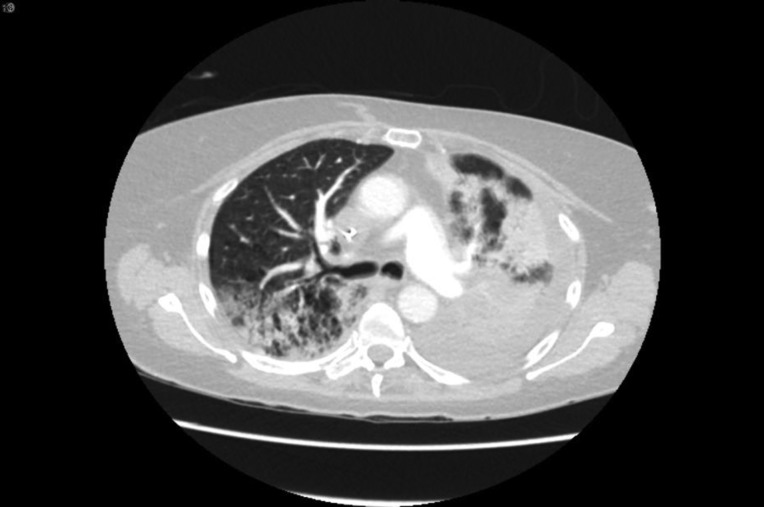
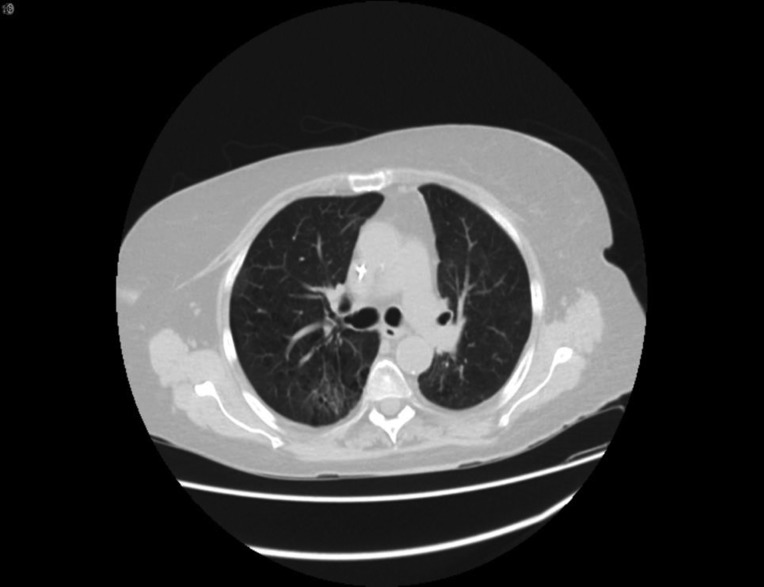
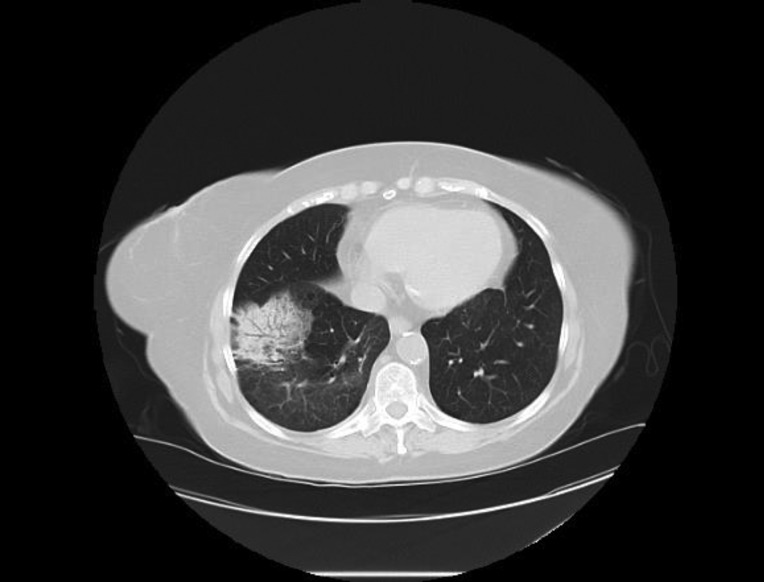
The patient is a 76‐year‐old woman and former smoker and was diagnosed with metastatic poorly differentiated lung adenocarcinoma to the brain. At time of diagnosis, a left frontal brain metastatic lesion and a 4.2‐cm left upper lobe (LUL) mass were found; she underwent Gamma Knife (Elekta; Stockholm, Sweden) radiation to both the brain lesion and the LUL mass, followed by carboplatin and pemetrexed chemotherapy. Follow‐up scan showed progression of LUL mass, and nivolumab was started. After her third dose, she reported worsening shortness of breath and an oxygen saturation of 82%. A computed tomography (CT) chest revealed findings consistent with immune checkpoint inhibitor (ICI)‐related pneumonitis (Fig. 1, pretreatment and Fig. 2, postnivolumab). Radiographically, there was noted to be extensive airspace consolidation and interlobular septal thickening involving the posterior right upper lobe, left upper lobe, and left lower lobe. Given the symptomatic and new hypoxia, the patient was admitted to the hospital, and she was started on intravenous methylprednisolone 1 mg/kg b.i.d., leading to a rapid improvement in her symptoms and radiographic improvement (Fig 3). Further nivolumab was held, and 2 weeks after completing a 4‐month steroid taper, she reported a new cough, upper respiratory symptoms, and wheezing, and home oxygen saturation measurements were down to 94%, consistent with recurrent pneumonitis or a “pneumonitis flare” (Fig. 4). An additional two attempts at tapering prednisone were unsuccessful, with recurrence of symptoms and CT findings of pneumonitis. The prednisone since has been maintained on prednisone 10 mg per day without recurrence of symptoms and no disease progression.
Figure 1.
Pretreatment.
Figure 2.
After programmed cell death protein 1 antibody treatment.
Figure 3.
On steroids.
Figure 4.
Pneumonitis recurrence of steroids.
Case Discussion
Pneumonitis is an adverse event of special interest, with fatalities observed in initial phase 1 trials [1]. The challenge is greater in patients with lung cancer given the baseline alteration in lung parenchyma related to underlying thoracic malignancy but preexisting chronic obstructive pulmonary disease, chronic pulmonary infections and associated scarring, and prior thoracic radiation therapy and associated fibrosis are also challenges. With greater experience and foreknowledge of this possible toxicity, early diagnosis and aggressive management have greatly reduced risk. In this case, the extensive pneumonitis and hypoxia warranted immediate admission and intravenous high‐dose steroids with rapid improvement. Despite a prolonged steroid taper, the pneumonitis recurred or “flared,” and ultimately, the patient was not able to taper off steroids completely.
Background
The mechanism of immune checkpoint inhibitor pneumonitis is not understood, and biopsy samples from ICI‐related pneumonitis have been mostly nonspecific with infiltration of dendritic cells, macrophages, and lymphocytes [2], [3]. It is postulated that the inflammatory response with anti‐CTLA‐4 leading to pneumonitis involves the infiltration of deregulated effector T cells in the interstitium of the lung [4]. Programmed cell death ligand (PD‐L) 2 may also play a role with anti‐ programmed cell death protein 1 (PD‐1) antibodies as it is responsible for regulating the development of respiratory tolerance in the lung [3], [5], [6].
Pulmonary Toxicity: Review of Pneumonitis Incidence
Pneumonitis is an uncommon immune‐related adverse event (irAE), but given its serious course, it has become one of particular interest. A meta‐analysis by Nishino et al. reviewing 20 anti‐PD‐1 studies with 4,496 patients (12 melanoma, 5 non‐small cell lung cancer [NSCLC], and 3 renal cell carcinoma [RCC]) showed that the overall incidence of all‐grade pneumonitis was less with anti‐PD‐1 monotherapy than with combination (2.7% and 6.6%, respectively) and even less with anti‐CTLA‐4 monotherapy (<1%) [7], [8]. However, overall, anti‐PD‐1 had a higher incidence of any grade pneumonitis versus anti‐PD‐L1 antibodies [9], [10]. With regard to the anti‐PD‐1 antibodies, the risk of pneumonitis was not different between nivolumab and pembrolizumab [9]. Whether risk of pulmonary toxicity is greater with certain malignancies has been a point of some debate. Nishino et el. [8] reported that tumor type does play a role in developing pneumonitis, showing a higher incidence of all‐grade pneumonitis with NSCLC and renal cell carcinoma compared with melanoma (4.1% and 4.1%, respectively, vs. 1.6%). In contrast, other analyses showed no statistically significant difference correlating the incidence between tumor types and all‐grade pneumonitis [9], [11], [12]. Of particular significance, pneumonitis‐related deaths may be greater in patients with NSCLC compared with other tumor types given these patients’ preexisting comorbidities [9], [13], [14], [15], [16]. One of the largest NSCLC data sets studied was a retrospective analysis of 550 patients treated with anti‐PD‐1 in KEYNOTE‐001 showing the incidence of any grade, and grade 3–5 pneumonitis was 3.8 and 3%, respectively [17]. The incidence of pneumonitis was higher in patients with a prior history of chronic obstructive pulmonary disease or prior chest radiation therapy. In contrast, prior smoking history and prior chemotherapy did not impact the pneumonitis incidence. The median time to onset of pneumonitis was 57 days with a considerable range (4–393). The fact that this wide of a range was also observed in additional studies underscores the constant vigilance required to monitor for toxicities at any point during the course of therapy (and even after therapy is completed) [8], [9], [15], [18], [19]. Immune checkpoint blockade with anti‐CTLA‐4 and anti‐PD‐1 has been shown to be a highly effective combination with U.S. Food and Drug Administration approvals in melanoma and renal cell carcinoma. As mentioned above, combination immune checkpoint blockade has been associated with increased autoimmune toxicities in general, including pneumonitis, and needs to be monitored carefully [7], [8], [11], [13], [14], [15], [20], [21].
There have been reported cases of grade 3 pneumonitis during anti‐PD‐1 treatment after thoracic radiotherapy, leading to the question of whether or not thoracic radiotherapy or chemoradiotherapy could be a direct risk factor in patients who go on to receive an ICI, as was presented in this case review [22]. Interestingly, in the PACIFIC study of durvalumab (anti‐PD‐1) versus placebo as consolidation treatment after concurrent chemotherapy and radiation therapy for stage III non‐small cell lung cancer, rates of grade 3–4 pneumonitis were similar in both groups (3.4% and 2.6%, respectively), as was any grade pneumonitis (33.9% and 24.8%, respectively) [23]. Of all the adverse events in the trial, pneumonitis, radiation pneumonitis, or pneumonia was the most common toxicity that led to the discontinuation of treatment [23].
Pulmonary Toxicity: Radiographic Patterns, Signs, and Symptoms, and Management of Pneumonitis
Patients can present with either very specific classic signs of pneumonitis to no signs at all with incidental findings on chest CT. Although chest x‐ray can be used as diagnostic workup per the ASCO guidelines, it should be noted that chest x‐rays are not sufficiently sensitive, and there is a possibility that no new radiographic findings will be found in patients with pneumonitis [15]. For many investigators, CT chest is the preferred imaging of choice. The treatment course a patient may need seems to correlate to radiographic patterns. For example, the pattern of an acute interstitial pneumonia (AIP), acute respiratory distress syndrome (ARDS), and cryptogenic organizing pneumonia (COP) pattern usually will indicate worsening respiratory symptoms requiring admission. On CT scan, AIP/ARDS will most likely present as diffuse ground glass opacities (GGO), consolidation, and lung volume loss, and COP pattern presents with multifocal consolidation and GGOs generally with peripheral distribution [24]. Nonspecific interstitial pneumonia pattern and hypersensitive pneumonitis patterns usually present with milder, low‐grade symptoms that can safely be treated in an outpatient setting [24]. These radiographic features seem to be similar across other studies and case reports [1], [15], [16], [19], [25], [26], [27]. Of note, patients with NSCLC were more likely to develop COP pattern pneumonitis, which can advance to a higher grade pneumonitis requiring high‐dose corticosteroid treatment [15], [24].
A radiographic finding of sarcoid‐like granulomatosis or sarcoid‐like lymphadenopathy, although rare, has been reported in patients receiving both anti‐CTLA‐4 and anti‐PD‐1 [28], [29], [30], [31], [32], [33], [34]. Nishino et al. reported four cases all with very similar radiographic findings that were consistent with sarcoid‐like granulomatosis in the absence of new or enlarging lymphadenopathy and that were asymptomatic clinically, which resolved with holding ICI treatment only [32]. This unique characteristic of a focal consolidation or “new lesion” with surrounding GGO is of high importance to identify for patient management that is less aggressive than treating pneumonitis.
The extremely variable imaging findings can make it challenging to diagnose, and clinicians should be alert to the typical signs and symptoms: dyspnea, cough, fever, chest pain, wheezing, or hypoxia (significant change in SP02 defined as ≥5% drop from baseline at rest) [15], [18], [35]. Onset of hypoxia has shown to be a more progressive symptom leading to respiratory failure [18], [36]. In general, the workup for patients with potential pneumonitis would include CT scan imaging, pulse oximetry and, for grade 2 or higher, an infectious workup [7]. Grade 1 and 2 pneumonitis can safely be managed in the outpatient setting with discontinuation of treatment and, depending on the extent of radiographic findings, either observation or starting corticosteroids. Given the potential rapid escalation of pneumonitis, grade 3 or higher usually require hospital admission and intravenous (IV) corticosteroids [7], [15], [20]. Most symptoms begin to improve within 2–7 days. However if this is not the case, additional immunosuppression should be considered, such as infliximab or mycophenolate [19]. The rationale for aggressive intervention with additional immunosuppression is the risk of chronic high‐dose steroid immunosuppression. Although pneumonitis can be life threatening, bacterial, viral, and fungal superinfection in the setting of high‐dose steroids can be equally dangerous. Of note, data reviewed by Brahmer et al. [7] found that patients who receive combination immunotherapy may be less likely to have a resolution of their irAE when compared with patients who received monotherapy. Outpatient treatment with corticosteroids is typically prednisone 1 mg/kg daily, with follow‐up imaging approximately 2–3 weeks later to ensure radiologic improvement and subsequent slow taper of steroids over a total period of 6 weeks. These evaluations and timelines are variable depending on the severity of pneumonitis. Refractory pneumonitis or pneumonitis flare can be a complication when trying to taper steroids requiring a close follow‐up. Our case review is an example of this scenario.
Bronchoscopy may be recommended in select cases in which differentiation between disease progression versus pneumonitis is required [7]. In most cases, however, the rapidity of new radiographic findings allow distinguishing from disease progression and delaying treatment for a bronchoscopic assessment would not be recommended. Even though pathology of those patients with pneumonitis may show lymphocytes, macrophages, and dendritic cells, there is no pathognomonic feature used to diagnose immune‐related pneumonitis [7], [15]. However, if the patient is not responding to the current treatment, a bronchoscopy may be warranted. Additionally, for patients receiving a prolonged course of corticosteroids, a bronchoscopy may be helpful to identify a superimposed infection.
There is suggestive evidence of a correlation between pneumonitis and efficacy of treatment. In one study of 915 patients treated with immune checkpoint blockade, 43 patients developed pneumonitis and of these, 25 had a complete response/partial response and 14 had stable disease [15], so one could speculate an association. However, responders typically have a greater duration of exposure, leading to bias of this data.
An academic concern of treating physicians is abrogating treatment benefit with steroid immunosuppression. However, this reservation is not founded, and early diagnosis and treatment with corticosteroids is imperative to curtail potentially life threatening pneumonitis. Data to date have not shown that immunosuppression with corticosteroids administered to treat an immune‐mediated adverse event reduces the efficacy of ICI treatment [20], [37].
Rechallenging patients after resolution of a grade 1 or 2 pneumonitis can be considered but may not be necessary and should be evaluated on a case‐by‐case basis taking into consideration whether the benefit outweighs the risk. Data in a group of patients with lung cancer showed that 33% of patients rechallenged with ICI after resolution of pneumonitis were at higher risk to develop a recurrence of pneumonitis or a new irAE [38]. It should be noted that there has been a clear distinction made between a pneumonitis flare and a “pneumonitis recurrence.” A pneumonitis flare occurs after or during a steroid taper and manifests with a similar radiographic pattern as the initial episode as in our case discussion, whereas a recurrent pneumonitis occurs after an ICI treatment rechallenge [24]. Of note, for those patients with a complete response or partial response prior to developing an irAE, progression‐free survival was similar in the discontinued group when compared with the retreatment group, suggesting it may be appropriate to observe and consider retreatment only if disease progression subsequently occurs [38].
Conclusion
With the growing approval of immune checkpoint blockades across many cancer types, immune‐mediated toxicities are becoming more commonplace and require a skill set distinct from traditional chemotherapy agents and targeted therapies. Pneumonitis continues to represent a significant patient risk, and constant vigilance for early detection is required. Treating physicians should monitor oxygen saturation at every visit and have a low threshold for obtaining a CT scan to rule out pneumonitis. Early intervention and patient and family education are critical in the management of pneumonitis.
Footnotes
For Further Reading: Nethanel Asher, Edith M. Marom, Guy Ben‐Betzalel et al. Recurrent Pneumonitis in Patients with Melanoma Treated with Immune Checkpoint Inhibitors. The Oncologist 2019;24:640–647.
Implications for Practice: This article sheds light on a poorly described immune‐related adverse event: recurrent pneumonitis despite complete discontinuation of immunotherapy (unprovoked), in patients with advanced melanoma.
Author Contributions
Conception/design: Vanessa A. Reed, Naiyer Rizvi
Provision of study material or patients: Vanessa A. Reed, Naiyer Rizvi
Manuscript writing: Vanessa A. Reed, Naiyer Rizvi
Final approval of manuscript: Vanessa A. Reed, Naiyer Rizvi
Disclosures
Naiyer Rizvi: Merck, AstraZeneca, Roche, Bristol‐Myers Squibb, Novartis, Pfizer, Eli Lilly & Co, Abbvie, Merck KGaA, Regeneron (C/A), Gritstone Oncology, ARMO Biosciences (OI), Neogenomics (SAB), Memorial Sloan Kettering Cancer Center (IP; patent PCT/US2015/062208). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Nishino M, Chambers ES, Chong CR et al. Anti‐PD‐1 inhibitor‐related pneumonitis in non‐small cell lung cancer. Cancer Immunol Res 2016;4:289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delaunay M, Cadranel J, Lusque A et al. Immune‐checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J 2017;50:1700050. [DOI] [PubMed] [Google Scholar]
- 3.Latchman Y, Wood CR, Chernova T et al. PD‐L2 is a second ligand for PD‐1 and inhibits T cell activation. Nat Immunol 2001;2:261–268. [DOI] [PubMed] [Google Scholar]
- 4.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabchi S, Messier C, Blais N. Immune‐mediated respiratory adverse events of checkpoint inhibitors. Curr Opin Oncol 2016;28:269–277. [DOI] [PubMed] [Google Scholar]
- 6.Xiao Y, Yu S, Zhu B et al. RGMb is a novel binding partner for PD‐L2 and its engagement with PD‐L2 promotes respiratory tolerance. J Exp Med 2014;211:943–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishino M, Giobbie‐Hurder A, Hatabu H et al. Incidence of programmed cell death 1 inhibitor‐related pneumonitis in patients with advanced cancer: A systematic review and meta‐analysis. JAMA Oncol 2016;2:1607–1616. [DOI] [PubMed] [Google Scholar]
- 9.Khunger M, Rakshit S, Pasupuleti V et al. Incidence of pneumonitis with use of programmed death 1 and programmed death‐ligand 1 inhibitors in non‐small cell lung cancer: A systematic review and meta‐analysis of trials. Chest 2017;152:271–281. [DOI] [PubMed] [Google Scholar]
- 10.Pillai RN, Behera M, Owonikoko TK et al. Comparison of the toxicity profile of PD‐1 versus PD‐L1 inhibitors in non‐small cell lung cancer: A systematic analysis of the literature. Cancer 2018;124:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel‐Rahman O, Fouad M. Risk of pneumonitis in cancer patients treated with immune checkpoint inhibitors: A meta‐analysis. Ther Adv Respir Dis 2016;10:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Liang F, Zhu J et al. Risk of pneumonitis associated with programmed cell death 1 inhibitors in cancer patients: A Meta‐analysis. Mol Cancer Ther 2017;16:1588–1595. [DOI] [PubMed] [Google Scholar]
- 13.Garon EB, Rizvi NA, Hui R et al.; KEYNOTE‐001 Investigators. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 14.Gettinger SN, Horn L, Gandhi L et al. Overall survival and long‐term safety of nivolumab (anti‐programmed death 1 antibody, BMS‐936558, ONO‐4538) in patients with previously treated advanced non‐small‐cell lung cancer. J Clin Oncol 2015;33:2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naidoo J, Wang X, Woo KM et al. Pneumonitis in patients treated with anti‐programmed death‐1/programmed death ligand 1 therapy. J Clin Oncol 2017;35:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishino M, Ramaiya NH, Awad MM et al. PD‐1 inhibitor‐related pneumonitis in advanced cancer patients: Radiographic patterns and clinical course. Clin Cancer Res 2016;22:6051–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahn MJ, Gandhi L, et Hamid O al. Risk of pneumonitis in patients with advanced NSCLC treated with pembrolizumab in KEYNOTE‐001. Ann Oncol 2015;26(suppl 9):ix140. [Google Scholar]
- 18.Chuzi S, Tavora F, Cruz M et al. Clinical features, diagnostic challenges, and management strategies in checkpoint inhibitor‐related pneumonitis. Cancer Manag Res 2017;9:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishino M, Sholl LM, Hodi FS et al. Anti‐PD‐1‐related pneumonitis during cancer immunotherapy. New Engl J Med 2015;373:288–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber JS, Hodi FS, Wolchok JD et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol 2017;35:785–792. [DOI] [PubMed] [Google Scholar]
- 21.Naidoo J, Page DB, Li BT et al. Toxicities of the anti‐PD‐1 and anti‐PD‐L1 immune checkpoint antibodies. Ann Oncol 2015;26:2375–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manapov F, Roengvoraphoj O, Dantes M et al. Pneumonitis in irradiated lungs after nivolumab: A brief communication and review of the literature. J Immunother 2018; 41:96–99. [DOI] [PubMed] [Google Scholar]
- 23.Antonia SJ, Villegas A, Daniel D et al.; PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med 2017;377:1919–1929. [DOI] [PubMed] [Google Scholar]
- 24.Nishino M, Hatabu H, Sholl LM et al. Thoracic complications of precision cancer therapies: A Practical guide for radiologists in the new era of cancer care. Radiographics 2017;37:1371–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balaji A, Verde F, Suresh K et al. Pneumonitis from anti‐PD‐1/ PD‐L1 therapy. Oncology (Williston Park) 2017;31:739‐746, 754. [PubMed] [Google Scholar]
- 26.Barjaktarevic IZ, Qadir N, Suri A et al. Organizing pneumonia as a side effect of ipilimumab treatment of melanoma. Chest 2013;143:858–861. [DOI] [PubMed] [Google Scholar]
- 27.O'Kane GM, Labbé C, Doherty MK et al. Monitoring and management of immune‐related adverse events associated with programmed cell death protein‐1 axis inhibitors in lung cancer. The Oncologist 2017;22:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berthod G, Lazor R, Letovanec I et al. Pulmonary sarcoid‐like granulomatosis induced by ipilimumab. J Clin Oncol 2012;30:e156–159. [DOI] [PubMed] [Google Scholar]
- 29.Cousin S, Toulmonde M, Kind M et al. Pulmonary sarcoidosis induced by the anti‐PD1 monoclonal antibody pembrolizumab. Ann Oncol 2016;27:1178–1179. [DOI] [PubMed] [Google Scholar]
- 30.Danlos FX, Pagès C, Baroudjian B et al. Nivolumab‐induced sarcoid‐like granulomatous reaction in a patient with advanced melanoma. Chest. 2016;149:e133–136. [DOI] [PubMed] [Google Scholar]
- 31.Montaudié H, Pradelli J, Passeron T et al. Pulmonary sarcoid‐like granulomatosis induced by nivolumab. Br J Dermatol 2017;176:1060–1063. [DOI] [PubMed] [Google Scholar]
- 32.Nishino M, Sholl LM, Awad MM et al. Sarcoid‐Like Granulomatosis of the lung related to immune‐checkpoint inhibitors: Distinct clinical and imaging features of a unique immune‐related adverse event. Cancer Immunol Res 2018;6:630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reuss JE, Kunk PR, Stowman AM et al. Sarcoidosis in the setting of combination ipilimumab and nivolumab immunotherapy: A case report & review of the literature. J Immunother Cancer 2016;4:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tirumani SH, Ramaiya NH, Keraliya A et al. Radiographic profiling of immune‐related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res 2015;3:1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassel JC, Heinzerling L, Aberle J et al. Combined immune checkpoint blockade (anti‐PD‐1/anti‐CTLA‐4): Evaluation and management of adverse drug reactions. Cancer Tret Rev 2017;57:36–49. [DOI] [PubMed] [Google Scholar]
- 36.Friedman CF, Proverbs‐Singh TA, Postow MA. Treatment of the immune‐related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncol 2016;2:1346–1353. [DOI] [PubMed] [Google Scholar]
- 37.Horvat TZ, Adel NG, Dang TO et al. Immune‐related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 2015;33:3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santini FC, Rizvi H, Wilkins O et al. Safety of retreatment with immunotherapy after immune‐related toxicity in patients with lung cancers treated with anti‐PD(L)‐1 therapy. Journal Clin Oncol 2017;35(suppl 15):9012a. [Google Scholar]