Abstract
Objective
To assess the association between exposure to monotherapy with 10 different antiepileptic drugs (AEDs) during the first 2 months of pregnancy and the risk of 23 major congenital malformations (MCMs).
Methods
This nationwide cohort study, based on the French health care databases, included all pregnancies ≥20 weeks and ending between January 2011 and March 2015. Women were considered to be exposed when an AED had been dispensed between 1 month before and 2 months after the beginning of pregnancy. The reference group included pregnant women with no reimbursement for AEDs. MCMs were detected up to 12 months after birth (24 months for microcephaly, hypospadias, and epispadias). Odds ratios (ORs) were adjusted for potential confounders for MCMs with at least 5 cases. Otherwise, we calculated crude ORs with exact confidence intervals (CIs).
Results
The cohort included 1,886,825 pregnancies, 2,997 of which were exposed to lamotrigine, 1,671 to pregabalin, 980 to clonazepam, 913 to valproic acid, 579 to levetiracetam, 517 to topiramate, 512 to carbamazepine, 365 to gabapentin, 139 to oxcarbazepine, and 80 to phenobarbital. Exposure to valproic acid was associated with 8 specific types of MCMs (e.g., spina bifida, OR 19.4, 95% CI 8.6–43.5), and exposure to topiramate was associated with an increased risk of cleft lip (6.8, 95% CI 1.4–20.0). We identified 3 other signals. We found no significant association for lamotrigine, levetiracetam, carbamazepine, oxcarbazepine, and gabapentin.
Conclusions
These results confirm the teratogenicity of valproic acid and topiramate. Because of the small numbers of cases and possible confounding, the other 3 signals should be interpreted with appropriate caution.
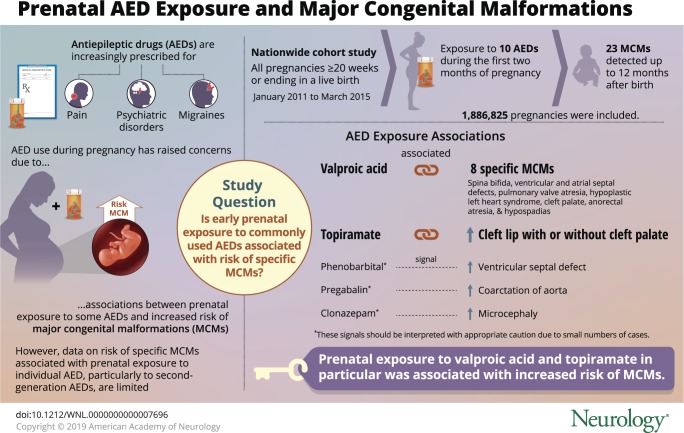 Antiepileptic drugs (AEDs), particularly newer AEDs, are increasingly prescribed for a wide range of medical conditions other than epilepsy such as pain, psychiatric disorders, and migraine.1 The prevalence of AED use during pregnancy has reached 21.9 per 1,000 pregnancies in the United States2 across all indications, while it ranges from 4.3 per 1,000 in the Netherlands to 6.0 in Wales3 and 6.7 in France.4
Antiepileptic drugs (AEDs), particularly newer AEDs, are increasingly prescribed for a wide range of medical conditions other than epilepsy such as pain, psychiatric disorders, and migraine.1 The prevalence of AED use during pregnancy has reached 21.9 per 1,000 pregnancies in the United States2 across all indications, while it ranges from 4.3 per 1,000 in the Netherlands to 6.0 in Wales3 and 6.7 in France.4
Safety studies on AED use during pregnancy are therefore of paramount importance, especially because prenatal exposure to some AEDs has already been associated with a risk of major congenital malformations (MCMs).5 The magnitude of this risk is known to be influenced by the type of AED, and the MCMs associated with teratogenic AEDs are limited to specific types of malformations.6 However, data on the risk of specific MCMs associated with prenatal exposure to individual AEDs, particularly to newer AEDs, are limited because few individual studies have sufficient power to detect excess risks of specific malformations.5–9
The French health care databases, covering 99% of the 67 million inhabitants in France,10 can therefore be useful for conducting such studies on rare drug exposures and rare neonatal outcomes. The objective of this study was to assess the association between prenatal exposure to monotherapy with the most commonly used AEDs during the first 2 months of pregnancy and the risks of 23 specific MCMs using the French health care databases.
Methods
Study design and data sources
We performed this nationwide cohort study using the French National Health Insurance claims information system (Système national d'information interrégimes de l'Assurance maladie), consisting mainly of 2 nationwide datasets linked by a unique patient identifier: the French national health insurance database (DCIR) and the French hospital discharge database (PMSI).11 The DCIR database contains all individualized and anonymous health care claims reimbursed by French National Health Insurance. In particular, these claims data include dispensed drugs coded according to the Anatomical Therapeutic Chemical classification and outpatient medical procedures coded according to the French medical classification for clinical procedures. The DCIR database also collects patient data such as age, sex, and eligibility for complementary universal health insurance (CMU-C), which provides free access to health care for low-income people.12 Eligibility for 100% health insurance coverage for serious and costly long-term diseases (LTDs), coded according to the ICD-10, is also recorded in the DCIR database. The PMSI database provides detailed medical information on all admissions to public and private hospitals in France, including discharge diagnosis ICD-10 codes, medical procedures coded according to the French medical classification for clinical procedures, and data related to pregnancy such as gestational age. In particular, almost all deliveries are recorded in the PMSI database because out-of-hospital delivery is rare in France, accounting for 0.4% of all births in 2005 to 2006.13
This linkage has previously been used to conduct large-scale pharmacoepidemiologic studies.14–16
Study population
All pregnancies ending between January 2011 and March 2015 with at least 20 weeks of gestation were eligible for inclusion, because pregnancies cannot be linked to neonatal data before 2011 and no birth stay was coded for pregnancies <20 weeks of gestation. If a woman had several pregnancies during the study period, all of them were taken into account. We identified pregnancies on the basis of their outcome from the PMSI database using discharge diagnoses and medical procedures indicative of completion of a pregnancy.4 The mother had to be enrolled in the national health insurance general scheme for salaried workers, which represents 75% of the French population, during the penultimate year before pregnancy.
We excluded twin pregnancies, which are associated with an increased risk of birth defects,17 and pregnancies that could not be linked to neonatal data. To avoid any potential differences in the duration of follow-up across exposure groups, resulting in underestimation of the number of MCMs among exposure groups with shorter follow-up, all live children were required not to be lost to follow-up during their first year of life. We also excluded all pregnancies with teratogenic infections (table e-1 available from Dryad, doi.org/10.5061/dryad.qg22n65) or exposure to teratogenic medications (table e-2 available from Dryad) during the first 2 months, as well as pregnancies with a documented chromosomal abnormality identified by hospital discharge diagnoses at birth coded as Q90 to Q99.
Exposure
Women were considered to be exposed when an AED (table e-3 available from Dryad, doi.org/10.5061/dryad.qg22n65) had been dispensed between 1 month before and 2 months after the beginning of pregnancy; AED prescriptions are dispensed with a 30-day supply. Monotherapy was defined as the absence of any other AED dispensed during the same period. AEDs rarely used in monotherapy during pregnancy (≤10 exposed pregnancies) were not included in this study.
We also calculated a cumulative dose over the first 2 months of pregnancy: the dose of AED dispensed was equally distributed over the 30 days after dispensing, and these daily doses were then added over the first 2 months of pregnancy. The reference group included pregnant women with no reimbursement for AEDs.
Outcomes
Because some MCMs remain unidentified at birth,18 we detected MCMs up to 12 months (24 months for microcephaly, hypospadias, and epispadias) after birth using hospital discharge diagnoses or specific medical procedures (table 1). These outcomes were selected from the list of MCMs of the European Surveillance of Congenital Anomalies (EUROCAT) network and had to be accurately identifiable in the PMSI database. We finally studied 23 specific MCMs, including some MCMs known to be associated with prenatal exposure to certain AEDs.
Table 1.
List of the 23 specific MCMs and related ICD-10 diagnosis codes and/or medical procedures
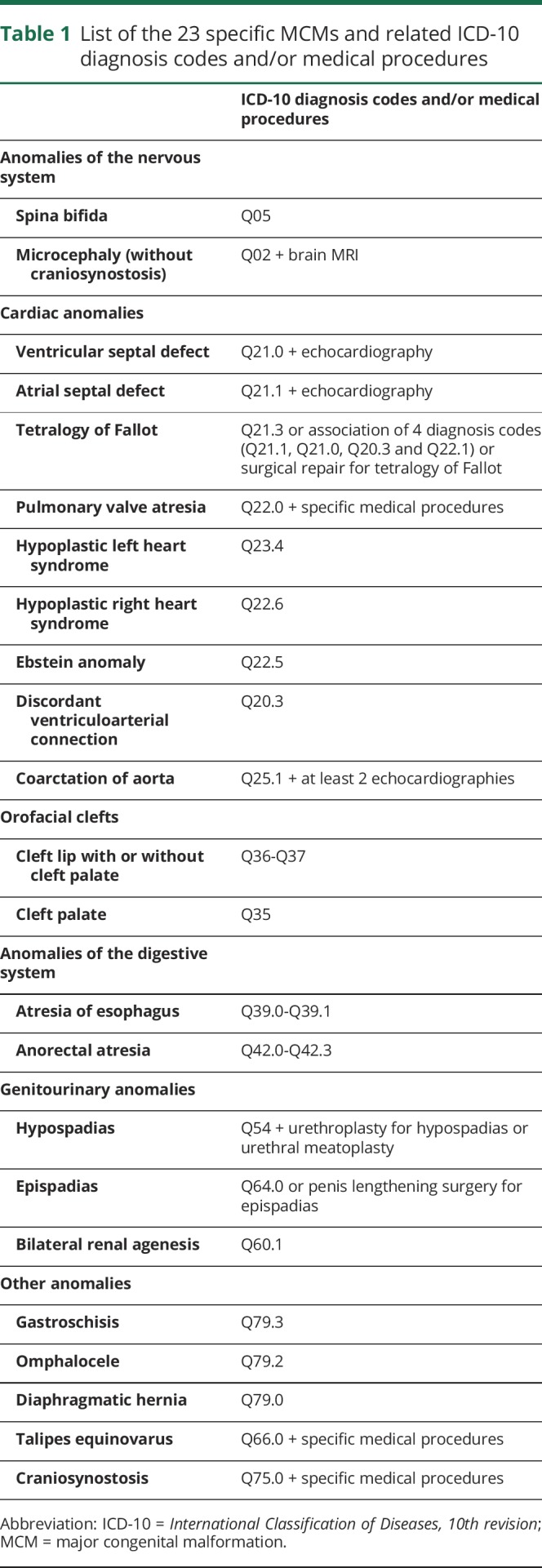
We constructed these algorithms in collaboration with a working group composed of experts in malformation coding and compared the frequency of each type of malformation observed in the study population to data from French local registries and the EUROCAT registry. Because anencephaly frequently results in early termination of pregnancy (before 20 weeks of gestation), the number of cases identified in the databases was much lower than the numbers derived from registries. This study therefore does not report the results for anencephaly. Because of imprecise or excess coding, we also do not report the results for dysmorphic facial features and congenital limb malformations other than talipes equinovarus.
Except for the most severe MCMs (spina bifida and bilateral renal agenesis), for which therapeutic abortions and stillbirths were also taken into account, we limited the study population to live births because of incomplete coding of malformations for therapeutic abortions.
Covariates
We identified potential confounding factors. Sociodemographic covariates included maternal age at birth (<25, 25–29, 30–35, and ≥35 years), mother's eligibility for CMU-C, and year of start of pregnancy. We defined preconception folic acid supplementation as at least 1 dispensing between 1 month before pregnancy and 3 months after the start of pregnancy. We identified pregestational diabetes by dispensing of antidiabetic drugs on at least 3 different dates (2 different dates when 3-month packs were dispensed) in the year before pregnancy,19 excluding dispensing between the sixth month and the end of a potential previous pregnancy to avoid considering gestational diabetes as pregestational diabetes.
We also searched the databases for LTD and hospital discharge diagnoses of epilepsy and mood (affective) disorders during the 3 years before pregnancy. All Anatomical Therapeutic Chemical codes, hospital discharge diagnoses, and LTD diagnoses are given in table e-4 (available from Dryad, doi.org/10.5061/dryad.qg22n65).
Statistical analysis
For each AED, we compared baseline covariates between exposed and unexposed pregnancies using the χ2 test. For each specific MCM, we calculated numbers of events and crude event rates in each AED exposure group and in the reference group. For MCMs with <5 cases per treatment group, we calculated crude odds ratios (ORs) with exact confidence intervals (CIs). For MCMs with at least 5 cases per treatment group, logistic regression models were conducted, and ORs were adjusted (aOR) for maternal age, mother's eligibility for CMU-C, year of start of pregnancy, preconception folic acid supplementation, and pregestational diabetes. Logistic regression models accounted for correlations within women with multiple pregnancies by using generalized estimating equations.
We conducted a sensitivity analysis to account for possible misclassification of exposure at the beginning of pregnancy: a woman was considered to be exposed when an AED had been dispensed at least once during the first 2 months of pregnancy.
Data extraction and statistical analysis were performed with SAS Enterprise Guide 4.3 software (SAS Institute, Inc, Cary, NC).
Standard protocol approvals, registrations, and patient consents
This observational cohort study based on the French health care databases was approved by the French Data Protection Agency (Commission nationale de l'informatique et des libertés) and did not require patient consent or ethics committee approval.
Data availability
Permanent access to the French health care databases is automatically granted to certain government agencies, public institutions, and public service authorities. Temporary access for studies and research is possible on request from the national health data institute (Institut National des Données de Santé).
Results
Of a total of 2,698,787 pregnancies potentially eligible for inclusion, we excluded 441,609 (16.4%) pregnancies that could not be linked to neonatal data, 48,289 (1.8%) twin pregnancies, 311,530 (11.5%) pregnancies for which the child was lost to follow-up, and 10,534 (0.4%) pregnancies with teratogenic infections, exposure to teratogenic medications, or a documented chromosomal abnormality (figure e-1 available from Dryad, doi.org/10.5061/dryad.qg22n65). The cohort included 1,886,825 pregnancies, representing 69.9% of all eligible pregnancies. Among these pregnancies, 1,870,234 (99.1%) ended in a live birth, 9,848 (0.5%) in a stillbirth, and 6,743 (0.4%) in a therapeutic abortion; 8,794 were exposed to AED monotherapy, 2,997 (34.1%) of which were exposed to lamotrigine, 1,671 (19.0%) to pregabalin, 980 (11.1%) to clonazepam, 913 (10.4%) to valproic acid, 579 (6.6%) to levetiracetam, 517 (5.9%) to topiramate, 512 (5.8%) to carbamazepine, 365 (4.2%) to gabapentin, 139 (1.6%) to oxcarbazepine, 80 (0.9%) to phenobarbital (table 2), and 41 (0.5%) to another AED monotherapy.
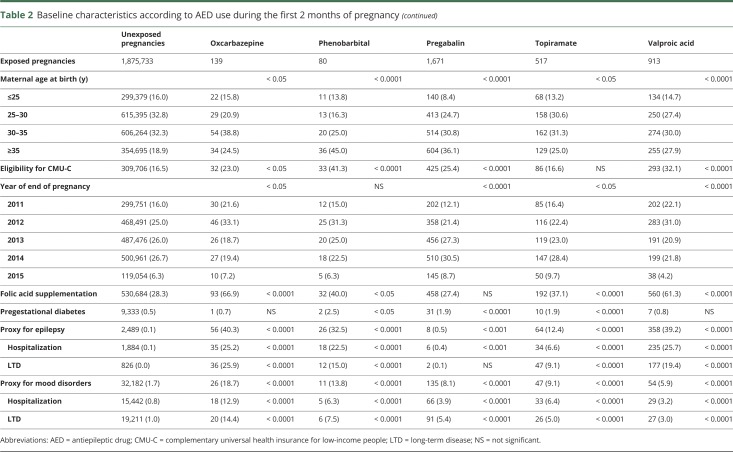
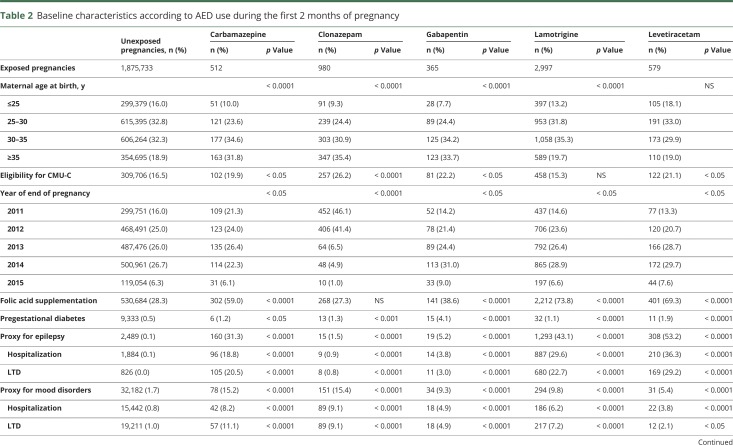
Table 2.
Baseline characteristics according to AED use during the first 2 months of pregnancy
Women exposed to AEDs, except for levetiracetam, were older than unexposed women. They more frequently had low incomes, except for women exposed to lamotrigine and topiramate, and were more commonly reimbursed for folic acid, except for women exposed to clonazepam and pregabalin. Pregestational diabetes was more frequent among women exposed to AEDs, except for women exposed to oxcarbazepine and valproic acid. Few women were hospitalized or had an LTD for epilepsy among those exposed to pregabalin, clonazepam, and gabapentin (table 2).
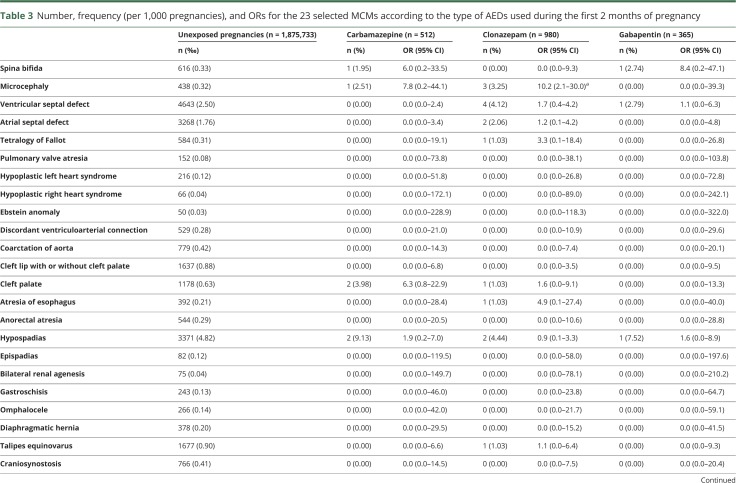
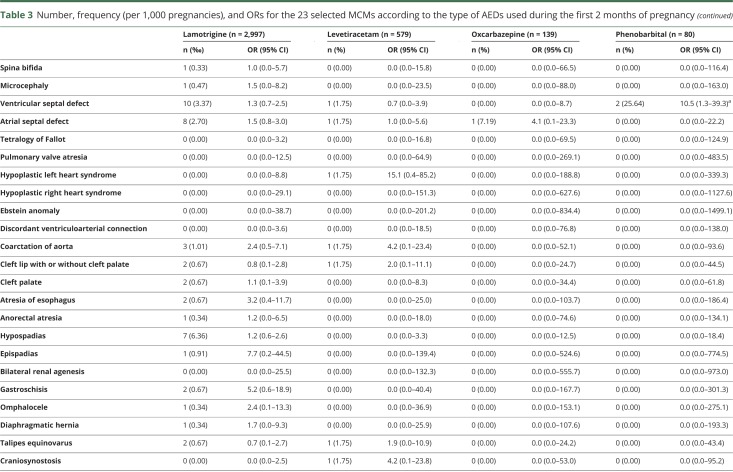
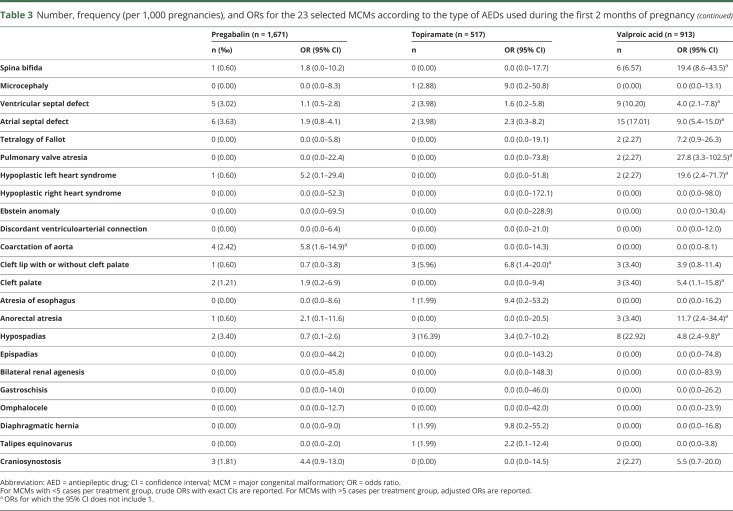
Exposure to valproic acid was associated with increased risks of spina bifida (aOR 19.4, 95% CI 8.6–43.5), ventricular (aOR 4.0, 95% CI 2.1–7.8) and atrial (aOR 9.0, 95% CI 5.4–15.0) septal defects, pulmonary valve atresia (OR 27.8, 95% CI 3.3–102.5), hypoplastic left heart syndrome (OR 19.6, 95% CI 2.4–71.7), cleft palate (OR 5.4, 95% CI 1.1–15.8), anorectal atresia (OR 11.7, 95% CI 2.4–34.4), and hypospadias (aOR 4.8, 95% CI 2.4–9.8). We found 4 other significant associations: exposure to clonazepam and the risk of microcephaly (OR 10.2, 95% CI 2.1–30.0), exposure to phenobarbital and the risk of ventricular septal defect (OR 10.5, 95% CI 1.3–39.3), exposure to pregabalin and the risk of coarctation of aorta (OR 5.8, 95% CI 1.6–14.9), and exposure to topiramate and the risk of cleft lip with or without cleft palate (OR 6.8, 95% CI 1.4–20.0). These 4 associations were based on small numbers of cases (3, 2, 4, and 3 cases, respectively). No significant association was found for the other AEDs (table 3).
Table 3.
Number, frequency (per 1,000 pregnancies), and ORs for the 23 selected MCMs according to the type of AEDs used during the first 2 months of pregnancy
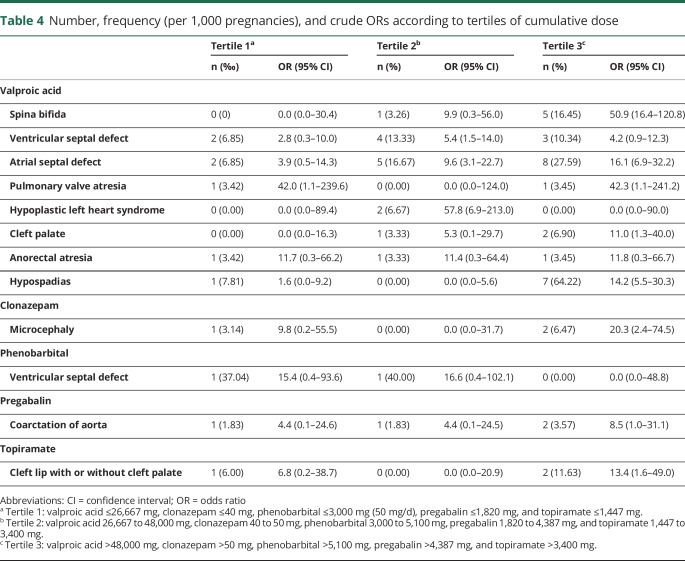
A dose-response relationship was observed for the MCMs most frequently associated with prenatal exposure to valproic acid. The highest ORs were observed in the last tertile of cumulative dose for spina bifida, atrial septal defect, cleft palate, and hypospadias. For the associations between clonazepam and microcephaly, pregabalin and coarctation of aorta, and topiramate and cleft lip with or without cleft palate, the highest ORs were also observed in the last tertile (table 4).
Table 4.
Number, frequency (per 1,000 pregnancies), and crude ORs according to tertiles of cumulative dose
In the sensitivity analysis with the dispensing window reduced to the first 2 months of pregnancy, exposure to valproic acid remained associated with the same MCMs as in the main analysis, except for pulmonary valve atresia. Among the other 4 signals, exposure to clonazepam remained significantly associated with an increased risk of microcephaly. Exposure to pregabalin became associated with an increased risk of craniosynostosis, and exposure to topiramate became associated with an increased risk of hypospadias (table e-5 available from Dryad, doi.org/10.5061/dryad.qg22n65).
Discussion
This study confirmed that exposure to valproic acid during the first 2 months of pregnancy was strongly associated with a wide range of malformations among those investigated, with a dose-response relationship for half of them. This well-known teratogenicity,7 together with the increased risk of developmental disorders for children exposed in utero,20,21 led the European Medicines Agency to announce tighter prescription criteria for valproate-containing medicines in May 2018, including a ban during pregnancy for migraine or bipolar disorder and for epilepsy except when no other effective treatment is available,22 whereas the US Food and Drug Administration issued a contraindication for the prevention of migraine in May 2013.23
The present study also found that prenatal exposure to topiramate was associated with an increased risk of cleft lip with or without cleft palate and, in the sensitivity analysis, with an increased risk of hypospadias. These results are consistent with previous findings. The first cases of oral clefts and hypospadias in infants prenatally exposed to topiramate were reported between 2006 and 2011 in studies based on small sample sizes.24–27 Concerning hypospadias, subsequent studies based on larger sample sizes reported consistent results.6,28,29 The association between topiramate and oral clefts was also confirmed by 1 meta-analysis30 and other studies based on larger sample sizes comparing infants of women exposed to topiramate monotherapy to infants of women unexposed to AEDs or exposed to lamotrigine.31–33 Compared to unexposed pregnancies, the relative risk (RR) for oral clefts associated with topiramate exposure found by Hernandez-Diaz et al.33 was 2.69 (95% CI 1.28–5.64) and 8.30 (95% CI 2.65–26.07) when the study population was restricted to women with epilepsy and when topiramate exposure was considered with or without other anticonvulsants. The same authors reported a dose-response relationship: the median daily dose was 200 mg for women with epilepsy and 100 mg for women without epilepsy, and the RR was 1.64 (95% CI 0.53–5.07) for daily doses ≤100 mg and 5.16 (95% CI 1.94–13.73) for daily doses >100 mg. The primary RR of this study was also pooled with 6 previous studies, resulting in an RR of 5.27 (95% CI 2.88–9.65).
We observed no increased risk for any of the specific MCMs investigated in the lamotrigine- and levetiracetam-exposed groups, which is consistent with the results of other studies.34
Some of the other signals have already been reported. Concerning pregabalin, a first study published in 2014 did not find any increased risk of MCMs among 30 prenatally exposed children,1 and a more recent study found 1 case of ventricular septal defect among 14 exposed children.35 Larger studies have also been conducted. In 1 study, the results of which may be prone to confounding, exposure to pregabalin was associated with an increased risk of birth defects, including cardiac malformations,36 but this association was not confirmed by a larger, better-designed study.37 Exposure to phenobarbital has also been shown to be associated with an increased risk of birth defects, with a higher frequency of cardiac malformations compared to other malformations.38 However, the associations between clonazepam and microcephaly and between valproic acid and both pulmonary valve and anorectal atresia have never been previously reported.
This first nationwide cohort study based on the French health care databases designed to assess drug safety during pregnancy has a number of strengths. Accurate identification of pregnancy periods should have limited misclassification of medication exposure during pregnancy.4 The increased risks of malformations observed in this study cannot be attributed to other known teratogenic medications or infections because only AED monotherapy was considered and women with concomitant teratogenic medications or infections were excluded. Almost 1.9 million pregnancies were included in the cohort, allowing a wide range of specific MCMs and AED classes to be studied. A large number of these associations have not been previously studied.34 This study therefore contributes to a better knowledge of AED use during pregnancy, which should guide prescribing decisions by allowing prescribers to compare the teratogenicity of an individual woman's treatment with alternative treatments.
However, this study has several limitations. First, exposure assessment was based on pharmacy claims, which do not indicate how the patient actually takes the medication. Although the time period during which a woman is pregnant was accurately identified, we cannot rule out exposure misclassification, especially for AED classes that are often discontinued before conception,39 which could explain why certain associations were no longer significant in the sensitivity analysis in which the dispensing window was reduced to the first 2 months of pregnancy. In particular, pregabalin was often discontinued before conception; the number of women exposed decreased from 1,671 (table 2) to 918 (table e-5 available from Dryad, doi.org/10.5061/dryad.qg22n65) when the dispensing window was reduced to the first 2 months of pregnancy.
Second, despite the use of a large cohort of almost 1.9 million pregnancies, only a small number of women were exposed to certain AEDs such as gabapentin or oxcarbazepine, and no clear safety conclusions can be drawn on the basis of the absence of signal from these exposure classes. Moreover, most of the signals identified were based on a small number of cases and should therefore be interpreted with appropriate caution.18 False-positive signals due to multiple testing also cannot be ruled out.
Third, even for MCMs with at least 5 cases per treatment group, residual confounding cannot be excluded, although the role of confounding in the assessment of AED teratogenicity has been reported to be limited.6 Over-the-counter folic acid supplements were not captured in the databases. However, given the high frequencies of folic acid supplementation observed in the cohort, particularly among women exposed to lamotrigine, levetiracetam, or oxcarbazepine, over-the-counter folic acid supplements should represent a small proportion of all folic acid dispensed. In addition, we could not adjust ORs for parental history of MCMs because this information is not available in the French health care databases. Although alcohol intake is also not available, we developed a modified version of an algorithm from a previously published study40: we constructed a proxy for alcohol intake in the year before pregnancy or during pregnancy on the basis of drug dispensing, hospital discharge diagnoses, LTD diagnoses, and the child's hospital discharge diagnoses at birth (table e-4 available from Dryad, doi.org/10.5061/dryad.qg22n65). This algorithm identified only 0.3% of women with alcohol-related diagnoses or drug reimbursements in the study cohort. In addition, because alcohol-related diagnoses are often coded at the same time as malformations, differential misclassification of alcohol intake cannot be ruled out, which is why, in this context, we did not use this proxy to adjust ORs for alcohol intake. However, we calculated e-values to assess the robustness of our results to potential unmeasured or uncontrolled confounding.41 Given the strength of the associations between the risk of MCMs and parental history of MCMs6 and alcohol intake42,43 found in the literature, it is unlikely that these factors could individually fully explain most of the observed ORs according to the e-values (table e-6 available from Dryad, doi.org/10.5061/dryad.qg22n65).
In addition, some AEDs are indicated for the treatment of not only epilepsy but also psychiatric disorders, pain, migraine, etc. In particular, the results of this study suggest that pregabalin, clonazepam, and gabapentin were marginally prescribed for the treatment of epilepsy. However, the French health care databases do not contain medical indications other than LTD and hospital discharge diagnosis ICD-10 codes, and these indications cannot be exhaustively assessed, which is why we did not limit the study population to women with epilepsy. However, it is now widely accepted that malformations are associated with the use of AEDs during pregnancy rather than with the underlying maternal illness being treated.44
Fourth, because the study outcomes were limited to a list of 23 MCMs, no conclusion can be drawn concerning all of the other MCMs. We also cannot rule out outcome misclassification because diagnoses and medical procedure codes used to identify the 23 selected MCMs in the PMSI database have not been externally validated. However, the PMSI database is used for planning and funding purposes and is subject to coding quality control. We also carefully identified the 23 selected MCMs in collaboration with an expert working group and, when necessary, required diagnoses to be followed by a medical procedure. The fact that MCMs known to be associated with valproic acid were also found to be associated with valproic acid in this study should increase confidence in the definition of these outcomes.
Differences between exposure groups, in terms of pregnancy management and especially in terms of screening, could also constitute another limitation regarding outcomes. More frequent screening for malformations among exposed mothers and babies could have resulted in overestimation of the association between AED exposure and the risk of malformations. However, when screening is performed before birth and results in therapeutic abortion, these associations would be biased in the opposite direction because data on malformations are not available for therapeutic abortions <22 weeks after the last menstrual period and are incomplete for therapeutic abortions ≥22 weeks after the last menstrual period18. If prenatal screening were performed more frequently among women exposed to AEDs during pregnancy, it would have resulted in underestimation of the association between AED exposure and the risk of severe malformations with a high propensity for termination of pregnancy.
Despite the limitations inherent to health care claims databases, this study highlights the importance of these data in assessing drug safety during pregnancy. It confirms the teratogenicity of valproic acid and the probable association between prenatal exposure to topiramate and the risk of cleft lip with or without cleft palate and hypospadias. These results also suggest that prenatal exposures to clonazepam, phenobarbital, and pregabalin were associated with an increased risk of 1 specific MCM, but as a result of possible exposure misclassification or confounding and small numbers of cases, these associations need to be interpreted with caution and confirmed by in-depth studies with a sample size allowing more definitive conclusions.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
Acknowledgment
The authors thank François Alla, Géric Maura, and Bénédicte Coulm for assistance in revising the manuscript for intellectual content and Anthony Saul, medical translator, for assistance in writing the manuscript.
Glossary
- AED
antiepileptic drug
- aOR
adjusted odds ratio
- CI
confidence interval
- CMU-C
complementary universal health insurance
- DCIR
French national health insurance database
- EUROCAT
European Surveillance of Congenital Anomalies
- ICD-10
International Classification of Diseases, 10th revision
- LTD
long-term disease
- MCM
major congenital malformation
- OR
odds ratio
- PMSI
French hospital discharge database
- RR
relative risk
Appendix. Authors
Study funding
The authors are employees of the French National Health Insurance (CNAM), the French National Agency for Medicines and Health Products Safety (ANSM), the French Public Health Agency or work at CHU Est Parisien, CHU Rennes, or CHU Clermont-Ferrand and received no funding.
References
- 1.Veiby G, Daltveit AK, Engelsen BA, Gilhus NE. Fetal growth restriction and birth defects with newer and older antiepileptic drugs during pregnancy. J Neurol 2014;261:579–588. [DOI] [PubMed] [Google Scholar]
- 2.Bobo WV, Davis RL, Toh S, et al. Trends in the use of antiepileptic drugs among pregnant women in the US, 2001–2007: a medication exposure in pregnancy risk evaluation program study. Paediatr Perinat Epidemiol 2012;26:578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charlton R, Garne E, Wang H, et al. Antiepileptic drug prescribing before, during and after pregnancy: a study in seven European regions. Pharmacoepidemiol Drug Saf 2015;24:1144–1154. [DOI] [PubMed] [Google Scholar]
- 4.Blotière P-O, Weill A, Dalichampt M, et al. Development of an algorithm to identify pregnancy episodes and related outcomes in health care claims databases: an application to antiepileptic drug use in 4.9 million pregnant women in France. Pharmacoepidemiol Drug Saf Epub 2018 May 15 [DOI] [PMC free article] [PubMed]
- 5.Tomson T, Battino D, Bonizzoni E, et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol Epub 2018 Apr 18. [DOI] [PubMed]
- 6.Hernández-Díaz S, Smith CR, Shen A, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology 2012;78:1692–1699. [DOI] [PubMed] [Google Scholar]
- 7.Jentink J, Loane MA, Dolk H, Barisic E, Morris JK, de Jong-van den Berg LT. Valproic acid monotherapy in pregnancy and major congenital malformations. N Engl J Med 2010;362:2185–2193. [DOI] [PubMed] [Google Scholar]
- 8.Campbell E, Kennedy F, Russell A, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and Ireland Epilepsy and Pregnancy Registers. J Neurol Neurosurg Psychiatry 2014;85:1029–1034. [DOI] [PubMed] [Google Scholar]
- 9.Cunnington MC, Weil JG, Messenheimer JA, Ferber S, Yerby M, Tennis P. Final results from 18 years of the International Lamotrigine Pregnancy Registry. Neurology 2011;76:1817–1823. [DOI] [PubMed] [Google Scholar]
- 10.Bezin J, Duong M, Lassalle R, Droz A, Blin P, Moore N. The national healthcare system claims databases in France, SNIIRAM and EGB: powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf 2017;26:954–962. [DOI] [PubMed] [Google Scholar]
- 11.Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: from the Système national d'information interrégimes de l'Assurance maladie (SNIIRAM) to the Système national des données de Santé (SNDS) in France. Rev Epidemiol Sante Publique 2017;65(suppl 4):S149–S167. [DOI] [PubMed] [Google Scholar]
- 12.Presentation de la CMU-C [online]. Available at: cmu.fr/cmu-complementaire.php. Accessed July 12, 2018.
- 13.Blondel B, Drewniak N, Pilkington H, Zeitlin J. Out-of-hospital births and the supply of maternity units in France. Health Place 2011;17:1170–1173. [DOI] [PubMed] [Google Scholar]
- 14.Basson M, Mezzarobba M, Weill A, Ricordeau H, Alla F, Carbonnel F. Severe intestinal malabsorption associated with olmesartan: a French nationwide observational cohort study. Gut 2016;65:1664–1669. [DOI] [PubMed] [Google Scholar]
- 15.Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ 2016;353:i2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudant J, Dupont A, Mikaeloff Y, Bolgert F, Coste J, Weill A. Surgery and risk of Guillain-Barré Syndrome: a French nationwide epidemiologic study. Neurology Epub August 24, 2018. [DOI] [PubMed]
- 17.Dawson AL, Tinker SC, Jamieson DJ, et al. Twinning and major birth defects, National Birth Defects Prevention Study, 1997–2007. J Epidemiol Community Health 2016;70:1114–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews EB, Tennis P. Promise and pitfalls of administrative data in evaluating pregnancy outcomes. Pharmacoepidemiol Drug Saf 2007;16:1181–1183. [DOI] [PubMed] [Google Scholar]
- 19.Billionnet C, Mitanchez D, Weill A, Nizard F, Hartemann A, Jacqueminet S. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia 2017;60:636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christensen J, Grønborg TK, Sørensen MJ, Schendel ET, Pedersen LH, Vestergaard M. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 2013;309:1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meador KJ, Baker GA, Browning N, et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol 2013;12:244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Medicines Agency. Human medicines—valproate and related substances [online]. Available at: ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Valproate_and_related_substances/human_referral_prac_000066.jsp&mid=WC0b01ac05805c516f. Accessed July 23, 2018.
- 23.US Food and Drug Administration. FDA Drug Safety Communication: valproate anti-seizure products contraindicated for migraine prevention in pregnant women due to decreased IQ scores in exposed children [online]. Available at: fda.gov/drugs/drugsafety/ucm350684.htm. Accessed August 6, 2018.
- 24.Morrow J, Russell A, Guthrie E, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry 2006;77:193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunt S, Russell A, Smithson WH, et al. Topiramate in pregnancy: preliminary experience from the UK Epilepsy and Pregnancy Register. Neurology 2008;71:272–276. [DOI] [PubMed] [Google Scholar]
- 26.Ornoy A, Zvi N, Arnon J, Wajnberg R, Shechtman S, Diav-Citrin O. The outcome of pregnancy following topiramate treatment: a study on 52 pregnancies. Reprod Toxicol Elmsford N 2008;25:388–389. [DOI] [PubMed] [Google Scholar]
- 27.Mølgaard-Nielsen D, Hviid A. Newer-generation antiepileptic drugs and the risk of major birth defects. JAMA 2011;305:1996–2002. [DOI] [PubMed] [Google Scholar]
- 28.Vajda FJE, O'Brien TJ, Graham J, Lander CM, Eadie MJ. Associations between particular types of fetal malformation and antiepileptic drug exposure in utero. Acta Neurol Scand 2013;128:228–234. [DOI] [PubMed] [Google Scholar]
- 29.Tennis P, Chan KA, Curkendall SM, et al. Topiramate use during pregnancy and major congenital malformations in multiple populations. Birth Defects Res A Clin Mol Teratol 2015;103:269–275. [DOI] [PubMed] [Google Scholar]
- 30.Veroniki AA, Cogo E, Rios P, et al. Comparative safety of anti-epileptic drugs during pregnancy: a systematic review and network meta-analysis of congenital malformations and prenatal outcomes. BMC Med 2017;15:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margulis AV, Mitchell AA, Gilboa SM, et al. Use of topiramate in pregnancy and risk of oral clefts. Am J Obstet Gynecol 2012;207:405.e1–405.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mines D, Tennis P, Curkendall SM, et al. Topiramate use in pregnancy and the birth prevalence of oral clefts. Pharmacoepidemiol Drug Saf 2014;23:1017–1025. [DOI] [PubMed] [Google Scholar]
- 33.Hernandez-Diaz S, Huybrechts KF, Desai RJ, et al. Topiramate use early in pregnancy and the risk of oral clefts: a pregnancy cohort study. Neurology 2018;90:e342–e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weston J, Bromley R, Jackson CF, et al. Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child. Cochrane Database Syst Rev 2016;11:CD010224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mostacci B, Bisulli F, Poluzzi E, et al. Emilia-Romagna Study on Pregnancy and Exposure to Antiepileptic drugs (ESPEA): a population-based study on prescription patterns, pregnancy outcomes and fetal health. J Neurol Neurosurg Psychiatry 2018;89:983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winterfeld U, Merlob P, Baud D, et al. Pregnancy outcome following maternal exposure to pregabalin may call for concern. Neurology 2016;86:2251–2257. [DOI] [PubMed] [Google Scholar]
- 37.Patorno E, Bateman BT, Huybrechts KF, et al. Pregabalin use early in pregnancy and the risk of major congenital malformations. Neurology Epub 2017 Apr 26. [DOI] [PMC free article] [PubMed]
- 38.Hill DS, Wlodarczyk BJ, Palacios AM, Finnell RH. Teratogenic effects of antiepileptic drugs. Expert Rev Neurother 2010;10:943–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grzeskowiak LE, Gilbert AL, Morrison JL. Exposed or not exposed? Exploring exposure classification in studies using administrative data to investigate outcomes following medication use during pregnancy. Eur J Clin Pharmacol 2012;68:459–467. [DOI] [PubMed] [Google Scholar]
- 40.Maura G, Billionnet C, Alla F, Gagne JJ, Pariente A. Comparison of treatment persistence with dabigatran or rivaroxaban versus vitamin K antagonist oral anticoagulants in atrial fibrillation patients: a competing risk analysis in the French National Health Care Databases. Pharmacotherapy 2018;38:6–18. [DOI] [PubMed] [Google Scholar]
- 41.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017;167:268–274. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, Qiu H, Qu P, Zhang R, Zeng L, Yan H. Prenatal alcohol exposure and congenital heart defects: a meta-analysis. PLoS One 2015;10:e0130681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grewal J, Carmichael SL, Ma C, Lammer EJ, Shaw GM. Maternal periconceptional smoking and alcohol consumption and risk for select congenital anomalies. Birth Defects Res A Clin Mol Teratol 2008;82:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holmes LB, Harvey EA, Coull BA, Huntington S, Hayes AM, Ryan LM. The teratogenicity of anticonvulsant drugs. N Engl J Med 2001;344:1132–1138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Permanent access to the French health care databases is automatically granted to certain government agencies, public institutions, and public service authorities. Temporary access for studies and research is possible on request from the national health data institute (Institut National des Données de Santé).