Abstract
Purpose
To highlight the striking similarities between the lesions of congenital toxoplasmosis (CT) and North Carolina Macular Dystrophy (NCMD) using multimodal imaging including spectral domain optical coherence tomography (SD-OCT).
Observations
We are comparing a case report of CT compared to that of NCMD. The case of a 64-year-old man with a lifelong history of decreased vision OD from toxoplasmosis and new onset of central retinal vein occlusion OS. Color fundus photography, spectral domain optical coherence tomography (SD-OCT), and intravenous fluorescein angiography (IVFA) were used as diagnostic imaging tools to demonstrate the similarities and differences between CT and NCMD. In this case, unilateral CT demonstrated a large, excavated, coloboma-like chorioretinal lesion identical to NCMD grade 3. Serology studies were positive for toxoplasmosis. The similarities of CT and NCMD grade 3 using SD-OCT are especially striking.
Conclusion and importance
Lesions of CT and NCMD grade 3 can appear identical on clinical exam and are indistinguishable from one another on SD-OCT. Because CT is a phenocopy of NCMD, many cases of the original NCMD family members had been misdiagnosed as CT. North Carolina Macular Dystrophy may be more common than previously realized and bilateral CT cases should be reexamined along with family members and genetic testing performed. Cases of bilateral CT actually may be NCMD cases. Now that the genetic and molecular mechanisms of NCMD are known, these may provide clues into the pathogenesis of CT.
Keywords: Congenital toxoplasmosis (CT), North Carolina macular dystrophy (NCMD/MCDR1), Optical coherence tomography (OCT), Phenocopy, PRDM13
1. Introduction
Ocular toxoplasmosis, a chorioretinal infection with the intracellular protozoan Toxoplasma gondii (T. gondii), is widely known as the most common cause of posterior segment infection worldwide.1,2 T. gondii may be transmitted to humans via the fecal-oral route, blood transfusion, organ transplantation, or transplacental transmission. Active toxoplasmic lesions in the peripheral retina are associated with symptomatic vitreous inflammation, which leads to blurry vision. Visual impairment may also be due to macular lesions or lesions involving the optic nerve. Inactive toxoplasmic lesions may present with scotomas directly related to the size and location of retinochoroidal scars.1 A unilateral decrease in visual acuity is the most common presenting symptom and the classic manifestation is a nidus of white, focal necrotizing retinochoroiditis adjacent to a variably pigmented chorioretinal scar.2 The diagnosis is made clinically and confirmed with T. gondii antibody titers of serum or intraocular fluids, or with polymerase chain reaction of aqueous and vitreous samples. Spectral domain optical coherence tomography (SD-OCT) findings of chorioretinal lesions depend on the stage of the disease, whether in the acute, treatment, or resolved phases.1 Features including hyper-reflective retinal structures, posterior hyaloid thickening and detachment, optical shadowing, and cystoid changes have been described.3 A variety of disease treatment options exist, the classic being triple-drug therapy of pyrimethamine, sulfadiazine, and corticosteroid.1
Congenital toxoplasmosis (CT) is acquired when a pregnant woman is exposed to tissue cysts or oocysts in uncooked meat or substances contaminated with cat feces.2 One factor influencing the risk and severity of disease is the age of pregnancy at maternal infection. However, the risk of vertical transmission when maternal infection occurs in the third versus the first and second trimesters can be increased by as much as 55%, and those fetuses infected earlier are most compromised.4 Additionally, Toxoplasma strain type affects the severity of CT.5 Congenital toxoplasmosis presents asymptomatically in most newborns, but thorough investigation may reveal severe ocular manifestations, including retinochoroiditis, microphthalmia, optic nerve atrophy, iris abnormalities, cataracts, and strabismus.6 Retinochoroiditis lesions are the most frequent manifestations.4 One large cohort study of 178 newborns with CT demonstrated that these lesions, although commonly viewed through clear vitreous, are frequently active.6 It has been shown that 65–85% of CT lesions are bilateral, 58% involve the macula and most lesions involve the papillomacular area.2,4 Diagnostic multimodal imaging of the retina included color fundus photographs (Zeiss, Oberkochen, Germany), fundus autofluorescence images (Zeiss, Oberkochen, Germany), fluorescein angiograms (Zeiss, Oberkochen, Germany), and SD-OCT images (Zeiss, Oberkochen, Germany).
North Carolina Macular Dystrophy (NCMD) is an autosomal dominant, congenital, completely penetrant bilateral macular dystrophy with great variable expressivity. Three grades have been defined by Small et al.7 Grade 1 manifests small drusen in the fovea; patients are asymptomatic. Grade 2 features confluent drusen with occasional central elevated vitelliform or fibrotic lesion. Grade 3 demonstrates a large, excavated lesion in the central macula, typically with fibrosis and sharp shelving edge temporally. The fibrosis and progressive vision loss experienced by a few patients are due to choroidal neovascular membranes (CNVMs). CNVMs in NCMD typically develop along the temporal edge, and vision may not be affected in these cases because fixation is typically along the nasal edge of the lesion. Vision loss can occur if the CNVM occurs along the nasal side of the lesion. Diagnosis is made with a combination of clinical findings of the individual and of family members and now with genetic testing. Examination of family members is critical as a third of affected NCMD subjects are asymptomatic with good vision. The causative mutations appear to cause overexpression of the gene PRDM13 due to point mutations in a DNASE1 hypersensitivity binding site, duplications on chromosome 6, or duplications of a DNASE1 hypersensitivity binding site on chromosome 5.8 Treatment can involve intravitreal anti-VEGF injections if choroidal neovascularization is present.
2. Case report findings
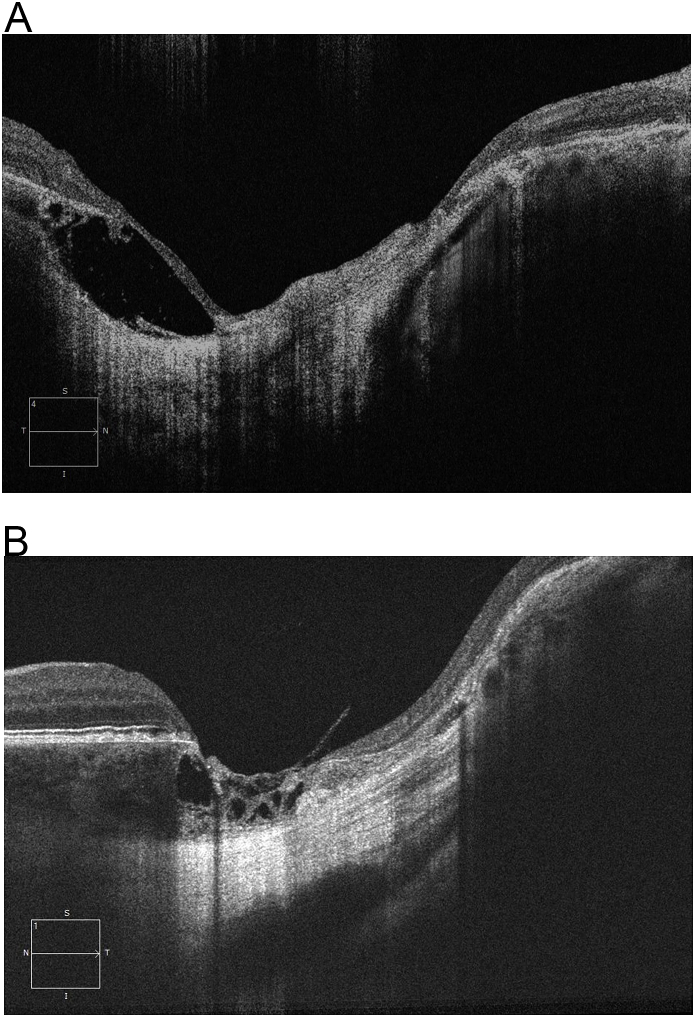
A 64-year-old male presented with new onset decreased vision in his previously dominant and presumably healthy left eye. Past ocular history was significant for bilateral cataracts, bilateral primary open angle glaucoma, pterygium in the right eye, and decreased vision in the right eye since birth. Past medical history was significant for systemic hypertension and kidney transplant. On physical exam, visual acuity was 20/80 in the right eye and count fingers in the left eye. Fundus exam of the right eye revealed a large excavated chorioretinal lesion with a well-defined, discrete edge and variable pigmentation within the macular coloboma-like lesion. Fundus exam of the left eye revealed cystoid macular edema, peripheral dot blot hemorrhages and edema consistent with a recent central retinal vein occlusion. SD-OCT of the right macula, within the coloboma like lesion, demonstrated thin, disorganized retina and a depression extending into the choroid and sclera with well-defined circumferential fibrosis (Fig. 1). Thinning and hyper-reflectivity of the retinal pigment epithelium, choroid, and sclera were noted. A lacuna of intrachoroidal fluid was also observed. The patient was diagnosed with an inactive congenital toxoplasmic scar in the right eye, which was eventually supported with positive serology testing (Toxoplasma Serological Profile (TSP) comprised of the Sabin-Feldman Dye Test (DT), double sandwich IgM ELISA, IgA ELISA, IgE ELISA, and AC/HS test); no treatment was required. The left eye was diagnosed with central retinal vein occlusion with cystoid macular edema; treatment involved multiple monthly intravitreal anti-VEGF injections with improvement to 20/40. Additionally, the NCMD subject (35 years old, male) has a grade 3 coloboma-like lesion of both maculae resulting in counting fingers vision in the right eye (OD) and 20/20 vision in the left eye (OS). Fundus photographs show bilateral grade 3 coloboma-like lesions that are more severe in the OD than in the OS. Additionally, in the right macula, there is submacular fibrosis extending under the nasal macula and superior to the optic nerve, suggesting that a previous episode of an actively leaking choroidal neovascularization (CNVM) occurred. Spectral domain optical coherence tomography (SD-OCT) images of the OD show a macular coloboma-like lesion with a discrete, well-demarcated absence of the photoreceptors and retinal pigment epithelium (RPE), and choroid encircled by subretinal fibrosis. Sanger DNA sequencing demonstrated the variant 2 (V2) point mutation (Chr6: 99593111) in the deoxyribonuclease 1 hypersensitivity-binding site in this affected participant. The macular lesion of the right eye of the CT subject was extremely similar to the lesion from NCMD grade 3 on OCT and it was difficult to distinguish the two lesions until genetic testing was performed (Fig. 1–a). An IRB approved signed informed consent was obtained from both subjects.
Fig. 1.
a: OCT images of a CT subject (Fig. 1) and a NCMD grade 3 subject (Fig. 1a).
3. Discussion and conclusion
In this case, the lesion incidentally found in the patient's right eye was typical for CT. Had the lesion been bilateral, distinguishing CT from NCMD would have been clinically difficult.8 The features described above can be characteristic of lesions manifested by both conditions, which cannot be distinguished using OCT (Fig. 1–a). Many of the original NCMD family members had been misdiagnosed as Congenital Ocular Toxoplasmosis by their local eye care physicians. For decades, ophthalmologists felt capable of clinically distinguishing NCMD grade 3 lesions from Toxoplasmosis. Having examined over 250 affected subjects with NCMD, my (KWS) confidence in making this clinical distinction has eroded. As more patients have presented, the clinical pictures and images have merged and the phenotypic similarities are undeniable. Images using OCTA of ocular toxoplasmosis chorioretinal lesions in the literature are lacking.
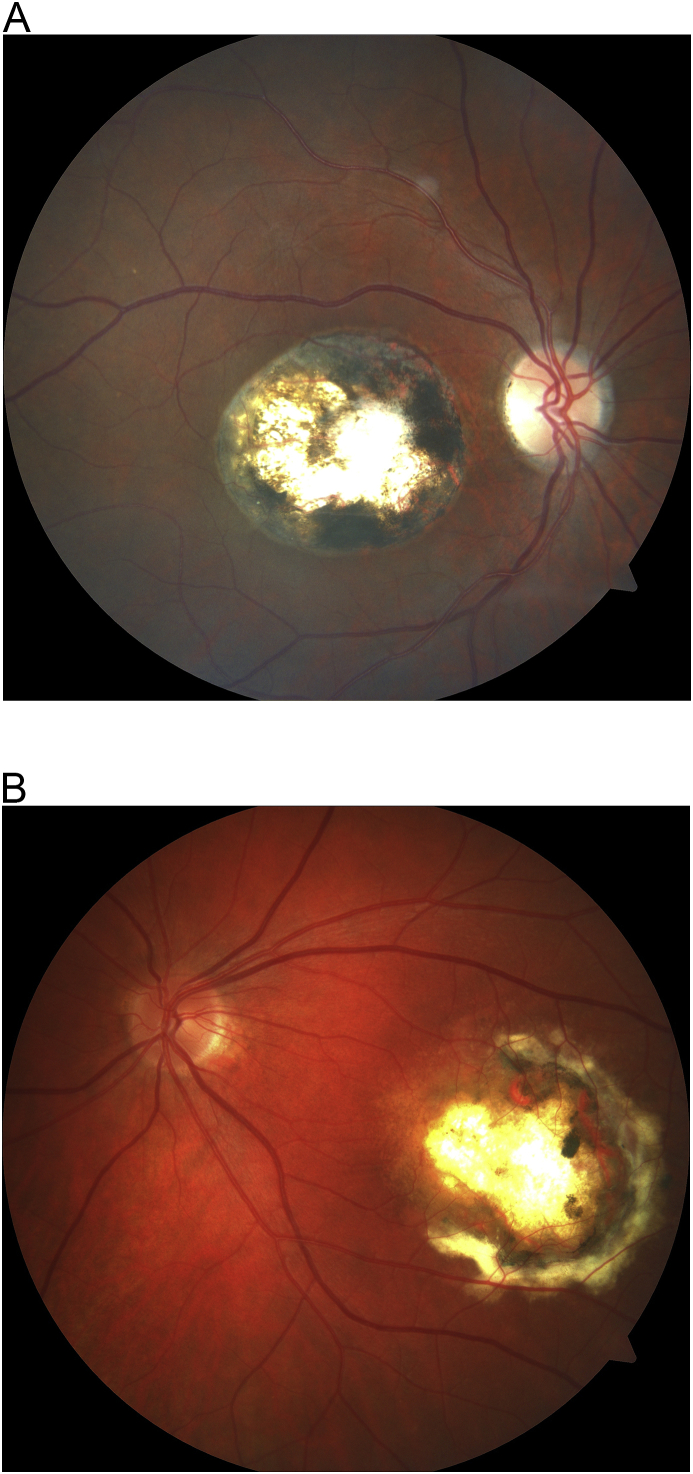
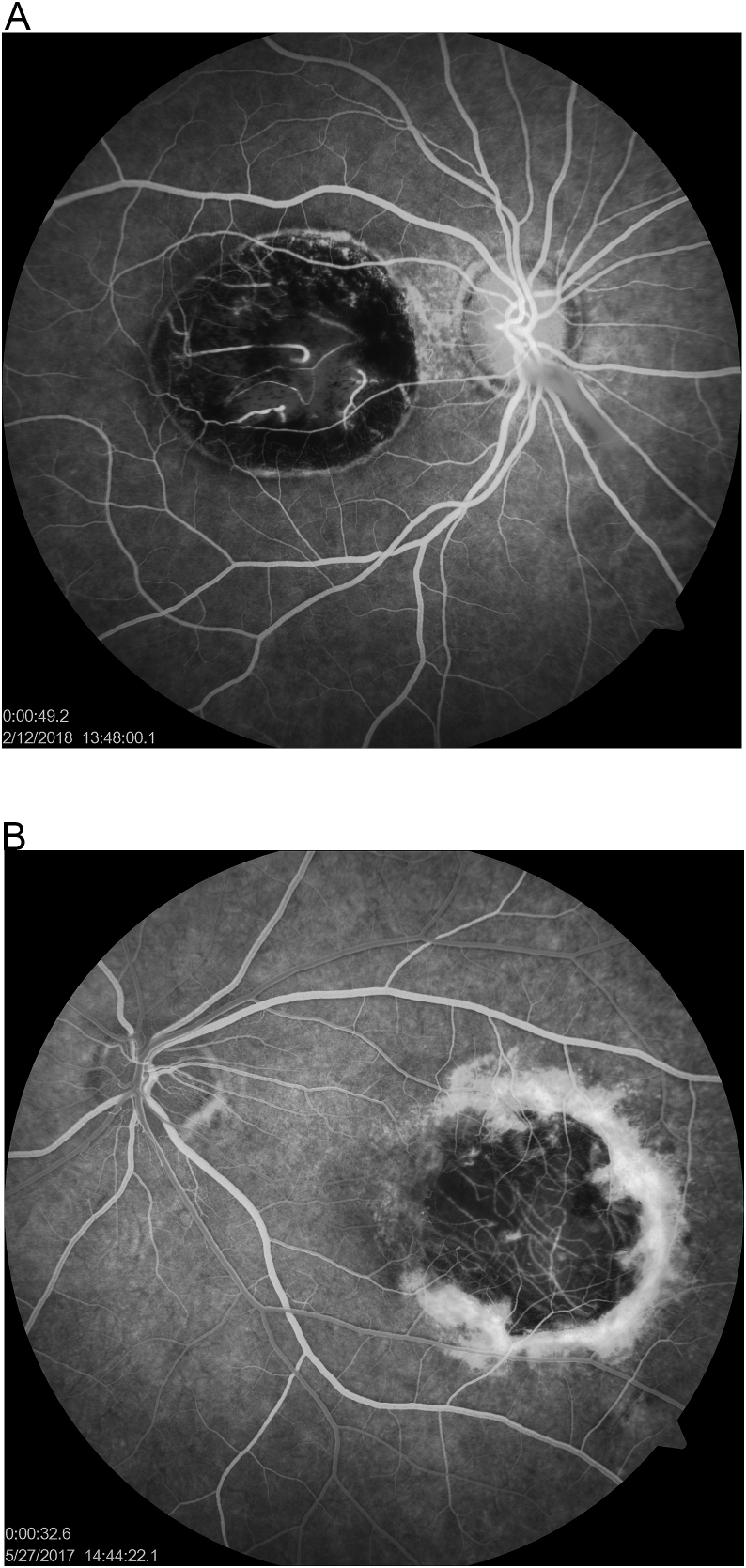
Certain features, however, may help clinicians to distinguish CT from NCMD. Firstly, as it can be seen on the fundus photos, CT lesions are typically circular in shape and demonstrate pigment distributed symmetrically around the defined edge of the scar (Fig. 2). NCMD grade 3 lesions, however, are more ovoid, with scarce pigment found nasally and within the coloboma-like lesion, but gradually increasing and sloping temporally (Fig. 2a). Secondly, CT can be unilateral, but NCMD grade 3 is always bilateral and typically symmetrical. Thirdly, toxoplasmic lesions can have evidence of active inflammation, but NCMD never does (Table 1). Finally, IVFA images show that toxoplasmic lesions may demonstrate a flat or elevated subretinal scarring/fibrosis; NCMD will only have this appearance if it is a grade 2 lesion with CNVMs (Fig. 3–a). The circular shape, the heavier pigment, and the unilaterality of the lesion were the best clues to the diagnosis of CT in our patient. Even serology testing might not prove to be helpful since 42.9% of randomly selected blood donors are seropositive for T. gondii.9 IgG suggests previous exposure/infection of CT; therefore, positive IgM suggests new active infection of T. gondii.9
Fig. 2.
a: Fundus images of a CT subject (Fig. 2) and a NCMD grade 3 subject (Fig. 2a).
Table 1.
Similarities and differences between NCMD and CT.
| CT | CT & NCMD | NCMD |
|---|---|---|
|
|
|
Fig. 3.
a: Intravenous fluorescein angiography (IVFA) images of a CT subject (Fig. 3) 49 seconds post dye injection and a NCMD grade 3 subject (Fig. 3a) 32 seconds post dye injection.
The spectral domain optical coherence tomography (SD-OCT) findings of CT and NCMD are strikingly similar and hard to distinguish (Fig. 1). The deep excavation into the choroid and sclera suggest the congenital/developmental nature of CT. Optical coherence tomography of CT also demonstrates the “intrachoroidal cavitation” which we refer to as a developmental lacuna in the choroid. Schoenberger et al. have previously described these lacunae as “cavitations.” However, the term cavitation implies the earlier embryonic existence of tissue, which then dissolved and collapsed much like a sinkhole geologically forms in Florida and other geographic regions consisting of soft limestone. The term cavitation implies that the actual development and mechanism is known and we feel is a bit presumptive given our serious deficiency in the embryogenesis of the primate macula.10
Clinically distinguishing CT from NCMD is not a mere academic exercise. A prominent retinal physician was successfully sued 25 years ago because of a misdiagnosis. The plaintiff had a child with visual impairment and was diagnosed with CT. The plaintiff wanted to know the chances of having another child with visual impairment. The unnamed retinal physician reportedly told her that her second child would not have visual impairment. Her second pregnancy resulted in her second child with visual impairment, which was subsequently diagnosed as NCMD. The plaintiff prevailed with her “wrongful birth” lawsuit. The retinal physician involved did not want to be named herein despite the fact that many of the original NCMD were misdiagnosed with CT originally (personal communication that requests to be anonymous).
We suggest that the similar phenotypes of CT and NCMD are due to overlapping mechanisms affecting retinal and macular development. These overlapping mechanisms may involve similar tissues, cells or molecular mechanisms at similar temporal-spatial times of macular embryogenesis. The mechanisms by which T. gondii cause the macular coloboma-like lesions have been presumed to be due to active in utero infection by transplacental migration of the organisms. However, there is no evidence of this in the literature showing oocysts or other evidence of organisms except in patients with active inflammation. No evidence exists in the literature that the colobomoa like lesions seen in CT contain any organisms at all as there are no examples in the literature of “satellite lesions” sprouting off of one of these coloboma-like lesion. One possible overlapping mechanism with CT and NCMD is that both are involving DNA methyltransferases and histone modifications.11 In NCMD, there are three point mutations in the non-coding region of a DNase1 hypersensitivity-binding site 13 Kb upstream of PRDM13. This retinal transcription factor has known DNA methyltranasferase activity and the DNAS1 binding is a region sensitive to DNA methylation and histone genomic organization. Conformational changes in histones and chromosomal structure cause this region to be exposed and vulnerable to binding and regulation of transcription factors. Possibly, T. gondii, or a yet to be defined transcription factor of T. gondii, might be involved in binding to this region as well. PRDM13 is a member of a large family of “helix-loop-helix” DNA-binding proteins that play key roles in controlling gene expression during development. Macular formation possibly relies on a precise interaction between transcription factors (like PRDM13) and their target genes. A change in the expression of a transcription factor due to mutations in its own regulatory regions could lead to impaired cell fate specifications in the developing macula.10 This could explain the phenotypic similarities between CT and grade 3 NCMD lesions.
Our case report highlights the phenotypic similarities clinically and by multimodal imaging between the lesions of CT and NCMD grade 3, and suggests a common pathogenic mechanism between the two disease entities. Clinicians must take care to thoroughly evaluate patients with such macular lesions and consider serology studies and genetic testing to confirm their clinical diagnosis. Cases of bilateral congenital Toxoplasmosis with macular lesion may in actuality be NCMD grade 3 and should be reevaluated with molecular testing for NCMD. Other family members should also be examined for evidence of NCMD. Clinicians should be reminded that more than half of NCMD subjects with grade 1 and 2 disease have good vision and are asymptomatic. Merely inquiring a patient of a “positive family history of eye disease” is inadequate. It seems likely that there are more cases of bilateral CT that are actually NCMD, especially those CT cases transmitted transplacentally.
Acknowledgements and disclosures
4. Patient consent
The patients consented to publication of the case in writing/orally.
IRB approval has been obtained.
Conflicts of interest
The following authors have no financial disclosures: KWS, ALV, CLK, FSS.
All authors attest that they meet the current ICMJE criteria for authorship.
Funding sources
Foundation Fighting Blindness Grant #: BR-GE-1216-0715-CSH.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2019.100521.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ozgonul C., Besirli C. Recent developments in the diagnosis and treatment of ocular toxoplasmosis. Ophthalmic Res. 2016;57(1):1–12. doi: 10.1159/000449169. [DOI] [PubMed] [Google Scholar]
- 2.Cantor L., Rapuano C., Cioffi G. American Academy of Ophthalmology; San Francisco: 2014. Basic and Clinical Science Course, 2015-2016; pp. 206–209. [Google Scholar]
- 3.Monnet D., Averous K., Delair E., Brézin A.P. Optical coherence tomography in ocular toxoplasmosis. Int J Med Sci. Mar. 2009;19:137–138. doi: 10.7150/ijms.6.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melamed J., Eckert G.U., Spadoni V.S., Lago E.G., Uberti F. Ocular manifestations of congenital toxoplasmosis. Eye. 12 June 2009;24(4):528–534. doi: 10.1038/eye.2009.140. [DOI] [PubMed] [Google Scholar]
- 5.Shobab L., Pleyer U., Johnsen J. Toxoplasma serotype is associated with development of ocular toxoplasmosis. J Infect Dis. 21 July 2013;208(9):1520–1528. doi: 10.1093/infdis/jit313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasconcelos-Santos D.V., Machado Azevedo D.O., Campos W.R. Congenital toxoplasmosis in southeastern Brazil: results of early ophthalmologic examination of a large cohort of neonates. Ophthalmology. Nov. 2009;116(11):2199–2205. doi: 10.1016/j.ophtha.2009.04.042. e1. [DOI] [PubMed] [Google Scholar]
- 7.Small K.W., Voo I., Flannery J., Udar N., Glasgow B.J. North Carolina macular dystrophy: clinicopathologic correlation. Trans Am Ophthalmol Soc. 2001;99:233–237. [PMC free article] [PubMed] [Google Scholar]
- 8.Small K.W., DeLuca A.P., Whitmore S.S. North Carolina macular dystrophy is caused by dysregulation of the retinal transcription factor PRDM13. Ophthalmology. Jan. 2016;123(1):9–18. doi: 10.1016/j.ophtha.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarkovic A., MacMurray C., Deva N., Ghosh S., Whitley D., Guest S. Seropositivity rates for Bartonella henselae, Toxocara canis and Toxoplasma gondii in New Zealand blood donors. Clin Exp Ophthalmol. 2007;35:131–134. doi: 10.1111/j.1442-9071.2006.01406.x. [DOI] [PubMed] [Google Scholar]
- 10.Schoenberger S.D., Agarwal A. Intrachoroidal cavitation in North Carolina macular dystrophy. JAMA Ophthalmol. 2013;131(8):1073–1076. doi: 10.1001/jamaophthalmol.2013.1598. [DOI] [PubMed] [Google Scholar]
- 11.Wei H., Jiang S., Chen L., He C., Wu S., Peng H. Characterization of cytosine methylation and the DNA methyltransferases of Toxoplasma gondii. Int J Biol Sci. 2017;13(4):458–470. doi: 10.7150/ijbs.18644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.