Abstract
It has been hypothesized that breast cancer and its chemotherapy can impart functional neural changes via an overlap with biological mechanisms associated with aging. Here we used fMRI to assess whether changes in neural activity accompanying visual episodic memory encoding and retrieval suggest altered activations according to patterns seen in functional imaging of cognitive aging. In a prospective longitudinal design, breast cancer patients (n=13) were scanned during memory encoding and retrieval before and after chemotherapy treatment, and compared to healthy-age matched controls (n=13). Our results indicate that despite equivalent behavioral performance, encoding and retrieval resulted in increased activation of prefrontal regions for the breast cancer group compared to controls for both before and after chemotherapy treatment. This was accompanied by decreased activity in posterior brain regions after chemotherapy, particularly those involved in visual processing, for the breast cancer group compared to controls. These findings are discussed as evidence for a possible anterior shift in neural processing to compensate for deficiencies in posterior brain regions, consistent with an accelerated aging account. Cancer and chemotherapy can impact brain regions underlying episodic memory, leading to additional recruitment of control regions, which may be linked to mechanisms related to aging.
Keywords: Breast Cancer, fMRI, episodic memory, chemotherapy
Background
Over a decade of functional neuroimaging studies in breast cancer patients have accumulated evidence of neural changes both before (Scherling, Collins, Mackenzie, Bielajew, & Smith 2012) and after chemotherapy treatment (Conroy, McDonald, Smith, et al. 2013; Kam et al. 2015; Silverman et al., 2007), even in the presence of equivalent behavioral performance (Conroy, McDonald, Ahles, West, & Saykin 2013; Deprez et al. 2014; Kam et al. 2015; López Zunini et al. 2013). Although the mechanisms that precipitate these cancer and cancer treatment related neural changes are not established, there may be a common biology between cancer, chemotherapy targets and the aging process (Ahles & Root 2018; Ahles, Root, & Ryan 2012; Badiola et al. 2015). For example, tumor growth and tumor cells induce cellular senescence, in the form of loss of cell function via accumulation of DNA damage and oxidative stress, which are also the main characteristics of normal aging (for review see Badiola et al. 2015). Research has suggested that adjuvant chemotherapy increases senescence markers associated with accelerated molecular aging (Sanoff et al. 2014). From this perspective, neural changes among breast cancer patients may be linked to common causes of aging. Age-related changes in brain activity supporting episodic memory are a hallmark of aging, which can inform targeted hypotheses regarding the nature of neural activity changes due to breast cancer and its chemotherapy treatment. Given this backdrop, investigating age related patterns of episodic memory related neural activity among breast cancer patients before and after their chemotherapy treatment was the goal of the current study.
Among the most consistent age-related changes in brain activity, accompanying cognitive functions including episodic memory, is the overactivation of frontal regions with under activation of occipitotemporal regions (Dennis et al. 2008; Grady, Bernstein, Beig, & Siegenthaler 2002; Gutchess et al. 2005; Leshikar, Gutchess, Hebrank, Sutton, & Park 2010) – a pattern referred to as the posterior-anterior shift in aging (PASA; Davis et al. 2008). This pattern has long been suggested to reflect compensation by higher-order executive functions in response to visual processing deficits, to the extent that a task demands visual processing for accurate performance (Grady et al. 1994). In a parallel with the functional neuroimaging of cognitive aging, functional overactivation of frontal regions has been repeatedly observed among breast cancer patients compared to healthy controls prior to (Cimprich et al. 2010; Scherling, Collins, Mackenzie, Bielajew, & Smith 2011) and after chemotherapy treatment (Ferguson, Mcdonald, Saykin, & Ahles 2007; Kesler, Bennett, Mahaffey, & Spiegel 2009; McDonald, Conroy, Ahles, West, & Saykin 2012; Silverman et al. 2007). However, few fMRI studies have examined brain activity accompanying episodic memory processes in breast cancer patients (de Ruiter et al. 2011; Kesler, Bennett, Mahaffey, & Spiegel 2009; López Zunini et al. 2013; Menning et al. 2017; Stouten-Kemperman et al. 2014), with even fewer examining activations prospectively (López Zunini et al. 2013; Menning et al. 2017). These previous prospective studies examined activation differences solely at retrieval using verbal but not pictorial stimuli (López Zunini et al. 2013) or solely for accurate memory judgments, which can obscure sustained compensation over time (Menning et al. 2017). The current study uses block-design fMRI to prospectively examine sustained task-level brain activations during episodic memory encoding and retrieval using visual stimuli in a breast cancer population. Consistent with fMRI literature showing overactivation in frontal regions, we sought to examine whether the breast cancer group would show increased recruitment in frontal regions compared to healthy controls. Consistent with the fMRI literature in cognitive aging, we investigated whether such overactivation might be compensatory for any under activation of occipitotemporal regions necessary to task performance.
Correspondingly, older adults increase brain activity in more demanding than less demanding memory tasks (e.g., Cabeza, Anderson, Locantore, & McIntosh 2002; Gutchess et al. 2007). Recent work among breast cancer patients showed no activation differences on an associative memory paradigm (Menning et al. 2017) that may reflect insufficient task demands on brain or cognitive level vulnerabilities. Here we use a “levels of processing” paradigm (Craik & Lockhart 1972) that changes the demands on attention at encoding and effort at retrieval (Craik 2002; Craik & Lockhart 1972) to manipulate the need for compensation in breast cancer patients. Memory following deep encoding is shown to produce longer-lasting, and stronger memory traces than shallow encoding because it demands more attentional resources to and consequently elaboration of items compared to the shallow level of analysis (Chun & Johnson 2011; Craik 2002). At retrieval, the weaker memory trace following shallow encoding demands more effortful retrieval attempts and, therefore processing resources, compared to items studied under deep encoding (Buckner, Koutstaal, Schacter, Wagner, & Rosen 1998). Thus, in addition to the primary goal of identifying neural activity changes that parallel aging, this addressed a secondary goal to elucidate if increasing task demands reveal greater need for compensatory recruitment of frontal regions.
Methods
Participants
Participants were 41 adult women recruited to participate in a larger Memorial Sloan Kettering Cancer Center (MSKCC) longitudinal study examining structural MRI and functional MRI changes over time (scanning at Weill Cornell Medical College). A total of 23 female breast cancer (BC) patients (age 47.3 ± 7.8 years) were recruited through the Evelyn H. Lauder Breast Center at Memorial Sloan Kettering Cancer Center, scanned post-surgical resection, but prior to the initiation of chemotherapy, and again within 1-month following the completion of chemotherapy treatment. Inclusion criteria of BC patients were 1) diagnosis of breast cancer, 2) post-resection, and 3) scheduled to undergo adjuvant chemotherapy, while exclusion criteria were 1) diagnosis of any central nervous system disease, or 2) history of neurological or psychiatric disorders. In the current study, 10 patients were not included in the final data set due to scanner malfunction (n=3), lost to follow-up (n=3), did not complete/contraindicated for the MRI (n=2), and/or excessive movement (n=2), leaving 13 BC patients (age 47.3 ± 7.3 years) for analyses. A total of 18 healthy control (HC) female participants matched on age (49.2 ± 8.1 years) and education were recruited through local newspaper advertising and Craig’s List, scanned at yoked intervals. Five control participants were not included in the final data set due to claustrophobia (n=1), lost to follow-up (n=3), or excessive movement (n=1), leaving 13 HC participants (age 48.5 ± 8 years) for analyses. The Institutional Review Board of MSKCC and Weill Cornell Medical College approved this study and all participants gave informed consent.
Stimuli and Experimental Procedure
All visual stimuli were presented on an MRI-compatible stimulus presentation and response collection hardware setup using e-prime experimental software (https://www.pstnet.com) and IFIS hardware (IFIS-SA, MRI Devices, Waukesha Wisconsin, USA; Psychology Software Tools, Pittsburgh, Pennsylvania, USA). Using a “levels of processing” paradigm, participants were shown scenes in black/white or color, and instructed in a “deep” encoding task to identify whether the image was a country or city scene, and in a “shallow” encoding task to identify whether the image was in black and white or color (Mandzia, Black, McAndrews, Grady, & Graham 2004). For the encoding condition, two runs consisted of total 96 visual scenes were presented (24 black and white countryside scenes; 24 black and white city scenes; 24 color countryside scenes; 24 color city scenes), in 12 blocks of 8 scenes each evenly divided between color and scene type, evenly divided into 2 runs, and 6 blocks each run. Blocks for deep and shallow conditions were alternated in the same order for all subjects. The presentation order of scenes was randomized across participants. Before each block, subjects were given 3 second written cue on whether to classify each scene as color versus black and white, or countryside versus city. Scenes were presented for 2000 ms with a fixation between each scene presented with a range of 1800ms – 3800ms (average of 2800 ms), resulting in 38.4 second block length, consistent with previous behavioral paradigms from our group (Perez et al. 2015; Weisholtz et al. 2015). A 24 second fixation period was presented between each block. The same two runs, blocks, and their trial structure were used for the recognition paradigm, with 48 targets from the encoding paradigm (randomly selected from the original 96 images at encoding) and 48 novel foil scenes presented, evenly divided within each block. The presentation order of scenes was randomized across participants. Subjects were asked to judge if the image was “old” (previously presented) or “new” (not previously presented) by pressing the corresponding button. The procedure was identical at baseline and at time 2, with two different stimuli sets for encoding counterbalanced between subjects and timepoints. Subsequent recognition memory performance for each encoding condition was calculated as d’, the ability to discriminate between old and new items, calculated as follows:
The behavioral data was analyzed using a three way-ANOVA with encoding condition (deep, shallow) and time (baseline, Time 2) as within-subject factors, and group (BC, HC) as the between subject factor.
fMRI Data Acquisition and Analysis
Image data were acquired with a GE Signa 3-Tesla MRI scanner (max gradient strength 40 mT/m, max gradient slew rate 150 T/m/s; General Electric Company, Waukesha, Wisconsin, USA). Whole brain functional MRI scans were collected using a gradient echo-planar imaging (EPI) sequence (repetition time (TR) = 1200 ms; echo time (TE) = 30 ms; 70° flip angle; 240mm field of view (FOV); 21 slices; 5-mm thickness with 1-mm interslice space; 64 × 64 matrix acquisition), with a modified z-shimming algorithm to reduce susceptibility artifact at the base of the brain. A reference T1-weighted anatomical image with the same axial slice placement and thickness as the EPI images was acquired to aid re-orientation and co-registration. A high-resolution T1-weighted anatomical image was acquired using a spoiled gradient recalled acquisition sequence (TR/TE = 30/8 ms, 45° flip angle, 220mm FOV, 140 1.5mm coronal slices; 256 × 256 matrix).
Modified SPM software (including elements from spm99 to spm12, Wellcome Department of Imaging Neuroscience) was used for processing the data (for details see Pan, Epstein, Silbersweig, & Stern 2011), which included extraction of physiological fluctuations such as cardiac and respiratory cycles from the EPI image sequence (Frank, Buxton, & Wong 2001); manual AC-PC re-orientation of all anatomical and EPI images; realignment of EPI images to correct for slight head movement between scans based on intracranial voxels; co-registration of functional EPI images to the corresponding high-resolution anatomical image based on the rigid body transformation parameters of the reference anatomical image to the latter for each individual subject; stereotactic normalization to a standardized coordinate space (Montreal Neurologic Institute MRI Atlas version of Talairach space) based on the high-resolution anatomical image; spatial smoothing with an isotropic Gaussian kernel (FWHM= 7.5 mm).
Following preprocessing, the statistical analyses were conducted using customized fmristat software (Worsley et al. 2002). A two-level voxel-wise linear mixed-effects model was utilized to examine the key group, encoding condition, and time contrasts of interest. First, a whole-brain voxel-wise multiple linear regression model was employed at the individual subject level which comprised the regressors of interest, which consist of the stimulus onset times convolved with a prototypical hemodynamic response function, the covariates of no interest (the first-order temporal derivative of the regressor of interest, global and physiological fluctuations, realignment parameters, scanning period means, and baseline drift up to the third order polynomials) and a first order auto-regression model of the residual time series to accommodate temporal correlation in consecutive scans. Second, at the group level, a mixed-effects model was used, which accounts for intra- and inter-subject variability, and allows for population-based inferences to be drawn. Age was used as a covariate of no interest in an analysis of covariance setting. The statistical inference at the group level was then drawn according to Gaussian random field theory. Initial voxel-wise threshold was p < 0.001; all comparisons reported were considered significant at family-wise error corrected (FWE) p < 0.05 in whole brain correction with a minimum cluster extent of k = 10 voxels (27mm3 per voxel).
For the encoding analyses, an additional HC was not included, and an additional 2 HC were not included in retrieval analyses due to excessive motion. Resulting Ns for each group during encoding were: BC = 13, HC = 12; during retrieval were: BC = 13, HC = 11. We carried out three analyses relating to the levels of processing manipulation: (1) to obtain a general map of activations during memory encoding and retrieval, the deep and shallow conditions were collapsed as an “All (deep+shallow)” > fixation contrast and differential activations based uniquely on the (2) deep > fixation or (3) shallow > fixation condition were then analyzed to determine the role of the level of processing on neural responses. The activations for All (deep+shallow) conditions were examined for between-group cross-sectional differences at baseline and Time 2 and group-by-time longitudinal differences (baseline > Time 2; Time 2 > baseline). For completeness, we also examined within-group longitudinal differences (baseline > Time 2; Time 2 > baseline) for exploratory purposes (or to follow-up the pattern of any interactions).
Results
Demographic Data
Demographic characteristics for each group and chemotherapy regimen for BC patients can be seen in Table 1. Groups did not significantly differ based on any demographic characteristics.
Table 1.
Sample Demographic Characteristics1
| Breast Cancer (n=13 Female) |
Healthy Control (n=13 Female) |
|
|---|---|---|
| Mean (SD) or N(%) | Mean (SD) or N(%) | |
| Age at baseline (years) | 47.85 (6.4) | 48.54 (8.0) |
| Education (years) | 17.23 (2.35) | 16.08 (1.71) |
| Handedness | ||
| Right | 13 (100) | 13 (100) |
| Left | 0 (0) | 0 (0) |
| Time between scans (days) | 152.69 (22.9) | 163.58 (17.98) |
| Race | ||
| White | 4 (30.8) | 11 (84.6) |
| Black or African-American | 2 (15.4) | 2 (16.7) |
| Asian | 5 (38.5) | 0 (0) |
| Hispanic or Latino | 2 (15.4) | 0 (0) |
| Native Hawaiian or Other Pacific Islander | 0 (0) | 0 (0) |
| American Indian or Alaska Native | 0 (0) | 0 (0) |
| Cancer stage | ||
| I | 3 (23.1) | |
| II | 6 (46.2) | |
| III | 2 (15.4) | |
| IV | 0 (0) | |
| No Stage recorded | 2 (15.4) | |
| Chemotherapy | ||
| Adriamycin, Cytoxan, and Taxol | 9 (69.2) | |
| Cyclophosphamide, methotrexate, and fluorouracil | 4 (30.8) | |
Groups did not significantly differ across demographic characteristics of age (t(24) = 0.243, p = 0.81); education (t(24) = −1.432, p = 0.165); time between scans (t(24) = 0.681, p = 0.502)
Behavioral Data
Encoding
There were no group differences in encoding accuracy (i.e., correctly attributing images to color or scene) at either time point or an interaction between groups and level of processing. There was a main effect of level of processing, F(1, 24) = 19.357, MSE = 0.074, p< 0.001, partial η2 = 0.446, with higher accuracy for deep (M±SEM: 0.952 ± 0.008) than shallow (M±SEM: 0.899 ± 0.014) encoded items.
Retrieval
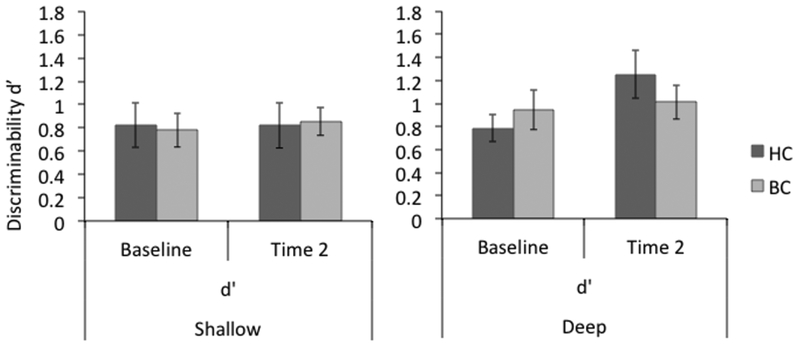
Consistent with a levels of processing effect, the deep encoding condition yielded higher discrimination accuracy than the shallow encoding condition as indicated by a significant main effect of encoding condition, F(1, 24) = 5.341, MSE = 0.833, p< 0.030, partial η2 = 0.182. No group X time interaction was found (Figure 1). Between group, cross-sectional analyses at baseline and Time 2 were not significant. Pairwise comparisons within groups showed the HC group improved recognition in the deep condition from baseline (M±SEM: 0.79 ± 0.12) to Time 2 (M±SEM: 1.25 ± 0.21) that approached significance; t(12) = −2.117, p = 0.056, while the BC group remained equivalent over time (baseline: M±SEM: 0.94 ± 0.17; Time 2: M±SEM: 1.01 ± 0.15).
Fig. 1.
Recognition accuracy following shallow or deep encoding conditions at baseline and Time 2. A group X level of processing X time ANOVA showed accuracy was significantly better for deep than shallow conditions, and significantly better at Time 2 than at baseline, but no statistically significant differences between groups. A separate analysis within groups, however, showed a near significant greater improvement in the deep condition over time for healthy controls but not breast cancer patients. This pattern is suggestive of an ability to improve due to practice for healthy controls that is undermined following chemotherapy for breast cancer. T-maps rendered on Montreal Neurologic Institute MRI Atlas using MRIcron (Rorden & Brett 2000) at voxel-wise p-values less than 0.001
fMRI data
Encoding
The regions of significant activation for these comparisons during encoding are listed in Table 2 in terms of their region names, peak coordinates, FWE corrected p-value, Z-values, and voxel cluster extent (k). BOLD activity t-maps of these regions are rendered on Montreal Neurologic Institute MRI Atlas using MRIcron (Rorden & Brett 2000) at voxel-wise p-values less than 0.001 (Figures 2A, 2B, and 3A).
Table 2.
Regions demonstrating significant differences during encoding
| Condition | Region | Hem | K | FWE-P | Max-Z | x | y | z | BA | |
|---|---|---|---|---|---|---|---|---|---|---|
| Group x Time | ||||||||||
| Baseline>Time 2 | ||||||||||
| BC>HC | All | Fusiform | R | 46 | .043 | 4.14 | 36 | −54 | −15 | 37 |
| Shallow | Fusiform | R | 90 | .01 | 4.53 | 36 | −54 | −15 | 37 | |
| * | .018 | 4.37 | 36 | −66 | −15 | 19 | ||||
| * | .023 | 4.30 | 33 | −69 | −12 | 19 | ||||
| Deep | Temp Pole Sup | R | 33 | .006 | 4.65 | 57 | 12 | −12 | 38 | |
| Time 2>Baseline | ||||||||||
| BC>HC | All | STG | L | 10 | .007 | 4.60 | 81 | −39 | 15 | 22 |
| Shallow | None | |||||||||
| Deep | None | |||||||||
| Between Groups | ||||||||||
| Baseline | ||||||||||
| BC>HC | All | Middle Frontal G. | R | 47 | .014 | 4.43 | 39 | 33 | 21 | 45/46 |
| Shallow | IFGoperc | L | 58 | .01 | 4.51 | −63 | 12 | 27 | 44 | |
| Middle Frontal G. | R | 40 | .038 | 4.17 | 39 | 33 | 21 | 45/46 | ||
| Deep | None | |||||||||
| HC>BC | All | None | ||||||||
| Shallow | None | |||||||||
| Deep | None | |||||||||
| Time 2 | ||||||||||
| BC>HC | All | Middle Frontal G. | R | 36 | .031 | 4.23 | 42 | 33 | 18 | 45 |
| Precentral G. | R | 27 | .038 | 4.17 | 36 | 0 | 45 | 6 | ||
| Shallow | Post PHG | R | 22 | .047 | 4.12 | 30 | −27 | −18 | 20 | |
| Deep | Precentral G. | R | 32 | .013 | 4.46 | 36 | 0 | 45 | 6 | |
| HC>BC | All | None | ||||||||
| Shallow | None | |||||||||
| Deep | None | |||||||||
| Within Groups | ||||||||||
| Baseline>Time 2 | ||||||||||
| BC | All | Inf. Parietal L. | L | 66 | .003 | 4.78 | −39 | −42 | 54 | 40 |
| Fusiform | R | 38 | .019 | 4.36 | 36 | −54 | −15 | 37 | ||
| * | .027 | 4.26 | 33 | −51 | −18 | 37 | ||||
| Shallow | None | |||||||||
| Deep | Inf. Parietal L. | L | 82 | .017 | 4.39 | −39 | −42 | 54 | 40 | |
| * | .027 | 4.27 | −39 | −36 | 51 | 40 | ||||
| Precentral G. | L | 47 | .019 | 4.36 | −33 | −9 | 63 | 6 | ||
| Postcentral G. | L | 33 | .042 | 4.14 | −45 | −24 | 66 | 3 | ||
| Temporal Pole Sup | R | 28 | .009 | 4.56 | 57 | 12 | −12 | 38 | ||
| HC | All | None | ||||||||
| Shallow | L | 51 | .05 | 4.10 | −42 | −21 | 72 | 4 | ||
| Precentral G. | ||||||||||
| Deep | None | |||||||||
| Time 2>Baseline | ||||||||||
| BC | All | Middle Frontal G. | L | 105 | .007 | 4.61 | −24 | 24 | 36 | 9 |
| * | .012 | 4.48 | −27 | 21 | 39 | 8/9 | ||||
| Shallow | Middle Frontal G. | L | 89 | .017 | 4.39 | −33 | 12 | 39 | 44 | |
| * | .018 | 4.38 | −24 | 24 | 36 | 9 | ||||
| Deep | None | |||||||||
| HC | All | None | ||||||||
| Shallow | None | |||||||||
| Deep | None | |||||||||
STG: Superior Temporal Gyrus; IFG: Inferior Frontal Gyrus; PHG: Parahippocampal Gyrus
Fig. 2.
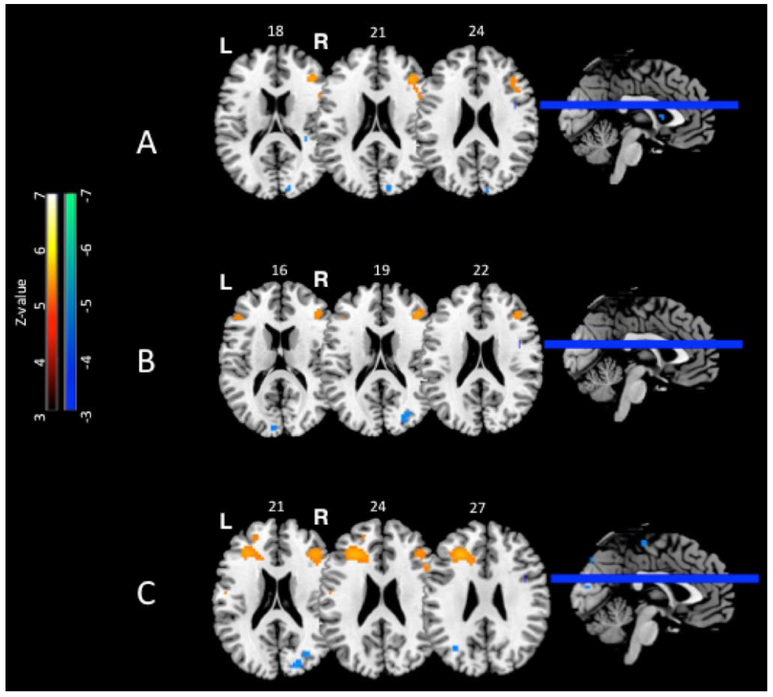
Cross sectional between-group differences. The number above each slice indicates the MNI z coordinate. Warm colors indicate increased activation while cool colors indicate decreased activation for the breast cancer versus healthy control group, revealing an overall pattern of decreased posterior activations that may necessitate compensatory increased activation in anterior brain regions for the breast cancer group. A. Encoding activation at baseline for the All (deep+shallow) contrast. Baseline, before chemotherapy, the breast cancer group showed greater activation than healthy controls in right prefrontal regions corresponding to BA 45/46 of lateral prefrontal cortex. B. Encoding activation at Time 2 for the All (deep+shallow) contrast Time 2, after chemotherapy, the breast cancer group showed greater activation than healthy controls in right prefrontal regions corresponding to BA 6 of premotor cortex and BA 45 of ventrolateral prefrontal cortex. C. Retrieval activation at Time 2 for All (deep+shallow) contrast. Time 2, after chemotherapy, the breast cancer group showed greater activation than healthy controls in bilateral middle frontal gyrus (BA 45/46). T-maps rendered on Montreal Neurologic Institute MRI Atlas using MRIcron (Rorden & Brett 2000) at voxel-wise p-values less than 0.001
Fig. 3.
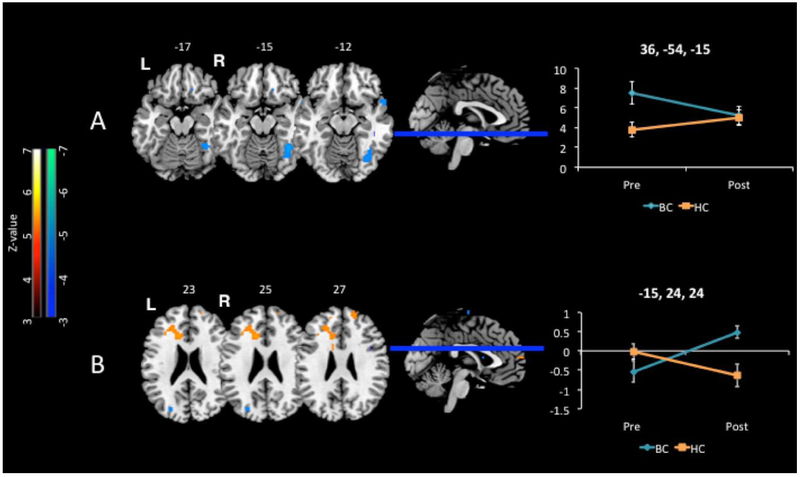
Group x time interactions for the All (deep+shallow) contrast. The number above each slice indicates the MNI z coordinate. Line graphs show BOLD % signal change on y-axis over time shown on the x-axis at the labeled x,y,z peak coordinates. Error bars represent standard error of the mean. A. During encoding the breast cancer group showed decreased activation over time in the right fusiform gyrus compared to healthy controls. As depicted in the line graph, this interaction was due to decreased activation of the fusiform gyrus within the breast cancer group over time, with no significant change in the healthy controls over time. B. During retrieval the breast cancer group showed increased activation over time in the left dorsal anterior cingulate cortex compared to healthy controls. As depicted in the line graph, this interaction was due to increased activation of the dorsal anterior cingulate at Time 2 for the breast cancer group compared to the health control group, suggesting healthy controls required less effortful control processes after repeated testing while the breast cancer group allocated greater effort over time. T-maps rendered on Montreal Neurologic Institute MRI Atlas using MRIcron (Rorden & Brett 2000) at voxel-wise p-values less than 0.001
Cross-Sectional Group Comparisons:
At baseline, the BC group exhibited greater activation in the right lateral prefrontal cortex (middle frontal gyrus; BA 45/46) for the All (deep+shallow) contrast compared to the HC group (Table 2; Figure 2A). This difference in right prefrontal activity was primarily driven by differences in the shallow encoding condition, with significantly greater activation for the BC group compared to HC group at identical local maxima (x, y, z: 39, 33, 21), as well as greater activity for the BC group in the left pars opercularis of the inferior frontal gyrus (BA 44; x, y, z: −63, 12, 27). There were no significant differences at baseline for the deep encoding condition, and there were no significant greater activations across the All, the deep and the shallow conditions when the HC group is compared to the BC group at baseline.
At Time 2, the BC group exhibited greater activation along right middle frontal and precentral gyrus (BA 45 and BA 6) in the All (deep+shallow) contrast compared to the HC group (Table 2; Figure 2B). The greater activation in area BA 6 was primarily driven by the deep encoding condition, which exhibited significant activation differences at the identical local maxima. During shallow encoding, the BC group exhibited greater activation in the posterior parahippocampal gyrus compared to the HC group. And at Time 2, the HC group did not show significantly greater activations than the BC group across the All, the deep and the shallow conditions.
Group X Time Interactions:
From Baseline to Time 2, the BC group exhibited lesser activation in the right fusiform gyrus (BA 37 and BA 19) for the All (deep+shallow; Figure 3A) and shallow encoding conditions, and in the right superior temporal pole (BA 38) for the deep encoding condition, compared to the HC group. The BC group exhibited greater activation in the left superior temporal gyrus (BA 22) for the All condition (Time 2 > Baseline), compared to the HC group.
Exploratory Within-Group Longitudinal Analysis:
Longitudinal analysis in the BC group indicates that right fusiform (BA 37) group X time interaction is driven by decreased activity from Baseline to Time 2 in the BC group, and additionally finds decreased activation from Baseline to Time 2 in the left inferior parietal lobule (BA 40) for the All (deep+shallow) condition, and decreased activity in the left inferior parietal lobule (BA 40), left precentral (BA 6) and postcentral gyrus (BA 3), and right temporal pole (BA 38) for the deep encoding condition in the BC group. The BC group exhibited relatively increased activity from Baseline to Time 2 in left middle frontal gyrus (BA 9 and BA 44) for the All(deep+shallow) and shallow encoding conditions. There were no within group activation differences over time for the HC group, except for decreased activity from Baseline to Time 2 in the left precentral gyrus (BA 4) for the shallow encoding condition.
Retrieval
The regions of significant activation for these comparisons during retrieval are listed in Table 3 in terms of their region names, peak coordinates, FWE corrected p-value, Z-values, and voxel cluster extent (k). BOLD activity t-maps of these regions are rendered on Montreal Neurologic Institute MRI Atlas using MRIcron (Rorden & Brett 2000) at voxel-wise p-values less than 0.001 for the purpose of presentation only (Figures 2C and 3B).
Table 3.
Regions demonstrating significant differences during retrieval
| Condition | Region | Hem | K | FWE-p | Max-Z | x | y | z | BA | |
|---|---|---|---|---|---|---|---|---|---|---|
| Group x Time | ||||||||||
| Baseline>Time 2 | ||||||||||
| BC>HC | All | None | ||||||||
| Shallow | None | |||||||||
| Deep | None | |||||||||
| Time 2>Baseline | ||||||||||
| BC>HC | All | dACG | L | 117 | .031 | 4.23 | −15 | 24 | 24 | 24 |
| Shallow | None | |||||||||
| Deep | None | |||||||||
| Between Groups | ||||||||||
| Baseline | ||||||||||
| BC>HC | All | None | ||||||||
| Shallow | None | |||||||||
| Deep | Fusiform | R | 67 | .05 | 4.10 | 30 | −42 | −15 | 37 | |
| Fusiform | L | 45 | .041 | 4.15 | −36 | −51 | −15 | 37 | ||
| HC>BC | All | None | ||||||||
| Shallow | None | |||||||||
| Deep | None | |||||||||
| Time 2 | ||||||||||
| BC>HC | All | MFG (dIPFC) | L | 115 | .002 | 4.86 | −27 | 33 | 27 | 46 |
| MFG | R | 41 | .027 | 4.26 | 39 | 33 | 21 | 45/46 | ||
| Shallow | Middle Frontal G. | L | 145 | .002 | 4.92 | −27 | 33 | 27 | 46 | |
| IFGtriang | L | * | .003 | 4.79 | −30 | 30 | 24 | 45 | ||
| dACG | L | * | .023 | 4.31 | −15 | 27 | 27 | 32 | ||
| Middle Frontal G. | R | 35 | .041 | 4.16 | 39 | 33 | 21 | 45/46 | ||
| Deep | None | |||||||||
| HC>BC | All | None | ||||||||
| Shallow | Calcarine | L | 84 | .043 | 4.14 | −9 | −72 | 15 | 17 | |
| Deep | None | |||||||||
| Within Groups | ||||||||||
| Baseline>Time 2 | ||||||||||
| BC | All | None | ||||||||
| Shallow | None | |||||||||
| Deep | None | |||||||||
| HC | All | None | ||||||||
| Shallow | Fusiform | L | 14 | .043 | 4.14 | −36 | −12 | −30 | 20 | |
| Time 2>Baseline | ||||||||||
| BC | All | Frontal Pole | R | 91 | .018 | 4.37 | 18 | 48 | 39 | 9 |
| Shallow | None | |||||||||
| Deep | Middle Frontal G. | L | 114 | .041 | 4.15 | −30 | 36 | 39 | 9 | |
| Inf. Temporal G. | L | 58 | .005 | 4.70 | −36 | −15 | −45 | 20 | ||
| HC | All | None | ||||||||
| Shallow | None | |||||||||
| Deeo | None | |||||||||
dACG: Dorsal Anterior Cingulate Gyrus; MFG: Middle Frontal Gyrus
Cross-Sectional Group Comparisons:
At baseline there were no differential activations noted for the All (deep+shallow) contrast or shallow recognition conditions. During the deep recognition condition there was significantly greater activation in the left and right fusiform gyrus (BA 37) for the BC group compared to the HC group.
At Time 2, there was significantly greater activation in the BC group compared to HC group in left and right middle frontal gyrus (BA 45/46) for the All (deep+shallow) contrast (Table 3; Figure 2C). This activation appeared to be driven by the shallow recognition condition, with the BC group exhibiting greater activation of bilateral middle frontal gyrus and the left pars triangularis of the inferior frontal gyrus (BA 45/46), as well as left anterior cingulate (BA 32) compared to the HC group. Additionally, for the shallow recognition condition, the HC group exhibited greater activation in the primary visual cortex compared to the BC group. There were no differences at Time 2 during the deep recognition condition between groups in either BC>HC or HC>BC comparisons.
Group X Time Interactions:
Compared to the HC group, the BC group exhibited greater activation in the left anterior cingulate cortex for the All(deep+shallow) contrast, that appeared to be driven by the greater activation for the BC compared to HC group at Time 2 seen in cross-sectional comparisons above (Table 3; Figure 3B). There were no other significant activation differences between groups over time for the shallow or deep recognition conditions.
Exploratory Within-Group Longitudinal Analysis:
The BC group exhibited increased activation from baseline to Time 2 within the right frontal pole (BA 9) for the All (deep+shallow) contrast, and the left middle frontal gyrus (BA 9) and inferior temporal gyrus (BA 20) for the deep recognition condition. There were no changes exhibited in the shallow recognition condition and no decreased activations from baseline to Time 2 within the BC group.
In contrast, the HC group exhibited decreased activation from baseline to Time 2 during the shallow recognition condition in the left fusiform gyrus (BA 20). There were no changes exhibited in the other recognition contrasts nor increased activations from baseline to Time 2 within the HC group.
Discussion
This study sought to identify whether breast cancer and/or chemotherapy treatment altered neural activity underlying episodic memory processes in a manner consistent with accelerated aging, using a design that manipulated processing demands at encoding and retrieval. The current prospective longitudinal study observed differential activity in several brain regions during episodic memory encoding and recognition for visual stimuli in breast cancer patients before and after treatment. Chief among these regions was the lateral prefrontal cortex along the middle frontal gyrus. The observation of increased encoding related activation for breast cancer patients compared to healthy controls at baseline in right BA 45/46, is consistent with previous pre-chemotherapy literature in breast cancer patients across a variety of tasks (Cimprich et al. 2010; McDonald, Conroy, Ahles, West, & Saykin 2012; Scherling, Collins, Mackenzie, Bielajew, & Smith 2011). The growing breast cancer literature showing behavioral deficits (Yao et al. 2016) and increased prefrontal activations before chemotherapy has been interpreted to suggest yet undefined mechanisms of cancer influence the need for compensatory recruitment to maintain equivalent performance to control subjects (Cimprich et al. 2010; McDonald et al. 2012), and possibly for factors such as fatigue, anxiety, or immediate effects following surgery (López Zunini et al. 2013; Scherling et al. 2011). Although the time since surgery was not measured here, structural and behavioral deficits, particularly in attention, have been documented when comparing before and after surgery in breast cancer survivors (Sato et al. 2015). Thus, we cannot rule out that increased activations seen here may be a consequence of such factors as surgical anesthesia rather than cancer, per se.
Alternatively, the overlap between the biologic mechanisms underlying cancer and aging have been proposed to contribute to both pre- and post-chemotherapy differences in cognition (Ahles et al. 2012; Mandelblatt et al. 2013). The findings presented here are consistent with functional neuroimaging studies of cognitive aging that have also documented increased activations compared to younger adults during episodic encoding and retrieval (Grady, McIntosh, Rajah, Beig, & Craik 1999; Logan, Sanders, Snyder, Morris, & Buckner 2002; Morcom, Good, Frackowiak, & Rugg 2003), thought to be a by-product of neural degradation leading to theories of dedifferentiation or compensation (e.g. Cabeza & Dennis 2012; Li et al. 2000, 2001; Park, Polk, Mikels, Taylor, & Marshuetz 2001; Reuter-Lorenz & Cappell 2008). Dedifferentiation suggests neural activity becomes inefficient, and therefore, less selective when recruiting brain regions to complete a task (Li et al. 2000, 2001. Compensation suggests neural activity can respond to such inefficiency by reorganizing to create new connections (Cabeza & Dennis 2012) that should vary with task demands (Reuter-Lorenz & Cappell 2008).
In the current study, the baseline pattern of activation changes seen across shallow encoding and deep retrieval may support either theoretical possibility. Using an event-related design that correlated encoding activation with subsequent successful retrieval found deep encoding engaged bilateral frontal regions, while shallow encoding engaged only left frontal regions in healthy young adults (Otten, Henson, & Rugg 2001). Our finding that breast cancer patients over-recruited bilateral frontal regions during shallow encoding may be indicative of a breakdown of regional selectivity for different encoding operations, such that the processing engaged by deep encoding was generalized to shallow encoding due to dedifferentiation. On the other hand, in the levels of processing framework, deep encoding results in greater attentional resources than shallow encoding (Craik 2002), and is thereby associated with greater neural activity in prefrontal regions (Kapur et al. 1994; Nyberg 2010; Otten et al. 2001). Correspondingly, deep encoding results in better memory retrieval than shallow encoding (Craik & Lockhart 1972), as was found also here, and is thereby associated with comparatively less retrieval effort and less neural activation of left dorsolateral prefrontal cortex (Buckner et al. 1998). From a compensation viewpoint, the breast cancer group showed greater activation during the conditions that should require less processing resources or effort, possibly to compensate for inefficient processing under lower task demands. Regardless of whether our findings are consequences of dedifferentiation or compensation, the idea remains that our findings do provide preliminary support for an overlap with changes consistent with an accelerated aging account.
Compensation may also explain our data following chemotherapy treatment, with similarly increased activation of right BA45/46 for breast cancer patients compared to healthy controls at encoding and recognition. Within group comparisons found increased dorsolateral prefrontal cortex (BA 9) activation over time in left hemisphere during encoding and right hemisphere during recognition for breast cancer patients. Given that regions of the prefrontal cortex are associated with cognitive control processes that facilitate encoding and recognition (Blumenfeld & Ranganath 2007; Bunge, Burrows, & Wagner 2004; Preston & Eichenbaum 2013; Tulving, Kapur, Craik, Moscovitch, & Houle 1994), these results could be consistent with the hypothesis that breast cancer patients are compensating through greater utilization of prefrontal control processes necessary to episodic memory.
Further support for compensation through the engagement of control processes comes from increased activation of the left dorsal anterior cingulate cortex over time for breast cancer patients compared to healthy controls seen during recognition. The dorsal anterior cingulate is thought to signal the need to readjust the direction of attention or control by lateral prefrontal regions to resolve conflict from interfering information (Botvinick, Cohen, & Carter 2004; Cohen, Botvinick, & Carter 2000). In particular, resolution of interference in memory is thought to be more necessary during recall, where one has to specify what is to be remembered among competing memories, than during recognition, where specific retrieval cues are provided (Levy & Anderson 2002). Here we used a recognition task, yet the breast cancer group recruited anterior cingulate processes, suggesting they may experience more interference when trying to retrieve from memory, particularly when discriminating between highly similar visual scenes (city targets from foils; country targets from foils). Also, the increased need for control processes are presumed to be necessary when the task cannot be completed through automatic processes (Shiffrin & Schneider 1984), such as perceived familiarity of an item (Jacoby 1991). Breast cancer survivors may, therefore, lose the ability to recruit or rely on these automatic retrieval processes leading to the need to allocate effortful, top down processes across retrieval tasks.
The pattern and possibility of compensatory activation among breast cancer patients in anterior regions may be in response to diminished input from posterior regions, such as that proposed by the posterior-anterior shift in aging. During encoding, there was decreased activation over time in the left inferior parietal lobule (BA 40), associated with visually guided attention, only within the breast cancer group, as well as decreased activation over time in the right fusiform gyrus, associated with visual processing of objects, for breast cancer patients compared to healthy controls. Functional neuroimaging studies of memory and aging have repeatedly found similar decreased activations of posterior regions accompanied by increased recruitment of anterior regions including prefrontal cortex (Anderson et al. 2000; Cabeza et al. 1997, 2004; Daselaar, Veltman, Rombouts, Raaijmakers, & Jonker 2003; Davis et al. 2008; Grady et al. 1994; Grady, Bernstein, Beig, & Siegenthaler 2002; Madden et al. 1999). This posterior-anterior shift in aging has been interpreted to suggest that a reliance on frontal regions allows the preservation of cognitive performance in the face of insufficient processing elsewhere in the brain (Davis et al. 2008; Grady et al. 1994). Mechanisms of cancer progression and chemotherapy treatment have been proposed to accelerate aging (Ahles & Root 2018; Ahles, Root, & Ryan 2012). Thus, our results are consistent with the idea that chemotherapy treatment may induce a posterior-anterior shift in neural processing, in line with accelerated aging, by allocating control mechanisms to compensate for insufficient processing in posterior regions such as the posterior parietal and fusiform cortex.
These reductions in activity of regions involved in various levels of visual processing is consistent with evidence of damage to occipital white matter tracts following breast cancer chemotherapy (Deprez et al. 2012). A systematic review of longitudinal neuropsychological findings in breast cancer supports a vulnerability in visual processing, such that the most reliable deficit across studies was in visuospatial function (Jim et al. 2012). Additionally, breast cancer chemotherapies result in ocular toxicities (Al-Tweigeri, Nabholtz, & Mackey 1996; Eisner & Luoh 2011; Raffa & Tallarida 2010) that are related to cognitive slowing and other neuropsychological impairments (Anstey, Butterworth, Borzycki, & Andrews 2006; Skeel, Schutte, van Voorst, & Nagra 2006; Wood et al. 2010). Such toxicity to the peripheral visual system may therefore be an overlooked contributor to cognitive impairment, particularly via decreased visual input to the brain (Raffa & Tallarida 2010). In other words, if vision is compromised due to chemotherapy, visual processing will be diminished leading to less activation of the brain regions involved with visual processing. Unfortunately, these data are preliminary evidence for parallels with cognitive aging, and we did not directly assess peripheral visual processing impairments. Whatever the mechanism, the pattern of activation differences reported here suggest a vulnerability to visual processing that necessitates compensation and further support for this comes from activation differences based on task differences described below.
The contrasts for shallow and deep encoding and recognition revealed additional activation changes unique to each task. The shallow conditions further revealed a posterior-anterior shift in neural processing, emphasizing alterations to visual processing regions that may underlie the need for compensation by anterior, executive control regions. Shallow encoding was associated with increased activation of the right parahippocampal gyrus after chemotherapy in BC patients compared to healthy controls, whereas recognition of shallowly encoded items was associated with decreased activation of the primary visual cortex after chemotherapy compared to healthy controls (coupled with increased activation of the bilateral BA45/46 and dorsal anterior cingulate cortex in the BC group at Time 2 compared to the HC group). Although parahippocampal operations are involved in scene processing during encoding (Prince, Dennis, & Cabeza 2009), the shallow encoding task required participants to attend to the color information, not the scenes depicted. Increased parahippocampal activation therein suggests patients were more likely to be distracted by processing the scenes, in line with distractibility as a common complaint for breast cancer survivors (Chen, Miaskowski, Liu, & Chen 2012; Downie, Mar Fan, Houédé-Tchen, Yi, & Tannock 2006; Kohli et al. 2007). The diminished activity in primary visual cortex during recognition further lends support to the aforementioned loss of functional activation in posterior regions in the brain, and suggests that recapitulation of color information necessary to remember shallowly processed items may have been particularly affected following chemotherapy.
Deep encoding was associated with decreased activation over time in the right temporal pole for the breast cancer but not healthy control group, whereas recognition for deeply encoded items was associated with increased activation in the left fusiform gyrus for the breast cancer compared to healthy control group only at baseline. Given the nature of the deep encoding task – namely, elaboration of meaning in the images – decreased temporal, semantic network processing in the breast cancer group may reflect a change in cognitive capacity or encoding strategy related to utilization of semantic knowledge (Leshikar, Duarte, & Hertzog 2012; Sperling et al. 2003). The change in cognitive capacity over time may also explain greater recognition related engagement of fusiform regions only seen at baseline, such that this form of compensation was still intact before chemotherapy but diminished following treatment. Taken together, the present findings suggest neural processing changes related to breast cancer and its treatment are also affected by the nature of operations engaged during episodic encoding and recognition.
Limitations
The prospective design provided the strength to characterize functional changes within the breast cancer group both before and after chemotherapy, but led to attrition over time, limiting ultimate group sample size. This limited the ability to confirm compensation to maintain intact task performance. For example, compensation suggests a positive correlation between increases in brain activity and intact behavioral performance, but the smaller N precludes such analysis as it may increase variability in our groups and obscure this pattern. Given that we did not perform this analysis or examine trials by memory type, future work in larger sample sizes should aim for such analyses to provide a more informative understanding of the nature of neural changes during episodic memory performance. Furthermore, previous work has shown some support for a specific role of cyclophosphamide, methotrexate, 5-fluorouracil (CMF) (Kesler et al. 2009). A small number of our patients were exposed to CMF here (i.e. there were differing chemotherapy protocols), but the N was too small to perform a subgroup analysis and understand how different chemotherapies contribute to changes in neural activation patterns. Lastly, the subjective experience of compensation, such as via self-reported complaints about memory accuracy or effort was not assessed here. Memory problems are a consistent subjective complaint among breast cancer survivors treated with chemotherapy (for review see Frank, Vance, Jukkala, & Meneses 2014), and self-report measures could have provided a more complete picture as to whether neural activity changes correspond with any self-reported awareness of the need for recruitment of compensatory neural resources.
Conclusion
The current study explored whether altered neural activations with intact behavioral performance among breast cancer patients may be due to accelerated aging. The current findings add to the growing literature that cancer and chemotherapy treatment alter neural recruitment in patients that contribute to cognitive complaints and behavioral deficits under some circumstances. Breast cancer patients may experience differences in both episodic memory encoding and retrieval prior to and after chemotherapy treatment, albeit under different contexts. These differences related to breast cancer and chemotherapy parallel findings in the literature of healthy aging, leading to suggestions that cancer, its treatments, and aging may be linked (Ahles et al. 2012; Mandelblatt et al. 2013). From this perspective, neural activation patterns observed here and in other studies may be understood in the context of theories of the cognitive neuroscience of aging. Our findings go beyond previous results by demonstrating that manipulating the nature of encoding operations has consequences for how breast cancer patients engage neural processes involved in episodic encoding and recognition before and after treatment. Furthermore, the evidence that episodic encoding and recognition was accompanied by increases in activity particularly in anterior regions with functional deficits seen in posterior regions of the brain for breast cancer patients is a parallel to functional neuroimaging evidence from memory and aging, supporting the hypothesis that cancer and its treatment may accelerate aging. Also given that chemotherapies impart ocular toxicities that can alter vision, under-recruitment of visual related processing regions reported here encourage future work to measure the presence of sensory processing deficits and when they contribute to downstream functional deficits in the brain. This emphasizes the need to move to studies that are based on more specific hypotheses that view the brain as a more integrated system, whereby compensation is necessitated by functional deficits elsewhere.
Acknowledgements
TAA and SDP were supported by the Starr Cancer Consortium (protocol #1-A17) and Amgen, Inc. DP would like to acknowledge the NIH/NCI Cancer Center Support Grant P30 CA008748 and the NCI award number T32 CA009461 under which authorship for this work was supported. The content is solely responsible of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
Denise Pergolizzi, James C. Root, Hong Pan, David Silbersweig, Emily Stern, Steven D. Passik and Tim A. Ahles declare that they have no conflict of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
Contributor Information
Denise Pergolizzi, Department of Psychiatry and Behavioral Sciences, Sloan Kettering Institute for Cancer Research.
James C. Root, Neurocognitive Research Laboratory; Department of Psychiatry and Behavioral Sciences, Memorial Sloan Kettering Cancer Center; Weill Cornell Medical College.
Hong Pan, Functional Neuroimaging Laboratory,; Departments of Psychiatry and Radiology, Brigham and Women’s Hospital Harvard Medical School.
David Silbersweig, Department of Psychiatry; Brigham Research Institute Neuroscience Research Center, Brigham and Women’s Hospital; Harvard Medical School.
Emily Stern, Functional and Molecular Neuroimaging; Departments of Radiology and Psychiatry, Brigham and Women’s Hospital.
Steven D. Passik, Collegium Pharmaceuticals.
Tim A. Ahles, Neurocognitive Research Laboratory; Department of Psychiatry and Behavioral Sciences, Memorial Sloan Kettering Cancer Center; Weill Cornell Medical College.
References
- Ahles TA, & Root JC (2018). Cognitive Effects of Cancer and Cancer Treatments. Annual Review of Clinical Psychology, 14(8), 425–451. 10.1146/annurev-clinpsy-050817-084903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Root JC, & Ryan EL (2012). Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. Journal of Clinical Oncology, 30(30), 3675–3686. 10.1200/JCO.2012.43.0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tweigeri T, Nabholtz JM, & Mackey JR (1996). Ocular toxicity and cancer chemotherapy. Cancer, 78(7), 1359–1373. [DOI] [PubMed] [Google Scholar]
- Anderson ND, Iidaka T, Cabeza R, Kapur S, McIntosh AR, & Craik FI (2000). The effects of divided attention on encoding- and retrieval-related brain activity: A PET study of younger and older adults. Journal of Cognitive Neuroscience, 12(5), 775–92. Retrieved from http://www.mitpressjournals.org/doi/abs/10.1162/089892900562598 [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Butterworth P, Borzycki M, & Andrews S (2006). Between- and within-individual effects of visual contrast sensitivity on perceptual matching, processing speed, and associative memory in older adults. Gerontology, 52(2), 124–130. 10.1159/000090958 [DOI] [PubMed] [Google Scholar]
- Badiola I, Santaolalla F, Garcia-Gallastegui P, Ana SR, Unda F, & Ibarretxe G (2015). Biomolecular bases of the senescence process and cancer. A new approach to oncological treatment linked to ageing. Ageing Research Reviews, 23(Pt B), 125–38. 10.1016/j.arr.2015.03.004 [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, & Ranganath C (2007). Prefrontal cortex and long-term memory encoding: An integrative review of findings from neuropsychology and neuroimaging. Neuroscientist, 13(3), 280–291. 10.1177/1073858407299290 [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, & Carter CS (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences, 8(12), 539–46. 10.1016/j.tics.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Wagner AD, & Rosen BR (1998). Functional-anatomic study of episodic retrieval using fMRI. I. Retrieval effort versus retrieval success. NeuroImage, 7(3), 151–162. 10.1006/nimg.1998.0327 [DOI] [PubMed] [Google Scholar]
- Bunge SA, Burrows B, & Wagner AD (2004). Prefrontal and hippocampal contributions to visual associative recognition: Interactions between cognitive control and episodic retrieval. Brain and Cognition, 56(2 SPEC. ISS.), 141–152. 10.1016/j.bandc.2003.08.001 [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, & McIntosh AR (2002). Aging Gracefully: Compensatory Brain Activity in High-Performing Older Adults. NeuroImage, 17(3), 1394–1402. 10.1006/nimg.2002.1280 [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, & Nyberg L (2004). Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cerebral Cortex, 14(4), 364–75. [DOI] [PubMed] [Google Scholar]
- Cabeza R, & Dennis NA (2012). Frontal lobes and aging. In Principles of Frontal Lobe Function (pp. 628–652). 10.1093/acprof:oso/9780195134971.001.0001 [DOI] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, … Craik FI (1997). Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. The Journal of Neuroscience, 17(1), 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ML, Miaskowski C, Liu LN, & Chen SC (2012). Changes in perceived attentional function in women following breast cancer surgery. Breast Cancer Research and Treatment, 131(2), 599–606. 10.1007/s10549-011-1760-3 [DOI] [PubMed] [Google Scholar]
- Chun MM, & Johnson MK (2011). Memory: Enduring traces of perceptual and reflective attention. Neuron, 72(4), 520–535. 10.1016/j.neuron.2011.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimprich B, Reuter-Lorenz P, Nelson J, Clark PM, Therrien B, Normolle D, … Welsh RC (2010). Prechemotherapy alterations in brain function in women with breast cancer. Journal of Clinical and Experimental Neuropsychology, 32(3), 324–331. 10.1080/13803390903032537 [DOI] [PubMed] [Google Scholar]
- Cohen JD, Botvinick M, & Carter CS (2000). Anterior cingulate and prefrontal cortex: who’s in control? Nature Neuroscience, 3(5), 421–423. 10.1038/74783 [DOI] [PubMed] [Google Scholar]
- Conroy SK, McDonald BC, Ahles TA, West JD, & Saykin AJ (2013). Chemotherapy-induced amenorrhea: A prospective study of brain activation changes and neurocognitive correlates. Brain Imaging and Behavior, 7(4), 491–500. 10.1007/s11682-013-9240-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy SK, McDonald BC, Smith DJ, Moser LR, West JD, Kamendulis LM, … Saykin AJ (2013). Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Research and Treatment, 137(2), 493–502. 10.1007/s10549-012-2385-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM (2002). Levels of processing: Past, present… and future? Memory, 10(5–6), 305–318. 10.1080/09658210244000135 [DOI] [PubMed] [Google Scholar]
- Craik FIM, & Lockhart RS (1972). Levels of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior, 11(6), 671–684. 10.1016/S0022-5371(72)80001-X [DOI] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SARB, Raaijmakers JGW, & Jonker C (2003). Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain : A Journal of Neurology, 126(Pt 1), 43–56. 10.1093/brain/awg005 [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, & Cabeza R (2008). Que PASA? The Posterior-Anterior Shift in Aging. Cerebral Cortex, 18(5), 1201–1209. 10.1093/cercor/bhm155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, van Dam FSAM, Nederveen AJ, … Schagen SB (2011). Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Human Brain Mapping, 32(8), 1206–1219. 10.1002/hbm.21102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, & Cabeza R (2008). Effects of aging on the neural correlates of successful item and source memory encoding. Journal of Experimental Psychology. Learning, Memory, and Cognition, 34(4), 791–808. 10.1037/0278-7393.34.4.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprez S, Amant F, Smeets A, Peeters R, Leemans A, Van Hecke W, … Sunaert S (2012). Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. Journal of Clinical Oncology, 30(3), 274–281. 10.1200/JCO.2011.36.8571 [DOI] [PubMed] [Google Scholar]
- Deprez S, Vandenbulcke M, Peeters R, Emsell L, Smeets A, Christiaens MR, … Sunaert S(2014). Longitudinal assessment of chemotherapy-induced alterations in brain activation during multitasking and its relation with cognitive complaints. Journal of Clinical Oncology, 32(19), 2031–2038. 10.1200/JCO.2013.53.6219 [DOI] [PubMed] [Google Scholar]
- Downie FP, Mar Fan HG, Houédé-Tchen N, Yi Q, & Tannock IF (2006). Cognitive function, fatigue, and menopausal symptoms in breast cancer patients receiving adjuvant chemotherapy: evaluation with patient interview after formal assessment. Psycho-Oncology, 15(10), 921–930. 10.1002/pon.1035 [DOI] [PubMed] [Google Scholar]
- Eisner A, & Luoh SW (2011). Breast cancer medications and vision: Effects of treatments for early-stage disease. Current Eye Research, 36(10), 867–885. 10.3109/02713683.2011.594202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson RJ, Mcdonald BC, Saykin AJ, & Ahles TA (2007). Brain Structure and Function Differences in Monozygotic Twins: Possible Effects of Breast Cancer Therapy. Journal of Clinical Oncology, 25(25), 3866–3870. 10.1200/JCO.2007.10.8639.Brain [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank JS, Vance DE, Jukkala A, & Meneses KM (2014). Attention and memory deficits in breast cancer survivors: Implications for nursing practice and research. Journal of Neuroscience Nursing, 46(5), 274–284. 10.1097/JNN.0000000000000078 [DOI] [PubMed] [Google Scholar]
- Grady CL, Bernstein LJ, Beig S, & Siegenthaler AL (2002). The effects of encoding task on age-related differences in the functional neuroanatomy of face memory. Psychology and Aging, 17(1), 7–23. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11931288 [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, … Haxby JV (1994). Age-related changes in cortical blood flow activation during visual processing of faces and location. The Journal of Neuroscience, 14(3 Pt 2), 1450–62. 10.1080/09541440042000304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Rajah MN, Beig S, & Craik FIM (1999). The effects of age on the neural correlates of episodic encoding. Cerebral Cortex, 9(8), 805–814. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Hebrank A, Sutton BP, Leshikar E, Chee MWL, Tan JC, … Park DC (2007). Contextual interference in recognition memory with age. NeuroImage, 35(3), 1338–1347. 10.1016/j.neuroimage.2007.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, & Park DC (2005). Aging and the neural correlates of successful picture encoding: Frontal activations compensate for decreased medial-temporal activity. Journal of Cognitive Neuroscience, 17(1), 84–96. 10.1162/0898929052880048 [DOI] [PubMed] [Google Scholar]
- Jacoby LL (1991). A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language, 30, 513–541. 10.1016/0749-596X(91)90025-F [DOI] [Google Scholar]
- Jim HSL, Phillips KM, Chait S, Faul LA, Popa MA, Lee Y-H, … Small BJ (2012). Meta-Analysis of Cognitive Functioning in Breast Cancer Survivors Previously Treated With Standard-Dose Chemotherapy. Journal of Clinical Oncology, 30(29), 3578–3587. 10.1200/JCO.2011.39.5640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam JWY, Brenner CA, Handy TC, Boyd LA, Liu-Ambrose T, Lim HJ, … Campbell KL (2015). Sustained attention abnormalities in breast cancer survivors with cognitive deficits post chemotherapy: An electrophysiological study. Clinical Neurophysiology 10.1016/j.clinph.2015.03.007 [DOI] [PubMed] [Google Scholar]
- Kapur S, Craik FI, Tulving E, Wilson AA, Houle S, & Brown GM (1994). Neuroanatomical correlates of encoding in episodic memory: levels of processing effect. Proceedings of the National Academy of Sciences of the United States of America, 91(6), 2008–11. 10.1073/pnas.91.6.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Bennett FC, Mahaffey ML, & Spiegel D (2009). Regional brain activation during verbal declarative memory in metastatic breast cancer. Clinical Cancer Research, 15(21), 6665–73. 10.1158/1078-0432.CCR-09-1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli S, Griggs JJ, Roscoe J. a., Jean-Pierre P, Bole C, Mustian KM, … Morrow GR (2007). Self-Reported Cognitive Impairment in Patients With Cancer. Journal of Oncology Practice, 3(2), 54–59. 10.1200/JOP.0722001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshikar ED, Duarte A, & Hertzog C (2012). Task-selective memory effects for successfully implemented encoding strategies. PLOS One, 7(5). 10.1371/journal.pone.0038160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshikar ED, Gutchess AH, Hebrank AC, Sutton BP, & Park DC (2010). The impact of increased relational encoding demands on frontal and hippocampal function in older adults. Cortex, 46(4), 507–521. 10.1016/j.cortex.2009.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, & Anderson MC (2002). Inhibitory process and the control of memory retrieval. Trends in Cognitive Sciences, 6(7), 299–305. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, & Frensch PA (2000). Unifying cognitive aging: From neuromodulation to representation to cognition. Neurocomputing, 32–33(November 2016), 879–890. 10.1016/S0925-2312(00)00256-3 [DOI] [Google Scholar]
- Li SC, Lindenberger U, & Sikström S (2001). Aging cognition: From neuromodulation to representation. Trends in Cognitive Sciences, 5(11), 479–486. 10.1016/S1364-6613(00)01769-1 [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, & Buckner RL (2002). Under-recruitment and nonselective recruitment: Dissociable neural mechanisms associated with aging. Neuron, 33(5), 827–840. 10.1016/S0896-6273(02)00612-8 [DOI] [PubMed] [Google Scholar]
- López Zunini RA, Scherling C, Wallis N, Collins B, MacKenzie J, Bielajew C, & Smith AM (2013). Differences in verbal memory retrieval in breast cancer chemotherapy patients compared to healthy controls: A prospective fMRI study. Brain Imaging and Behavior, 7(4), 460–477. 10.1007/s11682-012-9213-0 [DOI] [PubMed] [Google Scholar]
- Madden DJ, Gottlob LR, Denny LL, Turkington TG, Provenzale JM, Hawk TC, & Coleman RE (1999). Aging and recognition memory: Changes in regional cerebral blood flow associated with components of reaction time distributions. Journal of Cognitive Neuroscience, 11(5), 511–520. 10.1162/089892999563571 [DOI] [PubMed] [Google Scholar]
- Mandelblatt JS, Hurria A, McDonald BC, Saykin AJ, Stern RA, VanMeter JW, … Ahles T (2013). Cognitive Effects of Cancer and Its Treatments at the Intersection of Aging: What Do We Know; What Do We Need to Know? Seminars in Oncology, 40(6), 709–725. 10.1053/j.seminoncol.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandzia JL, Black SE, McAndrews MP, Grady C, & Graham S (2004). fMRI Differences in Encoding and Retrieval of Pictures Due to Encoding Strategy in the Elderly. Human Brain Mapping, 21(1), 1–14. 10.1002/hbm.10140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, & Saykin AJ (2012). Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. Journal of Clinical Oncology, 30(20), 2500–8. 10.1200/JCO.2011.38.5674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menning S, de Ruiter MB, Veltman DJ, Boogerd W, Oldenburg HSA, Reneman L, & Schagen SB (2017). Changes in brain activation in breast cancer patients depend on cognitive domain and treatment type. PLOS One, 12(3), e0171724 10.1371/journal.pone.0171724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcom AM, Good CD, Frackowiak RSJ, & Rugg MD (2003). Age effects on the neural correlates of successful memory encoding. Brain, 126(1), 213–229. 10.1093/brain/awg020 [DOI] [PubMed] [Google Scholar]
- Nyberg L (2010). Levels of processing: a view from functional brain imaging. Memory, 10(5–6), 345–8. 10.1080/09658210244000171 [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, & Rugg MD (2001). Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain, 124(Pt 2), 399–412. [DOI] [PubMed] [Google Scholar]
- Pan H, Epstein J, Silbersweig DA, & Stern E (2011). New and emerging imaging techniques for mapping brain circuitry. Brain Res Rev, 67(1–2), 226–251. doi: 10.1016/j.brainresrev.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Mikels JA, Taylor SF, & Marshuetz C (2001). Cerebral aging: integration of brain and behavioral models of cognitive function. Dialogues in Clinical Neuroscience, 3(3), 151–65. 10.1016/0025-5416(76)90216-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez DL, Pan H, Weisholtz DS, Root JC, Tuescher O, Fischer DB, … Stern E (2015). Altered threat and safety neural processing linked to persecutory delusions in schizophrenia: a two-task fMRI study. Psychiatry Research: Neuroimaging, 233(3), 352–366. 10.1016/j.pscychresns.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, & Eichenbaum H (2013). Interplay of hippocampus and prefrontal cortex in memory. Current Biology, 23(17), R764–R773. 10.1016/j.cub.2013.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince SE, Dennis NA, & Cabeza R (2009). Encoding and retrieving faces and places: Distinguishing process- and stimulus-specific differences in brain activity. Neuropsychologia, 47(11), 2282–2289. 10.1016/j.neuropsychologia.2009.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa RB, & Tallarida RJ (2010). Effects on the visual system might contribute to some of the cognitive deficits of cancer chemotherapy-induced ‘chemo-fog.’ Journal of Clinical Pharmacy and Therapeutics, 35(3), 249–255. 10.1111/j.1365-2710.2009.01086.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, & Cappell KA (2008). Neurocognitive ageing and the Compensation Hypothesis. Current Directions in Psychological Science, 17, 177–182. [Google Scholar]
- Rorden C, & Brett M (2000). Stereotaxic display of brain lesions. Behavioural Neurology, 12(4), 191–200. 10.1155/2000/421719 [DOI] [PubMed] [Google Scholar]
- Sanoff HK, Deal AM, Krishnamurthy J, Torrice C, Dillon P, Sorrentino J, … Muss HB (2014). Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. Journal of the National Cancer Institute, 106(4), 1–8. 10.1093/jnci/dju057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato C, Sekiguchi A, Kawai M, Kotozaki Y, Nouchi R, Tada H, … Ohuchi N (2015). Postoperative structural brain changes and cognitive dysfunction in patients with breast cancer. PLOS One, 10(11), 1–16. 10.1371/journal.pone.0140655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherling C, Collins B, Mackenzie J, Bielajew C, & Smith A (2011). Pre-chemotherapy differences in visuospatial working memory in breast cancer patients compared to controls: an FMRI study. Frontiers in Human Neuroscience, 5(November), 122 10.3389/fnhum.2011.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherling C, Collins B, Mackenzie J, Bielajew C, & Smith A (2012). Prechemotherapy differences in response inhibition in breast cancer patients compared to controls: a functional magnetic resonance imaging study. Journal of Clinical and Experimental Neuropsychology, 34(5), 543–60. 10.1080/13803395.2012.666227 [DOI] [PubMed] [Google Scholar]
- Shiffrin RM, & Schneider W (1984). Automatic and controlled processing revisited. Psychological Review, 91(2), 269–276. 10.1037/0033-295X.91.2.269 [DOI] [PubMed] [Google Scholar]
- Silverman DHS, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, … Ganz PA (2007). Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Research and Treatment, 103(3), 303–311. 10.1007/s10549-006-9380-z [DOI] [PubMed] [Google Scholar]
- Skeel RL, Schutte C, van Voorst W, & Nagra A (2006). The Relationship Between Visual Contrast Sensitivity and Neuropsychological Performance in a Healthy Elderly Sample. Journal of Clinical and Experimental Neuropsychology, 28(5), 696–705. 10.1080/13803390590954173 [DOI] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, … Albert MS (2003). fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry, 74(1), 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouten-Kemperman MM, de Ruiter MB, Koppelmans V, Boogerd W, Reneman L, & Schagen SB (2014). Neurotoxicity in breast cancer survivors ≥10 years post-treatment is dependent on treatment type. Brain Imaging and Behavior, (2015), 275–284. 10.1007/s11682-014-9305-0 [DOI] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Craik FI, Moscovitch M, & Houle S (1994). Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proceedings of the National Academy of Sciences of the United States of America, 91(6), 2016–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisholtz DS, Root JC, Butler T, Tüscher O, Epstein J, Pan H, … Stern E (2015). Beyond the amygdala: Linguistic threat modulates peri-sylvian semantic access cortices. Brain and Language, 151(3), 12–22. 10.1016/j.bandl.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J, Chaparro A, Anstey K, Lacherez P, Chidgey A, Eisemann J, … La P (2010). Simulated visual impairment leads to cognitive slowing in older adults. Optometry and Vision Science, 87(12), 1037–1043. 10.1097/OPX.0b013e3181fe64d7 [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, & Evans AC (2002). A general statistical analysis for fMRI data. NeuroImage, 15(1), 1–15. 10.1006/nimg.2001.0933 [DOI] [PubMed] [Google Scholar]
- Yao C, Rich JB, Tannock IF, Seruga B, Tirona K, & Bernstein LJ (2016). Pretreatment Differences in Intraindividual Variability in Reaction Time between Women Diagnosed with Breast Cancer and Healthy Controls. Journal of the International Neuropsychological Society, 22(05), 530–539. 10.1017/S1355617716000126 [DOI] [PubMed] [Google Scholar]