Abstract
Few treatments for human diseases have received as much investigation in the past 20 years as probiotics. In 2017, English-language meta-analyses totaling 52 studies determined the effect of probiotics on conditions ranging from necrotizing enterocolitis and colic in infants to constipation, irritable bowel syndrome, and hepatic encephalopathy in adults. The strongest evidence in favor of probiotics lies in the prevention or treatment of 5 disorders: necrotizing enterocolitis, acute infectious diarrhea, acute respiratory tract infections, antibiotic-associated diarrhea, and infant colic. Probiotic mechanisms of action include the inhibition of bacterial adhesion; enhanced mucosal barrier function; modulation of the innate and adaptive immune systems (including induction of tolerogenic dendritic cells and regulatory T cells); secretion of bioactive metabolites; and regulation of the enteric and central nervous systems. Future research is needed to identify the optimal probiotic and dose for specific diseases, to address whether the addition of prebiotics (to form synbiotics) would enhance activity, and to determine if defined microbial communities would provide benefit exceeding that of single-species probiotics.
Keywords: allergy, diarrhea, immunology, lactobacillus, microbiome, regulatory T cells
Do I contradict myself?
Very well then I contradict myself
(I am large, I contain multitudes.)
Walt Whitman, in Song of Myself, 1892
Probiotics have been defined as “live organisms that when administered in adequate doses confer a health benefit to the host”1 (FAO/WHO 2002) and are included in a number of fermentable foods, pills, powders, and liquid drops. Common probiotics are available in pharmacies, groceries, and online in the United States. They include but are not limited to Lactobacillus rhamnosus GG, Lactobacillus reuteri, Lactobacillus casei, Lactobacillus paracasei, Bacillus coagulans, Bacillus clausii, Bifidobacterium infantis, Bifidobacterium longum, Bifidobacterium infantis, Streptococcus thermophilus, Escherichia coli strain Nissle 1917, and yeasts, including Saccharomyces boulardii and Saccharomyces cerevisiae. Many probiotics contain mixtures of 2 or more individual species. Prebiotics are defined as metabolic substrates that promote the growth and/or activity of beneficial microorganisms, usually in the gastrointestinal tract.2 Although their definition has been debated and modified, the general consensus is that prebiotics are nondigestible by human gastrointestinal cells. Prebiotics include oligosaccharides, resistant starch, and soluble or insoluble fibers. Synbiotics are defined as mixtures of probiotics and prebiotics that beneficially affect the host by impacting the microbiome within the gastrointestinal tract.3
Each of the above definitions is loosely applied in most countries by the various food and pharmaceutical industries that produce these products, as they attempt to take advantage of the protean health advantages being documented by probiotic research. For example, many probiotic yogurts are marketed to promote human health. Live cultures are present in cheese, kimchee, kombucha tea, and miso soup. There are even probiotics designed to be administered to domestic pets. Prebiotics are added to formulas, cheese, and even juices. However, an improvement in health as a result of the selective stimulation of the growth of a defined population of intestinal bacteria—which is implied in the definition of prebiotics—is difficult to verify and is rarely done. Finally, prebiotics (typically fructooligosaccharides or inulin) are often added to probiotic foods at a low concentration to minimize gastrointestinal symptoms. Because of these low concentrations (<10%), there is most often no evidence to support an additive, synergistic, or even measurable effect of the product.3 Strong legislation is needed in order to authorize health claims for only selected strains with health-proven benefits.
The human microbiome refers to our commensal microbiota (bacteria, fungi, archaea, viruses, and protozoans), their genes, and gene products. The microbiome is of great interest to all researchers in medicine, affecting each major organ system, including even the cardiovascular system and the central nervous system (the latter leading to the term psychobiome). Humans harbor 1014 microbes, compared to 1013 of our own cells, and there are 1000 to 1500 unique species that colonize the human colon, of which the average person has approximately 160 species, contributing to about 3% of the human body mass.4 It seems ridiculous that one could consume a single probiotic at doses of 108 to 1010 colony-forming units (CFU) daily and expect that it could produce an effect on a health outcome. Evidence, however, is provided herein that shows that, indeed, probiotics at these doses do have proven health benefits in humans, and we will provide the evidence and potential mechanisms.
Evidence From Human Trials: Absent, Evolving, or Conclusive?
Few treatments for human diseases have received as much investigation in the past 20 years as probiotics. In the past single year, PubMed records 792 clinical trials of probiotics for human conditions. In examining the evidence for treatment efficacy, we are reminded that the strength of evidence (from weakest to strongest) is: case series → case control studies → cohort studies → randomized controlled trials (RCTs) → systematic reviews, and → (finally) meta-analysis. In 2017, English-language meta-analyses totaling 52 studies determined the effect of probiotics on conditions ranging from necrotizing enterocolitis and colic in infants to constipation, irritable bowel syndrome, and hepatic encephalopathy in adults (Table 1). Other studies focused on serum lipid levels, late-onset sepsis in preterm infants, blood glucose and hemoglobin A1C levels in people with type 2 diabetes, and disease activity in ulcerative colitis. Each of the above studies demonstrated efficacy of the probiotic for the condition studied. Importantly, there were also a few negative meta-analyses. Probiotic efficacy was not demonstrated in studies investigating the prevention of urinary tract infection, reducing the risk of developing bronchopulmonary dysplasia or retinopathy of prematurity, or in helping to eradicate bacterial vaginosis.
Table 1.
Meta-Analyses Reporting Probiotic Efficacy in 2017
| Condition | Probiotic | Reference |
|---|---|---|
| Abdominal pain in children | Variousa | 123 |
| Antibiotic-associated diarrhea (children) | 5 Bifidobacterium preparations | 124 |
| Blood glucose and A1C levels in type II diabetes mellitus | Various | 125 |
| Constipation | Lactobacillus, Bifidobacillus preparations | 126 |
| Halitosis | Lactobacillus | 127 |
| Helicobacter pylori eradication | Various | 128–130 |
| Hepatic encephalopathy | Variousa | 118 |
| Infant colic | Lactobacillus reuteri | 131 |
| Infection risk in the critically ill | Various | 132 |
| Irritable bowel syndrome | Saccharomyces cerevisiae, Bifidobacterium infantis, combinations | 23,24,133 |
| Late-onset sepsis in preterm infants | Various | 134,135 |
| Prevention of C. difficile infections in hospitalized patients | Various | 136 |
| Prevention of necrotizing enterocolitis | Various | 7,8 |
| Prevention of radiation-associated diarrhea | Lactobacillus ± Bifidobacterium bifidum | 137 |
| Small intestinal bacterial overgrowth | Various | 138 |
| Surgical site infections | Synbiotic | 139,140 |
| Total choleresterol and LDL-cholesterol lowering | Lactobacillus reuteri, Lactobacillus plantarum | 141,142 |
| Ulcerative colitis | VSL#3, various others | 117 |
LDL indicates low-density lipoprotein.
Cochrane review.
The authors have summarized the strongest evidence in favor of probiotics in the prevention or treatment of 5 disorders: necrotizing enterocolitis, acute infectious diarrhea, acute respiratory tract infections, antibiotic-associated diarrhea, and infant colic. We will briefly discuss these entities.
Netrotizing Enterocolitis (NEC)
NEC is the scourge of premature infants and neonatologists. It affects 5% to 10% of infants with birth weight between 500 and 1500 g, and approximately 50% of these infants require surgery.5 Mortality rates range from 20% to 30%, and NEC is the leading cause of short bowel syndrome in children. In a multicenter prospective 4-year trial, Warner et al6 studied the normal progression of microbial colonization in preterm infants and the disruption of this process prior to the onset of NEC. They found that during the first 60 days of life, infants not developing NEC experienced an expansion of fecal Negativicutes (composed largely of Veillonellaceae), whereas those developing NEC saw a reduction in Negativicutes and a more than doubling of the composition of Gammaproteobacteria (largely gram-negative aerobic bacilli) (Figure 1). Thus, NEC would appear to be a classical disease associated with gastrointestinal dysbiosis. (Dysbiosis is defined as the condition of having an abnormal microbial community, either in or on the body, as opposed to a disease caused by a single pathogen, such as Salmonella or Helicobacter pylori.)
Figure 1.
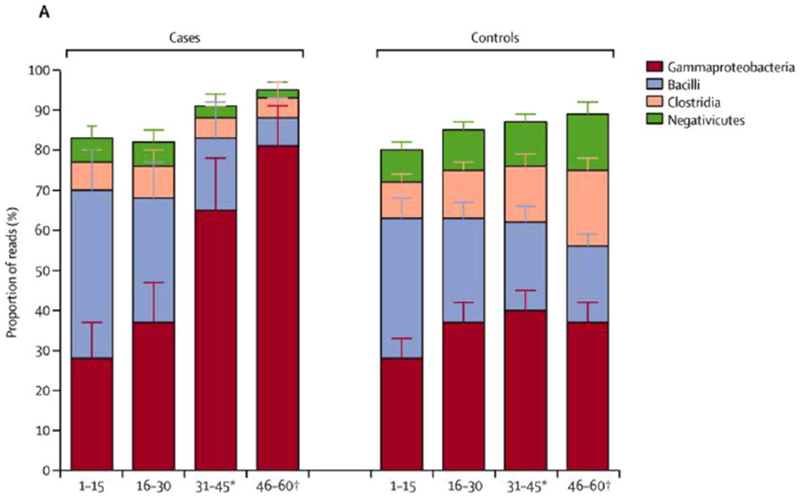
Major phyla in prospective multicenter study of microbiota of premature neonates during the first 60 days of life. Bar graphs show evolution toward increased negativicutes in control group, as contrasted to increased gamma proteobacteria in those developing NEC. From Warner et al.6
There have been at least 3 meta-analyses showing that probiotics prevent NEC. In 2012, Wang et al reported a meta-analysis of 20 RCTs in which probiotics were given individually to prevent NEC in very preterm infants. There were approximately 3700 infants included in studies from 1997 to 2011, and there was little heterogeneity, even though various probiotics were studied. They found that the relative risk ratio of developing NEC was 0.33 (95%CI, 0.24-0.46), indicating a powerful preventative effect. In 2017, 2 larger meta-analyses were published,7,8 both showing efficacy in preventing NEC. The largest analysis (n = 7345 infants) showed a similarly reduced odds ratio of developing NEC in probiotic-treated infants (0.36, 95%CI, 0.24-0.53). It also showed efficacy in preventing death of these infants in studies focusing on multiple strain probiotics, but not in those using a single strain.7 One limitation has been that these studies of probiotics in preventing NEC investigated a number of different probiotic preparations, and the best strain is unknown. To our knowledge, no studies have been completed in the United States. Safety issues in this highly vulnerable population are still of concern to the US Food and Drug Administration (FDA), which categorizes probiotics in the same group as vaccines. Preclinical and clinical trials are needed because of critical host/bacterial crosstalk in the very early stages of life, at the time it may be possible to permanently modify the quality of potentially invasive bacteria by an optimal local immune response. Looking at immunologic and microbiota effects of selected species in exclusively breastfed infants could be beneficial. L. rhamnosus GG and L. reuteri are among the best candidates. However, caution should be taken not to exceed doses of 109 CFU in premature neonates because of the risk of bacterial translocation.
With these safety considerations in mind, it nevertheless seems virtually certain at this point that a probiotic will be of major benefit in preventing NEC, especially when the infant is of very low birth weight (<1000 g). A quality improvement project in which a neonatal intensive care unit gave the probiotic L. reuteri to every premature infant admitted to the hospital reported significant and sustained improvements in the outcome of their babies after the new protocol was implemented, including most importantly a 6-fold lower risk of NEC.9
Acute Infectious Diarrhea
Acute diarrhea is usually viral in origin, and acute diarrheal infections peak in children between the ages of 6 months and 2 years old. Incidence is directly related to water purity, sanitation, and hygiene. Infants with acute diarrhea represent a group at high risk of diarrheal dehydration, the second-leading cause of mortality in children worldwide, with approximately 700,000 deaths annually.10
Probiotics have been studied for more than 30 years for children with acute diarrhea. The most widely studied probiotics are L. rhamnosus GG and L. reuteri. Recently, Szajewska et al11 summarized 15 studies of L. rhamnosus GG for acute diarrhea and concluded that L. rhamnosus GG reduces the severity of purging and the duration of diarrhea by approximately 1 day and is optimally effective at doses ≥1010 CFU. Using meta-analysis, Szajewska et al12 found that L. reuteri at a lower dose was effective in reducing the duration of diarrhea by approximately 1 day.
Deaths caused by diarrhea appear to be decreasing worldwide, most likely the result of improved hygiene, breastfeeding, and the rotavirus vaccine,10 although recent refugee crises may negate these benefits. However, the ability to reduce the duration of a 3- to 4-day illness by 1 day, as well as the severity of purging, by feeding a probiotic represents a significant advance. In fact, 1 academic hospital (Cincinnati Children’s Hospital) modified the hospital order set for children admitted with acute diarrhea to include L. rhamnosus GG as part of the treatment regimen; implementation of this quality improvement measure rose from approximately 1% to 100% of children admitted.13 The impact on outcomes of such measures will be of interest to all.
Upper Respiratory Infections
All probiotics induce an immune response, and probiotics increase immunoglobulin A (IgA)-secreting cells in respiratory and gastrointestinal mucosae.14 Day care center studies showed that consuming a daily probiotic by healthy children resulted in an approximately 25% reduction in the number of days of school missed.15 Systematic reviews of probiotics have shown that there is a reduction of the severity of symptoms associated with probiotics and a shorter duration of respiratory tract infection by approximately 1 day.16
Antibiotic-Associated Diarrhea (AAD)
Antibiotics are the most often prescribed medicines for children, with more than 50% of all children <18 years old receiving at least 1 course.17 The most widely prescribed antibiotics are amoxicillin, azithromycin, and amoxicillin/clavulanate. AAD is a very frequent side effect of antibiotic therapy, affecting approximately 11% of all children who receive antibiotics and 18% of those <2 years old.17 Initially, a meta-analysis of AAD by Hempel et al included 63 RCTs and 11811 subjects. The majority used Lactobacillus as the study product. Results showed that the relative risk of developing AAD when taking a probiotic was 0.58 (95%CI, 0.50-0.68; P < .001). However, there was heterogeneity in the pooled results. They concluded that probiotics are associated with a reduction in AAD; however, more research is needed to determine the best probiotic.
A more recent meta-analysis, extracted from 30 trials in China involving more than 7000 participants included 21 trials that focused on children. The probiotic always included Bifidobacilli, often in combination with other probiotic(s). Results confirmed the efficacy of probiotic prophylaxis, with an odds ratio of developing AAD of 0.34 (95%CI 0.23-0.43, P < 0.01) in the children receiving a Bifidobacillus-containing preparation—a powerful impact.
Irritable Bowel Syndrome (IBS)
Abdominal pain and IBS, collectively called functional bowel disorders, are the most common conditions leading to consultation by the pediatric gastroenterologist, and they affect about 10% of all school-aged children, similar to the prevalence in adults. There may be some regional variability; for example, IBS has been identified in 13% of children in China, 10% in the United States, and 6% in Sri Lanka.18 In adults, the prevalence of IBS is 18% in Latin America, 8% to 10% in North America and Asia, and 6% in Africa and the Middle East.19 IBS is defined by the Rome Criteria: recurrent abdominal pain at least 1 day weekly for >3 months that is (1) related to defecation, (2) a change in stool form, and/or (3) a change in stool frequency.
The etiology of IBS is multifactorial and includes a genetic predisposition, a reaction to life stresses, and/or the presence of an environmental trigger (such as infectious diarrhea). Several groups have identified an altered microbial community, as an additional and potentially central factor, in the feces of patients with IBS. The most notable patient population was those individuals with IBS characterized by diarrhea20,21 (Figure 2). These patients clearly have a distinct microbial population, characterized by reduced microbial diversity, reduced butyrate-producing organisms (a metabolite that enhances gut barrier function), and reduced methane producers (which dispose of gas).21 These observations make IBS a notable disease for targeted microbial manipulation.
Figure 2.
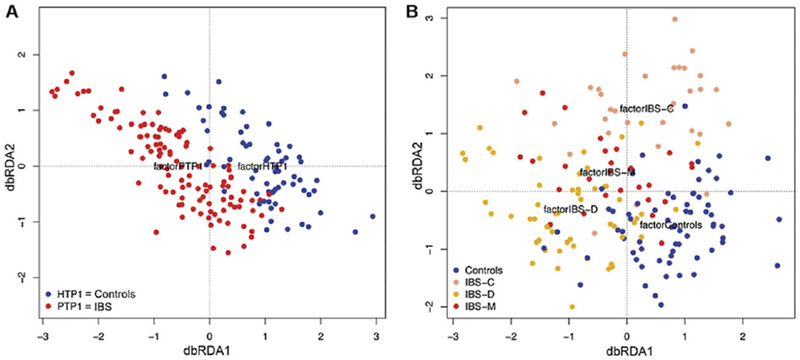
Two-dimensional principal components. Microbiota in IBS (A), divided by subgroup (B) in which C refers to constipation, D refers to diarrhea, and M refers to mixed diarrhea and constipation. From Pozuelo et al.21
Many RCTs have now been performed to determine if probiotic(s) are effective in patients with IBS. Ford et al22 from the United States and Canada identified 43 RCTs involving >3000 volunteers and showed that the relative risk (RR) of having persistent IBS symptoms was 0.79 (95%CI, 0.70-0.89) while taking probiotic. A recent update from China looking at 21 RCTs showed similarly an association between probiotic ingestion and an improvement in IBS symptoms, including an improvement in quality of life.23 The 2 studies differed in that one concluded that there were better results with a single probiotic, whereas the other found similar results with single- and multiple-organism probiotics.
Recently, there have been a number of RCTs that analyzed individual probiotics and showed efficacy in subjects with IBS, allowing meta-analysis. These probiotics included S. cerevisiae CNCM I-385620 and B. infantis 35624.24 The latter probiotic was effective only when used as a member of a multiorganism probiotic. In summary, individuals with IBS were found to respond to probiotics with a reduction in some symptoms, such as flatulence, abdominal pain, and constipation, but without change in other symptoms, such as bloating or urgency. L. reuteri benefitted children with functional abdominal pain, with a reduction in frequency of episodes and intensity of pain.25 Probiotics can benefit children with IBS, but with a lesser “effect size” compared to other treatments, such as low-dose antidepressants.26 There are important remaining questions, such as magnitude of effect, optimal dose, safety in vulnerable populations, and the most effective species and strain of probiotic.
Infant Colic
Defined as crying plus fussing for more than 3 hours daily, infants usually develop this condition between 3 weeks and 3 months of age.27 Colic affects up to 10% of normal infants. Crying occurs throughout the day but peaks in the hours between 5 and 11 PM27; this crying can be quantified by a “Barr diary,”28 which often shows increased crying after feedings. Colic may be a factor in child abuse and infanticide.29,30 In one investigation of 112 cases of abusive head trauma to infants, forensic interrogation revealed that shaking of the infant was violent and repetitive in most cases. The parent, usually a father, reported that he shook the infant in order to stop the baby from crying in 63% of cases, not intending to hurt the baby.31
Theories to account for the etiology of colic include parental psychological stress and inadequate resources to help them cope, cow milk protein allergy, the fourth-trimester theory (which postulates that the infant would prefer to stay in the protected “warm environment” of the amnionic fluid), and the inflammation-dysbiosis theory. Whereas there is some information to support each of these theories in a subset of patients, our data and the data of others suggests that dysbiosis and gut inflammation are reproducible findings. Savino et al,32,33 from Torino, Italy, found increased Escherichia coli and reduced lactobacilli; Rhoads et al34 identified increased Klebsiella and reduced microbial diversity in infants with colic in Houston, Texas; while Partty et al35 reported increased Bifidobacterium breve in infants with colic in Turku, Finland. Using a microarray technique and prospectively following a defined newborn population in Nijmegen, Holland, de Weerth et al36 found that the “top 10% of crybabies” harbored increased Proteobacteria, a group that would encompass both Klebsiella and E. coli. Two studies showed reduced lactobacilli,32,35 and 2 studies (1 our own) have shown elevated fecal calprotectin, a marker of gut inflammation.34,37
With these considerations in mind, at least 5 published studies have now investigated the role of a single probiotic, L. reuteri to alter the course in infants with colic. The preparation was originally isolated from a Peruvian mother’s breast milk, cured of an antibiotic-resistant plasmid, and is now provided as liquid drops in sunflower oil. Two meta-analyses concluded that in breastfed infants with colic, quantified “crying + fussing time” was reduced by approximately 1 hour per day within 2 weeks of administering the probiotic,38,39 with no significant side effects. However, 2 studies in formula-fed infants failed to show an advantage of probiotic over placebo.40,41 The negative findings could be related to a different probiotic that was studied, the “moving target effect” with the crying time decreasing with age naturally, a lack of optimal prebiotic in formula (eg, human milk contains oligosaccharides, which are prebiotics), or to a inadequate sample size. Currently, infants with colic are typically treated with acid blockers, which are ineffective,42 overused,43 over-dosed, and may contribute to cause small intestinal bacterial overgrowth.44 A new and safe treatment, such as L. reuteri, represents a welcome addition to the armamentarium of the pediatrician.
Allergic Diseases
The primary purpose of the immune system is to protect the host from the ever-changing microbes. In doing so, it has to balance a double-edged sword between defense against emerging organisms and tolerance to nonpathogenic antigens/allergens. This duty is challenging, and the immune system must recruit alliances in this never-ending battle. An obvious ally would be probiotics to help in the regulation between inflammation and allergic responses. The important role of microbes in regulating allergic development is supported by the hygiene hypothesis, and epidemiological data shows less susceptibility to allergic diseases in people living in rural and developing countries.45,46 Because of these data, there has been great interest in harnessing probiotics to prevent or treat allergic diseases such as asthma, eczema, and food allergy.
With the exception of atopic dermatitis, data from meta-analyses have revealed insufficient evidence for probiotics in preventing the development of asthma, allergic rhinitis, or food allergy.47,48 With regard to preventing or reducing the severity of atopic dermatitis, there is evidence suggesting a benefit of probiotics, but this effect is variable and not consistent between studies.49–51 In one recent meta-analysis examining 17 studies, results showed that when mothers were treated along with their infants with a probiotic, the infant had a significant reduction in RR for developing eczema compared to controls (RR, 0.78 [95%CI, 0.69-0.89]; P < 0.001), particularly those supplemented with a mixture of probiotics (RR, 0.54 [95%CI, 0.43-0.68]; P < 0.001).52
Thus far, the most intriguing benefit of probiotics is in their potential adjuvant effect for oral immunotherapy (OIT) of food allergy.53 In a recent double-blind, placebo-controlled randomized trial of the probiotic Lactobacillus rhamnosus CG MCC 1.3724 in conjunction with peanut oral immunotherapy in 56 children (1-10 years old) with peanut allergy, 82% achieved a sustained unresponsiveness to peanut challenge, versus 3.6% that were given placebo (P < .001).54 However, a definitive conclusion on the beneficial effect of the probiotic cannot be determined, because the trial lacked a control group receiving oral immunotherapy without the probiotic. Unfortunately, probiotics have failed to produce significant therapeutic efficacy for the treatment of allergic rhinitis and asthma in RCTs.55,56
Although current data from clinical trials indicate some beneficial effects of probiotics in the prevention and treatment of allergic diseases, the results are far from being robust enough to warrant a universal recommendation for their therapeutic usage. Clearly, there may be benefit in the prevention of atopic dermatitis (eczema). The outcomes from clinical trials with probiotics can be confounded by the different strains tested. Their activities and potency might vary among patients and their microbiota. It is conceivable that a specific strain of probiotic might be more viable and functional in the intestinal microbiome of one person and less so in another. There are many factors besides the interplay between different microbes that can impact the functionality of probiotics, such as the patient’s diet, immune system, mucosa, and emotional makeup. Another important consideration is the route of administration. Instead of ingestion, the probiotics might be more effective if delivered via nasal spray for allergic rhinitis, inhalation for asthma, or topically for atopic dermatitis. It is clear that more research is needed. The key is in deciphering the different mechanisms of how probiotics contribute to immunoregulation and homeostasis at the mucosal level.
Preclinical Trials and Mechanism of Action in Inflammatory Diseases
A number of mechanisms whereby probiotics regulate inflammation have been identified using animal models of inflammatory diseases (Figure 3). Important evidence attesting to the essential function of beneficial microbes come from studies of germ-free mice. Germ-free mice have reduced intestinal surface area, thin villi, increased cell-cycle time, a poorly developed intestinal microvasculature, and impaired peristalsis.57 Therefore, one must conclude that the resident microbial population contributes significant nutritional and local benefits to the host.
Figure 3.
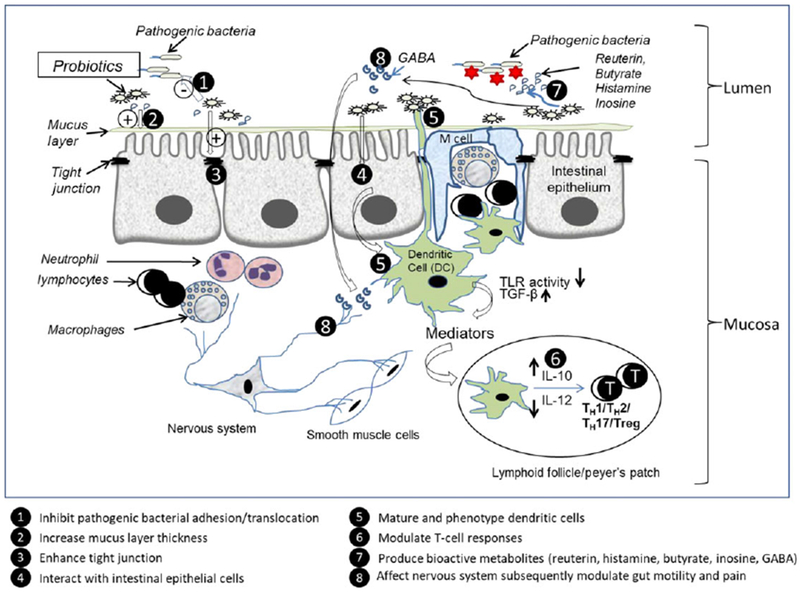
Documented mechanisms of action of probiotics. Probiotics may inhibit pathogenic bacterial adhesion, enhance barrier function, and interact with TLRs expressed on the intestinal epithelial cells and dendritic cells to produce cytokines/chemokines to further modulate T cells. Probiotics also can produce bioactive metabolites and affect nervous system subsequently modulate gut motility, reduce pain, and involve in gut-brain function (Illustration by Liu, Y.).
Probiotics Inhibit Pathogenic Bacterial Adhesion
Adhesion of pathogenic bacteria to mucosal surfaces mediated by the interaction between bacterial adhesins and specific mucosal receptors is considered to be the first step of intestinal infection.58 Some probiotics have the property of competitive exclusion, by which probiotics adhere to the intestinal mucosa to prevent the subsequent attachment of pathogens.59 For examples, specific Lactobacillus strains were found to inhibit the adhesion of pathogens such as enterotoxigenic E. coli to porcine enterocytes,60 diarrheagenic E. coli to human intestinal epithelium Caco-2 cell lines,59 and Salmonella typhimurium to intestinal mucus.61
Probiotics Enhance Intestinal Barrier Function
A hyperpermeable epithelial barrier in the gastrointestinal tract is proposed to be a major cause of chronic inflammation. Probiotics enhance the structure and function of intestinal epithelial barriers, including increasing mucin production, enhancing tight junctions, and modulating signaling pathways that affect cell proliferation and survival.62,63 Under normal physiological conditions, goblet cells continually produce mucins to replenish and maintain the mucus barrier; however, goblet cell function can be disrupted by various factors (such as microbes, microbial toxins, and cytokines) that can affect the integrity of the mucus barrier. This occurs in various pathological conditions such as chronic inflammatory diseases.64
L. plantarum (strain 299v) has the capacity to enhance the production and secretion of mucins (MUC2 and MUC3) from human intestinal (HT-29) epithelial cells.65 Probiotic mixtures also increase MUC2 gene expression and mucin protein secretion in rat colon.66 L. rhamnosus GG–derived soluble proteins (p40 and p70) ameliorate intestinal injury and inflammation by inhibiting epithelial cell apoptosis67 and increasing mucin production through transactivation of the epidermal growth factor receptor.68 Some probiotics have been demonstrated to protect tight junctions by changes in tight junction–related proteins, such as zonulin-1, occludins, and claudins, and by enhancing the electrical resistance of tight junctions contained in the apical junction complexes between adjacent polarized epithelia.69 Feeding Lactobacillus rhamnosus GG early in life to newborn mice enhanced epithelial cell proliferation, differentiation, tight junction formation, and mucosal IgA production.70 Other cellular and molecular mechanisms such as the release of metabolites and bioactive molecules, suppression of oxidative stress, interference with inflammatory pathways, and augmention of the levels of mucosal IgA help protect and repair epithelial barriers.71
Probiotics Modulate Both Innate and Adaptive Immune System
One of the most powerful effects of probiotics is to modulate the immune system. Probiotics strengthen both innate and adaptive immune responses through bacterial-epithelial-immune cell crosstalk.
Toll-Like Receptor Signaling.
Probiotics act as ligands for innate immune system receptors such as Toll-like receptors (TLRs) expressed on the intestinal epithelial cells and mucosal immune cells to influence important signaling pathways including nuclear transcription factor nuclear factor-kappa B (NF-κB), mitogen-activated protein kinases, phosphoinositide-3-kinase-protein kinase B/Akt (PI3K-PKB/Akt), and peroxisome proliferator–activated receptor-γ (PPAR-γ) pathways.62,63 Studies of our group demonstrated that human-derived L. reuteri DSM 17938 significantly increased survival and decreased the incidence and severity of experimental NEC via inhibition of TLR4 and NF-κB signaling in the intestine of newborn rats. This probiotic effect resulted in decreased gut secretion of tumor necrosis factor-α and interleukin 1β (IL-1β).72
We further observed that the beneficial regulation of L. reuteri DSM 17938 in intestinal inflammation during NEC is mediated by TLR2, which recognizes cell wall components of gram-positive bacteria. Recent studies reported that certain lactobacilli can protect from experimental colitis via mechanisms involving TLR2 and cyclooxygenase 2 (L. rhamnosus GG),73 TLR2-dependent induction of regulatory T cells (Tregs) (L. casei Lbs2),74 and TLR2-dependent inhibition of NF-κB signaling by macrophages (L. paracasei).75 Other studies using models of IBD also showed that probiotics can prevent activation of the NF-κB, resulting in decreased secretion of the chemokine IL-8, which is a potent neutrophil chemoattactant.63,76
The effects of various probiotics on the host are strain specific. Some probiotics (eg, Bifidobacterium lactis BB12 and Bacteroides vulgatus) are able to activate NF-κB and increase levels of the proinflammatory cytokine IL-6,63 but this could also be beneficial, as suggested by current evidence indicating that a low level of inflammation could have physiologic benefits, including maintenance of the epithelial barrier and priming of immune responses. Immunostimulatory phenotypes of probiotics or commensal bacteria might serve the important purpose of promoting host defense against pathogens.
Immune Cells.
Dendritic cells (DCs) are embedded within the epithelium (Figure 3), and they contain projections reaching into the lumen to sample bacteria. These cells can travel to mesenteric lymph nodes and beyond. Probiotics modulate DCs, which, in turn, influence T-cell populations. Various microbial factors interact with different DC surface pattern recognition receptors (such as TLRs) to determine DC maturation and subsequent DC-regulated differentiation of naive T cells into TH1, TH2, TH17 or Tregs.77 Intestinal DCs are central to maintaining immune tolerance in the gut, ultimately by generation of tolerogenic T-cell responses toward food antigens and the commensal microbiota, preventing unnecessary inflammation and hypersensitivity.63,78
It is known that a probiotic can be taken up by DCs, facilitating the maturation of DCs and the induction of Tregs.63,79 Tregs are critical for establishment of immune homeostasis and maintenance of tolerance,80 which is supported by evidence that, in humans, Treg deficiency due to mutations in the Foxp3 gene results in a rare condition called immune dysregulation, polyendocrinopathy, and enteropathy, with X-linked inheritance (IPEX syndrome). IPEX syndrome is associated with eczema, severe enteropathy, type 1 diabetes, thyroiditis, and inflammation in multiple organs.81 A critical role of Tregs in preventing colitis and IBD has also been supported by published murine studies.82,83 Weitkamp et al84 demonstrated that the proportion of Tregs was significantly decreased in premature infants with NEC. We studied an experimental NEC model and demonstrated that adoptive transfer of Tregs attenuated the severity of NEC, suggesting a role in controlling excessive inflammation.85 Our studies subsequently showed that oral administration of the probiotic L. reuteri DSM 17938 significantly increased the percentage of gut-derived CD103+DCs and Foxp3+Tregs while diminishing the percentage of inflammatory T-effector cells (including TH1, TH2 and TH17) in the intestinal mucosa of newborn mice during NEC.86,87
Intestinal CD103+DCs are critical for gut homeostasis. Acting in concert with conditioned mucosal CD103+DCs are dietary components (vitamin A), lipid ligands for PPAR-γ and aryl hydrocarbon receptor (AhR), intestinal epithelial cell–produced transforming growth factor-β (TGF-β); thymic stromal lymphopoietin (TSLP); retinoic acid; and enteroendocrine cell–produced neurotransmitters (vasoactive intestinal peptide).88 These conditioned CD103+DCs are subsequently capable of inducing Foxp3+Treg cell differentiation. The CD103+ receptor on DCs helps to modify its own paradigm in the sense of tolerance through an upregulation of different cytoplasmic proteins such as indoleamine 2,3-dioxygenase. As a consequence of this upregulation, the DC in close contact with the naive CD4+T cell will costimulate its cytotoxic T-lymphocyte-associated protein 4 receptor to promote Treg induction.89 CD103+DCs also drive the induction of α47 integrin and CCR9 on newly generated Tregs, which makes them home to gut-associated lymphoid tissue.90
In Treg deficiency–induced autoimmune disease, called the scurfy mouse (a mouse model of human IPEX syndrome), effector T cells proliferate out of control, resulting in TH1- and TH2-driven autoimmune disorders. In the mouse, as in the human, there is early-onset dermatitis, progressive multiorgan inflammation, and death within the first month of life caused by a lymphoproliferative syndrome.91 Oral administration of L. reuteri DSM 17938 markedly prolonged survival and reduced symptoms; treatment also reduced multiorgan inflammation by inhibiting TH1 and TH2 cells and their associated cytokines.92
Different probiotics influence the development and survival of different types of Tregs including TH3, Tr1, CD4+CD25+, CD8+ suppressor, and γδT cells,63 and assist the secretion of immunomodulatory cytokines including IL-10 (which can strongly suppress TH1-driving inflammation) and transforming growth factor-β (which can promote development of Tregs) by other cells.93 These observations suggest that probiotics have a widespread role in maintaining intestinal homeostasis and the balance between tolerance and reactivity to ingested food antigens and commensal microbes.
In addition to the immunomodulation of probiotics involving DCs and T cells, some probiotic strains have the ability to promote the differentiation of B cells into plasma cells and increase the production of secretory IgA.94 Secretory IgA provides a defense against pathogens by limiting bacterial association with the epithelium and preventing penetration of host tissue.95
Probiotics Produce/Promote Bioactive Metabolites That Have Anti-Inflammatory Properties
Specific probiotic and/or other gut bacteria modulated by probiotics are being found to produce multiple bioactive metabolites with anti-inflammatory properties. We will discuss 4 of these: reuterin, histamine, butyrate, and inosine.
Many human-derived L. reuteri strains produce the antimicrobial 3-carbon aldehyde reuterin with broad-spectrum in vitro antimicrobial activity against enteric pathogens and other intestinal bacteria.96 The vitamin B12-dependent production of reuterin occurs when L. reuteri ferments the substrate glycerol. Reuterin does not typically interfere with the growth of commensal lactic acid bacteria,96 but in the setting of Clostridium difficile infection (CDI), this compound changes in the composition and function of the microbial community that preferentially targets C. difficile outgrowth and toxicity.97
Some human-derived L. reuteri strains, for example, L. reuteri strain ATCC PTA 6475, synthesize histamine, which can suppress inflammation via type 2 histamine receptor activation in the mammalian intestine, resulting in suppression of chronic intestinal inflammation and colorectal tumorigenesis.98,99
Probiotics have the ability to ferment certain types of fibers, thus increasing the production of short-chain fatty acids (SCFAs), such as propionate, acetic acid, and butyrate. Short-chain fatty acids play multiple critical roles in host defense and immunity, including anticancer, anti-inflammation, and antioxidant activities, as well as outcompetition of enteric bacterial pathogens.100 Butyrate is particularly important as an inflammatory modulator, with anti-inflammatory effects on the intestinal epithelial cells, macrophages, and leukocytes. Butyrate interferes with inflammatory signal pathways to regulate cytokine production, inhibits histone deacetylase to regulate expression of numerous proinflammatory genes, and induces the differentiation and expansion of Tregs. Coming from the lumen of the colon, butyrate serves as the primary fuel source for colonocytes and facilitates the maintenance of the epithelial barrier.
In the Treg-deficient scurfy mouse, we showed that L. reuteri DSM 17938 prolongs survival and reduces inflammation in multiple organs (Figure 4). L. reuteri DSM 17938 restores levels of the purine metabolite inosine to inhibit Treg-deficiency–induced autoimmunity via adenosine 2A receptors (A2A). A key to Treg suppression of TH1/TH2 cells is an interaction between adenosine produced by Tregs (mediated by a CD39-CD73 pathway) and the A2A receptor predominantly expressed on neighboring TH1/TH2 cells in lymphoid organs such as the gastrointestinal tract. During Treg-deficiency, TH1 and TH2 cells lose their regulation by adenosine-A2A–mediated signaling, resulting in TH1 and TH2 cell–induced pathology. Inosine, a metabolite of adenosine, is increased by L. reuteri DSM 17938, and interacts with the A2A receptor to inhibit TH1 and TH2 development and differentiation (Figure 5). The direct mechanism of this change in inosine level has not been elucidated, but indirect evidence indicates that L. reuteri DSM 17938 may promote inosine absorption in the intestine, by improving villus length and increasing the gut levels of equilibrative nucleoside transporters that absorb adenosine and inosine. In addition, the genome of L. reuteri DSM 17938 contains the transfer RNA–specific adenosine deaminase gene; adenosine deaminase is required to synthesize inosine.
Figure 4.
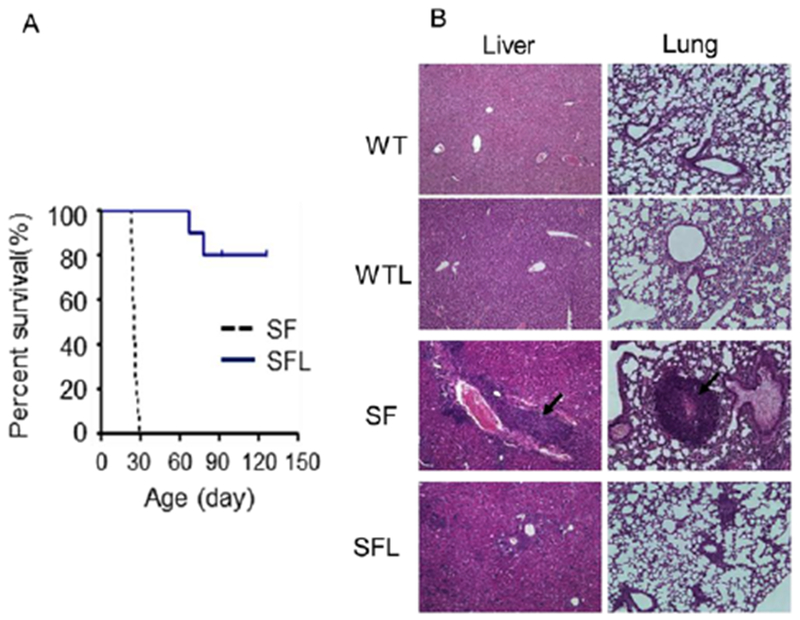
Lactobacillus reuteri DSM 17938 treatment increases survival (A) and reduces inflammation (B) in organs of SF mice (arrows indicate lymphocyte infiltration). From He et al.92
Figure 5.
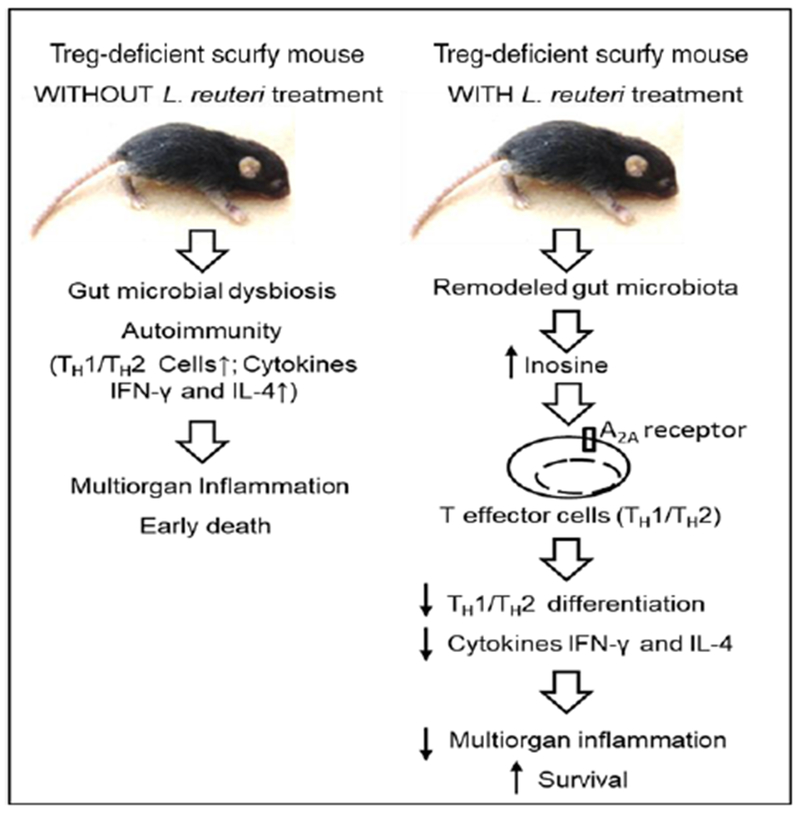
Mechanisms of Lactobacillus reuteri DSM 17938 protection against Treg-deficiency-mediated autoimmunity. Treg deficiency shapes gut microbiota and induces autoimmunity resulting in multi-organ inflammation and early death (left). Lactobacillus reuteri DSM 17938 remodels gut microbiota, alters the metabolites (inosine) and protects against Treg-deficiency-induced autoimmunity by suppressing TH1/TH2 cells via inosine-adenosine A2A interaction (right). From He et al.92
Probiotics Impact the Enteric and Central Nervous System
Many studies now provide evidence that probiotics have the capacity to activate specific opioid and cannabinoid receptors in the gut, which could reduce visceral pain by which the strains could be beneficial for patients with IBS and chronic IBD.101
Myths and Mysteries
Probiotics are the plat du jour of the 2010s. Sales of probiotic supplements exceeded US$1.3 billion in 2015, while in Asia the total was the equivalent of $US15 billion. Whereas a number of health claims are suggested by studies such as those mentioned above, some web sites claim much more. Probiotics.org cites >100 distinct benefits, including improved mental health; reduced dental caries; and beneficial effects in the treatment of lung cancer, pneumonia, and pleural effusion. It also cites reduced risk of cirrhosis, increased burn-healing rate, an antitumor effect in gastric cancer, and a reduced risk of cervical cancer. On the opposite side of these health claims is the FDA, which regulates probiotics as if they were vaccines. In 2016, the FDA stated: “To date, the U.S. Food and Drug Administration has not approved health claims for any probiotic.”102
The FDA and the European Medicines Agency have been concerned about safety and quality control, stating: “The current literature is not well equipped to answer questions on the safety of probiotic interventions with confidence.”102 There is justification for this concern. Safety concerns especially pertain to the newborn. The intestinal microbiota at birth commences when the neonate is colonized with myriad of strains originating from his or her own mother. These microbes aid in correcting the antenatal physiologic immune imbalance, allowing the fetus to be tolerated by the mother. The overall quality of the first colonization by invasive bacteria depends also on environmental influences (mode of delivery, where the birth takes place, perinatal medications, and early source of nutrition). Furthermore, the more invasive strains have a dramatic impact on postnatal immune development, with an optimal response against pathogens but also with an induced immune tolerance to successive selection of commensal microbes and environmental antigens, including dietary antigens.
These very complex immune adaptations between the host and its developing microbiome evolve and appear to reach a stable equilibrium sometime between 2 and 3 years of age.103 The intestinal physiologic resistance to further microbial colonization is effective at that time. Later in life, probiotics will further help to degrade dietary macromolecules and to modulate host immune responses but only when they are permanently given. As a consequence, probiotics may have more potency in affecting the immune system and ultimate microbiome when given in the early stage. However, absolute safety of giving probiotics at this time of immune immaturity—especially in premature neonates who are clearly immunocompromised—needs to be demonstrated. Safety and efficacy are both notably strain and dose dependent.
Probiotic adverse events include diarrhea, sepsis, subacute bacterial endocarditis, and meningitis, although these are exceedingly rare and not likely to be more frequent after probiotic than after a placebo.104 Contamination of a marketed product is of great concern, exemplified by the infection of an infant who was given a probiotic (ABC Dophilus) that contained both live bacteria and a Rhizopus fungus, the latter of which caused invasive gastrointestinal mucormycosis and death.105 One probiotic for children, S. boulardii (SB), is a yeast preparation isolated from the bark of the lychee fruit. SB has been extensively administered to immunocompromised patients, including premature infants, human immunodeficiency virus–infected adults, and individuals with Crohn disease. SB treatment has been associated with immunological benefits, and SB reduces the severity of infant diarrhea.106,107 However, SB is not a normal inhabitant of the human gut and will not become a stable member of the microbial community. This strain has been historically registered and marketed based on experimental data from animal studies. By now, there are numerous reports of fungemia (often with SB isolated) in critically ill or elderly patients.108–110
Below, we describe 6 of the myths and mysteries surrounding the probiotic “lore.”
1. All probiotics are similar.
In many of the meta-analyses cited above, different probiotics and combinations were effective, but careful scrutiny in preclinical randomized controlled trials often showed marked differences, even among different strains of the same species. This is likely true because different strains have different mechanisms. For example, as mentioned above, studies in the lab of Versalovic showed that L. reuteri strain ATCC PTA 6475 produces vitamin B12 and histamine; the probiotic reduced tumor necrosis factor-α production via a protein kinase A–dependent mechanism.111 Subsequently, in our lab, a different strain of L. reuteri DSM 17938, which does not produce vitamin B12 or increase histamine levels, did not reduce gut inflammation in rat intestine ex vivo, but prevented NEC87 and markedly attenuated multiorgan inflammation in the scurfy mouse.92 The mechanism involved, in contrast, was the activation of an adenosine A2A receptor.
Even though mechanisms may differ, it is notable that in all of the meta-analyses noted above, probiotics were either effective in treating the conditions targeted or not deleterious. In none of the studies did the condition worsen. It is possible but seems unlikely that this is because of publication bias, given the thousands of RTCs that have been reported and funnel-plot analyses showing no bias.
2. Probiotics affect diseases only via a placebo effect.
The placebo effect is always worth considering, but the authors note that all the above-cited studies were placebo controlled.
3. Probiotics have an enduring effect on symptoms via long-term colonization.
In most trials, the administered probiotic cannot be detected in the stool weeks after discontinuing the probiotic. In some trials, it was difficult to isolate the probiotic even while it is being consumed! In our safety trial of L. reuteri in adult volunteers, for example, only low levels of the probiotic could be detected in stool by PCR during treatment, and at 1 and 4 months after treatment, there was no more detectable L. reuteri.112 In a study in which we investigated a formula with or without L. rhamnosus GG, we could detect an increase in L. rhamnosus in the stool with a median value of 4% of total bacterial sequences after 2 weeks of consumption of L. rhamnosus GG (using 16S ribosomal DNA sequencing). However, while the infants were continuing to drink the formula, at 42 and 90 days, the population of L. rhamnosus decreased to <1%. We suspect that in both studies the probiotics were colonizing the small intestine and/or being outcompeted in the colon. Similarly, with minimal evidence of fecal excretion, daily oral L. reuteri evoked observable effects on fecal calprotectin,112 and in infants L. reuteri had potentially beneficial effects on the low neutrophil counts in peripheral blood that were seen in approximately 50% of the infants with colic.113 There may be probiotics that are able to establish long-term colonization under optimal conditions. For example, Panigrahi et al114 showed that in term infants in India, supplementation with L. plantarum and fructooligosaccharides daily for 7 days resulted in 100% colonization by 2 months, but fewer of these infants (32%) remained colonized by 6 months. It is unclear if these infants remained colonized later in life.
Perhaps a useful analogy for how a small number of probiotics could affect the microbiome of a host is one that gardeners would appreciate. A probiotic is not like a rapidly spreading, colorful, perennial flower, such as Impatiens walleriana. Probiotics are more like simple garden clover (Trifolium). Clover prevents weeds from forming, retains moisture for the other flowers, fixes nitrogen into the soil, and improves soil tilth (softness).115
4. Probiotics are inexpensive.
The cost of probiotics is remarkably variable. In the United States, a single product (L. rhamnosus GG) costs from $0.37 to $1.00 per capsule (Culturelle™; i-Health, Inc., Cromwell, Connecticut). A combination of L. acidophilus and B. longum BB-12 (Trubiotic™; Bayer, Leverkusen, Germany) ranged from $0.30 to $0.67 per capsule online. A multiorganism probiotic (Ultimate Flora™; Renew Life, Palm Harbor, Florida) is sold at concentrations ranging from 25 to 200 billion CFU per pill; online cost ranges from $0.46 to $1.40, in proportion to the number of CFU. [Note that the authors are not endorsing these specific products.] Probiotics are not covered by insurance companies or federal medical assistance plans. Thus, it is of concern that, at present, probiotics can be viewed at some level as a concierge medication.
5. Probiotics are dangerous in patients with severe diseases or in those with immune deficiencies.
Probiotics were originally felt to benefit mild digestive illnesses and were subsequently found to be beneficial in reducing infections in children in day care centers.116 However, now they have now been found to be beneficial in a number of severe conditions such as ulcerative colitis,117 hepatic encephalopathy,118 and rheumatoid arthritis.119 They are being actively studied in HIV-infected individuals as a benefit to standard highly active antiretroviral therapy.120–122 No severe adverse events have been identified in these studies that would be attributable to the probiotic. We believe probiotics may be more efficacious in children and adults with severe disorders.
In summary, evidence-based, mechanistic research on probiotics reveals that cultured microorganisms, when given in adequate quantities for sufficient periods of time, are beneficial in many human disease conditions and safer than most pharmaceuticals. These conditions include infantile colic, acute infectious diarrhea, and IBS. Probiotics also can prevent antibiotic-associated diarrhea and NEC. Much more research is needed, and clearly probiotics do have potential risks that need to be monitored. Future investigations are needed to identify the optimal probiotic and dose for specific diseases, first in animal models comparing various strains, and second in RCTs. Animal trials should use multiple “omics” to investigate not only the organisms but their effects on the global fecal community and their key metabolites, establishing mechanism of action. Their impact on the immune system is key to the understanding of how to therapeutically approach the globally increasing incidence of autoimmune diseases, including arthritis and other rheumatologic disorders, IBD, eosinophilic diseases, and multiple sclerosis.
These studies must also address whether the addition of a prebiotic would enhance the therapeutic effect of a probiotic and to determine if the administration of human-derived or synthetic “defined microbial communities” (as opposed to single organism probiotics) would provide additional benefit. Safety considerations will almost certainly favor a probiotic approach over the administration of fecal transplantation, because of lower risks to the host, greater ease of administration, and lower cost of production.
Acknowledgments
Disclosures
Research was partially supported by BioGaia AB, Sweden, in addition to federal and state governmental grants.
References
- 1.Reid G Probiotics: definition, scope and mechanisms of action. Best Pract Res Clin Gastroenterol 2016;30:17–25. [DOI] [PubMed] [Google Scholar]
- 2.Hutkins RW, Krumbeck JA, Bindels LB et al. Prebiotics: why definitions matter. Curr Opin Biotechnol. 2016;37:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol 2008;111:1–66. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med 2016;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warner BB, Deych E, Zhou Y, et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet. 2016;387:1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang HY, Chen JH, Chang JH, Lin HC, Lin CY, Peng CC. Multiple strains probiotics appear to be the most effective probiotics in the prevention of necrotizing enterocolitis and mortality: an updated meta-analysis. PLoS ONE. 2017;12:e0171579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu W, Sui W, Mu L, et al. Preventing necrotizing enterocolitis by food additives in neonates: a network meta-analysis revealing the efficacy and safety. Medicine (Baltimore). 2017;96:e6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter C, Dimaguila MA, Gal P, et al. Effect of routine probiotic, Lactobacillus reuteri DSM 17938, use on rates of necrotizing enterocolitis in neonates with birthweight <1000 grams: a sequential analysis. BMC Pediatr. 2012;12:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das JK, Bhutta ZA. Global challenges in acute diarrhea. Curr Opin Gastroenterol. 2016;32:18–23. [DOI] [PubMed] [Google Scholar]
- 11.Szajewska H, Skorka A, Ruszczynski M, Gieruszczak-Bialek D. Meta-analysis: Lactobacillus GG for treating acute gastroenteritis in children—updated analysis of randomised controlled trials. Aliment Pharmacol Ther. 2013;38:467–476. [DOI] [PubMed] [Google Scholar]
- 12.Szajewska H, Urbanska M, Chmielewska A, Weizman Z, Shamir R. Meta-analysis: Lactobacillus reuteri strain DSM 17938 (and the original strain ATCC 55730) for treating acute gastroenteritis in children. Benef Microbes, 2014;5:285–293. [DOI] [PubMed] [Google Scholar]
- 13.Parker MW, Schaffzin JK, Lo VA, et al. Rapid adoption of Lactobacillus rhamnosus GG for acute gastroenteritis. Pediatrics. 2013;131(suppl 1):S96–S102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gleeson M, Bishop NC, Oliveira M, Tauler P. Daily probiotic’s (Lactobacillus casei Shirota) reduction of infection incidence in athletes. Int J Sport Nutr Exerc Metab. 2011;21:55–64. [DOI] [PubMed] [Google Scholar]
- 15.Weizman Z The role of probiotics and prebiotics in the prevention of infections in child day-care centres. Benef Microbes. 2015;6:181–183. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Li X, Ge T, et al. Probiotics for prevention and treatment of respiratory tract infections in children: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016;95:e4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantegazza C, Molinari P, D’Auria E, Sonnino M, Morelli L, Zuccotti GV. Probiotics and antibiotic-associated diarrhea in children: a review and new evidence on Lactobacillus rhamnosus GG during and after antibiotic treatment. Pharmacol Res. 2017;128:63–72. [DOI] [PubMed] [Google Scholar]
- 18.Sandhu BK, Paul SP. Irritable bowel syndrome in children: pathogenesis, diagnosis and evidence-based treatment. World J Gastroenterol. 2014;20:6013–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sperber AD, Dumitrascu D, Fukudo S, et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut. 2017;66:1075–1082. [DOI] [PubMed] [Google Scholar]
- 20.Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:521–30, e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pozuelo M, Panda S, Santiago A, et al. Reduction of butyrate- and methane-producing microorganisms in patients with irritable bowel syndrome. Sci Rep. 2015;5:12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547–1561. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Li L, Guo C, et al. Effects of probiotic type, dose and treatment duration on irritable bowel syndrome diagnosed by Rome III criteria: a meta-analysis. BMC Gastroenterol. 2016;16:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan F, Ni H, Asche CV, Kim M, Walayat S, Ren J. Efficacy of Bifidobacterium infantis 35624 in patients with irritable bowel syndrome: a meta-analysis. Curr Med Res Opin. 2017;33:1191–1197. [DOI] [PubMed] [Google Scholar]
- 25.Weizman Z, Abu-Abed J, Binsztok M. Lactobacillus reuteri DSM 17938 for the management of functional abdominal pain in childhood: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2016;174:160–164. [DOI] [PubMed] [Google Scholar]
- 26.Ford AC, Quigley EM, Lacy BE, et al. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1350–1365. [DOI] [PubMed] [Google Scholar]
- 27.Wessel MA, Cobb JC, Jackson EB, Harris GS, Detwiler AC. Paroxysmal fussing in infancy, sometimes called colic. Pediatrics. 1954;14:421–435. [PubMed] [Google Scholar]
- 28.Barr RG, Rotman A, Yaremko J, Leduc D, Francoeur TE. The crying of infants with colic: a controlled empirical description. Pediatrics. 1992;90:14–21. [PubMed] [Google Scholar]
- 29.Barr RG. Crying as a trigger for abusive head trauma: a key to prevention. Pediatr Radiol. 2014;44(suppl 4):S559–S564. [DOI] [PubMed] [Google Scholar]
- 30.Levitzky S, Cooper R. Infant colic syndrome—maternal fantasies of aggression and infanticide. Clin Pediatr (Phila). 2000;39:395–400. [DOI] [PubMed] [Google Scholar]
- 31.Adamsbaum C, Grabar S, Mejean N, Rey-Salmon C. Abusive head trauma: judicial admissions highlight violent and repetitive shaking. Pediatrics. 2010;126:546–555. [DOI] [PubMed] [Google Scholar]
- 32.Savino F, Cresi F, Pautasso S, et al. Intestinal microflora in breastfed colicky and non-colicky infants. Acta Paediatr. 2004;93:825–829. [PubMed] [Google Scholar]
- 33.Savino F, Cordisco L, Tarasco V, Calabrese R, Palumeri E, Matteuzzi D. Molecular identification of coliform bacteria from colicky breastfed infants. Acta Paediatr. 2009;98:1582–1588. [DOI] [PubMed] [Google Scholar]
- 34.Rhoads JM, Fatheree NY, Norori J, et al. Altered fecal microflora and increased fecal calprotectin in infants with colic. J Pediatr. 2009;155:823–828. [DOI] [PubMed] [Google Scholar]
- 35.Partty A, Kalliomaki M, Endo A, Salminen S, Isolauri E. Compositional development of Bifidobacterium and Lactobacillus microbiota is linked with crying and fussing in early infancy. PLoS ONE. 2012;7:e32495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Weerth C, Fuentes S, Puylaert P, de Vos WM. Intestinal microbiota of infants with colic: development and specific signatures. Pediatrics. 2013;131:e550–e558. [DOI] [PubMed] [Google Scholar]
- 37.Savino F, Garro M, Montanari P, Galliano I, Bergallo M. Crying time and RORgamma/FOXP3 expression in Lactobacillus reuteri DSM17938-treated infants with colic: a randomized trial. J Pediatr. 2018;192:171–177. [DOI] [PubMed] [Google Scholar]
- 38.Harb T, Matsuyama M, David M, Hill RJ. Infant colic—what works: a systematic review of interventions for breast-fed infants. J Pediatr Gastroenterol Nutr. 2016;62:668–686. [DOI] [PubMed] [Google Scholar]
- 39.Xu M, Wang J, Wang N, Sun F, Wang L, Liu XH. The efficacy and safety of the probiotic bacterium Lactobacillus reuteri DSM 17938 for infantile colic: a meta-analysis of randomized controlled trials. PLoS ONE. 2015;10: e0141445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fatheree NY, Liu Y, Ferris M, et al. Hypoallergenic formula with Lactobacillus rhamnosus GG for babies with colic: a pilot study of recruitment, retention, and fecal biomarkers. World J Gastrointest Pathophysiol. 2016;7:160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sung V, Hiscock H, Tang ML, et al. Treating infant colic with the probiotic Lactobacillus reuteri: double blind, placebo controlled randomised trial. BMJ. 2014;348: g2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore DJ, Tao BS, Lines DR, Hirte C, Heddle ML, Davidson GP. Double-blind placebo-controlled trial of omeprazole in irritable infants with gastroesophageal reflux. J Pediatr. 2003;143:219–223. [DOI] [PubMed] [Google Scholar]
- 43.Lightdale JR, Gremse DA. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics. 2013;131:e1684–e1695. [DOI] [PubMed] [Google Scholar]
- 44.Sieczkowska A, Landowski P, Zagozdzon P, Kaminska B, Lifschitz C. The association of proton pump inhibitor therapy and small bowel bacterial overgrowth in children. Eur J Gastroenterol Hepatol. 2017;29:1190–1191. [DOI] [PubMed] [Google Scholar]
- 45.Daley D The evolution of the hygiene hypothesis: the role of early-life exposures to viruses and microbes and their relation-ship to asthma and allergic diseases. Curr Opin Allergy Clin Immunol. 2014;14:390–396. [DOI] [PubMed] [Google Scholar]
- 46.Lambrecht BN, Hammad H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol. 2017;18:1076–1083. [DOI] [PubMed] [Google Scholar]
- 47.Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev. 2007;CD006475. [DOI] [PubMed] [Google Scholar]
- 48.West CE, Jenmalm MC, Kozyrskyj AL, Prescott SL. Probiotics for treatment and primary prevention of allergic diseases and asthma: looking back and moving forward. Expert Rev Clin Immunol. 2016;12:625–639. [DOI] [PubMed] [Google Scholar]
- 49.Cuello-Garcia CA, Brozek JL, Fiocchi A, et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136:952–961. [DOI] [PubMed] [Google Scholar]
- 50.Dang D, Zhou W, Lun ZJ, Mu X, Wang DX, Wu H. Meta-analysis of probiotics and/or prebiotics for the prevention of eczema. J Int Med Res. 2013;41:1426–1436. [DOI] [PubMed] [Google Scholar]
- 51.Zhang GQ, Hu HJ, Liu CY, Zhang Q, Shakya S, Li ZY. Probiotics for prevention of atopy and food hypersensitivity in early childhood: a PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016;95:e2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zuccotti G, Meneghin F, Aceti A, et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy. 2015;70:1356–1371. [DOI] [PubMed] [Google Scholar]
- 53.Loh W, Tang M. Adjuvant therapies in food immunotherapy. Immunol Allergy Clin North Am. 2018;38:89–101. [DOI] [PubMed] [Google Scholar]
- 54.Tang ML, Ponsonby AL, Orsini F, et al. Administration of a probiotic with peanut oral immunotherapy: a randomized trial. J Allergy Clin Immunol. 2015;135:737–744. [DOI] [PubMed] [Google Scholar]
- 55.Vliagoftis H, Kouranos VD, Betsi GI, Falagas ME. Probiotics for the treatment of allergic rhinitis and asthma: systematic review of randomized controlled trials. Ann Allergy Asthma Immunol. 2008;101:570–579. [DOI] [PubMed] [Google Scholar]
- 56.Zajac AE, Adams AS, Turner JH. A systematic review and meta-analysis of probiotics for the treatment of allergic rhinitis. Int Forum Allergy Rhinol. 2015;5:524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar RD. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beachey EH. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981;143:325–345. [DOI] [PubMed] [Google Scholar]
- 59.Chauviere G, Coconnier MH, Kerneis S, Darfeuille-Michaud A, Joly B, Servin AL. Competitive exclusion of diarrheagenic Escherichia coli (ETEC) from human enterocyte-like Caco-2 cells by heat-killed Lactobacillus. FEMS Microbiol Lett. 1992;70:213–217. [DOI] [PubMed] [Google Scholar]
- 60.Spencer RJ, Chesson A. The effect of Lactobacillus spp. on the attachment of enterotoxigenic Escherichia coli to isolated porcine enterocytes. J Appl Bacteriol. 1994;77:215–220. [DOI] [PubMed] [Google Scholar]
- 61.Tuomola EM, Ouwehand AC, Salminen SJ. The effect of probiotic bacteria on the adhesion of pathogens to human intestinal mucus. FEMS Immunol Med Microbiol. 1999;26:137–142. [DOI] [PubMed] [Google Scholar]
- 62.Bermudez-Brito M, Plaza-Diaz J, Munoz-Quezada S, Gomez-Llorente C, Gil A. Probiotic mechanisms of action. Ann Nutr Metab. 2012;61:160–174. [DOI] [PubMed] [Google Scholar]
- 63.Thomas CM, Versalovic J. Probiotics-host communication: modulation of signaling pathways in the intestine. Gut Microbes. 2010;1:148–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cornick S, Tawiah A, Chadee K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers. 2015;3:e982426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dykstra NS, Hyde L, Mackenzie A, Mack DR. Lactobacillus plantarum 299v prevents caspase-dependent apoptosis in vitro. Probiotics Antimicrob Proteins. 2011;3:21–26. [DOI] [PubMed] [Google Scholar]
- 66.Caballero-Franco C, Keller K, De SC, Chadee K. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G315–G322. [DOI] [PubMed] [Google Scholar]
- 67.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L, Cao H, Liu L, et al. Activation of epidermal growth factor receptor mediates mucin production stimulated by p40, a Lactobacillus rhamnosus GG-derived protein. J Biol Chem. 2014;289:20234–20244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ukena SN, Singh A, Dringenberg U, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS ONE. 2007;2:e1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yan F, Liu L, Cao H, et al. Neonatal colonization of mice with LGG promotes intestinal development and decreases susceptibility to colitis in adulthood. Mucosal Immunol. 2017;10:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohland CL, MacNaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2010;298:G807–G819. [DOI] [PubMed] [Google Scholar]
- 72.Liu Y, Fatheree NY, Mangalat N, Rhoads JM. Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NF-kappaB signaling in the intestine. Am J Physiol Gastrointest Liver Physiol. 2012;302:G608–G617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ciorba MA, Riehl TE, Rao MS, et al. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut. 2012;61:829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thakur BK, Saha P, Banik G, et al. Live and heat-killed probiotic Lactobacillus casei Lbs2 protects from experimental colitis through Toll-like receptor 2-dependent induction of T-regulatory response. Int Immunopharmacol. 2016;36:39–50. [DOI] [PubMed] [Google Scholar]
- 75.Sun KY, Xu DH, Xie C, et al. Lactobacillus paracasei modulates LPS-induced inflammatory cytokine release by monocyte-macrophages via the up-regulation of negative regulators of NF-kappaB signaling in a TLR2-dependent manner. Cytokine. 2017;92:1–11. [DOI] [PubMed] [Google Scholar]
- 76.Fiocchi C Probiotics in inflammatory bowel disease: yet another mechanism of action? Gastroenterology. 2006;131:2009–2012. [DOI] [PubMed] [Google Scholar]
- 77.Yamane H, Paul WE. Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets. Immunol Rev. 2013;252:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mann ER, Landy JL, Bernardo D, et al. Intestinal dendritic cells: their role in intestinal inflammation, manipulation by the gut microbiota and differences between mice and men. Immunol Lett. 2013;150:30–40. [DOI] [PubMed] [Google Scholar]
- 79.Baba N, Samson S, Bourdet-Sicard R, Rubio M, Sarfati M. Commensal bacteria trigger a full dendritic cell maturation program that promotes the expansion of non-Tr1 suppressor T cells. J Leukoc Biol. 2008;84:468–476. [DOI] [PubMed] [Google Scholar]
- 80.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. [DOI] [PubMed] [Google Scholar]
- 81.Moraes-Vasconcelos D, Costa-Carvalho BT, Torgerson TR, Ochs HD. Primary immune deficiency disorders presenting as autoimmune diseases: IPEX and APECED. J Clin Immunol. 2008;28(suppl 1):S11–S19. [DOI] [PubMed] [Google Scholar]
- 82.Collins CB, Aherne CM, McNamee EN, et al. Flt3 ligand expands CD103(+) dendritic cells and FoxP3(+) T regulatory cells, and attenuates Crohn’s-like murine ileitis. Gut. 2012;61:1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. [DOI] [PubMed] [Google Scholar]
- 84.Weitkamp JH, Koyama T, Rock MT, et al. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut. 2013;62:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dingle BM, Liu Y, Fatheree NY, Min J, Rhoads JM, Tran DQ. FoxP3(+) regulatory T cells attenuate experimental necrotizing enterocolitis. PLoS ONE. 2013;8:e82963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Y, Fatheree NY, Dingle BM, Tran DQ, Rhoads M. Lactobacillus reuteri DSM 17938 changes the frequency of Foxp3+ regulatory T cells in the intestine and mesenteric lymph node in experimental necrotizing enterocolitis. PLoS ONE. 2013;8(2):e56547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Y, Tran DQ, Fatheree NY, Marc RJ. Lactobacillus reuteri DSM 17938 differentially modulates effector memory T cells and Foxp3+ regulatory T cells in a mouse model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2014;307:G177–G186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scott CL, Aumeunier AM, Mowat AM. Intestinal CD103+ dendritic cells: master regulators of tolerance? Trends Immunol. 2011;32:412–419. [DOI] [PubMed] [Google Scholar]
- 89.Kushwah R, Hu J. Role of dendritic cells in the induction of regulatory T cells. Cell Biosci. 2011;1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharma R, Sung SS, Fu SM, Ju ST. Regulation of multi-organ inflammation in the regulatory T cell-deficient scurfy mice. J Biomed Sci. 2009;16:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.He B, Hoang TK, Wang T, et al. Resetting microbiota by Lactobacillus reuteri inhibits T reg deficiency-induced autoimmunity via adenosine A2A receptors. J Exp Med. 2017;214:107–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Di GC, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol 2005;174:3237–3246. [DOI] [PubMed] [Google Scholar]
- 94.Corthesy B, Gaskins HR, Mercenier A. Cross-talk between probiotic bacteria and the host immune system. J Nutr. 2007;137:781S–790S. [DOI] [PubMed] [Google Scholar]
- 95.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. [DOI] [PubMed] [Google Scholar]
- 96.Casas IA, Dobrogosz WJ. Validation of the probiotic concept: Lactobacillus reuteri confers broad-spectrum protection against disease in humans and animals. Microb Ecol Health Dis. 2000;12:247–285. [Google Scholar]
- 97.Spinler JK, Auchtung J, Brown A, et al. Next-generation probiotics targeting Clostridium difficile through precursor-directed antimicrobial biosynthesis. Infect Immun. 2017;85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ganesh BP, Hall A, Ayyaswamy S, et al. Diacylglycerol kinase synthesized by commensal Lactobacillus reuteri diminishes protein kinase C phosphorylation and histamine-mediated signaling in the mammalian intestinal epithelium. [published online ahead of print July 26, 2017] Mucosal Immunol. 10.1038/mi.2017.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gao C, Ganesh BP, Shi Z, et al. Gut microbe-mediated suppression of inflammation-associated colon carcinogenesis by luminal histamine production. Am J Pathol, 2017;187:2323–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peng M, Biswas D. Short chain and polyunsaturated fatty acids in host gut health and foodborne bacterial pathogen inhibition. Crit Rev Food Sci Nutr. 2017;57:3987–4002. [DOI] [PubMed] [Google Scholar]
- 101.Rousseaux C, Thuru X, Gelot A, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13:35–37. [DOI] [PubMed] [Google Scholar]
- 102.Agency for Healthcare Research and quality (AHRQ). Safety of probiotics to reduce risk and prevent or treat disease: Executive Summary. Evidence-Based Practice http://www.ahrq.gov/sites/default/files/publications/files/probiotsum.pdf. Accessed April 2, 2018. [Google Scholar]
- 103.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut micro-biome viewed across age and geography. Nature. 2012;486:222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015;60(suppl 2):S129–S134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Delthia R After infant dies, health officials urge caution in children’s use of probiotic supplement. Newsday, News/Health 2014;http://www.newsday.com/news/health/fda-issues-warning-about-abc-dophilus-powder-health-after-infant-dies-1.9706489. Accessed April 2, 2018. [Google Scholar]
- 106.Das S, Gupta PK, Das RR. Efficacy and safety of Saccharomyces boulardii in acute rotavirus diarrhea: double blind randomized controlled trial from a developing country. J Trop Pediatr. 2016;62:464–470. [DOI] [PubMed] [Google Scholar]
- 107.Dinleyici EC, Kara A, Dalgic N, et al. Saccharomyces boulardii CNCM I-745 reduces the duration of diarrhoea, length of emergency care and hospital stay in children with acute diarrhoea. Benef Microbes. 2015;6:415–421. [DOI] [PubMed] [Google Scholar]
- 108.Lherm T, Monet C, Nougiere B, et al. Seven cases of fungemia with Saccharomyces boulardii in critically ill patients. Intensive Care Med. 2002;28:797–801. [DOI] [PubMed] [Google Scholar]
- 109.Martin IW, Tonner R, Trivedi J, et al. Saccharomyces boulardii probiotic-associated fungemia: questioning the safety of this preventive probiotic’s use. Diagn Microbiol Infect Dis. 2017;87:286–288. [DOI] [PubMed] [Google Scholar]
- 110.Thygesen JB, Glerup H, Tarp B. Saccharomyces boulardii fungemia caused by treatment with a probioticum. BMJ Case Rep. 2012. 10.1136/bcr.06.2011.4412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thomas CM, Hong T, van Pijkeren JP, et al. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS ONE. 2012;7:e31951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mangalat N, Liu Y, Fatheree NY, et al. Safety and tolerability of Lactobacillus reuteri DSM 17938 and effects on biomarkers in healthy adults: results from a randomized masked trial. PLoS ONE. 2012;7:e43910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fatheree NY, Liu Y, Taylor CM, et al. Lactobacillus reuteri for infants with colic: a double-blind, placebo-controlled, randomized clinical trial. J Pediatr. 2017;191:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Panigrahi P, Parida S, Pradhan L, et al. Long-term colonization of a Lactobacillus plantarum synbiotic preparation in the neonatal gut. J Pediatr Gastroenterol Nutr. 2008;47:45–53. [DOI] [PubMed] [Google Scholar]
- 115.Rajper MA, Udawatta RP, Kremer RJ, Lin C, Jose S. Effects of probiotics on soil microbial activity, biomass and enzymatic activity under cover crops in field and greehouse studies. Agroforestry Sys. 2016;90(5):811–827. [Google Scholar]
- 116.Weizman Z, Asli G, Alsheikh A. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics. 2005;115:5–9. [DOI] [PubMed] [Google Scholar]
- 117.Ganji-Arjenaki M, Rafieian-Kopaei M. Probiotics are a good choice in remission of inflammatory bowel diseases: a meta analysis and systematic review. J Cell Physiol. 2018;233:2091–2103. [DOI] [PubMed] [Google Scholar]
- 118.Dalal R, McGee RG, Riordan SM, Webster AC. Probiotics for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;2:CD008716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vaghef-Mehrabany E, Alipour B, Homayouni-Rad A, Sharif SK, Asghari-Jafarabadi M, Zavvari S. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition. 2014;30:430–435. [DOI] [PubMed] [Google Scholar]
- 120.d’Ettorre G, Ceccarelli G, Giustini N, et al. Probiotics reduce inflammation in antiretroviral treated, HIV-infected individuals: results of the “Probio-HIV” Clinical Trial. PLoS ONE. 2015;10:e0137200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gori A, Rizzardini G, Van’t Land B, et al. Specific prebiotics modulate gut microbiota and immune activation in HAART-naive HIV-infected adults: results of the “COPA” pilot randomized trial. Mucosal Immunol. 2011;4:554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim CJ, Walmsley SL, Raboud JM, et al. Can probiotics reduce inflammation and enhance gut immune health in people living with HIV: study designs for the probiotic Visbiome for inflammation and translocation (PROOV IT) pilot trials. HIV Clin Trials. 2016;17:147–157. [DOI] [PubMed] [Google Scholar]
- 123.Newlove-Delgado TV, Martin AE, Abbott RA, et al. Dietary interventions for recurrent abdominal pain in childhood. Cochrane Database Syst Rev. 2017;3:CD010972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu HB, Jiang RH, Sheng HB. Meta-analysis of the effects of Bifidobacterium preparations for the prevention and treatment of pediatric antibiotic-associated diarrhea in China. Complement Ther Med. 2017;33:105–113. [DOI] [PubMed] [Google Scholar]
- 125.Akbari V, Hendijani F. Effects of probiotic supplementation in patients with type 2 diabetes: systematic review and meta-analysis. Nutr Rev. 2016;74:774–784. [DOI] [PubMed] [Google Scholar]
- 126.Miller LE, Ouwehand AC, Ibarra A. Effects of probiotic-containing products on stool frequency and intestinal transit in constipated adults: systematic review and meta-analysis of randomized controlled trials. Ann Gastroenterol. 2017;30:629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Muniz FW, Friedrich SA, Silveira CF, Rosing CK. The impact of chewing gum on halitosis parameters: a systematic review. J Breath Res. 2017;11:014001. [DOI] [PubMed] [Google Scholar]
- 128.Feng JR, Wang F, Qiu X, et al. Efficacy and safety of probiotic-supplemented triple therapy for eradication of Helicobacter pylori in children: a systematic review and network meta-analysis. Eur J Clin Pharmacol. 2017;73:1199–1208. [DOI] [PubMed] [Google Scholar]
- 129.Lu M, Yu S, Deng J, et al. Efficacy of probiotic supplementation therapy for helicobacter pylori eradication: a meta-analysis of randomized controlled trials. PLoS ONE. 2016;11:e0163743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang F, Feng J, Chen P, et al. Probiotics in Helicobacter pylori eradication therapy: Systematic review and network meta-analysis. Clin Res Hepatol Gastroenterol. 2017;41:466–475. [DOI] [PubMed] [Google Scholar]
- 131.Schreck BA, Gregory PJ, Jalloh MA, Risoldi CZ, Hein DJ. Probiotics for the treatment of infantile colic: a systematic review. J Pharm Pract. 2017;30:366–374. [DOI] [PubMed] [Google Scholar]
- 132.Manzanares W, Lemieux M, Langlois PL, Wischmeyer PE. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Crit Care. 2016;19: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cayzeele-Decherf A, Pelerin F, Leuillet S, et al. Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: an individual subject meta-analysis. World J Gastroenterol. 2017;23:336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aceti A, Maggio L, Beghetti I, et al. Probiotics prevent late-onset sepsis in human milk-fed, very low birth weight preterm infants: systematic review and meta-analysis. [published on-line ahead of print on August 22, 2017] Nutrients. 2017;9 10.3390/nu9080904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rao SC, Athalye-Jape GK, Deshpande GC, Simmer KN, Patole SK. Probiotic supplementation and late-onset sepsis in preterm infants: a meta-analysis. Pediatrics. 2016;137: e20153684. [DOI] [PubMed] [Google Scholar]
- 136.Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology 2017;152:1889–1900. [DOI] [PubMed] [Google Scholar]
- 137.Liu MM, Li ST, Shu Y, Zhan HQ. Probiotics for prevention of radiation-induced diarrhea: a meta-analysis of randomized controlled trials. PLoS ONE. 2017;12:e0178870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhong C, Qu C, Wang B, Liang S, Zeng B. Probiotics for preventing and treating small intestinal bacterial overgrowth: a meta-analysis and systematic review of current evidence. J Clin Gastroenterol. 2017;51:300–311. [DOI] [PubMed] [Google Scholar]
- 139.Kasatpibal N, Whitney JD, Saokaew S, Kengkla K, Heitkemper MM, Apisarnthanarak A. Effectiveness of probiotic, prebiotic, and synbiotic therapies in reducing postoperative complications: a systematic review and network meta-analysis. Clin Infect Dis. 2017;64:S153–S160. [DOI] [PubMed] [Google Scholar]
- 140.Liu PC, Yan YK, Ma YJ, et al. Probiotics reduce postoperative infections in patients undergoing colorectal surgery: a systematic review and meta-analysis. Gastroenterol Res Pract. 2017;2017:6029075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hendijani F, Akbari V. Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: A systematic review and meta-analysis. Clin Nutr. 2018;37(2):532–541. [DOI] [PubMed] [Google Scholar]
- 142.Wu Y, Zhang Q, Ren Y, Ruan Z. Effect of probiotic Lactobacillus on lipid profile: a systematic review and meta-analysis of randomized, controlled trials. PLoS ONE. 2017;12:e0178868. [DOI] [PMC free article] [PubMed] [Google Scholar]


