Abstract
Introduction
Alcaligenes faecalis is a species of gram-negative, rod-shaped, aerobic bacteria commonly found in the environment. A. faecalis-associated nosocomial infections are common in hospitalized patients, but serious life threatening infections are rare. Here, we report a rare case of BSI with A. faecalis resistant to all available antibiotics; successfully treated with double-dose of tigecycline.
Presentation of case
A 60-year-old female presented with A. faecalis bloodstream infection, where the organism was completely resistant to all commercially available antibiotics including polymyxins and tigecycline. The physical condition of the patient was deteriorating and there were no active antibiotics available to prescribe based on sensitivities. Despite the organism’s resistance to tigecycline, double-dose of tigecycline therapy (100 mg twice daily, intravenously after a 200 mg single intravenous loading dose) was prescribed intentionally for the treatment of this infection. The organism was completely eradicated from the bloodstream of that patient within the 5 days of therapy-initiation.
Discussion
Double-dose of tigecycline maintains a higher serum drug concentration rather than the standard dose, and in this case, double-dose of tigecycline completely cleared the pandrug-resistant A. faecalis from the blood where initially, that organism was resistant to tigecycline. Previously, A. faecalis isolates were found resistant to fluoroquinolones, but here it was found very rarely resistant to even reserve antibiotics, polymyxins, carbapenems and tigecycline.
Conclusion
Pandrug-resistant A. faecalis-associated bloodstream infection is a very uncommon case and double-dose of tigecycline may be an effective option to treat it.
Keywords: Alcaligenes faecalis, Pandrug-resistance, Bloodstream infection, Double-dose, Tigecycline
Introduction
Alcaligenes faecalis is a gram-negative rod with flagella belonging to the family of Alcaligenaceae. It is a nonfermentative aerobic, nonencapsulated, oxidase-positive bacterium and named for its ability to produce an alkaline reaction in certain medium [1]. A. faecalis-induced nosocomial infections are common especially in immunocompromised critically ill patients, contamination of this pathogen is occurred through hospital equipments, direct contact and fluids [2,3]. Very rarely, incidences of A. faecalis-associated endocarditis, meningitis, peritonitis, chronic otitis, abscesses, pyelonephritis, bloodstream infections (BSI), skin and soft tissue infections and endophthalmitis were found in both hospital and community settings [[1], [2], [3]]. Pandrug-resistance (PDR) is defined in an organism resistant to all commercially available antimicrobials [4] and is rarely described in A. faecalis species to date. Here, we found a case of a pandrug-resistant A. faecalis-induced BSI which was treated with double-dose tigecycline, successfully.
Case report
A 60-year-old female with a history of diabetes mellitus and hypertension was admitted to our intensive care unit (ICU) through the emergency room (ER) with 6-day history of high grade fever (104°–105 °F), lower abdominal pain, and altered state of consciousness. During the ICU admission, her recorded urine output was <20 mL/h; body temperature was 104 °F; serum creatinine was 2.1 mg/dL with a baseline creatinine record of 0.9 mg/dL (range: 0.8–1.4 mg/dL); ammonia in serum was 38 μmol/L; serum potassium level was 2.9 mmol/L; C-reactive protein (CRP) level was 47 mg/L (range: <10.0 mg/L); procalcitonin (PCT) level was 2.17 ng/mL (range: <0.15 ng/mL) and blood oxygen saturation (SpO2) level was 84% (at room air). Her consciousness level in Glasgow Coma Scale (GCS) was measured as E-4, V-3 and M-4. Initially, she was supported by 2 L external oxygen. The carbapenem-resistant Enterobacteriaceae, Escherichia coli was detected in her urine culture within the first 24-h of ICU admission and that E. coli was found to be multidrug-resistant, only sensitive to polymyxins (polymyxin B and Colistin). No other organism was found in blood, tracheal aspirate, or sputum microbiological evaluation report (by microbroth dilution method) at that time. She was then treated with intravenous colistin (as monotherapy) as per the hospital dosing guideline and after 5 days later, a repeated urine culture sensitivity report showed a complete eradication of E. coli from urine. Her physical conditions and infection markers were improving gradually and two days later, she was transferred to the general ward with normal kidney function (creatinine: 1.3 mg/dL) and improved level of consciousness. After 4 days, she was transferred to ICU again due to increased body temperature (105 °F as recorded highest) with mild respiratory depression (SpO2 dropped to 87%) and disoriented level of consciousness (GCS: E-3, V-3 and M-5). At that time in ICU, her CRP level was 286.3; PCT level was 230 ng/mL and white blood cell (WBC) count was 13.9 K/μL. Her blood culture revelaed Alcaligenes faecalis, which was detected as a pandrug-resistant organism even to carbapenems, polymyxins and tigecycline. Sensitivities were determined using an automated microbroth dilution method (Fig. 1) through analyzing the minimum inhibitory concentrations (MIC) of those antibiotics following the standard of Clinical and Laboratory Standards Institute (CLSI) guidelines, and also by using disk diffusion method (Fig. 2) for reconfirming the susceptibility patterns of the antibiotics. According to the CS reports, there was no active antibiotic left to be prescribed for treating A. faecalis-associated bloodstream infection and patient condition was deteriorating rapidly. ICU-physicians and clinical pharmacist (Infectious Diseases) decided to use double-dose (a single 200 mg of tigecycline as a loading dose intravenously; and then 200 mg of tigecycline once daily intravenously for the next 14 days as maintenance dose) of tigecycline against A. faecalis though this pathogen was found resistant to tigecycline. In the disk diffusion susceptibility testing method of tigecycline against A. faecalis, the diameter of the zone of inhibition was measured 9 mm (zone-diameter range ≤14 mm, 15–18 mm and ≥19 mm represents the organism is resistant, intermediate-resistant and susceptible, respectively to tigecycline). Other culture sensitivity reports (sputum, tracheal aspirate and urine) were found free from any pathogen at that time. After 5 days treatment with double-dose tigecycline, the repeat blood CS was done and found the complete eradication of A. faecalis in the blood. During that time, Candida albicans was detected in the sputum CS report and other CS (tracheal aspirate and urine) reports were free from any pathogen. Her CRP level, PCT level and WBC count returned to normal level at the 9th day of tigecycline therapy and she was then shifted from ICU to ward again at the 11th day of tigecycline therapy with stable physical condition. After staying more 6 days in the hospital ward, she was then discharged to home with good physical condition.
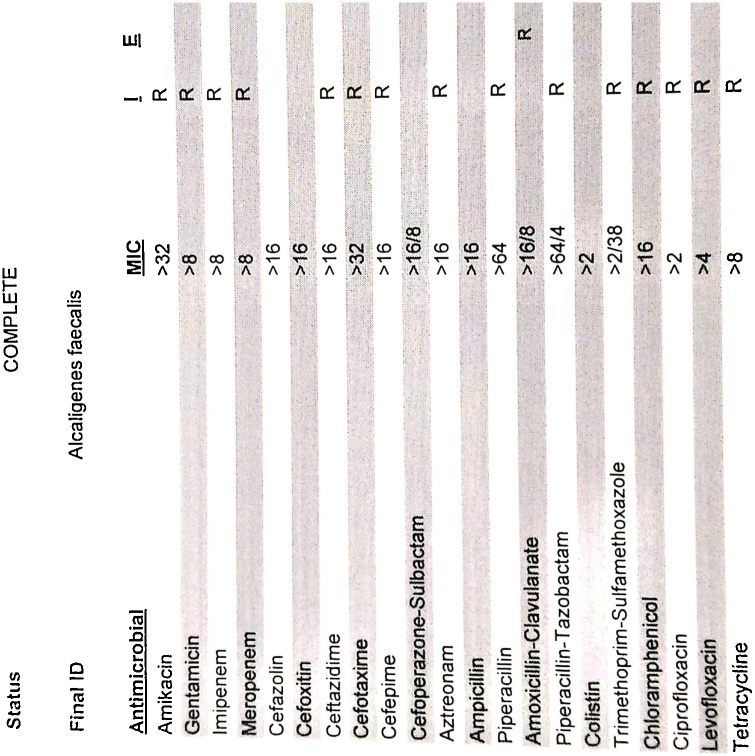
Fig. 1.
A. faecalis is resistant to all listed antibiotics (with MIC of the antibiotics; where ‘R’ represents ‘Resistance to antibiotic’). Automated microbroth dilution method was used.
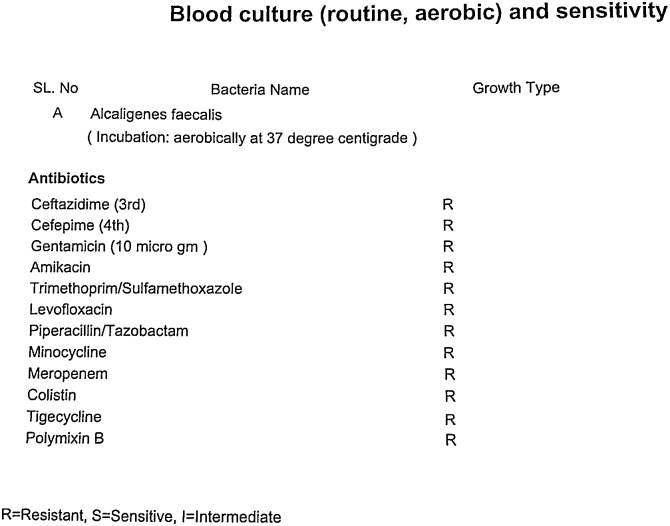
Fig. 2.
Pandrug-resistant A. faecalis in the blood sample showing resistance against all the listed antibiotics. Disk diffusion method was used where ‘R’ represents ‘Resistance’.
Discussion
A. faecalis is commonly found in soil, water, environments as well as in human intestinal flora, and was first discovered in human feces [1]. Nowadays, A. faecalis is widely used in the sewage treatment, and several strains of A. faecalis produce an important precursor, R-(—)-mandelic acid, essential for the production of various drugs and that is why, A. faecalis is also widely used in pharmaceutical industries [5]. Additionally, in the environmental industries, some strains of A. faecalis are used to degrade many organic contaminants in the biodegradation process of organic pollutants and industrial hazardous waste-materials [6]. Being a common bacterium in the human intestinal microbiota, A. faecalis occasionally moves to blood and respiratory tract, and causes infections [3]. Few rare cases of A. faecalis-associated peritonitis, eye infections and urinary tract infections are available [3,7,8]. In our case, A. faecalis infection was identified in the bloodstream of the patient staying in the hospital. Currently, serious life threatening infections with A. faecalis in the hospitalized patients is an emerging problem, globally [1,6]. A. faecalis-associated bloodstream infection in immunocompromised cancer patients was first reported in 2004, and that organism was found commonly resistant to ciprofloxacin, levofloxacin, aminoglycosides and monobactam [2]. Another study on 188 patients reported that A. faecalis was present in the ear discharge of 20 patients (10.6%), and 100% of A. faecalis isolates were sensitive to amikacin, all cephalosporins and colistin; 90% of A. faecalis isolates were sensitive to gentamicin, tobramycin and piperacillin/tazobactam; 75% of A. faecalis isolates were sensitive to trimethoprim/sulfamethoxazole; and 100% and 82.6% of A. faecalis isolates were resistant to ciprofloxacin and levofloxacin, respectively [7]. In our patient, the isolated A. faecalis from blood specimen was completely resistant to all commercially available antibiotics including polymyxin B, colistin and tigecycline (Fig. 1, Fig. 2). This super resistance-characteristic of A. faecalis to all common antibiotics including the reserve group antibiotics (polymyxins and tigecycline, mostly) was not reported elsewhere to date and the required treatment options against that pandrug-resistant organism was not recommended before. Conventional dosing regimen of tigecycline maintains a low serum-tigecycline concentration and still a controversial therapy in bloodstream infections [9]. Conventional therapy of tigecycline revealed a variable AUC/MIC ratio (33.4 ± 24.3; range 0.21–102) and the AUCSS (5.46 ± 1.62 mg.h/L; range 0.21–102) [10]. Study data of double-dose of tigeccycline are limited, but multiple study demonstrated a higher microbiological eradication rate and a better clinical outcome with double-dose of tigecycline than the recommended dose [10,11]. A study found that a daily double-dose regimen (100 mg every 12 hourly) of tigecycline evolved a higher total cumulative fraction of response (CFR) (90.2%) in hospital-acquired pneumonia (HAP) caused by carbapenemase-producing Klebsiella pneumoniae (CP-KP) than its conventional daily dosing regimen (50 mg every 12 hourly) (71.02%), and this findings showed the superior clinical efficacy of double-dose tigecycline [11]. Considering the current clinical evidences of double-dose tigecycline, the resistance pattern of that organism and clinical conditions of the patient, infectious disease-professionals decided to use the double-dose (100 mg twice daily, intravenously after a 200 mg single intravenous loading dose) of tigecycline in order to maximize the plasma concentration of tigecycline (tigecycline 100 mg single dose intravenously yields: maximum plasma concentration of 1.45 mg/L and area under the plasma concentration–time curve of 5.19 mg h/L) [12]. Successfully, the pandrug-resistant A. faecalis was completely eradicated from the bloodstream of that patient by applying double-dose of tigecycline and the microbiological cure-report came after 5 days of initiation of the tigecycline therapy.
Conclusion
We described an uncommon case of bloodstream infection with A. faecalis which was pandrug-resistant including polymyxins and tigecycline, and successfully treated with double-dose tigecycline therapy.
Funding
There are no sources of funding to declare.
Consent
Written informed consent was obtained from the patient for publication of this case report.
Authors’ contributions
Md Jahidul Hasan, Clinical Pharmacist: Collected the data and drafted the manuscript.
Lutfun Nahar Nizhu, Medical Specialist: Revised the manuscript critically for important intellectual contents.
Raihan Rabbani, MD: Revised the manuscript critically for important intellectual contents.
Acknowledgements
The authors are very grateful to the patient for giving her consent and to the authority of Square Hospitals Ltd. for their permission.
Contributor Information
Md Jahidul Hasan, Email: jahidul@squarehospital.com.
Lutfun Nahar Nizhu, Email: oporajita84@gmail.com.
Raihan Rabbani, Email: drraihan@squarehospital.com.
References
- 1.Tena D., Fernández C., Lago M.R. Alcaligenes faecalis: an unusual cause of skin and soft tissue infection. Jpn J Infect Dis. 2015;68(2):128–130. doi: 10.7883/yoken.JJID.2014.164. [DOI] [PubMed] [Google Scholar]
- 2.Aisenberg G., Rolston K.V., Safdar A. Bacteremia caused by Achromobacter and Alcaligenes species in 46 patients with cancer (1989–2003) Cancer. 2004;101(9):2134–2140. doi: 10.1002/cncr.20604. [DOI] [PubMed] [Google Scholar]
- 3.Kavuncuoglu F., Unal A., Oguzhan N., Tokgoz B., Oymak O., Utas C. First reported case of Alcaligenes faecalis peritonitis. Perit Dial Int. 2010;30(1):118–119. doi: 10.3747/pdi.2009.00058. [DOI] [PubMed] [Google Scholar]
- 4.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 5.Ju S., Lin J., Zheng J., Wang S., Zhou H., Sun M. Alcaligenes faecalis ZD02, a novel nematicidal bacterium with an extracellular serine protease virulence factor. Appl Environ Microbiol. 2016;82(7):2112–2120. doi: 10.1128/AEM.03444-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong L., Zhu S., Zhu L., Xie H., Wei K., Yan T. Colonization of Alcaligenes faecalis strain JBW4 in natural soils and its detoxification of endosulfan. Appl Microbiol Biotechnol. 2014;98(3):1407–1416. doi: 10.1007/s00253-013-5033-4. [DOI] [PubMed] [Google Scholar]
- 7.Filipe M., Reimer Å, Matuschek E., Paul M., Pelkonen T., Riesbeck K. Fluoroquinolone-resistant Alcaligenes faecalis related to chronic suppurative otitis media, Angola. Emerg Infect Dis. 2017;23(10):1740–1742. doi: 10.3201/eid2310.170268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pal S.S., Panigrahi P.K., Roy R., Nandi K., Das S. Endophthalmitis caused by Alcaligenes faecalis: a case series. Ocul Immunol Inflamm. 2013;21(6):446–448. doi: 10.3109/09273948.2013.817592. [DOI] [PubMed] [Google Scholar]
- 9.Stein G.E., Babinchak T. Tigecycline: an update. Diagn Microbiol Infect Dis. 2013;75(4):331–336. doi: 10.1016/j.diagmicrobio.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 10.MacGowan A.P. Tigecycline pharmacokinetic/pharmacodynamic update. J Antimicrob Chemother. 2008;62(Suppl. 1):i11–6. doi: 10.1093/jac/dkn242. [DOI] [PubMed] [Google Scholar]
- 11.Ni W., Li G., Zhao J., Cui J., Wang R., Gao Z. Use of Monte Carlo simulation to evaluate the efficacy of tigecycline and minocycline for the treatment of pneumonia due to carbapenemase-producing Klebsiella pneumoniae. Infect Dis (Lond) 2018;50(7):507–513. doi: 10.1080/23744235.2018.1423703. [DOI] [PubMed] [Google Scholar]
- 12.Rodvold K.A., Gotfried M.H., Cwik M., Korth-Bradley J.M., Dukart G., Ellis-Grosse E.J. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J Antimicrob Chemother. 2006;58(6):1221–1229. doi: 10.1093/jac/dkl403. [DOI] [PubMed] [Google Scholar]