Abstract
Objective
Adipocytokines are markers of fetal metabolism, but their association with childhood growth is unclear. This study examined associations of neonatal adipocytokines with longitudinal childhood adiposity measures in a prospective cohort of pregnant women and their children.
Methods
We measured leptin and adiponectin concentrations at delivery and children’s body mass index (BMI) z-scores between ages 4 weeks and 8 years. We estimated differences in BMI z-scores and rates of BMI z-score change by leptin (N = 257) and adiponectin (N = 271) terciles.
Results
Children in the middle (mean difference: 0.2; 95% CI: −0.1, 0.4) and highest (0.4; 95% CI: 0.1, 0.6) leptin terciles had greater BMI z-scores than children in the lowest tercile. Associations were null after adjustment for birthweight z-score. Children in the lowest adiponectin tercile had greater gains in BMI z-score (change per year: 0.10; 95% CI: 0.08, 0.13) than children in the middle (0.07; 95% CI: 0.04, 0.09) and highest terciles (0.04; 95% CI: −0.01, 0.05) (adiponectin x age interaction P < 0.001).
Conclusions
Lower adiponectin levels were associated with increased rates of BMI gains in the first 8 years of life. While leptin was positively associated with BMI, this association may be confounded by birthweight.
Keywords: Adipokines, Metabolism, Growth, Adiposity
Introduction
Childhood obesity increases the risk of cardiovascular disease, metabolic diseases, and obesity in adulthood. (1) Recent estimates from the National Health and Nutrition Examination Surveys show that 17 percent of United States children and adolescents are obese and nearly 6 percent are extremely obese. (2) While nutrition and physical activity play an important role in the risk of childhood obesity, there is compelling evidence that environmental influences during fetal development can alter the risk of obesity and cardiometabolic diseases. (3) For example, increased gestational weight gain is associated with increased risk of childhood obesity (4), while inadequate gestational weight gain during pregnancy has been associated with cardiometabolic risk in offspring (5).
Adipocytokines, including leptin and adiponectin, are hormones released primarily by adipose and placental tissue. (6, 7) Leptin, which mediates satiety and hunger through hypothalamic signaling, is positively associated with obesity and cardiometabolic risk in adults. (7–11) Adiponectin has anti-inflammatory properties and influencesglucose utilization and insulin sensitivity in peripheral tissues. (6, 8, 12) In adults, adiponectin concentrations are decreased in individuals with obesity and metabolic syndrome. (6, 8, 12) In newborns, both leptin and adiponectin are positively associated with birthweight, and thus, possibly act as biomarkers of adiposity and metabolism. (13, 14)
The impact of fetal adipocytokines on growth and obesity risk during childhood is unclear. Evidence from animal studies suggests that fetal leptin and adiponectin exposure induce changes in hypothalamic circuits and adipose tissue deposition, respectively, which may in turn increase offspring adiposity. (15, 16) Fewer studies have quantified the association between fetal adipocytokines and growth (i.e., change in anthropometry over time), and the results are inconclusive. (17–26) In one cohort, cord blood leptin was associated with slower weight gain between birth and age 2 years, but positively associated with both body mass index (BMI) and adiposity measures (fat mass and waist circumference) at age 9 years. (20, 21) In that same cohort, cord blood adiponectin was also positively associated with fat mass and waist circumference during adolescence. (20) In a second cohort, cord blood leptin was inversely associated with fat mass during adolescence. (18)
To fill this gap in the literature, we examined the association between neonatal adipocytokines and longitudinal measures of adiposity during childhood using data from an ongoing prospective cohort of pregnant women and their children. We hypothesized that cord blood leptin and adiponectin concentrations would be predictive of adiposity and rates of adiposity change during childhood. Further understanding the relationship of fetal adipocytokines and growth and adiposity gain during childhood may help to define these markers of adiposity risk and aide in the development of risk stratification and intervention strategies among high risk populations.
Methods
Study Population
We used data from the Health Outcomes and Measures of the Environment (HOME) Study, a prospective pregnancy and birth cohort in the Cincinnati, Ohio region. Between March 2003 and January 2006, we recruited pregnant women from 7 prenatal care clinics affiliated with 3 delivery hospitals, who were 16 ± 3 weeks gestation, ≥ 18 years old, English speaking, living in a home built prior to 1978, without a known diagnosis of cancer, diabetes, thyroid, seizure, or bipolar disorder, and without HIV infection. Women provided written informed consent for both themselves and their child. The Institutional Review Boards (IRB) of Cincinnati Children’s Hospital Medical Center (CCHMC) and the delivery hospitals approved the study. The Brown University IRB relied on the determinations made by the CCHMC IRB.
Among 401 women who enrolled in the study and did not drop out prior to delivery, we excluded those who had multiples, stillbirths, or infants with chromosomal/genetic abnormalities (N = 14, 3.5%), as well as newborns with insufficient umbilical cord serum for measurement of adipocytokines (N = 94, 23.4%), and those mother-child pairs with missing covariate information or without at least 1 subsequent follow-up visit (N = 22, 5.5%). An additional 14 children (3.5%) missing neonatal leptin measurements were excluded from the leptin analyses, but were included in the adiponectin analyses. A total of 271 children returned for 1,338 follow-up visits at an average of 35 days (SD 5, N = 262) and 1.1 (SD 0.1, N = 240), 2.1 (SD 0.1, N = 207), 3.2 (SD 0.1, N = 184), 4.2 (SD 0.1, N = 137), 5.3 (SD 0.2, N = 147), and 8.2 (SD 0.6, N = 161) years of age.
Neonatal Adipocytokine Measurement
We measured neonatal leptin and adiponectin concentrations in previously frozen venous umbilical cord serum obtained at delivery using an ELISA sandwich assay and a BioTeck microtiter FLx 808 plate reader. Quality control samples and reagent blanks were included in each analytic batch, with coefficients of variation for repeated, blinded, quality control samples of 11% (leptin) and 17% (adiponectin). Levels of detection were 0.8 ng/mL (leptin) and < 2 μg/mL (adiponectin).
Child Anthropometry
We abstracted gestational age, weight, and length at birth from medical records, and calculated gestational age- and sex-standardized birthweight z-scores. (27) We conducted home visits at age 4 weeks, and children and their parents returned to the study clinic at ages 1, 2, 3, 4, 5, and 8 years. Using a digital scale, we measured weight with the child wearing only a dry diaper or undergarments. At the 4 week and 1 year visits, we measured recumbent length with a length board, and measured standing height with a wall-mounted stadiometer, without the child’s shoes or head coverings for all other visits. We calculated age- and sex-specific BMI z-scores according to World Health Organization standards (i.e., Standard Deviation Scores [SDS]). (28) At age 8, we measured waist circumference using a measuring tape placed around a horizontal plane defined by the right and left iliac crests, with the child wearing only undergarments. We also measured body fat percentage at age 8 years using a Tanita (Arlington Heights, IL, USA) children’s body fat monitor.
Covariates
Trained research staff collected data on potential confounders of neonatal adipocytokines and childhood growth using computer-assisted questionnaires and medical chart abstraction. Maternal sociodemographic factors included age, race, education, and income. Perinatal factors included parity, maternal BMI at 16 weeks gestation, gestational diabetes mellitus, and maternal serum cotinine concentration at approximately 16 weeks gestation (a biomarker of tobacco exposure).
Statistical Analysis
We began by calculating univariate statistics of neonatal adipocytokine concentrations according to covariate categories. We then examined the relationship of leptin and adiponectin concentrations with BMI z-scores during childhood. We categorized leptin and adiponectin concentrations into terciles, using the lowest terciles as the reference category for the analyses.
To examine the association between neonatal adipocytokines and longitudinal BMI z-scores during childhood, we used multivariable linear regression with generalized estimating equations and an exchangeable covariance matrix. This allowed us to estimate differences in BMI z-scores as a function of child age, while also accounting for the with-in child correlation of the repeated BMI z-scores. We estimated the average annual linear BMI z-score slope (i.e., rate of BMI z-score change) between age 4 weeks and 8 years for children in each of the adipocytokine terciles. To do this, we included child age, the adipocytokine terciles, and an age × adipocytokine interaction term in the model. We kept the adipocytokine × age interaction term in the final linear model if the P-value for the interaction term was < 0.05, and then estimated yearly changes in BMI z-score for each adipocytokine tercile using the linear combinations estimates from the model. If the P-value for the interaction term was not statistically significant, we did not include the adipocytokine × age interaction term in the final model, and subsequently estimated differences in BMI z-scores across all ages between the adipocytokine terciles, using the lowest tercile as the reference.
We selected potential confounders to include in the final model based on the prior literature. (6, 29) Our primary analysis was adjusted for maternal age, race, income, parity, maternal BMI, cotinine concentrations, child age, and child sex. Inclusion of other covariates, such as gestational age, maternal gestational diabetes mellitus, maternal glucose concentrations after oral glucose tolerance testing, and delivery mode, did not change the regression estimates and were therefore not included in the final model.
Secondary Analyses
We evaluated if sex modified the association between neonatal adipocytokines and BMI z-score changes during childhood using both a stratified analysis and all second and third order product interaction terms between child age, child sex, and adipocytokine terciles. To evaluate for confounding by birthweight, gestational weight gain, and maternal hyperglycemia, we additionally adjusted for birthweight z-score (27), weight gain for gestational age z-score (30), and maternal glucose concentrations after oral glucose tolerance testing in separate models. To test the robustness of our assumptions, we conducted several sensitivity analyses of our primary results. We excluded participants with conditions that may affect fetal growth: maternal obesity (defined as BMI > 30 kg/m2, N = 61), gestational diabetes mellitus (N = 12), preterm birth (defined as gestational age < 37 weeks, N = 15), and small for gestational age births (defined as gestational age- and sex-standardized birthweight z-score < 10th percentile, N = 20). We also conducted a sensitivity analysis excluding the 4-week anthropometry measures, as our initial exploratory analysis suggested that this may be an influential time point in the association between adipocytokine concentrations and growth in these data.
Finally, we examined associations between neonatal adipocytokine concentrations and adiposity measures at age 8 years. We classified children as being overweight or obese at age 8 years if their age- and sex-specific BMI z-score was ≥ 1 SDS, according to World Health Organization standards. (28) We calculated risk of overweight/obesity at age 8 years according to neonatal leptin (N = 154) and adiponectin (N = 161) terciles using modified Poisson regression with robust standard errors. Using linear regression, we estimated differences in BMI z-score, waist circumference, and body fat percentage across terciles of neonatal leptin and adiponectin. All 8-year adiposity measure models were adjusted for maternal age, race, income, parity, maternal BMI, cotinine concentrations, and child sex. The waist circumference and body fat percentage models were additionally adjusted for child age at the time of the 8-year study visit.
Results
Women in the study sample were predominantly white (67%), college educated (55%), and multiparous (54%) (Table 1). Most children in the study were born at term (95%) and had a birthweight z-score between the 10th and 90th percentile (78%). The distribution of covariates among women and children in the study sample did not differ significantly from the full HOME Study cohort (Table S1). Median neonatal serum leptin and adiponectin concentrations were 9.8 ng/mL and 42 μg/mL, respectively (Table 1). There was a weak positive correlation between cord serum leptin and adiponectin (Pearson correlation coefficient = 0.16, P-value = 0.01).
Table 1.
Neonatal cord serum adipocytokine concentrations according to participant covariates (The HOME Study, 2003–2006)
| Characteristics | N (%) | Leptin (ng/mL) Median (25th, 75th) | Adiponectin (μg/mL) Median (25th, 75th) |
|---|---|---|---|
| Overall | 271 | 9.8 (5.5, 16) | 42 (30, 53) |
| Maternal age (years) | |||
| 18–25 | 53 (20) | 7.6 (4.6, 11) | 36 (26, 45) |
| > 25–35 | 176 (65) | 11 (6.1, 17) | 44 (32, 54) |
| > 35 | 42 (15) | 11 (7.2, 15) | 44 (34, 56) |
| Maternal race | |||
| Non-Hispanic White | 183 (67) | 10 (5.4, 17) | 45 (34, 56) |
| Non-Hispanic Black | 70 (26) | 9.9 (6.7, 15) | 35 (27, 48) |
| Other | 18 (7) | 7.3 (5.1, 8.1) | 38 (27, 49) |
| Maternal education | |||
| High school or less | 55 (20) | 9.4 (6.7, 17) | 37 (26, 55) |
| Tech school or some college | 67 (25) | 8.8 (5.4, 17) | 38 (30, 47) |
| Bachelors or more | 149 (55) | 11 (5.3, 15) | 45 (34, 56) |
| Household income ($/year) | |||
| < 20,000 | 79 (29) | 11 (5.4, 15) | 47 (37, 58) |
| 20–40,000 | 99 (37) | 11 (7.1, 17) | 45 (34, 55) |
| 40–80,000 | 44 (16) | 8.5 (5.2, 16) | 36 (27, 45) |
| > 80,000 | 49 (18) | 9.5 (5.2, 16) | 33 (25, 45) |
| Parity | |||
| 0 | 120 (44) | 9.8 (5.4, 16) | 43 (33, 54) |
| 1 | 89 (33) | 8.5 (5.3, 15) | 43 (32, 53) |
| 2+ | 62 (23) | 11 (6.6, 18) | 36 (26, 52) |
| Maternal body mass index (kg/m2) | |||
| < 25 (normal) | 119 (44) | 8.3 (4.3, 13) | 42 (33, 53) |
| 25–30 (overweight) | 91 (34) | 9.6 (5.5, 17) | 45 (32, 56) |
| > 30 (obese) | 61 (22) | 14 (8.0, 21) | 40 (26, 50) |
| Maternal serum cotinine (ng/mL) | |||
| < 0.015 (unexposed) | 107 (40) | 8.7 (4.2, 16) | 43 (34, 56) |
| 0.015–3 (secondhand) | 137 (50) | 10 (7.3, 16) | 42 (30, 52) |
| > 3 (active) | 27 (10.0) | 8.2 (5.5, 16) | 33 (21, 59) |
| Delivery type | |||
| Vaginal | 198 (73) | 9.0 (5.5, 15) | 43 (31, 55) |
| Caesarian section | 73 (27) | 12 (5.7, 19) | 40 (27, 48) |
| Infant sex | |||
| Female | 148 (55) | 12 (7.3, 18) | 43 (31, 55) |
| Male | 123 (45) | 8.1 (3.9, 14) | 42 (30, 53) |
| Gestational age (weeks) | |||
| < 37 (preterm) | 15 (5) | 2.8 (1.5, 6.2) | 24 (16, 42) |
| ≥ 37 (term) | 256 (95) | 10 (6.4, 16) | 43 (32, 54) |
| Birthweight z-score (percentile) | |||
| < 10th (small for gestational age) | 20 (7) | 4.9 (2.6, 8.1) | 40 (28, 47) |
| 10th – 90th (appropriate for gestational age) | 210 (78) | 9.0 (5.5, 15) | 43 (30, 53) |
| > 90th (large for gestational age) | 41 (15) | 16 (13, 28) | 42 (32, 53) |
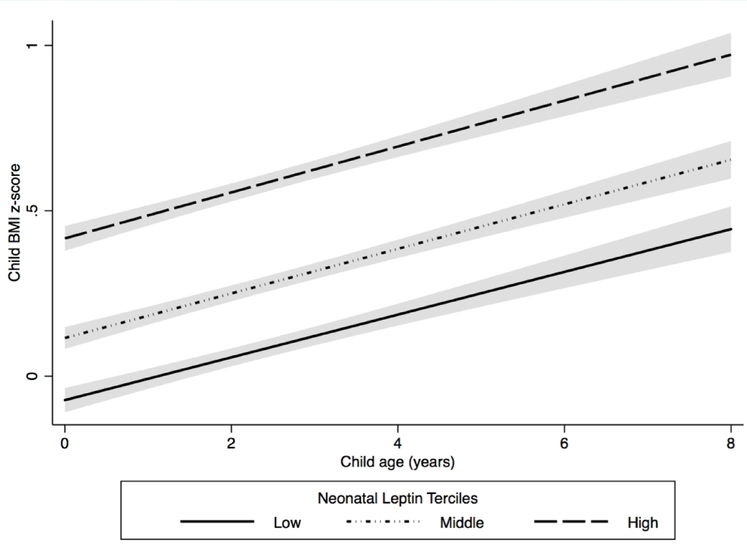
After adjusting for maternal age, race, income, parity, maternal BMI, cotinine concentrations, child age, and child sex, there was a positive association between neonatal cord serum leptin concentrations and child BMI z-scores (Figure 1, Table S2). The rate of change in BMI z-scores did not vary by neonatal serum leptin concentrations (leptin x age interaction P-value = 0.48). Children in the middle (mean BMI z-score difference: 0.2 SDS units; 95% Confidence Interval (CI): −0.1, 0.4) and highest (mean BMI z-score difference: 0.4 SDS units; 95% CI: 0.1, 0.6) leptin terciles had greater BMI z-scores between age 4 weeks and 8 years than children in the lowest tercile (P-value for trend = 0.001).
Figure 1. Estimated child body mass index (BMI) z-scores between age 4 weeks and 8 years by neonatal leptin tercile (N = 257) (The HOME Study, 2003–2006).
Cord serum leptin concentration ranges for each tercile: low (0.2 – 7.1 ng/mL, N = 84), middle (7.2 – 14 ng/mL, N = 85), and high (14 – 95 ng/mL, N = 88). Model was adjusted for maternal race, age, maternal BMI, parity, cotinine concentrations, household income, child age, and child sex. The solid and dashed lines represent estimated mean child BMI z-scores between age 4 weeks and 8 years. Shading represents the 95% confidence intervals.
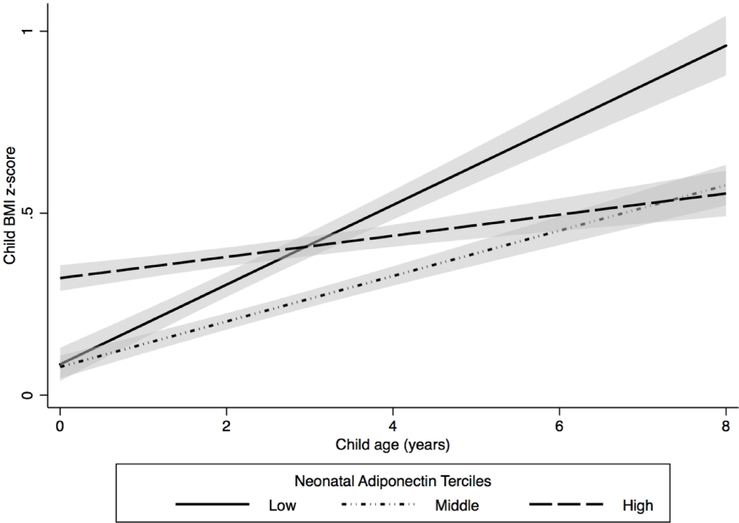
We found that the rate of change in BMI z-score differed by neonatal adiponectin tercile (adiponectin × age interaction P-value < 0.001). Between age 4 weeks and 8 years, children in the lowest adiponectin tercile had greater gains in BMI z-score (BMI z-score change per year: 0.10 SDS units; 95% CI: 0.08, 0.13) than children in the middle (BMI z-score change per year: 0.07 SDS units; 95% CI: 0.04, 0.09) and highest terciles (BMI z-score change per year: 0.02 SDS units; 95% CI: −0.001, 0.05) (Figure 2, Table S3).
Figure 2. Estimated child body mass index (BMI) z-scores between age 4 weeks and 8 years by neonatal adiponectin tercile (N = 271) (The HOME Study, 2003–2006).
Cord serum adiponectin concentration ranges for each tercile: low (2.2 – 34 μg/mL, N = 86), middle (34 – 49 μg/mL, N = 94), and high (49 – 143 μg/mL, N = 91). Model was adjusted for maternal race, age, maternal BMI, parity, cotinine concentrations, household income, child age, child sex, and adiponectin x age interaction term (P-value for interaction term < 0.001). The solid and dashed lines represent estimated mean child BMI z-scores between age 4 weeks and 8 years. Shading represents the 95% confidence intervals.
Secondary Analysis
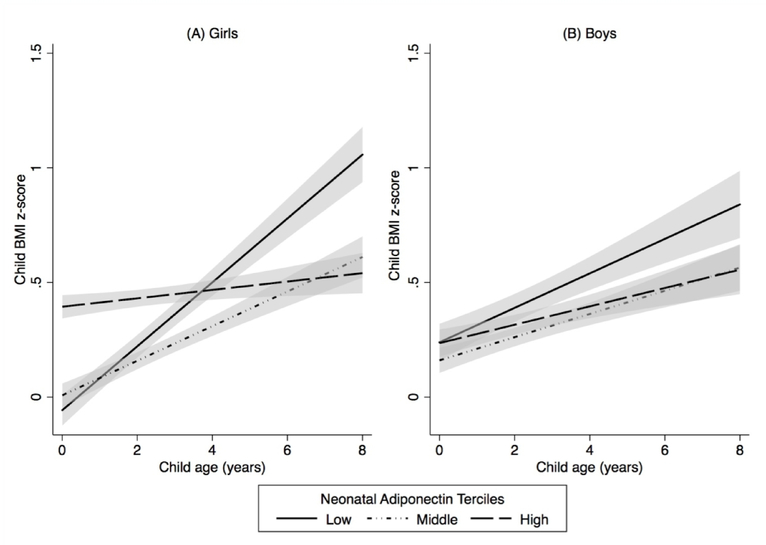
The association between neonatal leptin concentrations and childhood BMI z-scores was similar in boys and girls (leptin × sex interaction term P-value = 0.85) (Table S2). In our sex-stratified analysis, differences in yearly gains in BMI z-score by neonatal adiponectin tercile were stronger in girls (adiponectin × age interaction term for girls P-value < 0.001), but still present in boys (adiponectin x age interaction term for boys P-value = 0.19) (Figure 3, Table S3).
Figure 3. Estimated child body mass index (BMI) z-scores between age 4 weeks and 8 years by neonatal adiponectin tercile, stratified by child sex: (A) Girls (N = 148) and (B) Boys (N = 123) (The HOME Study, 2003–2006).
Model was adjusted for maternal race, age, maternal BMI, parity, cotinine concentrations, household income, child age, and adiponectin x age interaction term (P-value for interaction term < 0.001 for girls and 0.19 for boys). The solid and dashed lines represent estimated mean child BMI z-scores between age 4 weeks and 8 years. Shading represents the 95% confidence intervals.
When we additionally adjusted for birthweight z-score, the differences in BMI z-score across leptin terciles became null. Compared to children in the lowest leptin tercile, mean BMI z-score difference for children in the middle leptin tercile was − 0.10 SDS units (95% CI: − 0.32, 0.15) and − 0.08 SDS units (95% CI: − 0.34, 0.19) for children in the highest leptin tercile (Table S4). In addition, the association between birthweight z-score and BMI z-score during childhood did not change with additional adjustment for leptin concentrations at birth. The adjusted absolute difference in BMI z-score for each unit increase in birthweight z-score was 0.40 SDS units (95% CI: 0.30, 0.48), and 0.42 SDS units (95% CI: 0.31, 0.53) without and with adjustment for neonatal leptin concentration, respectively. In contrast, the association between neonatal serum adiponectin concentrations and gains in BMI z-score did not change when we additionally adjusted for birthweight z-score (Table S5).
Overall, our results were similar when we excluded women with obesity or gestational diabetes, and infants born preterm or small for gestational age from the analysis (Table S4 and Table S5). Our results were also similar when the models were additionally adjusted for gestational weight gain z-score and maternal glucose concentrations (Table S4 and S5). The associations of neonatal leptin and adiponectin with BMI z-scores and changes in BMI z-scores, respectively, were attenuated when we excluded the 4-week growth measures from the analysis (Table S4 and Table S5).
Most measures of adiposity and the risk of overweight or obesity at age 8 did not differ by neonatal leptin tercile (Table 2). Consistent with our longitudinal analysis, children in the highest adiponectin tercile had a 38% (risk ratio: 0.62; 95% CI: 0.38, 1.02) decreased risk of obesity compared to those in the lowest adiponectin tercile. Children in the highest adiponectin tercile also had decreased waist circumference (difference: −2.6 cm; 95% CI: −6.0, 0.7) and body fat percentage (difference: −1.9 percent; 95% CI: −4.3, 0.4) compared to children in the lowest adiponectin tercile (Table 2).
Table 2.
Adjusted associations of neonatal cord serum leptin (N = 154) and adiponectin (N = 161) concentrations and childhood adiposity measures at age 8 years (The HOME Study, 2003–2006)a
| BMI z-score difference β (95% CI) | Waist circumference (cm) difference β (95% CI) | Body fat percentage difference β (95% CI) | RR of overweight or obesity (95% CI)b | ||
|---|---|---|---|---|---|
| Leptin | Lowest Tercile | Reference | Reference | Reference | Reference |
| Middle Tercile | 0.35 (−0.12, 0.82) | 3.1 (−0.5, 6.6) | 1.5 (−1.0, 3.9) | 1.01 (0.57, 1.77) | |
| Highest Tercile | 0.25 (−0.25, 0.75) | 1.6 (−2.2, 5.4) | 0.3 (−2.3, 2.9) | 0.82 (0.45, 1.50) | |
| P-value for trend | 0.06 | 0.05 | 0.48 | 0.70 | |
| Adiponectin | Lowest Tercile | Reference | Reference | Reference | Reference |
| Middle Tercile | 0.01 (−0.46, 0.47) | 0.0 (−3.4, 3.5) | −0.3 (−2.8, 2.1) | 0.74 (0.45, 1.23) | |
| Highest Tercile | −0.29 (−0.74, 0.16) | −2.6 (−6.0, 0.7) | −1.9 (−4.3, 0.4) | 0.62 (0.38, 1.02) | |
| P-value for trend | 0.70 | 0.81 | 0.80 | 0.19 |
Abbreviations: Body mass index (BMI), Confidence Interval (CI), Risk Ratio (RR)
Model adjusted for maternal race, age, income, parity, maternal BMI, cotinine concentrations, and child sex. Waist circumference and body fat percentage model also adjusted for child age.
Overweight or obesity defined as having a sex- and age-standardized World Health Organization BMI z-score ≥ 1 standard deviation
Discussion
In this prospective longitudinal cohort study, both leptin and adiponectin concentrations at birth were associated with childhood BMI z-scores from infancy through school age. Specifically, higher leptin concentrations were associated with higher BMI z-scores during the first 8 years of life, while lower adiponectin concentrations were associated with greater gains in BMI z-score from age 4 weeks to age 8 years.
To our knowledge, this is the first study to report that higher cord serum adiponectin concentrations are associated with slower weight gain during childhood. Weight gain is an important outcome to consider given that we and others have observed that patterns of weight gain during early childhood are associated with risk of overweight and obesity later in childhood. (31–33) Few studies have examined the association between adiponectin at birth and adiposity during childhood or adolescence. In a German cohort, Meyer et al (26) found a positive association between cord plasma adiponectin concentrations and adiposity at ages 3 and 4 years, but not at age 5 years. In a Boston, MA cohort (19), cord plasma adiponectin concentrations were positively associated with skin fold thickness, but not BMI at age 3 years; however, this association was null in early adolescence. (18) Finally, cord plasma adiponectin concentrations were positively associated with fat mass and waist circumference at age 17 years in a United Kingdom cohort. (20)
Previous studies have also reported both positive (20) and inverse (17, 22, 25) associations of neonatal leptin with childhood adiposity. Only two studies have followed children past age 7 years. (18, 20) Similar to the present study, Simpson et al (20) found that cord plasma leptin concentrations were positively associated with BMI, waist circumference, and fat mass at age 9 years; however, these associations were null at age 17 years. Differences in the study results across the literature may arise from population-specific factors that influence growth, and variations in the timing and methods of adiposity assessment.
The mechanism of action of fetal adipocytokines that gives rise to alterations in growth and obesity risk during childhood is largely unknown. Evidence from rodent models show that leptin influences the programming of hypothalamic circuits during gestation, which may influence ex utero energy metabolism through satiety and hunger signaling. (15) Additionally, leptin may increase brown adipose tissue deposition and enhance the activity of thermoregulatory circuits, which may in turn influence adipose tissue metabolism and deposition during infancy and childhood. (34)
Our findings are not consistent with previous animal studies showing that fetal leptin administration of undernourished dams reduces offspring adiposity. (35, 36) We speculate that this is because we measured leptin at delivery, which is a marker of neonatal adipose tissue levels. This speculation is also consistent with our findings showing that the association between leptin and childhood BMI was null after adjusting for birthweight, as well as the lack of change in the association between birthweight z-score and childhood BMI z-score after adjusting for neonatal leptin. Moreover, leptin-associated differences in BMI z-score were attenuated when the 4-week BMI measures were excluded from the analysis. We believe that this is a case of confounding, where birthweight is a crude marker of adiposity (and leptin levels) at birth, and a predictor of later life adiposity. (37) In Project Viva, Li et al (18) found that cord plasma leptin concentrations were inversely associated with adiposity measures and BMI z-scores from early childhood through adolescence when adjusted for birthweight z-score.
In regards to the mechanism by which adiponectin influences growth, fetal adiponectin has been shown to enhance fetal fat deposition at birth in mouse models (16). Moreover, adiponectin may play a role in both insulin sensitization and in the availability of nutrients such as fatty acids, which may in turn affect both fetal and childhood growth. (6, 38) Our finding that low adiponectin concentrations at birth are predictive of greater adiposity gains in childhood may represent fetal adaptation to altered nutrient supply and impaired insulin sensitization during gestation. In contrast to our findings with leptin, the association of adiponectin with adiposity gain during childhood is not just reflective of size at birth, but perhaps the effect of fetal programing.
This study has several strengths and limitations. Strengths include its prospective design and longitudinal anthropometry measures from infancy through school age. These repeated anthropometry measures allowed us to model adiposity trajectories over time, while adjusting for potential confounders using the extensive covariate information available. While we have included multiple potential confounders in our analysis, there may be residual confounding from unmeasured factors. For instance, factors such as maternal nutrition, gestational weight gain, and placental function may influence fetal growth and adipocytokines levels. Moreover, physical activity and eating behaviors during childhood may influence childhood growth. (29, 39) In addition, our use of a linear model does not allow for inferences of growth patterns during specific periods of time. Future studies could consider focusing on the association of neonatal adipocytokines with specific periods of childhood growth using more advanced growth modeling techniques.
Additional limitations include modest sample size and inability to measure maternal adipocytokine concentrations, which have been associated with childhood adiposity in prior studies. (18) Maternal adipocytokines cannot cross the placenta, but may influence placental adipocytokine production during gestation. (6, 7) We speculate that inclusion of maternal adipocytokines in the analysis would produce similar results to those that we observed. Moreover, we estimated fetal adipocytokine concentrations at one point in time. Limited information is available regarding changes in adipocytokine concentrations after delivery. (23, 40) Some studies suggest that trajectories of adipocytokine concentrations may be an important factor for growth and adiposity development in childhood, but this remains an avenue for future research. (17, 23)
Conclusion
In this longitudinal cohort study of pregnant women and their children, neonatal serum adipocytokine concentrations were predictive of adiposity and growth from infancy through school age. Higher leptin concentrations at birth were associated with increased BMI during the first 8 years of life, while lower adiponectin concentrations at birth were associated with greater BMI gains during infancy and childhood. Further examination of the role of adipocytokines in both developmental programming and as markers of obesity risk may aide in the development of intervention strategies to target high-risk populations.
Supplementary Material
Study Importance Questions.
Adipocytokines, such as leptin and adiponectin, are markers of fetal metabolism.
Few studies have examined the association of fetal adipocytokine concentrations with longitudinal measures of childhood adiposity.
This study shows that neonatal leptin and adiponectin concentrations at birth may be predictive of growth and adiposity gains during childhood.
Findings suggest that low neonatal adiponectin is associated with body mass index gains during childhood, while associations between neonatal leptin and growth may be confounded by size at birth.
Acknowledgements
We would like to thank Theresa Kenney for conducting the adipocytokine measurements.
Funding: This work was supported by NIEHS grants R01 ES025214, R01 ES020349, and P01 ES011261.
Footnotes
Disclosure: JMB was financially compensated for serving as an expert witness for plaintiffs in litigation related to tobacco smoke exposures. The remaining authors declared no conflicts of interest.
References
- 1.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002;360(9331):473–82. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. JAMA. 2016;315(21):2292–9. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutton EF, Gilmore LA, Dunger DB, Heijmans BT, Hivert MF, Ling C, et al. Developmental programming: State-of-the-science and future directions-Summary from a Pennington Biomedical symposium. Obesity (Silver Spring). 2016;24(5):1018–26. doi: 10.1002/oby.21487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaillard R, Welten M, Oddy WH, Beilin LJ, Mori TA, Jaddoe VW, et al. Associations of maternal prepregnancy body mass index and gestational weight gain with cardio-metabolic risk factors in adolescent offspring: a prospective cohort study. BJOG. 2016;123(2):207–16. doi: 10.1111/1471-0528.13700. [DOI] [PubMed] [Google Scholar]
- 5.Tam CHT, Ma RCW, Yuen LY, Ozaki R, Li AM, Hou Y, et al. The impact of maternal gestational weight gain on cardiometabolic risk factors in children. Diabetologia. 2018;61(12):2539–48. doi: 10.1007/s00125-018-4724-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aye IL, Powell TL, Jansson T. Review: Adiponectin--the missing link between maternal adiposity, placental transport and fetal growth? Placenta. 2013;34 Suppl:S40–5. doi: 10.1016/j.placenta.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briffa JF, McAinch AJ, Romano T, Wlodek ME, Hryciw DH. Leptin in pregnancy and development: a contributor to adulthood disease? Am J Physiol Endocrinol Metab. 2015;308(5):E335–50. doi: 10.1152/ajpendo.00312.2014. [DOI] [PubMed] [Google Scholar]
- 8.Van de Voorde J, Pauwels B, Boydens C, Decaluwe K. Adipocytokines in relation to cardiovascular disease. Metabolism. 2013;62(11):1513–21. doi: 10.1016/j.metabol.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Sainz N, Barrenetxe J, Moreno-Aliaga MJ, Martinez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism. 2015;64(1):35–46. doi: 10.1016/j.metabol.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Yun JE, Kimm H, Jo J, Jee SH. Serum leptin is associated with metabolic syndrome in obese and nonobese Korean populations. Metabolism. 2010;59(3):424–9. doi: 10.1016/j.metabol.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Galletti F, Barbato A, Versiero M, Iacone R, Russo O, Barba G, et al. Circulating leptin levels predict the development of metabolic syndrome in middle-aged men: an 8-year follow-up study. J Hypertens. 2007;25(8):1671–7. doi: 10.1097/HJH.0b013e3281afa09e. [DOI] [PubMed] [Google Scholar]
- 12.Gil-Campos M, Canete RR, Gil A. Adiponectin, the missing link in insulin resistance and obesity. Clin Nutr. 2004;23(5):963–74. doi: 10.1016/j.clnu.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Karakosta P, Chatzi L, Plana E, Margioris A, Castanas E, Kogevinas M. Leptin levels in cord blood and anthropometric measures at birth: a systematic review and meta-analysis. Paediatric and Perinatal Epidemiology. 2011;25(2):150–63. doi: 10.1111/j.1365-3016.2010.01163.x. [DOI] [PubMed] [Google Scholar]
- 14.Sivan E, Mazaki-Tovi S, Pariente C, Efraty Y, Schiff E, Hemi R, et al. Adiponectin in human cord blood: relation to fetal birth weight and gender. J Clin Endocrinol Metab. 2003;88(12):5656–60. doi: 10.1210/jc.2003-031174. [DOI] [PubMed] [Google Scholar]
- 15.Zeltser LM. Developmental influences on circuits programming susceptibility to obesity. Front Neuroendocrinol. 2015;39:17–27. doi: 10.1016/j.yfrne.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiao L, Yoo HS, Madon A, Kinney B, Hay WW Jr., Shao J. Adiponectin enhances mouse fetal fat deposition. Diabetes. 2012;61(12):3199–207. doi: 10.2337/db12-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boeke CE, Mantzoros CS, Hughes MD, Rifas-Shiman SL, Villamor E, Zera CA, et al. Differential Associations of Leptin with Adiposity Across Early Childhood. Obesity. 2013;21(7):1430–7. doi: 10.1002/oby.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li LJ, Rifas-Shiman SL, Aris IM, Young JG, Mantzoros C, Hivert MF, et al. Associations of maternal and cord blood adipokines with offspring adiposity in project viva: Is there an interaction with child age? Int J Obes (Lond). 2017. doi: 10.1038/ijo.2017.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, Gillman MW. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics. 2009;123(2):682–9. doi: 10.1542/peds.2008-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson J, Smith AD, Fraser A, Sattar N, Lindsay RS, Ring SM, et al. Programming of Adiposity in Childhood and Adolescence: Associations With Birth Weight and Cord Blood Adipokines. J Clin Endocrinol Metab. 2017;102(2):499–506. doi: 10.1210/jc.2016-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong KK, Ahmed ML, Sherriff A, Woods KA, Watts A, Golding J, et al. Cord blood leptin is associated with size at birth and predicts infancy weight gain in humans. ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. J Clin Endocrinol Metab. 1999;84(3):1145–8. doi: 10.1210/jcem.84.3.5657. [DOI] [PubMed] [Google Scholar]
- 22.Karakosta P, Roumeliotaki T, Chalkiadaki G, Sarri K, Vassilaki M, Venihaki M, et al. Cord blood leptin levels in relation to child growth trajectories. Metabolism-Clinical and Experimental. 2016;65(6):874–82. doi: 10.1016/j.metabol.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Volberg V, Heggeseth B, Harley K, Huen K, Yousefi P, Dave V, et al. Adiponectin and Leptin Trajectories in Mexican-American Children from Birth to 9 Years of Age. Plos One. 2013;8(10). doi: 10.1371/journal.pone.0077964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeung EH, Sundaram R, Xie Y, Lawrence DA. Newborn adipokines and early childhood growth. Pediatr Obes. 2018. doi: 10.1111/ijpo.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer DM, Brei C, Stecher L, Much D, Brunner S, Hauner H. Leptin in Maternal Plasma and Cord Blood as a Predictor of Offspring Adiposity at 5 Years: A Follow-up Study. Obesity (Silver Spring). 2017. doi: 10.1002/oby.22037. [DOI] [PubMed] [Google Scholar]
- 26.Meyer DM, Brei C, Stecher L, Much D, Brunner S, Hauner H. Cord blood and child plasma adiponectin levels in relation to childhood obesity risk and fat distribution up to 5 y. Pediatr Res. 2017;81(5):745–51. doi: 10.1038/pr.2016.275. [DOI] [PubMed] [Google Scholar]
- 27.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vickers MH. Developmental programming and adult obesity: the role of leptin. Curr Opin Endocrinol Diabetes Obes. 2007;14(1):17–22. doi: 10.1097/MED.0b013e328013da48. [DOI] [PubMed] [Google Scholar]
- 30.Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. Am J Clin Nutr. 2013;97(5):1062–7. doi: 10.3945/ajcn.112.051706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braun JM, Kalkwarf HJ, Papandonatos GD, Chen A, Lanphear BP. Patterns of early life body mass index and childhood overweight and obesity status at eight years of age. BMC Pediatr. 2018;18(1):161. doi: 10.1186/s12887-018-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glavin K, Roelants M, Strand BH, Juliusson PB, Lie KK, Helseth S, et al. Important periods of weight development in childhood: a population-based longitudinal study. BMC Public Health. 2014;14:160. doi: 10.1186/1471-2458-14-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kain J, Martinez M, Close M, Uauy R, Corvalan C. The association of excessive growth with development of general and central obesity at 7 years of age in every period after birth in Chilean children. Nutrition. 2016;32(4):426–31. doi: 10.1016/j.nut.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Caron A, Lee S, Elmquist JK, Gautron L. Leptin and brain-adipose crosstalks. Nat Rev Neurosci. 2018;19(3):153–65. doi: 10.1038/nrn.2018.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stocker CJ, Wargent E, O’Dowd J, Cornick C, Speakman JR, Arch JR, et al. Prevention of diet-induced obesity and impaired glucose tolerance in rats following administration of leptin to their mothers. Am J Physiol Regul Integr Comp Physiol. 2007;292(5):R1810–8. doi: 10.1152/ajpregu.00676.2006. [DOI] [PubMed] [Google Scholar]
- 36.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, et al. The effect of neonatal leptin treatment on postnatal weight gain in male rats is dependent on maternal nutritional status during pregnancy. Endocrinology. 2008;149(4):1906–13. doi: 10.1210/en.2007-0981. [DOI] [PubMed] [Google Scholar]
- 37.Belbasis L, Savvidou MD, Kanu C, Evangelou E, Tzoulaki I. Birth weight in relation to health and disease in later life: an umbrella review of systematic reviews and meta-analyses. BMC Med. 2016;14:15. doi: 10.1186/s12916-016-0692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Ippolito S, Tersigni C, Scambia G, Di Simone N. Adipokines, an adipose tissue and placental product with biological functions during pregnancy. Biofactors. 2012;38(1):14–23. doi: 10.1002/biof.201. [DOI] [PubMed] [Google Scholar]
- 39.Gillman MW. Early infancy - a critical period for development of obesity. Journal of Developmental Origins of Health and Disease. 2010;1(5):292–9. doi: 10.1017/s2040174410000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Mazary A-AM, Nasif KA, Abdel-Hakeem GL, Sherif T, Farouk E, El-Gezawy EM. Adiponectin, leptin and insulin levels at birth and in early postnatal life in neonates with hypoxic ischemic encephalopathy. Journal of Diabetes and Metabolic Disorders. 2015;14. doi: 10.1186/s40200-015-0219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.