Abstract
INTRODUCTION:
Females face a significantly higher risk of presenting with voice problems than males. This discrepancy has been associated with a number of differences in respiratory behavior and the physiology of the laryngeal and endocrine systems.
METHODS:
In conjunction with established raw spirometry measures, the Vocal Fatigue Index was used to determine (1) if there is a relationship between base pulmonary function and vocal fatigue among teachers; and (2) if that relationship is different in females from males. One hundred and twenty-two elementary and middle school teachers (96 females, 26 males) from the Jordan School District in northern Utah participated in the study.
RESULTS:
Vocal Fatigue Index (VFI) factors were predictors of the outcomes of several raw spirometry measures for female participants, but the same predictive relationship was not found for male participants. Additionally, there appeared to be no relationship between VFI and spirometry measures in females when using normalized, rather than raw, spirometry metrics.
CONCLUSIONS:
The results suggest that the pulmonary physiology that would result in reduced raw pulmonary function, in combination with other differences associated with gender, may lead to a greater incidence of vocal fatigue among female teachers than their male counterparts.
Keywords: vocal fatigue, spirometry, speech breathing, vocal fatigue index
BACKGROUND
Among occupational voice users, teachers have received a large portion of the research focus. This is due in part to the relatively high incidence of vocal complaints and diagnosed voice problems among teachers 1, 2, as well as the high cost of these disorders both financially and educationally 3. Some risk factors that have been associated with occupation-related laryngeal complaints include vocal loading 4, recovery period 5, gender 6, and environmental factors (e.g., room acoustics, airborne particulates, irritants) 7, 8. Further, previous research suggests that teachers of lower grade levels reported higher amounts of vocal fatigue 9,10, possibly because of the need to use their voices more often and with a higher vocal effort and greater pitch variation in order to conduct their teaching activities and maintain the attention of their students inside the classroom.
One common complaint of those with voice problems is vocal fatigue, which often corresponds with increased vocal effort, loss of endurance, and physiological changes 11,12, p. 255. Regrettably, vocal fatigue has been widely used as a “catch all” term of both a symptom and a diagnosis that includes many complaints, including laryngeal discomfort, increased or elevated vocal/respiratory effort, or poor vocal quality 13. Vocal fatigue has been implicated as an early indicator of some voice disorders 14,15.
It has been a challenge to identify consistently and measure definitively the severity of vocal fatigue. Current standardized voice assessment tools such as the Vocal Handicap Index (VHI) 16 and voice-related quality of life (V-RQOL) 17 are general and were not designed to be sensitive to vocal fatigue specifically. In response, Nanjundeswaran et al. 18 proposed the Vocal Fatigue Index (VFI), a 19-question scale that was developed to identify those suffering from vocal fatigue and provide insight into its presentation. The index is composed of three individual factors with associated scores. Factor 1 (11 response items) relates to tiredness of voice, with items such as “I avoid social situations when I know I have to talk more” or “I find it difficult to project my voice with voice use.” Factor 2 contains five items relating to physical discomfort, with items such as “I experience throat pain at the end of the day with voice use” or “My voice feels sore when I talk more.” Factor 3 relates to improvement of symptoms with rest and contains three items such as “My voice feels better after I have rested.” Nanjundeswaran et al. 18 found that in the voice disorder population, Factors 1 (avoidance) and 3 (recovery) were the most reliable predictors of patients reporting vocal fatigue. As the VFI is an index, it may indicate the severity of the fatigue, where items are equally weighted.
Although the VFI was originally tested with 175 individuals (comprising patients presenting at vocal health clinics, N=105, and healthy controls, N=70), it was not tested specifically with occupational voice users who are at risk of developing health issues but may not, as yet, have visited voice clinics. Hunter and Banks 19 sampled 640 teachers across the United States and found some key trends that may indicate higher risks of developing vocal fatigue. First, school teachers on average were found to have higher VFI scores than the healthy controls of Nanjundeswaran et al. while having lower scores than individuals diagnosed with voice disorders. Additionally, the sample of female teachers appeared to have an elevated VFI score relative to male teachers in Factor 1 (tiredness, avoidance) and even more in Factor 2 (discomfort). Factor 1 values for both male and female teachers were about half way between healthy controls (lower end) and dysphonic individuals. However, female teachers’ values for Factor 2 were nearly that of dysphonic individuals indicating that the high voice requirements of teachers results in elevated VFI values generally but are especially evident in female teachers for Factor 2.
Many underlying physiological mechanisms may contribute to vocal health problems. A common part of any vocal health intervention is to ensure adequate breath support during voice production, indicating a correlation between pulmonary use or ability and vocal health concerns or voice quality. In the laboratory, Iwarsson et al. 20 studied the effect of lung volume on glottal voice source characteristics reporting that when individuals phonated using the higher end of their lung volume, they tended to have higher subglottal pressure, greater flow amplitude, and a lower closed quotient in the glottal cycle. Additionally, when using greater lung volumes, they had greater glottal leakage and greater relative estimated glottal area. Further, a separate study showed that a decline in pulmonary function appeared to be a primary cause for vocal complaints in the elderly population 21.Moreover, it has been suggested that teachers with voice disorders use smaller lung volumes at the beginning and end of a speech breath group than teachers without disorders 22.
Given this link between pulmonary use during speech and vocal health concerns, it is possible the gender difference in VFI scores discussed above 19 may be affected by gender-based differences in pulmonary function and/or breath support during phonation. In a review of gender physiological differences that may contribute to vocal health, Hunter et al. 6 suggested that slightly smaller average lung volumes in females could reduce breathing capacity and thus result in more glottal involvement in phonation than in males. This could explain the results seen by Stathopoulos and Sapienza 23 who found a significant decrease in the glottal airflow maximum flow declination rate in women compared to men, which implies a reduced vocal efficiency. Stathopoulos and Sapienza also reported that the magnitudes of laryngeal, pharyngeal and vocal fold pressures were greater in women than men across vocal intensity tasks, which suggests a greater glottal load.
Based on the literature reviewed above, the following hypothesis is presented: Reduced pulmonary function (due to factors such as smaller lungs or learned behavior) in otherwise healthy individuals leads to reduced vocal efficiency and, therefore, higher possibility of reporting elevated vocal fatigue, particularly in high voice use individuals where the effects of a reduced vocal efficiency could build up over time. On this basis, two research questions are proposed: (1) To what extent is there a relationship between the VFI sub-scores and raw pulmonary function test metrics? and (2) Is this relationship moderated by gender? In summary, the aim of the current study is to test the above hypothesis and address the questions by evaluating the relationship between general pulmonary function (as measured by a spirometer) and vocal fatigue (as measured by the VFI) among teachers (high voice use individuals).
METHODS
Participants
Recruitment was limited to full-time elementary and middle school teachers (as opposed to high school teachers) because previous studies suggest teachers of younger grades are at greater risk of developing vocal fatigue 9,10. To be included in the study, subjects had to be teaching actively at the time of data collection with at least one year of full-time teaching experience prior to enrolling in the study. Exclusion criteria included significant history of gastrointestinal surgery (or disease already being treated); greater than mild reflux requiring medical intervention (as determined by symptoms); speech or voice disorders unrelated to reflux (defined by the research team); reported or known neurological or structural abnormalities of vocal folds unrelated to reflux; previous laryngeal surgeries; hearing loss outside the normal amount for age; cardiac abnormalities; and/or recent history of smoking. Exclusion criteria was determined by the research team by means of (1) interview prior to enrollment and (2) subject responses to a vocal health questionnaire.
One hundred and twenty-two subjects met all eligibility requirements and participated in the study. Subject gender and age distributions (96 female, 26 male; aged from 21 to 70 y/o) were targeted to roughly match their distributions among teachers in the state of Utah 24. In addition to identifying as full-time teachers, 8 subjects also identified as administrators and/or support staff and 1 subject identified as a trained singer.
Environment
All data were collected on site at each participant’s place of work. The research team traveled to the school with all equipment, which was set up in a quiet common area such as a library or empty classroom. This protocol likely resulted in higher participation at each location because teachers could participate at their convenience (before/after school, at lunch) without the research team needing to relocate to participants’ classrooms.
Equipment
Spirometry measures were obtained using the Micro Direct MicroLab portable spirometer (Micro Direct, Lewiston, ME). The spirometer was fitted with the manufacturer’s disposable filter (SpiroSafe) designed to prevent the spread of airborne pathogens without impeding airflow, allowing for rapid reuse of the spirometer without the need for sanitizing the mouthpiece between subjects.
Procedure
After providing consent, subjects completed a series of questionnaires that elicited demographic information, vocal history, and information about their teaching/work environment. Subjects also completed the Vocal Fatigue Index, a self-report questionnaire described above (Nanjundeswaran et al. 18).
After completion of the questionnaires, all subjects performed a common pulmonary function test using a spirometer to collect the following information: forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), peak expiratory flow (PEF), forced expiratory time (FET), and the ratio of forced expiratory volume in the first second to forced vital capacity (FEV1/FVC). Pulmonary function test metrics are usually presented in terms of a percent of predicted normal values 25. Predicted normal values (hereafter called normalized values) come from large scale studies of healthy individuals while controlling for such parameters as height, age and gender. In effect, this allows for persons with smaller size or younger age to be compared to those of larger size or different age. This methodology is helpful when considering general health, however, per the hypothesis of the current study, the raw (not predicted) values were of most interest when considering just overall lung function. Nevertheless, both sets (raw and normalized) were used in the analysis below. Normalized values were provided by the default database comparison built into the spirometer.
The spirometry technique followed international standards 26: subjects were instructed to breathe in as fully as possible then exhale as quickly and as forcefully as possible for as long as possible. After completing this phase, the subjects were then asked to perform a steady inhalation to completely full lung volume. Subjects were coached on this task by a member of the research team, and the procedure was repeated until three acceptable trials were performed, as determined by the spirometer’s internal software. If any two trials varied by more than 20%, the trial was rejected and the procedure was repeated.
Statistical Analysis
Statistical analysis was conducted using R version 3.1.2. Within both gender categories, VFI factor scores were converted to z-scores before model fitting. Linear regression models were fitted to each spirometry parameter separately (both the raw and normalized values) as these parameters were correlated. The assumptions of the linear regression procedures were met. Nested model comparisons (using likelihood ratio tests) were used to determine whether age should be included in each model with the spirometry parameter. Model comparisons using the same likelihood ratio tests were used to ascertain that there was no interaction of age and VFI factors so that these interactions could be excluded from the final models.
RESULTS
Overall average VFI scores for the three factors for all participants are presented in Table I. Factor values tended to be higher for females than males. In particular, VFI Factor 2 (which relates to pain or discomfort during phonation) seemed the most prominent and ranged from 0 to 16 for females with a mean of 5.98, and 0 to 11 for males with a mean of 3.85. A Welch two sample t-test with unequal variances indicated that females obtained higher Factor 2 scores than males (after Benjamini-Hochberg correction: t = 2.52, df = 46.8, p < 0.05). However, while there was no reliable difference for the other factors (p > 0.05), this result should be treated with caution as sample sizes were unequal.
Table I.
All subject mean VFI scores for reference (non-standardized).
| Number | Factor 1 | Factor 2 | Factor 3 | |
|---|---|---|---|---|
| All Subjects | 122 | 15.92 s.d. 7.18 | 5.52 s.d. 4.34 | 3.98 s.d. 2.92 |
| Females | 96 | 16.35 s.d. 7.39 | 5.98 s.d. 4.42 | 3.87 s.d. 3.00 |
| Males | 26 | 14.35 s.d. 6.21 | 3.85 s.d. 3.65 | 2.61 s.d. 2.61 |
Using the Spearman rank-order correlation method, Factors 1 and 2 were moderately positively correlated (r = 0.77, p < 0.0001) for females, while Factors 1 and 3 and Factors 2 and 3 were weakly correlated (Factor 1-Factor 3, r = 0.22, p < 0.05; Factor 2-Factor 3, r = 0.25, p < 0.05). For males, Factors 1 and 2 were moderately positively correlated (r = 0.71, p < 0.0001), but Factors 1 and 3 and Factors 2 and 3 were not (Factor 1-Factor 3, r = 0.29, p = 0.15; Factor 2-Factor 3, r = 0.16, p=0.42).
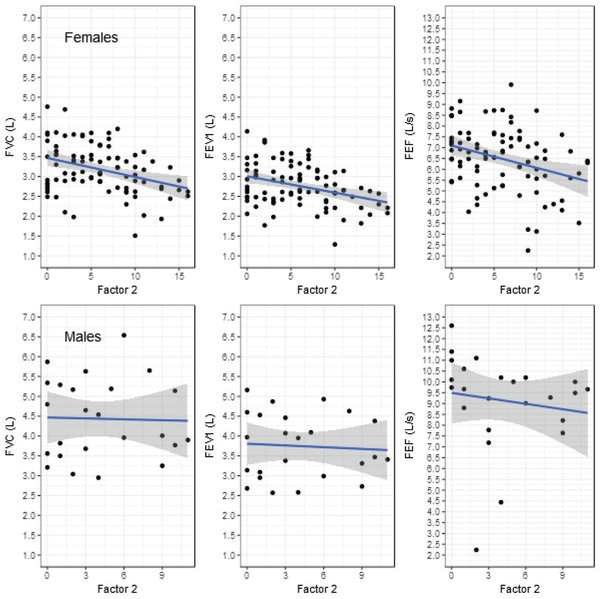
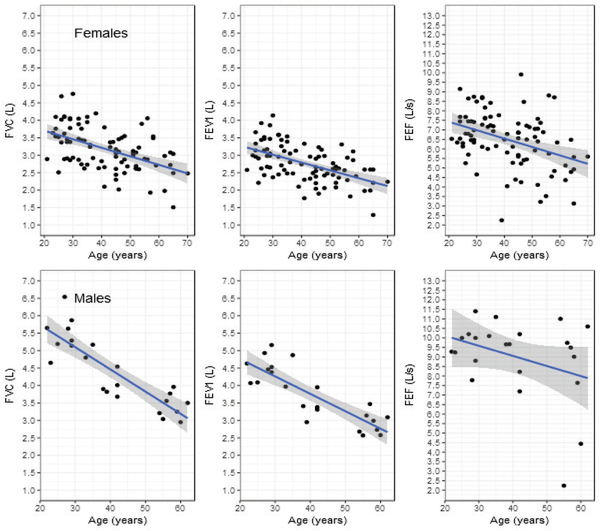
The variables considered included VFI Factors 1, 2, and 3, teacher gender, teacher age, and spirometry metrics (raw and normalized). For females, Factor 2 was a predictor of raw FVC, FEV1 and PEF. The effects of Factor 1 on raw spirometry values approached significance, while age reliably predicted raw spirometry values. There was no reliable relationship between spirometry parameters and Factor 3. Figure 1 shows the relationship between Factor 2 and raw pulmonary metrics for females (upper) and males (lower); Figure 2 presents the data between raw pulmonary metrics and age. For every unit increase in the standardized Factor 2 score, raw forced vital capacity (FVC) decreased by 0.18 L (SE = 0.06, t = -3.18, p < 0.01; Figure 1, upper panel); and for every increase in age of one year, FVC decreased by 0.02 L (SE = 0.00, t = -5.08, p < 0.0001; Figure 2, upper panel). For every unit increase in the standardized Factor 2 score, forced expiratory volume in the first second (FEV1) decreased by 0.15 L (SE = 0.05, t = -3.14, p < 0.01); and for every increase in age of one year, FEV1 decreased by 0.02 L (SE = 0.00, t = -5.67, p < 0.0001). For every unit increase in the standardized Factor 2 score, peak expiratory flow (PEF) decreased by 0.40 L/s (SE = 0.14, t = -2.87, p < 0.01); and for every increase in age of one year, PEF decreased by 0.04 L/s (SE = 0.01, t = -3.55, p < 0.01).
Figure 1.
Scatterplots of VFI Factor 2 scores (x-axis) and raw spirometry values (y-axis; FVC left, FEV1 middle, PEF, right) for females (Upper) and males (Lower). The linear regression fitted line (solid line) is shown with the 95% confidence region (grey).
Figure 2.
Scatterplots of age in years (x-axis) vs. raw FVC, FEV1 and PEF values (y-axis) for female teachers (Upper) and raw FVC and FEV1 vs. age (years) for male teachers (Lower). The linear regression fitted line (solid line) is shown with the 95% confidence region (grey).
For males, none of the VFI Factors corresponded to raw spirometry values. However, age was a reliable predictor of FVC and FEV1 such that as age increased, raw pulmonary function as indicated by these parameters decreased (FVC, β1 = −0.06, SE = 0.01, t = −7.47, p < 0.0001; FEV1, β1 = −0.05, SE = 0.01, t = −6.80, p < 0.0001; PEF, β1 = −0.05, SE = 0.03, t = −1.65, p = 0.11). The way in which male teachers’ FVC and FEV1 values from the pulmonary test change with age is shown in Figure 2 (lower panel). For neither females nor males was there an identifiable relationship between spirometry parameter FET and age or between FET and VFI sub-scores.
When the pulmonary parameters of interest were replaced by the normalized values (adjusted per height, weight, etc.), Factor 2 was no longer a reliable predictor for either females or males (Table II). Further, Factor 1 was not a reliable predictor of normalized pulmonary function for females or males (Table III). In both cases, age was still a reliable predictor of the normalized FVC, FEV1, and PEF. Additionally, there was a reliable positive correlation (Kendall’s tau) between height and FEV1, FVC and PEF (for both raw and predicted values) for both males and females. However, there was not a reliable correlation of height and VFI Factors.
Table II.
Linear regression model results for VFI Factor 2 and age by normalized FVC, FEV1 and PEF for females.
| Estimate | SE | t | p | ||
|---|---|---|---|---|---|
| FVC | (Intercept) | 4.50 | 0.18 | 25.72 | <0.0001 |
| Factor2 | −0.03 | 0.05 | −0.54 | 0.59 | |
| Age | −0.02 | 0.01 | −4.49 | <0.0001 | |
| FEV1 | (Intercept) | 4.00 | 0.13 | 30.40 | <0.0001 |
| Factor2 | −0.02 | 0.04 | −0.58 | 0.56 | |
| Age | −0.02 | 0.01 | −7.43 | <0.0001 | |
| PEF | (Intercept) | 8.20 | 0.31 | 26.57 | <0.0001 |
| Factor2 | −0.02 | 0.09 | −0.24 | 0.81 | |
| Age | −0.03 | 0.01 | −3.88 | <0.001 |
Table III.
Linear regression model results for VFI Factor 1 and age by normalized FVC, FEV1 and PEF for females.
| Estimate | SE | t | p | ||
|---|---|---|---|---|---|
| FVC | (Intercept) | 4.15 | 0.20 | 21.13 | <0.0001 |
| Factor1 | −0.11 | 0.06 | −1.84 | 0.07 | |
| Age | −0.02 | 0.01 | −5.21 | <0.001 | |
| FEV1 | (Intercept) | 3.66 | 0.16 | 22.56 | <0.0001 |
| Factor1 | −0.09 | 0.05 | −1.84 | 0.07 | |
| Age | −0.02 | 0.01 | −5.85 | <0.0001 | |
| PEF | (Intercept) | 8.20 | 0.47 | 17.56 | <0.0001 |
| Factor1 | −0.30 | 0.14 | −2.16 | <0.05 | |
| Age | −0.04 | 0.01 | −3.96 | <0.001 |
DISCUSSION
Average VFI Factor scores from the teachers who participated in this study implied elevated levels of vocal fatigue (Factors 1 and 2) in comparison with the healthy control of Nanjundeswaran et al. 18. Supporting the findings of a larger review of school teachers and the VFI by Hunter and Banks 19, the teachers in this study were approximately three times more likely to report tiredness and voice avoidance symptoms (Factor 1) and more than three times as likely to report physical discomfort symptoms in the voice (Factor 2), while patients with dysphonia from Nanjundeswaran et al 18 reported nearly 5 times higher scores than the healthy controls.
Increases in VFI Factor 2 (related to pain or discomfort during phonation) corresponded to lower pulmonary function raw metrics FVC (vital capacity - volume), PEF (peak expiratory flow), and FEV1 (airway ease – forced volume in first second) for female teachers only. However, scores did not correspond to FET (forced expiratory time) for either females or males. Of the metrics, FVC is the most easily related to voice production. FVC represents the maximum usable volume of air a person can utilize with maximum inhalation and exhalation. Normal adult capacity is between 3–5 liters. Therefore, a larger volume (assuming otherwise similar physiology) would allow for a longer phonation between inhalations, and perhaps less vocal effort to support a long phonation.
As sufficient and coordinated respiratory control and breath management (e.g. adequate subglottal pressure and airflow) are required to manage efficient voice production, the respiratory system is crucial to maintaining vocal health. Factors such as poor vocal breath support behavior in healthy individuals (which could have a link to smaller lung volume) may lead to reduced vocal efficiency and, therefore, a higher possibility of reporting elevated vocal fatigue. This is particularly the case for high voice use populations where the effects of a reduced vocal efficiency could build up over time. It is likely that the differences in lung function as measured by the spirometry test in the current study are indicative of gender differences in breath usage to support voice. The lower raw pulmonary function test metrics in females would likely be associated with greater laryngeal effort as evidenced by elevated VFI Factor 2 scores. Hence, without additional underlying pathology, the key indicator of vocal fatigue caused by reduced general lung volume may be pain or discomfort during phonation.
On average, females have smaller lung volumes and maximum breathing capacity than males, with the primary differentiation happening after puberty 27. Further, the male lungs have been reported to have a higher static recoil during exhalation 23, suggesting that women require a higher percentage of lung volume use to create an equivalent lung pressure, which is a necessary driving force of vocal fold vibration. Considering the capacity and recoil differences, an average woman may need to compensate during speech by using more inhalation respiratory effort (larger percentage of tidal volume) to vocalize on a par (loudness and duration) with an average man. Such a compensation would require concerted effort to maintain and an increased rate of fatigue, potentially putting females at risk of developing vocal nodules and other voice disorders 28. Women who did not compensate with increased respiratory effort would likely have insufficient airflow and would, therefore, have to compensate with increased laryngeal effort. This laryngeal compensation would increase contact force per unit area on the medial edges of the vocal folds 29, which is ultimately a less healthy vocalization style. Therefore, differences in the respiratory system could be one of the factors contributing to the higher instance of vocal nodules in female compared to male teachers 30. These results give insight into previous findings that have suggested that teachers with voice disorders use smaller lung volumes at the beginning and end of a speech breath group than teachers without disorders 22.
When the raw pulmonary parameter values, which corresponded to VFI Factors 1 and 2, were replaced by the normalized values (essentially adjusting for height, age, etc.), there was no longer any reliable evidence of a relationship between pulmonary function and VFI. This indicates that from the perspective of lung health for sustaining life (e.g., when body size, gender, age is no longer a factor), vocal fatigue symptoms are not related to pulmonary function health. For example, a participant may have smaller pulmonary ability due to smaller lungs (raw spirometer metrics) yet still have healthy lungs for their body size and age (compared to the average raw values of a cohort of health persons with similar age, height, etc.). However, that individual may be at a disadvantage in some situations when it comes to supporting vocalization and may require more concerted effort to support within breath group voice production if an extended vocalized phrase for a breath group is desired. In most situations this is likely inconsequential. However, within high vocal demand populations like teachers, and over time, this extra effort requirement would likely result in higher vocal fatigue.
Finally, the current study indicated that gender is a moderating variable in the relationship between age and the functioning of the respiratory system as measured by a spirometer. Specifically, age reliably predicted the raw values of spirometry parameters’ FEV1, and FVC. There is evidence of decreased respiratory support in the aging population 21, suggesting the possibility of decreased respiratory function and a lowered maximum phonation duration with older age. These results are not unexpected and show that the samples collected are comparable to the larger population when it comes to an inevitable decline in pulmonary function in later life 31. Interestingly, there was a gender difference with age as PEF was a reliable predictor for female teachers. This relationship needs to be further explored in future studies with a larger aging population and equal numbers of male and female teachers.
As with any study, there are limitations (such as uncertainty in measurement, sample size and unequal samples distributions). While there were statistical significances found, it is uncertain about the realized significance or if the participating teachers are truly representative sample of all teachers; nevertheless, the results do support general assumptions in the literature about the connection between voice vocal health and lung function. In this study, the focus was limited only on pulmonary function test metrics and the vocal fatigue index. While the results showed significant predictability, it is possible that there are other large factors underlying the gender differences presented that were not measured. Another potential limitation could be due to the differences between reporting discomfort across gender; for example, previous studies have discussed such reporting differences (such as pain and discomfort) affect female responses generally 19 and the VFI specifically 6. Nonetheless, such an influence would likely result in the males offering a reduced range in the VFI. However, the male results (e.g. Figure 1, lower charts) for FVC and FEV1 do not appear to be suppressed version of the female responses. Further research is needed to further investigate these results.
CONCLUSIONS
The current study examined the relationship between general pulmonary function and vocal fatigue among teachers, particularly exploring potential gender differences in this relationship. The results of this study indicate that raw metrics quantifying pulmonary function are related to reports of vocal fatigue in female teachers in a manner not evident for their male counterparts. Specifically, vocal fatigue symptoms related to laryngeal pain and discomfort were shown to be related to spirometer lung function test results.
Therefore, in light of these findings, a spirometer based pulmonary function screening of females who are experiencing vocal fatigue could be used to differentiate further between those whose vocal fatigue should be addressed with traditional functional voice therapy and those who would benefit from a breath training regimen or, in more severe instances, a consultation with a pulmonologist. The result would be a more efficient and effective use of voice clinic scheduling and, ideally, the identification and correction of vocal inefficiencies without the need for therapeutic intervention.
In normal vocal communication, persons with smaller lung function or capacity would likely not experience elevated vocal fatigue. However, in the case of high vocal demand professions (school teachers or other occupational voice users who speak for long periods of time and at higher levels), the discussed effects may result in less efficient vocalization, higher reports of vocal fatigue, and possibly even a higher prevalence of voice disorders.
ACKNOWLEDGEMENT
The researchers acknowledge the approval of the Jordan, UT School District and the principals at the elementary and middle schools where data collection took place. Research reported in this publication was partially supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under Award Number R01DC012315. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
LITERATURE REVIEW
- 1.Hunter EJ, Titze IR. Variations in intensity, fundamental frequency, and voicing for teachers in occupational versus nonoccupational settings. J Speech Lang Hear Res. 2010;53(4):862–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sliwinska-Kowalska M, Niebudek-Bogusz E, Fiszer M, et al. The prevalence and risk factors for occupational voice disorders in teachers. Folia Phoniatr Logop. 2006;58(2):85–101. doi: 10.1159/000089610 [DOI] [PubMed] [Google Scholar]
- 3.Cantor Cutiva LC, Burdorf A. Medical Costs and Productivity Costs Related to Voice Symptoms in Colombian Teachers. J Voice. 2015;29(6):776.e15–776.e22. doi: 10.1016/j.jvoice.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 4.Gaskill CS, O’Brien SG, Tinter SR. The Effect of Voice Amplification on Occupational Vocal Dose in Elementary School Teachers. J Voice. 2012;26(5):667.e19–667.e27. doi: 10.1016/j.jvoice.2011.10.010 [DOI] [PubMed] [Google Scholar]
- 5.Hunter EJ, Titze IR. Quantifying vocal fatigue recovery: Dynamic vocal recovery trajectories after a vocal loading exercise. Ann Otol Rhinol Laryngol. 2009;118(6):449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter EJ, Tanner K, Smith ME. Gender differences affecting vocal health of women in vocally demanding careers. Logoped Phoniatr Vocol. 2011;36(3–4):128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottalico P, Graetzer S, Hunter EJ. Effects of speech style, room acoustics, and vocal fatigue on vocal effort. J Acoust Soc Am. 2016;139(5):2870–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams NR. Occupational voice disorders due to workplace exposure to irritants—a review of the literature. Occup Med. 2002;52(2):99–101. doi: 10.1093/occmed/52.2.99 [DOI] [PubMed] [Google Scholar]
- 9.Angelillo IF, Maio GD, Costa G, Angelillo IF, Barillari U. Prevalence of occupational voice disorders in teachers. J Prev Med Hyg. 2009;50(1). http://www.jpmh.org/index.php/jpmh/article/view/152. [PubMed] [Google Scholar]
- 10.Banks R, Cantor Cutiva LC, Hunter EJ. What work related factors influence the use of voice amplification in the classroom? Lang Speech Hear Serv Sch [Google Scholar]
- 11.Chang A, Karnell MP. Perceived phonatory effort and phonation threshold pressure across a prolonged voice loading task: a study of vocal fatigue. J Voice. 2004;18(4):454–466. doi: 10.1016/j.jvoice.2004.01.004 [DOI] [PubMed] [Google Scholar]
- 12.Solomon NP. Vocal fatigue and its relation to vocal hyperfunction †. Int J Speech Lang Pathol. 2008;10(4):254–266. doi: 10.1080/14417040701730990 [DOI] [PubMed] [Google Scholar]
- 13.Welham NV, Maclagan MA. Vocal fatigue: current knowledge and future directions. J Voice. 2003;17(1):21–30. doi: 10.1016/S0892-1997(03)00033-X [DOI] [PubMed] [Google Scholar]
- 14.Ghassemi M, Stan JHV, Mehta DD, et al. Learning to Detect Vocal Hyperfunction From Ambulatory Neck-Surface Acceleration Features: Initial Results for Vocal Fold Nodules. IEEE Trans Biomed Eng. 2014;61(6):1668–1675. doi: 10.1109/TBME.2013.2297372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCabe DJ, Titze IR. Chant Therapy for Treating Vocal Fatigue Among Public School Teachers: A Preliminary Study. Am J Speech Lang Pathol. 2002;11(4):356. [Google Scholar]
- 16.Jacobson BH, Johnson A, Grywalski C, et al. The voice handicap index (VHI) development and validation. Am J Speech Lang Pathol. 1997;6(3):66–70. [Google Scholar]
- 17.Hogikyan ND, Sethuraman G. Validation of an instrument to measure voice-related quality of life (V-RQOL). J Voice. 1999;13(4):557–569. doi: 10.1016/S0892-1997(99)80010-1 [DOI] [PubMed] [Google Scholar]
- 18.Nanjundeswaran C, Jacobson BH, Gartner-Schmidt J, Verdolini Abbott K. Vocal Fatigue Index (VFI): Development and Validation. J Voice. 2015;29(4):433–440. doi: 10.1016/j.jvoice.2014.09.012 [DOI] [PubMed] [Google Scholar]
- 19.Hunter EJ, Banks RE. Gender Differences in the Reporting of Vocal Fatigue in Teachers as Quantified by the Vocal Fatigue Index. Ann Otol Rhinol Laryngol. 2017;126(12):813–818. doi: 10.1177/0003489417738788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwarsson J, Sundberg J. Effects of lung volume on vertical larynx position during phonation. J Voice. 1998;12(2):159–165. doi: 10.1016/S0892-1997(98)80035-0 [DOI] [PubMed] [Google Scholar]
- 21.Mueller PB. The Aging Voice. Semin Speech Lang. 1997;18(02):159–169. doi: 10.1055/s-2008-1064070 [DOI] [PubMed] [Google Scholar]
- 22.Lowell SY, Barkmeier-Kraemer JM, Hoit JD, Story BH. Respiratory and Laryngeal Function During Spontaneous Speaking in Teachers With Voice Disorders. J Speech Lang Hear Res. 2008;51(2):333–349. doi: 10.1044/1092-4388(2008/025) [DOI] [PubMed] [Google Scholar]
- 23.Stathopoulos ET, Sapienza C. Respiratory and Laryngeal Function of Women and Men During Vocal Intensity Variation. J Speech Lang Hear Res. 1993;36(1):64–75. doi: 10.1044/jshr.3601.64 [DOI] [PubMed] [Google Scholar]
- 24.Utah State office of Education. 2011–2012 Educator Population Analysis; 2011. http://www.schools.utah.gov/data/Educational-Data/Educators/2011-2012-All-Populations-Summary.aspx.
- 25.Gutierrez C, Ghezzo RH, Abboud RT, et al. Reference Values of Pulmonary Function Tests for Canadian Caucasians. Canadian Respiratory Journal. doi: 10.1155/2004/857476 [DOI] [PubMed] [Google Scholar]
- 26.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 27.Needham CD, Rogan MC, McDonald I. Normal Standards for Lung Volumes, Intrapulmonary Gas-mixing, and Maximum Breathing Capacity. Thorax. 1954;9(4):313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sapienza CM, Stathopoulos ET, Brown WS. Speech breathing during reading in women withvocal nodules. J Voice. 1997;11(2):195–201. doi: 10.1016/S0892-1997(97)80078-1 [DOI] [PubMed] [Google Scholar]
- 29.Jiang JJ, Titze IR. Measurement of vocal fold intraglottal pressure and impact stress. J Voice. 1994;8(2):132–144. doi: 10.1016/S0892-1997(05)80305-4 [DOI] [PubMed] [Google Scholar]
- 30.Preciado-López J, Pérez-Fernández C, Calzada-Uriondo M, Preciado-Ruiz P. Epidemiological study of voice disorders among teaching professionals of La Rioja, Spain. J Voice. 2008;22(4):489–508. doi: 10.1016/j.jvoice.2006.11.008 [DOI] [PubMed] [Google Scholar]
- 31.Poon AH, Houseman EA, Ryan L, Sparrow D, Vokonas PS, Litonjua AA. Variants of Asthma and Chronic Obstructive Pulmonary Disease Genes and Lung Function Decline in Aging. J Gerontol Ser A. 2014;69(7):907–913. doi: 10.1093/gerona/glt179 [DOI] [PMC free article] [PubMed] [Google Scholar]