Abstract
Purpose
The ability to predict and detect clinical and subclinical nephrotoxicity early in the course of therapy has the potential to improve long-term outcomes in cancer patients receiving cisplatin chemotherapy. Pharmacokinetic parameters could serve as predictors of cisplatin-induced nephrotoxicity.
Methods
Participants [n=13] were treated with a one-hour cisplatin infusion [30–75 mg/m2]. Blood was collected pre-dose and up to 6 h post-dose. Urinary biomarkers [KIM-1, calbindin, clusterin, GST-pi, β2M, albumin, NGAL, osteopontin, clusterin, MCP-1, cystatin C and TFF3] were measured at baseline, day 3 and day 10. Total and unbound platinum concentrations were measured using ICP/MS. Noncompartmental analysis was performed and correlation and regression analyses evaluated the relationships between platinum pharmacokinetics and nephrotoxicity.
Results
Peak platinum urinary concentrations correlated with urinary levels of KIM-1, calbindin, clusterin, GST-pi, β2M, albumin, NGAL, osteopontin, clusterin, cystatin C and TFF3 at day 10. Unbound platinum plasma concentrations at 2 h also correlated with urinary clusterin, β2M, cystatin C, NGAL, osteopontin, and TFF3 at day 3. Regression analyses suggested 2 h total plasma platinum concentrations greater than 2,000 ng/ml and peak urinary platinum concentrations above 24,000 ng/ml may serve as potential approximations for elevated risk of nephrotoxicity. Platinum area under the plasma concentration time curve was associated with serum creatinine and estimated glomerular filtration rate.
Conclusions
Peak plasma and urinary platinum concentrations and pharmacokinetic parameters were associated with risk of subclinical cisplatin-induced kidney injury as assessed using novel urinary biomarkers. Future studies will examine these relationships in larger clinical populations of cisplatin-induced acute kidney injury.
Keywords: Cisplatin, Pharmacokinetics, Biomarkers, Nephrotoxicity
Introduction
Cisplatin is a drug used extensively to treat head and neck, testicular, lung, and ovarian cancers, among others. Although a potent and effective drug, cisplatin-induced nephrotoxicity remains a limitation to its clinical use [1]. Up to one-third of patients receiving cisplatin treatment develop acute kidney injury [AKI] during the course of treatment [2]. Despite efforts to confront this challenge, such as the development of platinum analogs and the use of hydration during therapy, nephrotoxicity remains a limitation in cisplatin-based therapies. The ability to predict which patients are most susceptible and to detect kidney injury early following treatment may improve outcomes in cancer patients treated with cisplatin.
Determination of kidney injury traditionally has relied on serum creatinine [SCr] and estimated glomerular filtration rate [eGFR] [3]. However, the use of SCr can be insensitive and delayed. In addition, eGFR values are often maintained within normal ranges even after a 50% decrease in renal functioning mass [4]. Because of the inherent limitations of SCr [secretion by transporters, dependency on muscle mass, etc.] and Cr-based calculations for detecting kidney injury, recent efforts have focused on the development and validation of a novel set of biomarkers. In 2008, the FDA approved seven novel urinary proteins [kidney injury molecule 1 [KIM-1], clusterin, albumin, total protein, beta 2-microglobulin [β2M], cystatin C, and trefoil factor 3 [TFF3]] for preclinical assessment of nephrotoxicity [5]. In rats, these biomarkers were found to outperform SCr and blood urea nitrogen [BUN] in detecting early toxicity [6, 7]. Clinical studies in humans have shown high sensitivity in detecting clinical AKI with these biomarkers, as assessed by area under the receiver operating characteristic curves [8]. Time-dependent changes in the urinary excretion of kidney injury biomarkers in patients without overt nephrotoxicity [e.g., subclinical toxicity] were recently described following treatment with cisplatin [9].
Pharmacokinetic parameters may also represent important biomarkers for predicting cisplatin kidney injury. Previous studies have demonstrated associations between two pharmacokinetic parameters, maximal plasma concentrations [Cmax] and area under the plasma concentration versus time curve [AUC], and cisplatin-induced nephrotoxicity [10–12]. While these studies evaluated relationships between traditional clinical meaures [SCr and BUN] and pharmacokinetic parameters, relationships between novel urinary biomarkers and cisplatin disposition have not been described. Elucidating these relationships could improve cisplatin dosing regimens and facilitate early detection of clinical and subclinical nephrotoxicity. In the present study, we performed a noncompartmental pharmacokinetic analysis of cisplatin disposition in cancer patients and evaluated relationships between key pharmacokinetic parameters and traditional and novel biomarkers of kidney injury.
Methods
Study Patients
Patients [n=13] undergoing outpatient chemotherapy with intravenous cisplatin [doses greater than 25 mg/m2] from the University of Colorado Cancer Center were recruited for this study. Notable exclusion criteria included history of organ transplantation/kidney dialysis, diagnosis of kidney cancer, previous exposure to platinum-based chemotherapy, and concurrent exposure to other known nephrotoxins (with the exception of contrast agents) within 30 days prior to cisplatin treatment. Clinical data (demographics, cisplatin doses, type of cancer, contrast agent administration, and labs) were collected on each patient. All subjects provided informed consent and protocols were approved by the Institutional Review Board. Additional information pertaining to study design and inclusion/exclusion criteria have been previously described [9].
Specimen Collection
Blood was collected just prior to and at 0.5, 1, 1.5, 2, 3, 4 and 6 h after beginning a one-hour cisplatin intravenous infusion. Urine was collected at 0–2 h, 2–4 h, and 4–6 h, and spot urines were collected at days 3 and 10. Urinary biomarkers [KIM-1, calbindin, clusterin, glutathione s-transferase pi [GST-pi], β2M, albumin, neutrophil gelatinase-associated lipocalin [NGAL], osteopontin, clusterin, monocyte chemoattractant protein-1 [MCP-1], cystatin C and and trefoil factor 3 [TFF3]] were measured at baseline, day 3 [range: 2–5 days] and day 10 [range: 5–10 days]. eGFR [reported in mL/min/1.73 m2] was calculated using the Modification of Diet in Renal Disease [MDRD] equation [13]. SCr was obtained between 7–21 days after the first dose per the decision of the treating physician. Urinary biomarkers were quantified using Bio-Plex multiplex assays [BioRad], as previously described [9]. Absolute biomarker concentrations were used for the current analyses, given that time-dependent changes in absolute biomarker concentrations were similar to those normalized to urinary creatinine [9].
Platinum Measurement
Total and unbound platinum [Pt] were measured in plasma and total Pt was measured in urine. To measure unbound Pt concentrations, proteins were precipitated from blood samples through the addition of cold trichloroacetic acid [TCA, 20%]. The precipitation method is cost-effective and has been shown to produce similar results compared to routine plasma ultrafiltration in the literature [14] and in pilot studies in our laboratories. After placing on ice for 10 min, samples were centrifuged at 4000 x g for 10 min at 4°C. The supernatant was assayed for unbound Pt. Total and unbound Pt concentrations were quantified utilizing inductively coupled plasma mass spectrometry [ICP/MS]. Briefly, EMD OmniTace Ultra high-purity nitric acid [VWR, Radnor, PA] was added to plasma/urine for total Pt or the supernatant for unbound Pt analysis. A CEM Mars X microwave system [CEM Corp, Matthews, NC] was then used to digest the mixture prior to ICP/MS [Nu Instruments Attom HR-ICPMS] [15]. Pt concentrations were determined using mass to charge ratio (m/z) of 195. The Pt dynamic range was 3 orders of magnitude of linearity, with standards of 0.05 to 5 ppb Pt in solution.
Pharmacokinetic Analysis
A noncompartmental pharmacokinetic analysis of total and unbound Pt was performed using Phoenix , with the linear up-log down method for AUC calculations. AUC0–6, AUC0−∞, Cmax, elimination rate constant [λ], half-life [t1/2], total body clearance [Cl] and volume of distribution [Vd] were calculated. Amount of total Pt in urine was determined by multiplying urinary concentration by volume of each collection period [0–2, 2–4, 4–6 h]. Renal clearance was calculated by dividing total amount in urine [0–6 h] by AUC0–6.
Other Assessments
Spearman correlations between Pt pharmacokinetic parameters and urine Pt assessments versus urinary biomarkers were performed to determine significant associations [P<0.05]. For each biomarker that was found to be statistically significant, linear regression was used to generate equations for the outcome of Pt urinary concentration and pharmacokinetic parameters. Utilizing previous reports of the range of biomarkers in normal and cancer populations [4], predicted urinary concentrations at the lower and upper limit of the reported ranges were obtained. A forward/backward selection process was used [SAS®, Cary, NC] to generate multiple regression equations of the most influential biomarkers that predict urinary Pt concentrations and other Pt pharmacokinetic parameters. First, collinearity of each biomarker in the model was assessed by evaluating the variance inflation factor [VIF] [16]. Biomarkers with VIF greater than 10 were eliminated from the model in a stepwise manner, leaving only the biomarkers with VIF below 10 for the final forward/backward selection analysis.
A median split was used to categorize subjects as having “high” or “low” urinary concentrations of each biomarker. For each biomarker, the mean 2 h total plasma Pt concentration was calculated to generate predictions of an estimated Pt plasma concentration that corresponded to higher risk of kidney toxicity.
Results
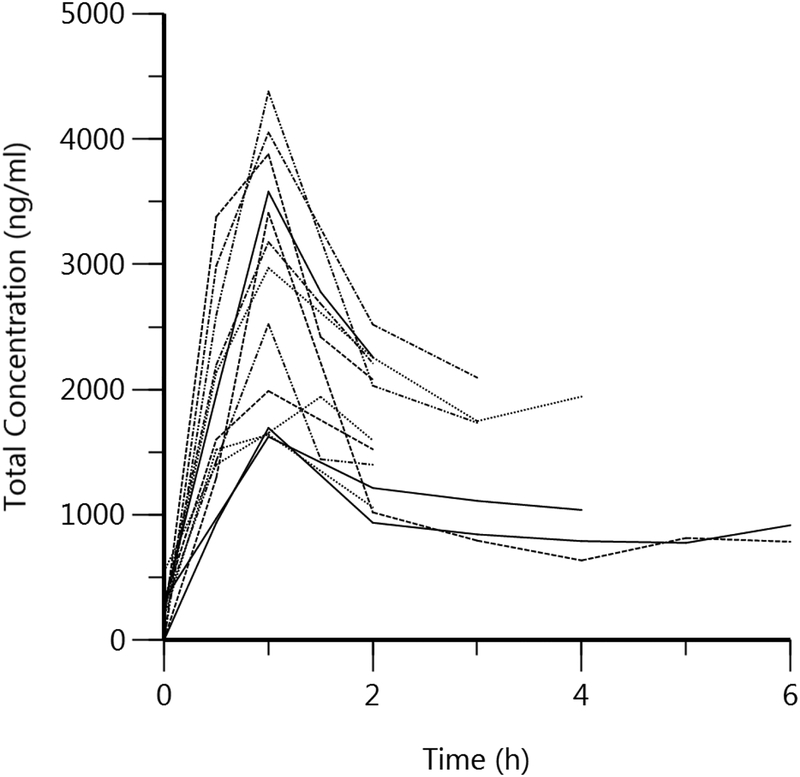
Patient characteristics are shown in Table 1. The mean (SD) age of participants was 58 (10) years and the majority were Caucasian [11/13] and male [8/13]. Mean cisplatin dose was 53.5 (20) mg/m2. The distribution of cancer types were head/neck (n = 6), digestive (n = 3), genital (n = 1), bone (n = 1), bladder/pelvis (n = 1). Intravenous contrast agents were administered to a total of three patients. Individual patient concentration vs. time curves for total and unbound Pt are depicted in Figure 1. Noncompartmental pharmacokinetic results for total and unbound Pt are shown in Table 2. Renal clearance was estimated as 3.4 ± 1.2 l/h. Unbound Pt exhibited a higher Cl and Vd, shorter T1/2, and lower AUC and Cmax values as compared to total Pt.
Table 1.
Patient Characteristics [n=13 subjects]
| Demographics | |
|---|---|
| Mean ± SD or N [%] | |
| Age [years] | 58 ± 10 |
| Ethnicity | |
| Caucasian | 11 [85%] |
| Other | 2 [15%] |
| Gender | |
| Male | 8 [61%] |
| Female | 5 [39%] |
| Body Surface Area [m2] | 1.9 ± 0.29 |
| Cisplatin Dose [mg/m2] | 53.5 ± 19 |
| Baseline SCr [mg/dL] | 0.91 ± 0.2 |
| Change in SCr after first dose | 0.02 ± 0.12 |
| Baseline BUN [mg/dL] | 15 ± 5 |
| Change in BUN after first dose [mg/dL] | −0.2 ± 4 |
| Baseline eGFR [mL/min/1.73m2] | 88 ±18 |
| Change in eGFR after first dose [mL/min/1.73m2] |
−2 ± 16 |
Abbreviations: BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; SCr, serum creatinine; SD, standard deviation.
Fig 1.
Plasma Concentration vs. Time Curve of 1a) Total Platinum and 1b) Unbound Platinum for 13 patients
Table 2.
Platinum [Pt] Pharmacokinetic Parameters [n=13 patients]
| Pharmacokinetic Parameter | Total Pt | Unbound Pt |
|---|---|---|
| Cmax [ng/ml] | 2,836 ± 992 | 874 ± 369 |
| λ [h−1] | 0.11 ± 0.09 | 1.05 ± 0.70 |
| Half-life [h] | 13.5 ± 16.0 | 1.3 ± 1.3 |
| AUC0–6 [ng*h/ml] | 5,151 ± 1950 | 1,037 ± 425 |
| AUC0−∞ [ng*h/ml] | 33,058 ± 22,878 | 1,280 ± 435 |
| Volume of Distribution [l] | 55.3 ± 15.1 | 135 ± 51 |
| Total Body Clearance [l/h] | 5.4 ± 3.6 | 91 ± 18.0 |
| Renal Clearance [l/h] | 3.4 ± 1.2 | Not measured |
| Cmax [at 0–2 h] in Urine [μg/ml] | 20.9 ± 12.2 | Not measured |
Dtabta represents mean ± standard deviation
Abbreviations: λ, elimination rate constant; AUC, area under the plasma concentration vs. time curve; Cmax, maximum concentration in plasma
Assessments of Relationships between Pt Pharmacokinetics and Biomarkers
Urinary biomarker concentrations at baseline and Days 3 and 10 post-dose are shown in Supplementary Table 1. Urinary creatinine data is also provided. Correlations between peak Pt urinary concentrations, occurring within the first two hours of collection [0–2 h], and urinary biomarkers demonstrated highest correlations at day 10. Total peak Pt urinary concentrations were strongly correlated with KIM-1, calbindin, clusterin, β2M, albumin, NGAL, osteopontin, clusterin, cystatin C and TFF3 at day 10 [Table 3]. Unbound Pt plasma concentrations at 2 h were correlated with urinary clusterin [r = 0.73, P = 0.02], β2M [r=0.81, P=0.0072], cystatin C [r = 0.83, P = 0.005], NGAL [r = 0.68, P = 0.04], osteopontin [r = 0.92, P =0.0005], TFF3 [r = 0.78, P = 0.01], albumin [r =0.78, P = 0.01] at day 3. Unbound Pt plasma concentrations at 2 h were correlated with osteopontin [r = 0.68, P = 0.04] and GST-pi [r = 0.73, P =0.02] at day 10 [Table 3].
Table 3.
Correlations between Total Peak [2-hour] Plasma and Urinary Platinum Concentrations and Urinary Biomarker Concentrations at Days 3 and 10
| Day 3 | Day 10 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urinary Pt | Plasma Pt | Urinary Pt | Plasma Pt | |||||||||
| R2 | R | P-value | R2 | R | P-value | R2 | r | P-value | R2 | r | P-value | |
| TFF-3 | 0.081 | 0.283 | 0.46 | 0.618 | 0.786 | 0.014 | 0.811 | 0.9 | <0.001 | 0.160 | 0.400 | 0.286 |
| Albumin | 0.203 | 0.45 | 0.224 | 0.613 | 0.783 | 0.013 | 0.797 | 0.893 | <0.001 | 0.339 | 0.583 | 0.099 |
| NGAL | 0.007 | 0.083 | 0.433 | 0.466 | 0.683 | 0.042 | 0.797 | 0.893 | <0.001 | 0.380 | 0.617 | 0.077 |
| Osteopontin | 0.123 | 0.35 | 0.356 | 0.840 | 0.917 | <0.001 | 0.785 | 0.886 | <0.001 | 0.466 | 0.683 | 0.042 |
| Calbindin | 0.16 | 0.4 | 0.286 | 0.514 | 0.717 | 0.030 | 0.777 | 0.882 | <0.001 | 0.339 | 0.583 | 0.099 |
| Cystatin C | 0.09 | 0.3 | 0.433 | 0.693 | 0.833 | 0.005 | 0.742 | 0.862 | 0.001 | 0.360 | 0.600 | 0.088 |
| IL-18 | 0.048 | −0.219 | 0.572 | 0.130 | 0.361 | 0.339 | 0.742 | 0.862 | 0.001 | 0.414 | 0.644 | 0.061 |
| KIM-1 | 0.284 | 0.533 | 0.139 | 0.902 | 0.95 | <0.001 | 0.641 | 0.8 | 0.005 | 0.233 | 0.483 | 0.188 |
| Clusterin | 0.054 | 0.233 | 0.546 | 0.537 | 0.733 | 0.025 | 0.582 | 0.763 | 0.008 | 0.09 | 0.300 | 0.433 |
| β2M | 0.49 | 0.7 | 0.036 | 0.667 | 0.817 | 0.007 | 0.250 | 0.500 | 0.170 | 0.033 | 0.183 | 0.637 |
| GST-pi | 0.001 | −0.033 | 0.932 | 0.321 | 0.567 | 0.111 | 0.527 | 0.433 | 0.244 | 0.537 | 0.733 | 0.025 |
| MCP-1 | 0.0003 | −0.016 | 0.966 | 0.494 | 0.703 | 0.035 | 0.027 | 0.164 | 0.337 | 0.1470 | 0.383 | 0.309 |
Abbreviations: β2M, beta 2-microglobulin; GST-pi, glutathione S-transferase-pi; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; MCP-1, monocyte chemoattractant protein-1; NGAL, neutrophil gelatinase-associated lipocalin; TFF3, trefoil factor 3.
Unbound Pt AUC0–6 negatively correlated with the absolute change in BUN after the first dose [r = −0.60, P = 0.03]. Total AUC0−∞ correlated with change in SCr [r = 0.93, P =0.008] and change in eGFR [r = −0.81, P = 0.04] after the first dose. Cmax positively correlated with total urinary Pt in the first two hours of collection [r = 0.61, P = 0.04] and negatively correlated with baseline KIM-1 [r = −0.64, P = 0.03] and GSTpi [r = −0.65, P = 0.03].
Predictions of Urinary and Plasma Pt Concentration and Risk of Kidney Injury
A multiple regression analysis was conducted to determine predictive outcomes of urinary total Pt concentrations with combinations of the seven FDA-approved biomarkers that showed significant relationships with Pt concentrations [Equation 1]. Due to high collinearity between the biomarkers and a small sample size, only one biomarker was selected for inclusion in each model following forward/backward selection. Urinary albumin was found to be the strongest determinant of urinary Pt concentrations. Similar multiple regression analyses for unbound plasma Pt at the 2 h estimations were also performed [Equation 2]. TFF3 was found to be the strongest determinant of unbound plasma Pt concentration at 2 h. Point estimates of albumin and TFF3 are described in Supplementary Table 2.
| Equation 1 |
| Equation 2 |
Estimated total plasma Pt concentrations that would have a high risk of kidney toxicity based on urinary biomarkers were assessed. Median splits were performed on each biomarker to stratify subjects as having “high” or “low” urinary concentrations of each biomarker. Interestingly, the same subjects were categorized as “high” for clusterin, cystatin C, osteopontin and NGAL. Mean 2 h total Pt plasma concentration for these biomarkers was 1,900 ng/ml. Mean urinary total Pt concentration was 24,010 ng/ml. The same subjects were categorized as “high” for TFF3 and β2M. For these biomarkers, mean 2 h total plasma Pt concentration was 2,080 ng/ml. Mean urinary total Pt concentration for these biomarkers was 27,800 ng/ml. Therefore, a 2 h total plasma Pt concentration of approximately 2,000 ng/ml and a peak urinary total Pt concentration reaching 24,000 ng/ml may be indicative of higher risk of subclinical kidney toxicity.
Discussion
In this study, a noncompartmental analysis was conducted to evaluate the relationships between Pt pharmacokinetic parameters and novel urinary biomarkers of nephrotoxicity. Total and unbound Pt exhibited distinct pharmacokinetic profiles, suggesting the potential for molecular species-specific differences in off-target toxicities. We found that AUC0−∞ was associated with SCr and eGFR. In addition, peak urinary total Pt concentrations correlated with KIM-1, calbindin, clusterin, GST-pi, β2M, albumin, NGAL, osteopontin, clusterin, cystatin C and TFF3 at day 10. TFF3 and albumin were most predictive of plasma and urinary Pt concentrations, respectively. Median splits of biomarker values suggest 2 h total plasma Pt concentrations greater than 2,000 ng/ml and peak urinary Pt concentrations above 24,000 ng/ml are potential approximations for increased risk of nephrotoxicity. In summary, plasma and urinary Pt concentrations in the first 2 h after a cisplatin dose, in addition to the pharmacokinetic parameters AUC0−∞ and Cmax, represent biomarkers that could identify individuals with increased susceptibility to cisplatin-induced kidney injury. Elevations of markers of subclinical kidney injury would also inform clinicians to be aware of concurrent exposures to other nephrotoxins and conditions predisposing to injury.
Dosing of platinum derivatives have historically relied on AUC and GFR measurements that are inputted into the Calvert formula [17]. Although our study confirmed the association between AUC and SCr/eGFR, the current study suggests that therapeutic drug monitoring of peak platinum levels may be more important to detect earlier kidney injury. The urinary biomarkers measured in this study reflect injury along the proximal and distal tubule of the nephron, both of which are targets for cisplatin toxicity [17]. Significant associations were detected for both sites of toxicity, including some biomarkers that can reflect injury at both sites [e.g., NGAL]. A previous study by our group examined time-dependent changes in these biomarkers from patients receiving cisplatin [9]. Results showed that although traditional clinical biomarkers [e.g. SCr, BUN] were largely unchanged, β2M was three-fold higher by day 3 and KIM-1, TFF3 and calbindin were elevated two- to eight-fold by day 10. Therefore, certain biomarkers might be more sensitive in detecting cisplatin-induced kidney injury, even in the absence of clinically detectable AKI, as defined by obvious changes in SCr. Evidence suggests that 20% of patients who do not meet crietria for SCr-based diagnoses of AKI are likely to have acute tubular damage [18]. Meta-anlayses have shown that urinary biomarkers allow earlier detection of kidney injury, before clinical kidney dysfunction is apparent [19]. Thus, protective strategies and interventions can be under-taken, such as reducing exposure to other AKI risk factors and potentially nephrotoxic medications surrounding the timeframe of treatment with cisplatin.
Previous studies have examined relationships between Pt pharmacokinetics and traditional measures of nephrotoxicity in patients with overt kidney injury [10, 12, 20, 21]. These studies implicated peak unbound plasma and total urine Pt concentrations, AUC0−∞, Cmax, time above toxic concentration [as assessed by nephrotoxicity markers] and dosing schedule [continuous 72 h infusion vs. single 6 h infusion vs. repetitive 1 h infusions] as important predictors of nephrotoxicity. One study reported optimal Pt Cmax levels of between 1.5 – 2 μg/ml, similar to the current study’s cut-off value of 2 μg/ml [12]. Evidence from these studies supported cisplatin continuous infusions vs. intermittent bolus injections to reduce Cmax and risk of nephrotoxicity. Similarly, our results indicate peak total urinary and plasma Pt concentrations as robust predictors, but in the realm of subclinical nephrotoxicity.
Finally, we were able to generate regression relationships between various biomarkers and urinary or plasma Pt concentrations. TFF3 and albumin were strong determinants of peak plasma and urinary concentrations, respectively. Using these relationships and reference biomarker ranges, total urinary Pt concentrations above 24,000 ng/ml corresponded to the upper limit of these ranges and could thus be used as a cut-off for increased risk of nephrotoxicity. Total circulating Pt concentrations above 2,000 ng/ml paralleled those individuals categorized as having “high” levels of urinary biomarkers. The highest levels of certain biomarkers correlated with one another, evidenced by the same subjects being categorized as “high” versus “low” after median splits of each biomarker. The use of urinary albumin as a biomarker may represent a level of confusion, however, since platinum can be bound to albumin (and other proteins). Although the current study was limited by patient number, evaluating combinations of biomarkers in regression relationships may provide additional information pertaining to nephrotoxicity.
The incidence of overt acute kidney injury with cisplatin-based therapy ranges from 8–40%, and is purportedly contingent on dose, dose frequency and peak plasma concentrations [22]. In the current study, no patient exhibited clinical signs of overt acute kidney injury, utilizing the SCr and eGFR criteria designated by KDIGO, as defined by increases in SCr >0.3 mg/dL over 48 h or 1.5 times baseline after one dose [3]. Several scenarios could explain this observation in the current study, including limited sample size, timing of blood collection after cisplatin infusion, moderate cisplatin doses, and improvements in supportive care. Despite these limitations, results nonetheless demonstrated strong relationships between urinary total Pt concentrations and biomarkers of kidney injury, suggesting the occurrence of subclinical nephrotoxicity following cisplatin treatment and support for the potential use of urinary total Pt excretion, plasma unbound and total Pt concentrations, and AUC0−∞ or Cmax as additional biomarkers of cisplatin-induced kidney injury.
Clinical studies have reported nephrotoxic outcomes 10 days after cisplatin treatment [22]. A 10-day timeframe to detect kidney injury is longer than warranted and has necessitated the development of new methods and biomarkers for earlier detection of nephrotoxicity. In this study, we found that 2 h plasma concentrations and peak urinary total Pt concentrations within 2 h of cisplatin dosing were associated with novel urinary biomarkers at 10 days and could thus be predictive of earlier kidney injury. To this end, evaluation of peak urinary concentrations after the first dose can inform subsequent dosing strategies. Additional studies with larger samples are needed to further refine these relationships and to generate a range of urinary total Pt concentrations that are predictive of cisplatin-induced kidney injury. One final aspect of consideration is the risk to benefit ratio for cisplatin dosing. Future studies and/or pharmacokinetic-pharmacodynamic modeling will be required to determine how dosing modifications to reduce kidney injury may impact treatment responses to cisplatin. This could be especially relevant for patients who require higher cisplatin doses for their cancer treatment.
Supplementary Material
Acknowledgements
The authors wish to thank the participants in this study. Grant funding support for this study was provided by National Institutes of Health funding numbers T32ES007148, R21DK093903, P30ES005022 and P30CA072720.
Footnotes
Author Contributions
MI analyzed data and wrote the manuscript. CC, NM, MG, CLO, DWB contributed to conduct of the patient study. YH and SLH contributed to the analysis plan. BG, XW, and BB performed biomarker assays and/or platinum analyses. MSJ and LA conceived, designed and conducted the study and oversaw all components of the analytical and manuscript plan. All authors contributed to editing the manuscript.
Conflict of Interest
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Ethical approval
“All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
References
- 1.Pabla N and Dong Z, Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int, 2008. 73(9): p. 994–1007. [DOI] [PubMed] [Google Scholar]
- 2.Arany I and Safirstein RL, Cisplatin nephrotoxicity. Semin Nephrol, 2003. 23(5): p. 460–4. [DOI] [PubMed] [Google Scholar]
- 3.Khwaja A, KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract, 2012. 120(4): p. c179–84. [DOI] [PubMed] [Google Scholar]
- 4.Devarajan P, The use of targeted biomarkers for chronic kidney disease. Adv Chronic Kidney Dis, 2010. 17(6): p. 469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dieterle F, et al. , Renal biomarker qualification submission: a dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat Biotechnol, 2010. 28(5): p. 455–62. [DOI] [PubMed] [Google Scholar]
- 6.Shinke H, et al. , Urinary kidney injury molecule-1 and monocyte chemotactic protein-1 are noninvasive biomarkers of cisplatin-induced nephrotoxicity in lung cancer patients. Cancer Chemother Pharmacol, 2015. 76(5): p. 989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaidya VS, et al. , Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol, 2010. 28(5): p. 478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinches M, et al. , Evaluation of novel renal biomarkers with a cisplatin model of kidney injury: gender and dosage differences. Toxicol Pathol, 2012. 40(3): p. 522–33. [DOI] [PubMed] [Google Scholar]
- 9.George B, et al. , Profiling of Kidney Injury Biomarkers in Patients Receiving Cisplatin: Time-dependent Changes in the Absence of Clinical Nephrotoxicity. Clin Pharmacol Ther, 2016. 101(4): p. 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erdlenbruch B, et al. , Pharmacokinetics of cisplatin and relation to nephrotoxicity in paediatric patients. Eur J Clin Pharmacol, 2001. 57(5): p. 393–402. [DOI] [PubMed] [Google Scholar]
- 11.Nagai N and Ogata H, Quantitative relationship between pharmacokinetics of unchanged cisplatin and nephrotoxicity in rats: importance of area under the concentration-time curve (AUC) as the major toxicodynamic determinant in vivo. Cancer Chemother Pharmacol, 1997. 40(1): p. 11–8. [DOI] [PubMed] [Google Scholar]
- 12.Nagai N, et al. , Relationship between pharmacokinetics of unchanged cisplatin and nephrotoxicity after intravenous infusions of cisplatin to cancer patients. Cancer Chemother Pharmacol, 1996. 39(1–2): p. 131–7. [DOI] [PubMed] [Google Scholar]
- 13.Skali H, et al. , Prognostic assessment of estimated glomerular filtration rate by the new Chronic Kidney Disease Epidemiology Collaboration equation in comparison with the Modification of Diet in Renal Disease Study equation. Am Heart J, 2011. 162(3): p. 548–54. [DOI] [PubMed] [Google Scholar]
- 14.Ma J, et al. , Comparison of ethanol plasma-protein precipitation with plasma ultrafiltration and trichloroacetic acid protein precipitation for the measurement of unbound platinum concentrations. Cancer Chemother Pharmacol, 1996. 38(4): p. 391–4. [DOI] [PubMed] [Google Scholar]
- 15.Xie R, et al. , A study of the interactions between carboplatin and blood plasma proteins using size exclusion chromatography coupled to inductively coupled plasma mass spectrometry. Anal Bioanal Chem, 2007. 387(8): p. 2815–22. [DOI] [PubMed] [Google Scholar]
- 16.O’brien RM. A Caution Regarding Rules of Thumb for Variance Inflation Factors. Quality & Quantity. 2007. October 1;41(5):673–90. [Google Scholar]
- 17.van Warmerdam LJ, et al. , The use of the Calvert formula to determine the optimal carboplatin dosage. J Cancer Res Clin Oncol, 1995. 121(8): p. 478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haase M, Kellum JA, and Ronco C, Subclinical AKI--an emerging syndrome with important consequences. Nat Rev Nephrol, 2012. 8(12): p. 735–9. [DOI] [PubMed] [Google Scholar]
- 19.Haase M, et al. , Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis, 2009. 54(6): p. 1012–24. [DOI] [PubMed] [Google Scholar]
- 20.Yasumasu T, et al. , Comparative study of cisplatin and carboplatin on pharmacokinetics, nephrotoxicity and effect on renal nuclear DNA synthesis in rats. Pharmacol Toxicol, 1992. 70(2): p. 143–7. [DOI] [PubMed] [Google Scholar]
- 21.Fukushima K, et al. , Population Pharmacokinetic-Toxicodynamic Modeling and Simulation of Cisplatin-Induced Acute Renal Injury in Rats: Effect of Dosing Rate on Nephrotoxicity. J Pharm Sci, 2016. 105(1): p. 324–32. [DOI] [PubMed] [Google Scholar]
- 22.Madias NE and Harrington JT. Platinum nephrotoxicity. Am J Med, 1978. 65(2): p. 307–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.