Abstract
Background:
Bioprosthetic heart valves undergo structural degeneration and calcification. Similarities exist in the histopathologic features of explanted bioprosthetic valves and rejected pig tissues and organs after xenotransplantation into nonhuman primates. The development of more durable bioprosthetic valves, namely from genetically-modified pigs, could negate the need for the insertion of mechanical prostheses in children and young adults with the requirement for life-long anticoagulation, and might avoid the need for reoperation in elderly patients.
Methods:
We reviewed the literature (MedlinePlus, PubMed, Google Scholar) through September 1, 2018, under 4 key terms: (1) bioprosthetic heart valves; (2) xenograft antigens; (3) immunologic responses to bioprosthetic valves; and (4) genetic modification of xenografts.
Results:
Advances in tissue and organ xenotransplantation have elucidated important immunologic barriers that provide innovative approaches to prevent structural degeneration of bioprosthetic heart valves. The current evidence suggests that bioprosthetic valves derived from genetically-modified pigs lacking xenogeneic antigens (namely Gal, Neu5Gc, and Sda), termed triple-knockout pigs, would function considerably longer than current wild-type (genetically-unmodified) porcine valves in human recipients.
Conclusions:
Preclinical and clinical studies determining the safety and efficacy of triple-knockout porcine bioprosthetic valves will likely establish that they are more resistant to human immune responses and thus less susceptible to structural degeneration.
Valve replacement remains a cornerstone of cardiothoracic surgery. For example, between 2005 and 2013, the number of isolated aortic valve replacements nearly doubled to 30,679 in the United States alone [1]. Bioprosthetic heart valves are increasingly used [2]. In the United States, nearly half of patients aged 55-64 now elect for tissue valve replacements [2]. This number is likely to increase as the mortality benefit of mechanical prostheses disappears by 55 years of age [3].
Still, bioprostheses, regardless of tissue origin, remain prone to degeneration and calcification [4,5]. Reoperation rates range from 30% to 80% at 15 years; outcomes are worse in younger patients [5-7]. The development of more durable bioprostheses, namely from genetically-modified pigs, could negate the need for the insertion of mechanical prostheses in children and young adults with the requirement for life-long anticoagulation. A durable bioprosthetic valve would also avoid the need for reoperation in elderly patients, which carries an increased surgical risk [8].
Surgical replacement remains standard of care for stenotic or incompetent heart valves, but is particularly challenging in frail and elderly patients in whom transcatheter aortic valve replacement (TAVR) is increasingly used. Regardless, endovascularly-deployed valves are constructed of similar materials as other bioprosthetic valves, namely porcine or bovine tissues [2,8]. Improving long-term outcomes will require addressing the underlying reasons for bioprosthetic valve failure, which include structural degeneration, calcification, leaking, and tearing [9].
Since the first xenograft bioprosthetic valve was inserted in 1965, significant advances have been made in understanding the mechanisms responsible for graft failure [10,11]. Here we review progress in combatting the pathobiology of bioprosthetic valve degeneration, with emphasis on recent advances in genetic-modification that could provide a more optimal graft for implantation into humans.
Material and Methods
We reviewed the literature through PubMed, Google Scholar, and MedlinePlus for publications relevant to (1) bioprosthetic heart valves; (2) xenograft antigens; (3) immunologic responses to bioprosthetic heart valves; and (4) genetic modifications in xenografts. In total, 230 primary and review articles were obtained, of which we have included the 80 most relevant and contemporary for reference.
Results
1. Historical advances in valve development
After pioneering work by Charles Hufnagel (Supplemental FigureS1A) in Boston and Gordon Murray (Supplemental FigureS1B) in Toronto, who inserted aortic valve homografts (allografts) into the descending aorta in patients with aortic regurgitation, Donald Ross (Supplemental FigureS1C) in the United Kingdom, and Brian Barratt-Boyes (Supplemental FigureS1D) in New Zealand, were first to place homograft valves in the subcoronary position [12-15]. Their results sparked enthusiasm to further develop biologic valves. Although technically more demanding to implant, the natural valve did not obstruct flow like mechanical ball-and-cage designs, and importantly negated the need for anticoagulation.
In 1965, the French surgeon, Alain Carpentier (Supplemental FigureS1E), performed the first successful porcine xenograft valve replacement [10]. After dismal failure rates of 40% at 6 months, and ~55% at 1 year, he importantly identified histologic evidence of host immune responses to the valve [16]. A number of protocols were developed to pretreat the valves in order to eliminate or denature antigens, which stabilized the grafts chemically and mechanically [17]. Valves were placed into a glutaraldehyde solution to neutralize free amino acids, improving functional rates at 5 years to 77%, 89%, and 96% in the mitral, aortic, and tricuspid positions, respectively [18]. Nevertheless, it soon became clear that, despite these treatments, biologic valves deteriorated over time.
Improving long-term outcomes after bioprosthetic valve insertion
Xenograft bioprostheses are most commonly constructed from porcine aortic valves or porcine (or bovine) pericardium [17]. Glutaraldehyde-fixed valve leaflets or sheets of pericardium are fastened to a stent covered by fabric that has been developed to reduce thrombosis [6,17]. Nevertheless, degeneration is progressive. In older adults, histologic evidence of calcification can be seen within 3 years, and by 10 years, 20-30% of bioprostheses become dysfunctional [5,19]. In some cases, fewer than 10% of patients survive with the original prosthesis at 20 years [20].
Durability (and, of course, survival) varies by age [4]. Higher rates of surgical reintervention are required in children and young adults, which is probably associated with a more vigorous immune response and calcium metabolism [22]. Up to 45% of patients <40 years of age experience bioprosthetic valve structural degeneration within 15 years of implantation, compared to <10% of patients older than 70 [4]. As such, bioprostheses (as opposed to mechanical prostheses) are recommended in patients (i) whose presumed life expectancy is less than the anticipated longevity of the bioprosthetic valve, (ii) when the likelihood (or risk) of reintervention is otherwise low, and (iii) in other relevant situations (including patient preference) [2]. Structural degeneration and calcification, leading to stenosis or leakage, is the predominant mechanism of failure.
Pathobiological mechanisms of valvular degeneration
After implantation of a bioprosthetic valve, host tissue overgrowth (pannus) comprised of myofibroblasts, fibroblasts, and capillary endothelial cells, is expected. This tissue overgrowth is important for healing and eventually creating a non-thrombogenic surface at the suture line [9]. However, pathologic overgrowth can contribute to bioprosthesis failure [9,17].
Furthermore, calcification contributes to stenosis or insufficiency, and can occur within the pannus or elsewhere [17,22]. Recent attempts to use anti-calcifying agents may combat passive processes of calcification caused by fixation and processing (i.e., disrupting calcium-exchange and exposing extracellular phospholipids). However, active immunity also amplifies calcification and valve degeneration, indicating a need to reduce the immunogenicity of bioprostheses prior to implantation [6,17,22]. This is the focus of this review.
Establishing the role for immunogenicity in bioprosthetic valve degeneration
Many of the earliest reports of bioprosthetic valve failures identified histologic evidence of pseudointimal proliferation, inflammation, thrombosis, and fibrocalcification, particularly in explants from children [11,16,18]. A case report in 1982 linked aortic stenosis to host immune responses and graft rejection [23]. By 1988, electron microscopy exhibited crystalline structures on explanted bioprostheses, and demonstrated monocyte phagocytosis of collagen, further implicating an immunologic role in calcification [24]. In 1990, bovine pericardial valves were reported to induce a cellular and humoral response [25]. Using widely-disparate (discordant) animal models to mimic bioprosthetic valve degeneration in humans, the role of immunity was becoming apparent [26]. Even human allografts were found to elicit an immune response [21,27].
In 1996, Hoekstra and colleagues provided evidence of inflammation in human allograft explants [28]. While both allogeneic and xenogeneic tissues degraded after glutaraldehyde-fixation, calcium deposition and macrophage infiltration were comparatively increased in xenografts, suggesting that immunologic infiltration of glutaraldehyde-fixed bioprosthetic valves was a result of heightened antigenic incompatibility [29,30]. It was later demonstrated that bioprostheses elicit a unique immune response compared to homografts as well as aortic valves isolated from transplanted hearts with features of chronic rejection [31]. To some extent, immunosuppressive therapy reduces the cellular infiltrate that correlates with bioprosthetic valve calcification. However, the inherent risks of such therapy makes it an unrealistic option for most patients receiving bioprosthetic valves [32].
Similar pathobiological inflammation and calcification is characteristic of other chronic diseases that can be attenuated, which has substantiated the importance of preventing immunologic responses to bioprosthetic valves, and has remained a focus of investigation to improve graft longevity [33].
Humoral and cellular immunity in bioprosthetic valve degeneration
Devitalization protocols (glutaraldehyde fixation, etc.) inherently disrupt matrix crosslinking. The products of new cellular, molecular, and ionic interactions accelerates the immunologic and inflammatory responses contributing to bioprosthesis mineralization [9]. Paradoxically, while glutaraldehyde concentrations have been reduced over time to limit tissue damage, higher glutaraldehyde concentrations may prevent calcification [17,34]. Thus, there is an elusive balance in pretreatment protocols to prevent the host immune response while preserving the integrity of the graft.
It is of key importance to recognize that glutaraldehyde does not reduce the expression of xenoantigens on a variety of bioprosthetic valves (Figure1, Figure2A,B) [35]. Thus, while optimizing bioprosthetic valve fixation is important for preventing immune responses and improving graft longevity, it is inadequate (and could potentially be eliminated). Additional techniques are needed to combat xenogeneic antibody responses, because although glutaraldehyde treatment reduces the antibody response, it fails to eliminate binding to key xenoantigens (Figure2C) [35].
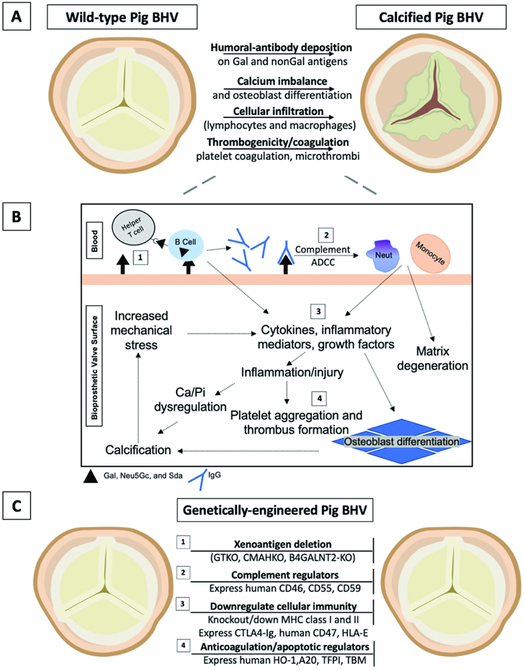
Figure 1. Overview of immunologic responses and proposed genetic modifications to combat bioprosthetic valve degeneration and calcification.
(A) Mechanisms of degeneration and calcification in wild-type (genetically-unmodified) pig BHVs, and (B) a schematic overview of immune-mediated degeneration and calcification. (C) Established genetic modifications that may inhibit these processes and improve commercial BHV durability (see Table 1 for feasibility, advantages, and disadvantages).
(1) Xenoantigens are retained despite BHV fixation and decellularization. Genetic deletion of three key xenoantigens, Gal, Neu5Gc, and Sda (GTKO, CMAHKO, β4GALNT2-KO, respectively) prevents most antibody-dependent cell-mediated cytotoxicity (ADCC) and complement activation. ADCC results from host immune cells (e.g., macrophages) that bind antibodies through their Fc receptors. Host antigen-presenting cells, including B cells and dendritic cells, opsonize xenoantigens for presentation to helper T cells, which activate B cell maturation, class switching, and additional antibody production.
(2) Transgenic expression of human complement regulators further attenuates the innate immune response by preventing activation of the cell-killing membrane attack complex. Products of the complement cascade also activate neutrophil and macrophage infiltration, promoting inflammation and phagocytosis that degenerates the surrounding matrix.
(3) Macrophages, neutrophils, and lymphocytes play a major role in cellular immunity against implanted foreign materials. Preventing recruitment and activation could inhibit the production of inflammatory cytokines that incite fibroblast and valve interstitial cell osteoblastic differentiation. Genetically-engineered porcine BHVs express proteins that may reduce macrophage activity (hCD47), cytotoxicity (CTLA4-Ig), and natural killer cell immunity (HLA-E). Knockdown (or knockout) of MHC molecules (swine leukocyte antigens) reduces porcine protein presentation and recognition required for the innate and adaptive cellular immune responses.
(4) Similarly, transgenic expression of anti-apoptotic (A20 and HO-1) or anti-coagulation proteins (TBM, ECPR, CD39) can minimize cell destruction and vascular inflammation that serves as a nidus for calcium nucleation and thrombus formation, respectively. Deposition of calcium orthophosphates alter the hemodynamics and increase mechanical stress, further disrupting the homeostatic environment of the bioprosthetic valve and surrounding tissues. (2-4) It is important to note that glutaraldehyde-fixed pig BHVs may not retain biologic activity comparable to fresh pig tissues; therefore, expression of these molecules may be negated in pretreated valves.
A20 = tumor necrosis factor-alpha-induced protein 3; β4GALNT2-KO = Sda knockout; BHV = bioprosthetic heart valve; CD39 = ectonucleoside triphosphate diphosphohydrolase-1; CD46 = membrane cofactor protein; CD55 = decay-accelerating factor; CD59 = protectin or membrane inhibitor of reactive lysis; CMAHKO = Neu5Gc-knockout; CTLA4-Ig = cytotoxic T-lymphocyte antigen 4-Ig; ECPR = endothelial protein C receptor; GTKO = Gal-knockout; hCD47 = human integrin-associated protein; HO-1 = hemeoxygenase-1; MHC = major histocompatibility complex; Neut = neutrophil; TBM = thrombomodulin; TFPI = tissue factor pathway inhibitor.
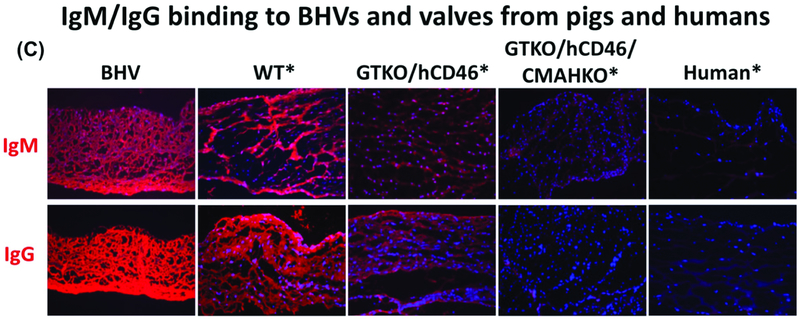
Figure 2: Xenoantigen expression and human antibody binding on human, wild-type pig, and genetically-modified pig valves.
Glutaraldehyde fixation of wild-type (WT) and three different BHVs does not eliminate structural glycoprotein/xenoantigen components, as indicated by immunofluorescence staining. Gal and Neu5Gc are likely to be, at least partially, responsible for a xenogeneic host response.
(A) Immunofluorescence staining for Gal (red) on various valves. In BHVs, Gal expression is still quite strong compared to WT pig valves, whereas GTKO, GTKO/hCD46/CMAHKO, and human tissue are negative. Of note, BHVs stain positive for nuclei (blue; DAPI). While BHVs are considered ‘devitalized’, the nuclear remnants may continue to act as a source of xenoantigens.
(B) Neu5Gc epitopes (red) are not decreased in BHVs compared to WT or GTKO/hCD46 valves. In comparison, Neu5Gc was not detected in GTKO/hCD46/CMAHKO pigs, or human valves.
(C) Valves were incubated with human serum for 1 hour, and stained with biotin-conjugated anti-human IgM and IgG antibodies, followed by incubation with Alexa Fluor 647-conjugated streptavidin. Immunofluorescence staining of human IgM and IgG (red) binding did not differ between BHV and WT tissues, but decreased to indicate reduced binding to GTKO/hCD46 pig tissue. Antibody binding was further decreased on GTKO/hCD46/CMAHKO pig valves, which look comparable to human valves. (Magnification x200. *Tissue was fixed with 0.2% glutaraldehyde for 48 hours prior to staining. BHV = Bioprosthetic heart valve; BHV1 = Carpentier Edwards Model 2605 bovine-stented supra-annular valve [Edwards Lifesciences, Irvine, CA]; BHV2 = Medtronic Mosaic 305 porcine-stented Cinch valve [Medtronic, Minneapolis, MN]; BHV3 = Medtronic Freestyle 995 Porcine-stentless valve.) (Modified from Lee and colleagues [35] with permission from John Wiley and Sons)
In 2001, Human and Zilla demonstrated that antibodies binding fixed bioprosthetic tissue could promote calcification, establishing what may be the first direct evidence of antibody-mediated degeneration [36]. Human anti-pig antibodies bind porcine xenoantigens, activating host immune responses that injure graft endothelial cells, likely leading to calcification and graft degradation [37]. Host IgM/IgG antibody binding initiates macrophage infiltration, collagen degradation, and mineralization (Figure1) [25,35,38]. Furthermore, monocytes and lymphoid cells produce cytokines that may contribute to tissue calcification [33,39]. As such, the rapid degeneration of bioprostheses in young individuals may therefore result from a more robust immune system [21].
2. Establishing the role of key xenoantigens in bioprosthetic valve degeneration
Lessons from organ xenotransplantation
Bioprosthetic valve replacement is a unique form of xenotransplantation that has evolved over more than 50 years [11]. Advances in tissue and whole-organ xenotransplantation in the past 30 years has elucidated important immunologic barriers, which may provide innovative approaches for preventing bioprosthetic valve degeneration.
Similarities exist in the histopathologic features of explanted bioprosthetic valves and pig tissues and organs rejected after xenotransplantation into nonhuman primates [35,38,39]. Currently, bioprostheses are prepared from genetically-unmodified (wild-type) pigs, and retain surface xenoantigens that can induce an immune response (Figure2A,B) [35,40]. Reducing the xenoantigenicity of bioprosthetic valves through genetic-modification of the pig source is a major prospect for improving valve durability [41].
Humans produce natural preformed antibodies to three major carbohydrate antigens expressed on pig vascular endothelial cells, namely galactose-α1,3-galactose (Gal), N-glycolylneuraminic acid (Neu5Gc), and Sda [42-46]. Anti-Gal antibodies are believed to develop during infancy as a response to colonization of the gastro-intestinal tract by various bacterial and viral flora (that express Gal) [47]. When pig organs are transplanted into humans or Old World nonhuman primates, expression of Gal results in almost uniform hyperacute rejection [48].
In 2003, with the production of α1,3-galactosyltransferase gene-knockout (GTKO) pigs (genetically-modified not to express Gal), the immunologic barrier of hyperacute rejection was largely overcome [49,50]. The transplantation of organs from pigs with multiple genetic modifications has significantly improved xenograft survival [51]. For example, with the addition of immunosuppressive regimens to prevent T cell-dependent elicited antibody responses, survival of non-life-supporting heterotopic pig hearts and life-supporting kidneys is now being measured in months or even years, rather than minutes as originally reported (Figure3) [48,52,53].
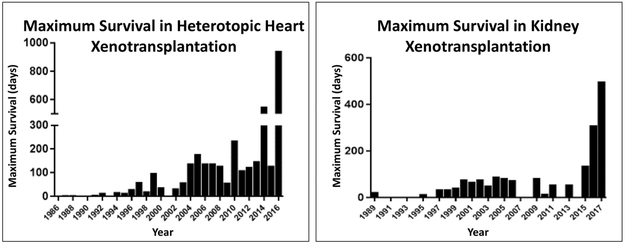
Figure 3: Survival after pig organ transplantation in nonhuman primates, 1986-2017.
(Left) After heterotopic (non-life-supporting) pig heart xenotransplantation (in the abdomen) in nonhuman primates, maximum survival has improved from <8 hours in 1986 to 945 days.
(Right) After life-supporting pig kidney xenotransplantation, maximum survival has improved from 23 days in 1989 to >1 year.
NB. Organ transplantation from genetically-engineered pigs expressing a human complement-regulatory protein was first reported in 1995, and organ transplantation from GTKO pigs was first reported in 2005. GTKO pigs expressing both human complement- and coagulation-regulatory proteins were first tested in 2011.
(Reviewed in reference [51])
Two key genetic approaches are responsible for these encouraging results—(i) deletion of pig xenoantigens against which humans (and nonhuman primates) have natural (preformed) antibodies, and (ii) transgenic expression of ‘protective’ human complement- and/or coagulation-regulatory proteins in the source pigs [51]. We suggest that the ability to genetically-modify pig sources lacking multiple key xenoantigens will soon overcome the barriers of valve degeneration in glutaraldehyde-fixed bioprosthetic valves. If fixation is no longer required, then the additional transgenic expression of human complement, coagulation, or other regulatory proteins may eventually eliminate the need for all cellular devitalization and pretreatment protocols. We suggest it is timely to seriously consider genetically-modified pigs as sources of clinical bioprosthetic heart valves.
However, it must be noted that even autologous (i.e. immunologically inert) valve replacements (such as the ‘Ross’ procedure, which replaces the patient’s aortic valve with their own pulmonary valve) still experience structural valve degeneration and calcification over time. Thus, while genetic modifications are an encouraging initial prospect for overcoming the immunologic barriers of degeneration, it would not be a panacea for the additional mechanical, degenerative, and atherosclerotic processes responsible for graft failure [6].
Gal antigens in bioprosthetic heart valves
Interest in the Gal antigen with respect to bioprosthetic valve degeneration increased in 2005, when Konakci, et al. demonstrated that Gal antigens were expressed on bioprosthetic heart valves, and that patients receiving bioprostheses (but not mechanical prostheses) had abundant anti-Gal antibodies. This suggested to them that anti-Gal antibodies may be implicated in bioprosthetic valve degeneration [38]. Several other groups have subsequently documented the role of Gal in the structural degeneration of bioprostheses [39]. By 2010, it was demonstrated that anti-Gal antibodies significantly increased in patients with bioprosthetic valves [54].
Anti-nonGal antibodies in xenograft rejection
Natural human antibodies to the two other major carbohydrate xenoantigens (Neu5Gc and Sda) have been identified, but the cytotoxicity associated with these antibodies is relatively reduced compared to anti-Gal antibodies (Figure4A,B) [44,55-57].
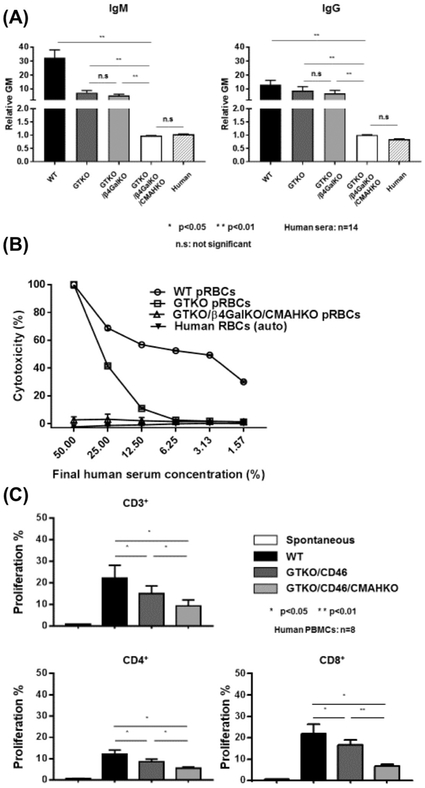
Figure 4:
(A) Human IgM (left) and IgG (right) antibody binding to wild-type (WT), Gal knockout (GTKO), Gal/Sda double-knockout (GTKO/β4GalKO), and Gal, Sda, Neu5Gc triple-knockout (GTKO/β4GalKO/CMAHKO) pig red blood cells (pRBCs). Binding to TKO pig RBCs was not significantly different from human IgM and IgG binding to human RBCs of blood type O. (B) Pooled human serum complement-dependent cytotoxicity (hemolysis) to WT, GTKO, double-knockout, and triple-knockout pig RBCs. Cytotoxicity of the same serum to autologous human O RBCs was tested as a control. (C) Human T cell proliferative response to WT, GTKO/hCD46, and GTKO/hCD46/CMAHKO pig peripheral blood mononuclear cells (PBMCs) in mixed leukocyte reaction. (Triple-knockout pig PBMCs were not available to us at the time.)
Similar to Gal, Neu5Gc antigens are widely expressed on porcine vascular endothelium and bioprosthetic valves (Figure2B) [35,58,59]. In 2013, Jeong, et al. demonstrated that antigens containing Neu5Gc were present on porcine valves, which may indicate that Neu5Gc is expressed on different molecules (glycolipids or glycoproteins) in different tissues. In particular, Neu5Gc may be expressed on glycoproteins of porcine valves [57,60]. Because it is possible that antibodies only recognize certain structural forms of Neu5Gc, identifying the predominant forms on bioprostheses will be important [57]. However, definitive conclusions remain to be drawn regarding the immunogenicity of Neu5Gc in bioprosthetic valve degeneration, which will require further analysis of patients with porcine-derived tissue grafts.
It is important to note that many studies investigating the role of Neu5Gc have been completed in vitro [35,59]. Although nonhuman primates have been established as reliable surrogate hosts in xenotransplantation models, baboons and Old World monkeys differ from humans in one important immunologic respect. Old World primates express Neu5Gc, and therefore do not make anti-Neu5Gc antibodies, and thus cannot provide a model for studying Neu5Gc in xenotransplantation (as they do for Gal) [59,61]. In contrast, New World nonhuman primates do not express Neu5Gc (similar to humans) and, although generally small, could serve an important role in preclinical pigto- nonhuman primate trials [59,62].
3. Preventing immunologic rejection of bioprosthetic valves through genetic modifications
Initial production of Gal-deficient pigs
In 2005, Kuwaki, et al. demonstrated that GTKO porcine hearts transplanted into baboons do not undergo hyperacute rejection [50]. It was hypothesized that pigs genetically-modified to eliminate Gal might be a source for future bioprosthetic valves [63].
In 2011, Lila and McGregor demonstrated that GTKO porcine pericardium (pre-treated with human anti-Gal antibodies) xenografted into rats and rabbits calcified less than pericardium from wild-type pigs [64]. In 2013, this group placed porcine valves into nonhuman primates, and demonstrated that serum anti-Gal IgG significantly increased after implanting wild-type valves (compared to GTKO valves) over a one-year period [65]. However, the authors did not report the gross pathology and histopathology of the valves, which would have provided valuable evidence for the definitive role of rejection.
Combining genetic modifications to further reduce immunogenicity of bioprosthetic valves
Aortic endothelial cells from pigs genetically-modified to both eliminate Gal expression and express hCD46 (a human complement-regulatory protein; GTKO/hCD46 pigs), have been shown to reduce human platelet aggregation [59,66]. Important studies on double-knockout (i.e., GTKO + knockout of Neu5Gc, termed GTKO/CMAHKO) porcine peripheral blood mononuclear cells demonstrated a striking reduction in their antigenicity (both humoral and cellular) to a level not significantly different to that of allograft cells (Figure4) [67]. Double-knockout pigs with additional transgenic expression of human CD46 (GTKO/hCD46/CMAHKO) have been demonstrated to be largely resistant to xenograft rejection [58,59,67,68].
Indeed, Lee demonstrated that GTKO/hCD46/CMAHKO porcine valves and pericardium reduced human serum antibody binding to levels comparable to that of human valves (Figure2) [35,57,59]. The human humoral and cellular immune responses to these pig cells are weak (Figure4C) [58,69,70]. However, it is important to note that if glutaraldehyde-fixation remains essential in the preparation of porcine bioprostheses, the biologic activity of hCD46 may be negated [11].
Similarly, cells from triple-knockout pigs with an additional deletion of the enzyme responsible for expression of Sda (β-1,4-N-acetyl-galactosaminyl transferase 2; β4GalNT2-KO) on a GTKO/CMAHKO background are associated with even greater reduction in the human humoral and cellular responses. For example, human antibody binding to triple-knockout pig red blood cells is comparable to that of human blood type O red blood cells (Figure4A,B). However, the effect on tissues from which bioprosthetic valves could be fashioned (e.g., pericardium) had not been investigated [35,57,67] until recently when Zhang and colleagues showed that triple-knockout porcine pericardium attracts minimal human IgM/IgG binding [37,70].
Importantly, the biophysical properties of the pericardium were preserved, suggesting that triple-knockout pigs would serve as ideal bioprosthetic valve sources (Figure5) [37]. Still, whether triple-knockout porcine pericardium and valve leaflets will continue to preserve their structural integrity under in vivo hemodynamics remains to be elucidated.
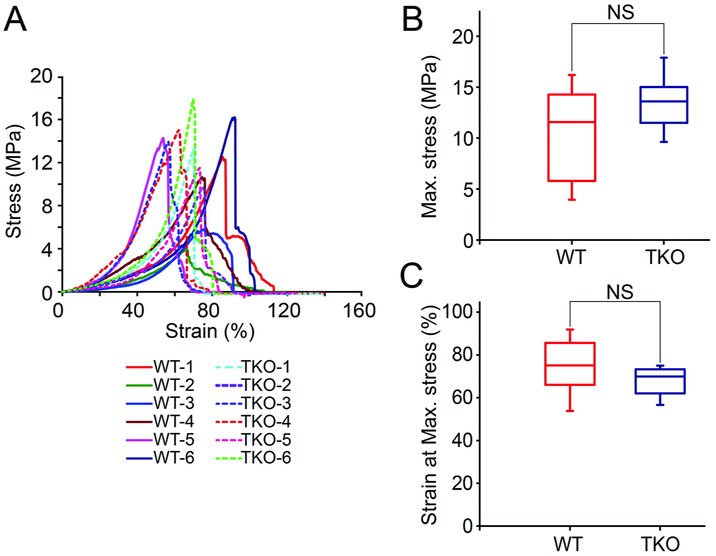
Figure 5: Biomechanical properties of triple-knockout porcine pericardium are similar to those of wild-type pig pericardium.
Uniaxial stress test of wild-type (WT) and triple-knockout (TKO) pig pericardium (n = 3/group).
(A) Pericardium was harvested from 6 WT and 6 TKO pigs and tested for stress strain.
(B) Box plot of the maximum stress (10.56±4.39 MPa and 13.54 ± 2.61 MPa for the WT and TKO, respectively).
(C) Box plot of the strain at the maximum stress (WT was 74.62±12.43%, TKO was 67.81±6.44%).
(NS = not significant; TKO = triple-knockout (GTKO/β4GalNT2-KO/CMAHKO); WT = wild-type) (Reproduced from Zhang and colleagues [37] with permission from Elsevier)
4. Moving towards clinical application
Future investigations
Continuing to define the role of triple-knockout source pigs, it would be prudent to compare xenograft rejection of pig tissues (ideally valve leaflets, pericardium, or an aortic patch capable of comparing the elicited antibody and T cell proliferative response) from GTKO (as controls) and triple-knockout pigs into New World monkeys that make anti-Neu5Gc and anti-Sda antibodies similar to humans [35, 62,71,72]. It will be important to ensure that any genetic modifications completely delete antigens, and do not ‘unmask’ additional epitopes that might adversely accelerate an immunologic response and valve degeneration [35,37,68]. Similarly, attempts to eliminate or substitute glutaraldehyde fixation for other processing techniques must ensure appropriate protection from free amino acids and other immunogenic antigens denatured by such established, albeit imperfect, protocols [17].
A number of other transgenic modifications (e.g., expression of human complement- and coagulation-regulatory, anti-inflammatory, and ‘self-recognition’ proteins) and methods for T cell costimulation blockade studied in tissue and organ xenotransplantation may be applied to bioprosthetic valves (Table 1) [73]. However, given that expression of human transgenes in fixed and processed valves would prove challenging, we believe that initial steps in establishing the safety and efficacy of genetically-modified pigs as sources of bioprosthetic heart valves should focus on the relatively easy and established application of xenoantigen deletion (i.e., Gal, Neu5Gc, and Sda).
Table 1:
Selected genetic-engineering approaches to reduce the human pathobiological responses to transplanted pig hearts (though not all may be relevant to bioprosthetic valves)
|
Potential Genetic Modifications |
Feasibility | Advantages | Disadvantages |
|---|---|---|---|
| Xenoantigen deletion | ++++ | Relatively easy and established application of xenoantigen deletion Modest cost burden after establishing an initial herd |
Investment for clinical trial application Regulatory approval New World primates needed to investigate Neu5Gc |
| α1,3-galactosyltransferase gene-knockout (GTKO) | ++++ | ||
| Cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH) gene-knockout (CMAHKO, i.e., KO of Neu5Gc) | ++++ | ||
| β4GalNT2 (β1,4 N-acetylgalactosaminyltransferase) gene-knockout (β4GalNT2-KO, i.e., KO of Sda) | ++++ | ||
| Human complement-regulatory gene expression | +++ | Relatively easy and established application of transgene expression. Provides protection from human complement activation. | Glutaraldehyde-fixation would inhibit function of at least some transgenic ‘protective’ proteins |
| CD46 (membrane cofactor protein) | +++ | ||
| CD55 (decay-accelerating factor) | +++ | ||
| CD59 (protectin or membrane inhibitor of reactive lysis) | +++ | ||
| Suppression of cellular (lymphocyte/macrophage) responses by human transgene expression or human gene deletion or downregulation | +++ | Relatively established application of transgene expression Local immunosuppression of adaptive immune response T cell costimulation blockade |
Glutaraldehyde-fixation would inhibit function of at least some transgenic ‘protective’ proteins Less established in preclinical models Risk of systemic immunosuppression |
| Expression of hCD47 (human integrin associated protein; species-specific interaction with SIRP-α inhibits phagocytosis) | +++ | ||
| Expression of cytotoxic T-lymphocyte antigen 4-Ig (CTLA4-Ig) (results in a local immunosuppressive effect) | +++ | ||
| MHC class I-knockout (MHC-I-KO) (results in no expression of swine leukocyte antigen [SLA] class I) | ++ | ||
| MHC class II transactivator knockdown (CIITA-DN) (results in reduced expression of SLA class II). | ++ | ||
| Expression of hHLA-E, hHLA-G, or hHLA-Cw3 (inhibits human natural killer cell cytotoxicity) | ++ | ||
| Human anticoagulation and anti-inflammatory gene expression | + | Relatively easy and established application of transgene expression. Provides protection from human coagulation activation. | Potential risk of bleeding |
| Thrombomodulin (TBM) | + | ||
| Endothelial protein C receptor (EPCR) | + | ||
| Tissue factor pathway inhibitor (TFPI) | + | ||
| CD39 (ectonucleoside triphosphate diphosphohydrolase-1) | + | ||
| Human anti-inflammatory and anti-apoptotic gene expression | + | Protection against local inflammatory and apoptotic stimuli | Questionable preclinical significance and efficacy Limited preclinical experience in xenotransplantation |
| Hemeoxygenase-1 (HO-1) | + | ||
| A20 (tumor necrosis factor-alpha-induced protein 3) | + |
= Highly feasible, well-established model, and readily applicable;
= Moderately feasible, well-established model, challenges to application in fixed valve tissues;
= Feasible, established model, with questionable clinical efficacy;
= Feasible, limited application in preclinical models, theoretical efficacy
Congenital heart disease: a potential for initial clinical application
Despite progress in bioprosthesis processing and design, neonates and infants often face sub-optimal outcomes after valve replacement, in part because of the frequent lack of availability of homografts of a suitable size [74]. This limitation is particularly serious in very young (<1 year) and low-weight (<3kg) children, in whom subsequent reintervention-free survival is poor [75]. Similarly, extracardiac conduits have allowed routine repair of complex congenital heart defects in the first year of life, but are again limited in availability. As a result, a number of synthetic and/or biologic alternatives to restore pulmonary and/or systemic hemodynamics have been developed [76,77]. Despite promising results overall (particularly in bovine jugular vein, homograft vein, and porcine-valved Dacron conduits), an optimal approach remains unclear [77]. Complicating matters, allosensitization after graft failure may limit heart allotransplantation, which is often required in such vulnerable children, and may increase transplant morbidity and mortality [78].
Pigs could provide an unlimited range of sizes of bioprosthetic valves that would be available whenever required, and triple-knockout porcine bioprostheses would have reduced immunogenicity, and therefore are likely to be associated with reduced valve/conduit degeneration [79].
Comment
The current evidence suggests that triple-knockout porcine bioprosthetic heart valves would function considerably longer than current wild-type bioprosthetic valves in human recipients. Preclinical and clinical studies determining the safety and efficacy of triple-knockout bioprosthetic valves will almost certainly establish that porcine bioprostheses are more resistant to human immune responses and are thus less susceptible to degeneration. It will be important to confirm that genetically-modified porcine valves retain their biophysical properties over a long period of time [37]. We suggest that nonhuman primates with similar immune systems to humans, particularly New World monkeys, can provide an initial model for safety and efficacy studies of triple-knockout bioprostheses prior to clinical trials [35,37,59,68].
In contrast to pigs bred as sources of vital organs (e.g., the heart), that will be required to be housed under strict biosecured conditions (to prevent infection in the pigs), once a genetically-modified pig herd is established for bioprosthetic valve use, the pigs could be housed in facilities no different from those of the wild-type pigs currently used as sources of bioprosthetic valves today [37]. The costs of breeding and housing will therefore be modest. If these bioprostheses improve longevity and significantly reduce the need for their replacement, potential healthcare savings would be considerable [80].
Moreover, the children and young adults most likely to benefit from the implantation of genetically-engineered porcine bioprosthetic heart valves, for whom there is no ideal valve replacement today, would be spared the complications of mechanical prostheses and lifelong anticoagulation. Developing genetically-modified bioprosthetic valves to reduce immunogenicity and graft degeneration is now possible. Such an advance would constitute a momentous milestone in the progress of biomedical engineering and cardiothoracic surgery.
Supplementary Material
Supplementary Figure S1: Pioneers in heart valve replacement with homografts or bioprosthetic heart valves.
(A) Charles Hufnagel, (B) Gordon Murray, (C) Donald Ross, (D) Brian Barratt-Boyes, (E) Alain Carpentier
Abbreviations
- β4GalNT2
β1,4 N-acetylgalactosaminyltransferase
- CMAH
cytidine monophosphate-N-acetylneuraminic acid hydroxylase
- Gal
galactose-α1,3-galactose
- GTKO
α1,3-galactosyltransferase gene-knockout
- Neu5Gc
N-glycolylneuraminic acid
- Sda
the product of β4GalNT2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.D'agostino RS, Jacobs JP, Badhwar V, et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2018 Update on Outcomes and Quality. Ann Thorac Surg 2018;105:15–23. [DOI] [PubMed] [Google Scholar]
- 2.Head S, Mevlut C, Kappetein P. Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J 2017;38:2183–2191. [DOI] [PubMed] [Google Scholar]
- 3.Goldstone AJ, Chiu P, Baiocchi M, et al. Mechanical or biologic prostheses for aortic-valve and mitral-valve replacement. N Engl J Med 2017;377:1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohn LH, Collins JJ, Disesa VJ, et al. Fifteen-year experience with 1678 Hancock porcine bioprosthetic heart valve replacements. Ann Surg 1989;210:435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamieson W, Munro A, Miyagishima R, Allen P, Burr LH,Tyers GF. Carpentier-Edwards standard porcine bioprosthesis: clinical performance to seventeen years. Ann Thorac Surg 1995;60:999–1006. [DOI] [PubMed] [Google Scholar]
- 6.Pibarot P, Dumesnil JG. Prosthetic heart valves: selection of the optimal prosthesis and long-term management. Circulation 2009;119:1034–1048. [DOI] [PubMed] [Google Scholar]
- 7.Bourguignon T, Bouquiaux-stablo AL, Candolfi P, et al. Very long-term outcomes of the Carpentier-Edwards Perimount valve in aortic position. Ann Thorac Surg 2015;99:831–837. [DOI] [PubMed] [Google Scholar]
- 8.Maganti M, Rao V, Armstrong S, Feindel CM, Scully HE, David TE. Redo valvular surgery in elderly patients. Ann Thorac Surg 2009;87:521–525. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqui RF, Abraham JR, Butany J. Bioprosthetic heart valves: modes of failure. Histopathology. 2009;55:135–144. [DOI] [PubMed] [Google Scholar]
- 10.Binet JP, Carpentier A, Langlois J, Duran C, Colvez P. Implantation of heterogenic valves in the treatment of aortic cardiopathies. CR Acad Sci Hebd Seances Acad Sci D 1965;261:5733–5734. [PubMed] [Google Scholar]
- 11.Manji RA, Lee W, Cooper DKC. Xenograft bioprosthetic heart valves: Past, present and future. Int J Surg 2015;23:280–284. [DOI] [PubMed] [Google Scholar]
- 12.Hufnagel CA, Harvey WP, Rabil PJ, McDermott TF. The surgical correction of aortic regurgitation. Preliminary report. Bull Georgetown Univ Med Center 1953;6:60–61. [PubMed] [Google Scholar]
- 13.Murray G Homologous aortic valve segment transplants as surgical treatment for aortic and mitral insufficiency. Angiology 1956;7:466–471. [DOI] [PubMed] [Google Scholar]
- 14.Barratt-Boyes BG. Homograft aortic valve replacement in aortic incompetence and stenosis. Thorax 1964;19:131–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross DN. Homograft replacement of the aortic valve. Lancet 1962;2:487. [DOI] [PubMed] [Google Scholar]
- 16.Carpentier A, Lemaigre G, Robert L, Carpentier S, Dubost C. Biological factors affecting long-term results of valvular heterografts. J Thorac Cardiovasc Surg 1969;58:467–83. [PubMed] [Google Scholar]
- 17.Singhal P, Luk A, Butany J. Bioprosthetic Heart Valves: Impact of Implantation on Biomaterials. ISRN Biomaterials 2013:1–14. [Google Scholar]
- 18.Carpentier A, Deloche A, Relland J, et al. Six-year follow-up of glutaraldehyde-preserved heterografts. With particular reference to the treatment of congenital valve malformations. J Thorac Cardiovasc Surg 1974;68:771–782. [PubMed] [Google Scholar]
- 19.Schoen FJ, Kujovich JL, Webb CL, Levy RJ. Chemically determined mineral content of explanted porcine aortic valve bioprostheses: correlation with radiographic assessment of calcification and clinical data. Circulation 1987;76:1061–1066. [DOI] [PubMed] [Google Scholar]
- 20.Oxenham H, Bloomfield P, Wheatley DJ, et al. Twenty year comparison of a Bjork-Shiley mechanical heart valve with porcine bioprostheses. Heart 2003;89:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke DR, Campbell DN, Hayward AR, Bishop DA. Degeneration of aortic valve allografts in young recipients. J Thorac Cardiovasc Surg 1993;105:934–42. [PubMed] [Google Scholar]
- 22.Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg 2005;79:1072–1080. [DOI] [PubMed] [Google Scholar]
- 23.Talbert WM Jr, Wright P. Acute aortic stenosis of a porcine valve heterograft apparently caused by graft rejection: case report with discussion of immune mediated host response. Tex Heart Inst J 1982;9:225–229. [PMC free article] [PubMed] [Google Scholar]
- 24.Stein PD, Wang CH, Riddle JM, Magilligan DJ Jr. Leukocytes, platelets, and surface microstructure of spontaneously degenerated porcine bioprosthetic valves. J Card Surg 1988;3:253–261. [DOI] [PubMed] [Google Scholar]
- 25.Dahm M, Lyman WD. Schwell AB, Factor SM, Frater RW, Immunogenicity of glutaraldehyde-tanned bovine pericardium. J Thorac Cardiovasc Surg 1990;99:1082–1090. [PubMed] [Google Scholar]
- 26.Schoen FJ, Hirsch D, Bianco RW, Levy RJ. Onset and progression of calcification in porcine aortic bioprosthetic valves implanted as orthotopic mitral valve replacements in juvenile sheep. J Thorac Cardiovasc Surg 1994;108:880–887. [PubMed] [Google Scholar]
- 27.Gonzalez-Lavin L, Bianchi J, Graf D, Amini S, Gordon CI. Degenerative changes in fresh aortic root homografts in a canine model: evidence of an immunologic influence. Transplant Proc 1988;20:815–819. [PubMed] [Google Scholar]
- 28.Hoekstra F, Knoop C, Vaessen L, et al. Donor-specific cellular immune response against human cardiac valve allografts. J Thorac Cardiovasc Surg 1996;112:281–286. [DOI] [PubMed] [Google Scholar]
- 29.Vincentelli A, Latremouille C, Zegdi R, et al. Does glutaraldehyde induce calcification of bioprosthetic tissues? Ann Thorac Surg 1998;66:S255–S258. [DOI] [PubMed] [Google Scholar]
- 30.Grabenwoger M, Fitzal F, Gross C, et al. Different modes of degeneration in autologous and heterologous heart valve prostheses. J Heart Valve Dis 2000;9:104–109. [PubMed] [Google Scholar]
- 31.Wilhelmi MH, Mertsching H, Wilhelmi M, et al. Role of inflammation in allogeneic and xenogeneic heart valve degeneration: immuno- histochemical evaluation of inflammatory endothelial cell activation. J Heart Valve Dis 2003;12:520–526. [PubMed] [Google Scholar]
- 32.Chang Q, Jing H, Sun M, Xu P. Exploring the role of short-course cyclosporin a therapy in preventing homograft valve calcification after transplantation. Cell Immunol 2014;287:36–45. [DOI] [PubMed] [Google Scholar]
- 33.Cho HJ, Cho HJ, KIM HS. Osteopontin: a multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr Atheroscler Rep 2009; 11:206–213. [DOI] [PubMed] [Google Scholar]
- 34.Zilla P, Weissenstein C, Human P, Dower T, von Oppell UO. High glutaraldehyde concentrations mitigate bioprosthetic root calcification in the sheep model. Ann Thorac Surg 2000;70:2091–2095. [DOI] [PubMed] [Google Scholar]
- 35.Lee W, Long C, Ramsoondar J, et al. Human antibody recognition of xenogeneic antigens (NeuGc and Gal) on porcine heart valves: could genetically modified pig heart valves reduce structural valve deterioration? Xenotransplantation 2016;23:370–380. [DOI] [PubMed] [Google Scholar]
- 36.Human P, Zilla P. Inflammatory and immune processes: the neglected villain of bioprosthetic degeneration? J Long Term Eff Med Implants 2001;11:199–220. [PubMed] [Google Scholar]
- 37.Zhang R, Wang Y, Chen L, et al. Reducing immunoreactivity of porcine bioprosthetic heart valves by genetically-deleting three major glycan antigens. GGTA1/β4GalNT2/CMAH. Acta Biomater 2018;72:196–205. [DOI] [PubMed] [Google Scholar]
- 38.Konakci KZ, Bohle B, Blumer R et al. Alpha-Gal on bioprostheses: xenograft immune response in cardiac surgery. Eur J Clin Invest 2005;35:17–23. [DOI] [PubMed] [Google Scholar]
- 39.Manji RA, Hara H, Cooper DK. Characterization of the cellular infiltrate in bioprosthetic heart valves explanted from patients with structural valve deterioration. Xenotransplantation 2015;22:406–407. [DOI] [PubMed] [Google Scholar]
- 40.Manji RA, Zhu LF, Nijjar NK, et al. Glutaraldehyde-fixed bioprosthetic heart valve conduits calcify and fail from xenograft rejection. Circulation 2006;114:318–327. [DOI] [PubMed] [Google Scholar]
- 41.McGregor CGA, Byrne G. Prosthesis type for aortic- and mitral-valve replacement. N Engl J Med. 2018;378:776–779. [DOI] [PubMed] [Google Scholar]
- 42.Good AH, Cooper DK, Malcolm AJ, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplant Proc 1992;24:559–562. [PubMed] [Google Scholar]
- 43.Cooper DK, Good AH, Koren E, et al. Identification of alpha-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transpl Immunol 1993;1:198–205. [DOI] [PubMed] [Google Scholar]
- 44.Bouhours D, Pourcel C, Bouhours JE. Simultaneous expression by porcine aorta endothelial cells of glycosphingolipids bearing the major epitope for human xenoreactive antibodies (Gal alpha 1-3Gal), blood group H determinant and N-glycolylneuraminic acid. Glycoconj J 1996;13:947–953. [DOI] [PubMed] [Google Scholar]
- 45.Byrne GW, Stalboerger PG, Du Z, Davis TR, McGreggor CG. Identification of new carbohydrate and membrane protein antigens in cardiac xenotransplantation. Transplantation 2011;91:287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Byrne G, Ahmad-Villers S, Du Z, McGreggor C. B4GALNT2 and xenotransplantation: A newly appreciated xenogeneic antigen. Xenotransplantation 2018:e12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galili U, Mandrell RE, Hamadeh RM, et al. Interaction between human natural anti-α-galactosyl immunoglogulin G and bacteria of the human flora. Infect Immun 1988;56:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lexer G, Cooper DK, Rose AG, et al. Hyperacute rejection in a discordant (pig to baboon) cardiac xenograft model. J Heart Transplant 1986;5:411–418. [PubMed] [Google Scholar]
- 49.Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science 2003;299:411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat. Med 2005;11:29–31. [DOI] [PubMed] [Google Scholar]
- 51.Cooper DK, Ezzelarab MB, Hara H, et al. The pathobiology of pig-to-primate xenotransplantation: a historical review. Xenotransplantation 2016;23:83–105. [DOI] [PubMed] [Google Scholar]
- 52.Mohiuddin MM, Singh AK, Corcoran PC, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant 2014;14:488–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iwase H, Hara H, Ezzelarab M, et al. Immunologic and physiologic observations in baboons with life-supporting genetically-engineered pig kidney grafts. Xenotransplantation 2017;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park CS, Park SS, Choi SY, Yoon SH, Kim WH, Kim YJ. Anti alpha-gal immune response following porcine bioprosthesis implantation in children. J Heart Valve Dis 2010;19:124–130. [PubMed] [Google Scholar]
- 55.Rood PP, Hara H, Busch JL, et al. Incidence and cytotoxicity of antibodies in cynomolgus monkeys directed to nonGal antigens, and their relevance for experimental models. Transplant Int 2006;19:158–165. [DOI] [PubMed] [Google Scholar]
- 56.Hara H, Long C, Lin YJ, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transplant Int 2008;21:1163–1174. [DOI] [PubMed] [Google Scholar]
- 57.Lee W, Hara H, Cooper DK, Manji RA. Expression of NeuGc on pig heart valves. Xenotransplantation 2015;22:153–154. [DOI] [PubMed] [Google Scholar]
- 58.Lutz AJ, Li P, Estrada JL, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation 2013;20:27–35. [DOI] [PubMed] [Google Scholar]
- 59.Lee W, Hara H, Ezzelarab MB, et al. Initial in vitro studies on tissues and cells from GTKO/CD46/NeuGcKO pigs. Xenotransplantation 2016;23:137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jeong HJ, Adhya M, Park HM, Kim YG, Kim BG. Detection of Hanganutziu-Deicher antigens in O-glycans from pig heart tissues by matrix-assisted laser desorption/ ionization time-of-flight mass spectrometry. Xenotransplantation 2013;20:407–417. [DOI] [PubMed] [Google Scholar]
- 61.Galili U, Shoet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem 1988;263:17755–17762. [PubMed] [Google Scholar]
- 62.Springer SA, Diaz SL, Gagneux P. Parallel evolution of a self-signal: humans and new world monkeys independently lost the cell surface sugar Neu5Gc. Immunogenetics 2014;66:671–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cooper DK, How important is the anti-Gal antibody response following the implantation of a porcine bioprosthesis? J Heart Valve Dis 2009;18:671–672. [PubMed] [Google Scholar]
- 64.McGregor CG, Carpentier A, Lila N, Logan JS, Byrne GW. Cardiac xenotransplantation technology provides materials for improved bioprosthetic heart valves. J Thorac Cardiovasc Surg 2011;141:269–275. [DOI] [PubMed] [Google Scholar]
- 65.McGregor CG, Kogelberg H, Vlasin M, Byrne GW. Gal-knockout bioprostheses exhibit less immune stimulation compared to standard biological heart valves. J Heart Valve Dis 2013;22:383–390. [PubMed] [Google Scholar]
- 66.Iwase H, Ekser B, Hara H, et al. Regulation of human platelet aggregation by genetically modified pig endothelial cells and thrombin inhibition. Xenotransplantation 2014;21:72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burlak C, Paris LL, Lutz AJ et al. Reduced binding of human antibodies to cells from GGTA1/CMAH KO pigs. Am J Transplant 2014;14:1895–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang ZY, Burlak C, Estrada JL, Li P, Tector MF, Tector AJ. Erythrocytes from GGTA1/CMAH knockout pigs: implications for xenotransfusion and testing in non-human primates. Xenotransplantation 2014;21:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilhite T, Ezzelarab C, Hara H, et al. The effect of Gal expression on pig cells on the human T-cell xenoresponse. Xenotransplantation 2012;19:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Estrada JL, Martens G, Li P, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation 2015;22:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iwase H, Ekser B, Satyananda V, et al. Initial in vivo experience of pig artery patch transplantation in baboons using mutant MHC (CIITA-DN) pigs. Transpl Immunol 2015;32:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Q, Shaikh S, Iwase H, et al. An investigation of carbohydrate antigen expression and anti-pig antibodies in New World capuchin monkeys. Xenotransplantation. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ezzelarab MB, Ekser B, Echeverri G, et al. Costimulation blockade in pig artery patch xenotransplantation-a simple model to monitor the adaptive immune response in nonhuman primates. Xenotransplantation 2012;9:221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharabiani MT, Dorobantu DM, Mahani AS, et al. Aortic valve replacement and the Ross operation in children and young adults. J am Coll Cardiol 2016;26:2858–2870. [DOI] [PubMed] [Google Scholar]
- 75.Karamlou T, Jank K, Williams WG, et al. Outcomes and associated risk factors for aortic valve replacemnt in 160 children: a competing risks-analysis. Circulation 2005;112:3462–3469. [DOI] [PubMed] [Google Scholar]
- 76.Erez E, Bush D, Tam VK, Doublin NA, Stakes J. Outcome in infants less than 3 kilograms for placement of saphenous venous homografts as systemic-to-pulmonary arterial shunts. Cardiol Young 2008;18:386–391. [DOI] [PubMed] [Google Scholar]
- 77.Vitanova K, Cleuziou J, Horer J, et al. Which type of conduit to choose for right ventricular outflow tract reconstruction in patients below 1 year of age? Eur J Journal Cardiothorac Surg 2014;46:961–966. [DOI] [PubMed] [Google Scholar]
- 78.Jacobs JP, Quintessenza JA, Boucek RJ, et al. Pediatric cardiac transplantation in children with high panel reactive antibody. Ann Thorac Surg 2004;78:1703–1709. [DOI] [PubMed] [Google Scholar]
- 79.Wells WJ, Arroyo H Jr, Bremner RM, Wood J, Starnes VA. Homograft conduit failure in infants is not due to somatic outgrowth. J Thorac Cardiovasc Surg 2002;124:88–96. [DOI] [PubMed] [Google Scholar]
- 80.Osnabrugge RL, Speir AM, Head SJ, et al. Costs for surgical aortic valve replacement according to preoperative risk categories. Ann Thorac Surg 2013;96:500–506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Pioneers in heart valve replacement with homografts or bioprosthetic heart valves.
(A) Charles Hufnagel, (B) Gordon Murray, (C) Donald Ross, (D) Brian Barratt-Boyes, (E) Alain Carpentier