Abstract
Aberrant right subclavian artery with a left aortic arch is rare, but it is the most common congenital aortic arch anomaly. It can present as an incidental finding later in life or be symptomatic at a young age. Here, we describe a case of an aberrant right subclavian artery discovered in a 4 month old with respiratory distress and feeding difficulties. She underwent an extensive aerodigestive evaluation including bronchoscopy, both flexible and rigid, upper GI endoscopy, modified barium swallow with esophageal sweep, chest imaging, CT thorax and echocardiogram. The final decision per the management team was to observe the patient in order to allow more growth. She ultimately improved with age and remains asymptomatic.
1. Introduction
Aberrant right subclavian artery is the most common anomaly arising from the aorta. Its incidence is between 0.5% and 2% worldwide [1,2]. It usually arises distal to the left subclavian artery from a dilated segment of the proximal descending aorta, and crosses in the posterior part of the mediastinum to the right upper extremity. In majority of the cases it crosses behind the esophagus or occasionally between trachea and the esophagus [3,4]. This can result in dysphagia, respiratory distress and stridor. Here we report the case of an aberrant right subclavian artery in a baby crossing behind the esophagus leading to respiratory and digestive issues.
2. Case report
A 2 week old girl was admitted with respiratory failure. She had been born full term with no respiratory related issues, but had experienced trouble gaining weight since birth. She was initially admitted at 2 weeks of age to the hospital after an episode of perioral cyanosis and dyspnea after feeding. Her oxygen saturation in the ER was 50%–60% with significant tachypnea at 80 breaths per minute. Initial chest x-ray showed diffuse bilateral haziness. Her initial complete blood count was normal. Due to her tenuous respiratory status she was intubated and ventilated for 8 days. She had a full sepsis work up which was negative. After extubation, her respiratory rate decreased to 50–60 breaths per minute. She had chest wall retraction with bilateral lung crackles and required supplemental oxygen 0.5–1 Lpm to maintain SpO2 >95%.
At one year of age she was finally weaned off oxygen. However, she continued to develop significant respiratory distress and hypoxemia with upper respiratory tract infections. Additionally, she had delay of her developmental milestones and mild hypotonia. There was no family history of immunodeficiency, surfactant deficiency or underlying genetic disorders. She always had trouble gaining weight and would experience coughing, choking and frequent spit up with liquid feeds. On her follow up physical exam in the clinic, she was not dysmorphic but always had mild bilateral subcostal chest wall retractions with bilateral inspiratory crackles which slowly improved over time. Her abdomen was soft with no evidence of organomegaly. There was no digital clubbing.
Due to her unusual presentation with recurrent respiratory illnesses and feeding difficulty, it was necessary for an aerodigestive approach to evaluate her. Chest x-ray showed mild central right lung haziness and bronchial wall thickening. Fig. 1.
Fig. 1.
Arrow on the chest image shows mild right lung haziness.
Chest computed tomography (CT) with IV contrast confirmed a left side aortic arch with an aberrant right subclavian artery with a retroesophageal course, Fig. 2A. There was no aneurysmal dilatation suggestive of Kommerell's diverticulum.
Fig. 2a.
CT scan thorax in axial view: blue arrow shows the retroesophageal course of the right subclavian artery. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
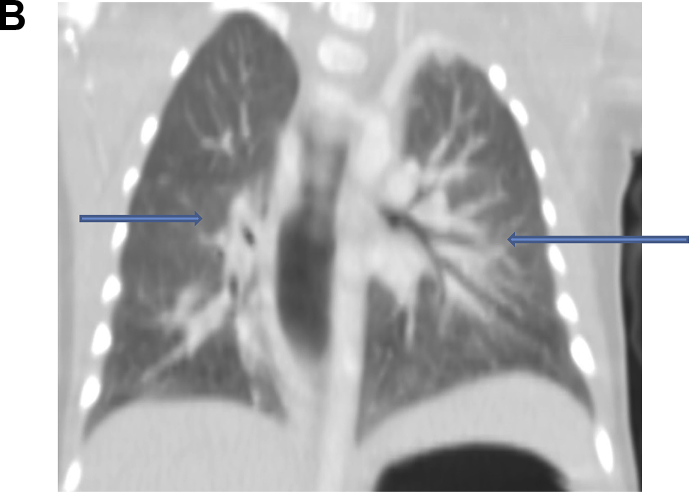
The lungs show bilateral perihilar ground glass haziness in the lower lobes Fig. 2B.
Fig. 2b.
CT scan of the lung coronal view: both blue arrows show bilateral ground glass opacities. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
A sweat chloride test was normal and no CFTR mutations were found on direct mutation, full gene sequencing and duplication/deletion analysis. Surfactant mutations for ABCA3, SPB and SPC were negative. Pancreatic fecal elastase was negative. FISH analysis showed normal 22q11 with no evidence of deletion or duplication.
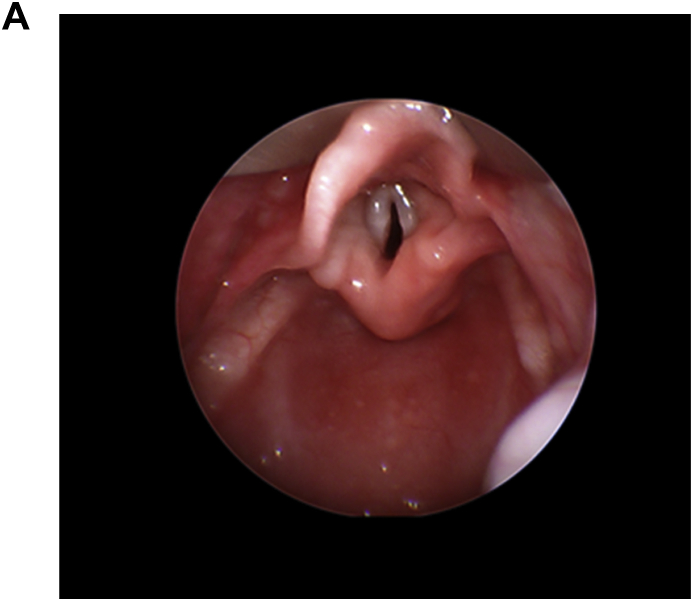
Flexible bronchoscopy was completed which showed a pulsatile posterior membranous distal tracheal wall with a mild distal posterior tracheal bulge with copious amount of frothy white secretions in the right and left main stem bronchi. Rigid laryngoscopy did not reveal a laryngeal cleft or tracheoesophageal fistula. The right main stem bronchus did appear to be of smaller caliber than the left main stem bronchus Fig. 3a, Fig. 3b(A and B).
Fig. 3a.
Telescopic laryngoscopy Normal larynx.
Fig. 3b.
Rigid Bronchoscopy: arrow points to the mild bulge of the posterior wall of the distal trachea.
The bronchoalveolar lavage was non inflammatory with predominance of lipid laden macrophages.
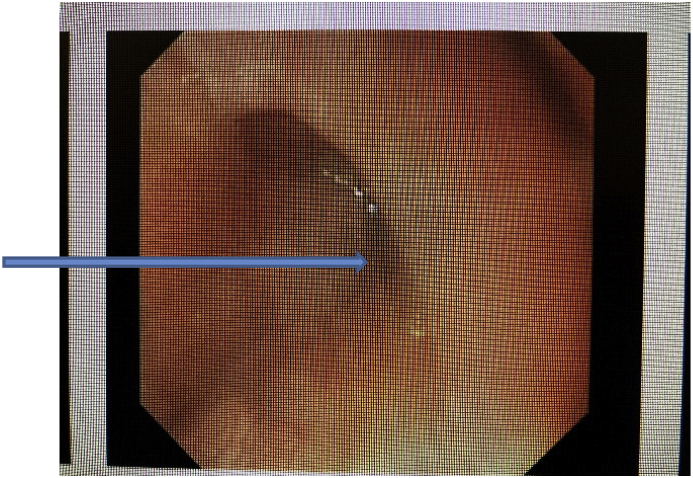
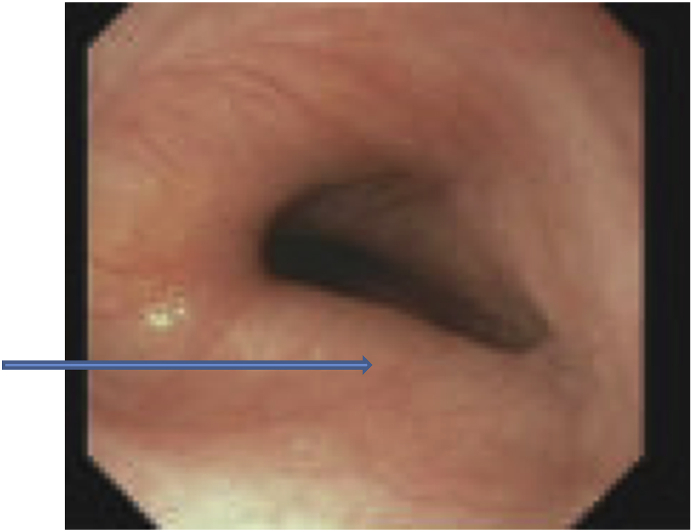
Concurrent esophagogastroduodenoscopy revealed a compressed portion in the upper to mid third of esophagus. The esophagus could be minimally distended in this region, but not to the same caliber as the rest of the esophagus. Dilation or significant pressure was not required to advance distally. The mucosa was visually normal, and the proximal esophagus was not noted to be dilated. Fig. 4.
Fig. 4.
Arrow points to the moderate to severe esophageal compression seen on EGD.
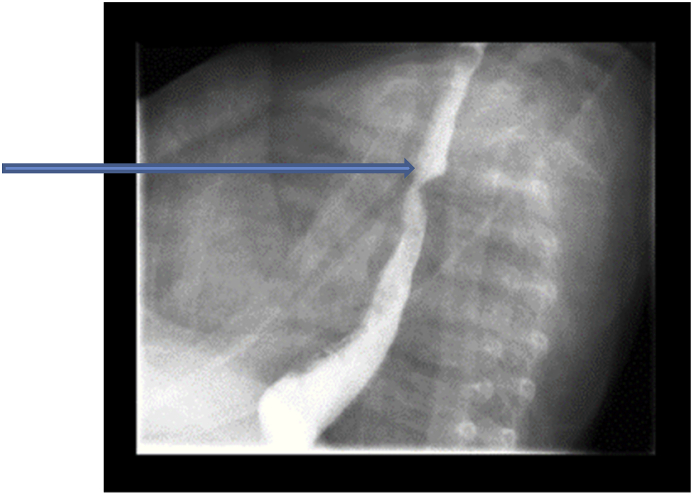
Following completion of the combined endoscopic procedures, and with continued cough related to feeding, a video fluoroscopic swallow study was performed. The study confirmed the presence of a compression, consistent with an aberrant right subclavian artery. Fig. 5.
Fig. 5.
Arrow points to the barium swallow showing a posterior esophageal indentation.
A repeat endoscopy with bronchoscopy performed approximately one year later due to vague reports of difficulty swallowing solids, showed improved esophageal lumen in the region of the aberrant subclavian artery, without mucosal disease or proximal esophageal dilation. Fig. 6.
Fig. 6.
Arrows points to the improved esophageal luminal diameter at age 16 months.
During the most recent pulmonary clinic follow up the patient was doing well. She showed good weight gain, was tolerating solids, and continued to be on room air with a normal respiratory exam.
3. Discussion
Developmentally, aberrant subclavian artery is one of the most common arch anomalies. It arises as the last brachiocephalic branch of the aorta and makes a U-turn to reach the right half of the body [17]. It is present in 0.7–2% of the population with a left aortic arch and 0.04–0.4% with a right aortic arch and aberrant right subclavian artery [9,14].
In 80% of cases the aberrant subclavian artery crosses behind the esophagus (18). In 15% it crosses in between the trachea and the esophagus and in 5% it crosses anterior to the trachea [16]. Although most patients are asymptomatic, but 25%–37% patients have congenital cardiac defects such as conotruncal abnormalities or other chromosomal abnormalities [[9], [10], [11],14,20].
The most commonly reported chromosomal abnormality with an aberrant right subclavian is Trisomy 21. In a large case series of 106 fetuses with Trisomy 21, the rate of this vascular anomaly was 25% in the second trimester ultrasound [15,20].
In a longitudinal study by Jan et [[9], [18]], there was a slight female gender predominance of this vascular anomaly, which has been reported in many other studies. They also report that the best way to identify this vascular anomaly is by echocardiogram, CT scan or barium esophagram [9].
Most cases of aberrant right subclavian artery are asymptomatic, especially if there are no additional aortic arch/vessel anomalies. It is typical to have respiratory symptoms in infancy but dysphagia can occur at an older age when solid or semisolids are introduced, commonly known as dysphagia lusoria [6,14,21]. According to Klinkhamer [17], severe cases of tracheoesophageal compression occur if the right and left carotid arteries arise from the aortic arch too close together or have a common origin which can result in compression of the trachea due to inability of the trachea to mobilize or escape the compression. The aberrant subclavian artery can be associated with an aneurysmal dilatation at the base known as Kommerell's diverticulum, which can rupture if left untreated [6,14].
Extrinsic compression of the airway and esophagus caused by an aberrant right subclavian artery is rare in infancy. This disorder ranges from being asymptomatic to circumstances of respiratory distress, stridor, wheeze, dyspnea, recurrent respiratory tract infections or feeding problems which may necessitate surgical intervention [9,17]. Some patients may experience feeding problems. This may increase their risk for recurrent aspiration and respiratory tract infections [9]. Jan et al., found that neonates with an aberrant right subclavian artery had a smaller birth size but long term growth rates were unaffected. They proposed a non-surgical approach for asymptomatic infants with isolated aberrant right subclavian vessel. They noted that larger studies are needed to assess the long term outcomes of affected infants [9].
The extrinsic compression of the esophagus causing a bulge to the posterior aspect of trachea can cause wheezing, stridor, recurrent pneumonia, cough and cyanosis [5,16].
It is important to consider a complete aerodigestive work up, CT scan, upper GI tract endoscopy, and airway evaluation.
According to Naqvi et al., aberrant right subclavian artery may have dysphagia lusoria, which was described by Bayford in 1787 [7,17] as ‘difficulty in swallowing due to quirk of nature’. Dysphagia may be initially with solids but progress to liquids as well. This is more common in adults when the tracheal rings have more cartilage and are less compressive [17]. The presence of Komerrell's diverticulum may worsen the dysphagia and needs to be evaluated [6]. It is also important to exhibit caution when considering a thickened liquid trial given clinical symptoms of coughing and congestion with feeding. In this case, a thickened liquid would have exacerbated respiratory symptoms.
Barium esophagram is a simple and very valuable test in identifying many vascular anomalies such as pulmonary slings, complete aortic ring or aberrant vessels compressing the esophagus. It typically shows a lateral indentation in the case of an aberrant subclavian artery [8,12]. Given the nonspecific symptoms of coughing and congestion with feeding, a modified barium swallow study with esophageal sweep should be considered. Given the known link between pharyngeal and esophageal dysphagia, their simultaneous investigation can prove more efficacious for our patients in relation to time, cost and length of work up to diagnosis. Watts et al., [19] reported that one in four patients (26%) had an esophageal cause of their dysphagia that would have gone unnoticed in a traditional MBS without following the bolus to the lower esophageal sphincter.
There is a case report by Still et al. [7] of a patient with history of TEF repair presenting with recurrent pneumonia and chronic barky cough due to possible post-surgical retrotracheal course of the aberrant right subclavian artery resulting in anterior wall esophageal and posterior tracheal wall compression. There is a case report of a development of a TEF in an adult from an aberrant right subclavian following a retroesophageal course with esophageal occlusion and small perforation of the esophagus into the trachea leading to chronic malnutrition and aspiration pneumonitis in the absence of a diverticulum [6].
The treatment of an aberrant right subclavian artery can vary by age and additional associated vascular malformations. In healthy infants and young children with less severe disease, where malformations are not likely obvious, the multidisciplinary approach of the aerodigestive team will lead to early and accurate diagnosis, and dramatically improve outcomes.
In older adults treatment options vary from esophageal dilatations to the use of proton pump inhibitors and occasionally thoracic vascular surgery based on the location and any other nerve or thoracic duct anomalies [13,22].
This case highlights the importance of an aerodigestive team in the care of a young infant with an unusually severe lung disease and oxygen dependence. The patient's symptoms of dysphagia and poor weight gain led to airway and endoscopic evaluations. With time and growth her proximal esophageal compression improved and she was able to tolerate solid foods without aspiration.
Funding source
No funding was secured for this study.
Potential conflicts of interest
The authors have indicated they have no potential conflicts of interest to disclose.
Author disclosure
Drs Baig, Fortner, Rivera, Gupta, Sher, Mortelitti and Ms. Merrow have disclosed no financial relationships relevant to this article. This commentary does not contain a discussion of an unapproved/investigative use of a commercial product/device.
Acknowledgements
The authors would like to express their gratitude to Ms. La Shaun Jones, administrative assistant for her help with the manuscript.
References
- 1.Freed K., Low V.H. The aberrant subclavian artery. AJR Am. J. Roentgenol. 1997;168:481–484. doi: 10.2214/ajr.168.2.9016231. [DOI] [PubMed] [Google Scholar]
- 2.Polednak A.P. Prevalence of the aberrant right subclavian rtery reported in a published systematic review ofcadaveric studies: the impact of an outlier. Clin. Anat. 2017;30:1024–1028. doi: 10.1002/ca.22905. [DOI] [PubMed] [Google Scholar]
- 3.Natsis K., Didagelos M., Gkiouliava A., Lazaridis N., Vyzas V., Piagkou M. The aberrant right subclavian artery: cadaveric study and literature review. Surg. Radiol. Anat. 2017;39:559–565. doi: 10.1007/s00276-016-1796-5. [DOI] [PubMed] [Google Scholar]
- 4.Epstein D.A., Debord J.R. Abnormalities associated with aberrant right subclavian arteries—a case report. Vasc. Endovasc. Surg. 2002;36:297–303. doi: 10.1177/153857440203600408. [DOI] [PubMed] [Google Scholar]
- 5.Barone C., Carucci N., Romano C. A rare case of esophageal dysphagia in children: aberrant right subclavian artery. Case Rep Pediatr. 2016 doi: 10.1155/2016/2539374. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naqvi S.E., Beg M.H., Thingam Ski, Ali E. Aberrant right subclavian artery presenting as tracheoesophageal fistula in a 50-year-old lady: case report of a rare presentation of a common arch anomaly. Ann. Pediatr. Cardiol. 2017;10:190–193. doi: 10.4103/apc.APC_158_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Still G.G., Li S., Wilson M., Wong L., Sammut L. Retrotracheal aberrant right subclavian artery: congenital anomaly or post-surgical complication. Global Pediatric Health. Feb 2018;5:1–5. doi: 10.1177/2333794X18762689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ming J., Chen C.-T., Wang J.-N., Wu M.H., Lin C.-H., Yang Y.-J. Upper airway obstruction caused by Vascular anomalies in children. Acta Paediatr. Sin. 1996;37(2) [PubMed] [Google Scholar]
- 9.Jan S.-L., Lin M.-C., Chan S.-C. Mid-term follow up study of neonatal isolated aberrant right subclavian artery. Cardiol. Young. 2018;28:1024–1030. doi: 10.1017/S1047951118000872. [DOI] [PubMed] [Google Scholar]
- 10.Atanas0va D., Markov D., Pavlova E. Aberrant right subclavian artery – a new ultrasound marker for chromosomal fetal abnormalities. Akush. Ginekol. (Mosc.) 2015;54:12–17. [PubMed] [Google Scholar]
- 11.Scala C., Maggiore L.R., Cadiani M. Aberrant right subclavian artery in fetuses with DS: a systematic review and meta-analysis. Ultrasound obstetric Gynecol. 2015;46:266–276. doi: 10.1002/uog.14774. [DOI] [PubMed] [Google Scholar]
- 12.Chen F.-L., Liaw Y.-P., Hsu S.-Y., Nfor O. Association between vascular rings and learning performance: a cross sectional study. J. Clin. Ultrasound.Nov/December. 2017;45(9) doi: 10.1002/jcu.22502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arakoni R., Merrill R., Simon E. Foreign body sensation: a rare case of dysphagia lusoria in a healthy female. AJEM (Am. J. Emerg. Med.) 2018;36:2134. doi: 10.1016/j.ajem.2018.08.029. e1-e2. [DOI] [PubMed] [Google Scholar]
- 14.Myers P.O., Fasel J.H.D., Kalangos A., Gailloud P. Arteria lusoria: developmental anatomy, clinical, radiological and surgical aspects. Annales de cardiologie et d’ange’iolgie. 2010;59:147–154. doi: 10.1016/j.ancard.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Paladini D., Sglavo G., Pastore G., Masucci A., D'Armiento M.R., Nappi C. Aberrant right subclavian artery: incidence and correlation with other markers of Down syndrome in second-trimester fetuses. Ultrasound Obstet. Gynecol. 2012;39:191–195. doi: 10.1002/uog.10053. [DOI] [PubMed] [Google Scholar]
- 16.Derbel B., Saaidi A., Kasraoui R., Chaouch J., Aouini F., Romdhane N., Manna J., Tunisie T. Ann. Vasc. Surg. 3. Vol. 26. April 2012. April 2012. Aberrrant Right Subclavian Artery or Ateria Lusoria: A Rare Case of Dyspnea in Children; pp. 419.e1–419.e4. [DOI] [PubMed] [Google Scholar]
- 17.Klinkhamer A. Aberrant right subclavian artery. Clinical and Roentgenologic aspects. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1966;97:438–446. doi: 10.2214/ajr.97.2.438. [DOI] [PubMed] [Google Scholar]
- 18.Natsis K., Didagelos M., Gkiouliava A., Lazaridis N., Vyzas V., Piagkou M. The aberrant right subclavian artery: cadaveric study and literal review. Surg. Radiol. Anat. 2017;39:559–565. doi: 10.1007/s00276-016-1796-5. [DOI] [PubMed] [Google Scholar]
- 19.Watts S., Gaziano J., Jacobs J., Richter J. Improving the diagnostic capability of the modified barium swallow study through standardization of an esophageal sweep protocol. Dysphagia. 2019;34:34–42. doi: 10.1007/s00455-018-09966-5. [DOI] [PubMed] [Google Scholar]
- 20.Zapata, Edwards, Titus Aberrant Right subclavian artery with left aortic arch: associated Cardiac Anomalies. Pediatr. Cardiol. 1993;14:159–161. doi: 10.1007/BF00795645. [DOI] [PubMed] [Google Scholar]
- 21.Polguj, Chrzanowski, Kasprazk, Stefacncyk, Topol, Majos The Aberrant right Subclaivan Artery (Ateria Lusoria): the Morphological and Clinical Aspects of one of the most important variations-A systemic study of 141 reports. Sci. World J. 2014;2014:292734. doi: 10.1155/2014/292734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epstien, DeBord Abnormalities associated with aberrant right subclavian arteries: a case report. Vasc. Endovasc. Surg.J. 2002;36:297–303. doi: 10.1177/153857440203600408. [DOI] [PubMed] [Google Scholar]