Abstract
Background
The aim of this study was to evaluate the effectiveness of gracilis muscle transposition (GMT) to treat recurrent anovaginal, rectovaginal, rectourethral, and pouch–vaginal fistulas in patients with inflammatory bowel disease (IBD).
Methods
A retrospective study was conducted in patients with IBD who had GMT performed by a single surgeon between 2000 and 2018. Follow-up data regarding healing rate, complications, additional procedures, and stoma closure rate was collected.
Results
A total of 30 women and 2 men had GMT. In all patients fistula was associated with Crohn's disease. In 1 female patient, contralateral gracilis transposition was required after a failed attempt at repair. The primary healing rate was 47% (15/32) and the definitive healing rate (healed by the time of data collection and after secondary procedures) was 71% (23/32). Additional surgical procedures due to fistula persistence or recurrence were performed on 17 patients (53%).At least 7 patients (21%) suffered complications including one wound infection with ischemia of the gracilis muscle. Stoma closure was successful in 18 of 31 cases of patients with stoma (58% of the patients).
Conclusions
GMT for the treatment of recurrent and complex anorectal fistulas in patients with IBD patient is eventually successful in almost 2/3 of patients.
Keywords: Crohn disease, Fecal incontinence, Surgical flaps, Surgical stomas
Introduction
Treatment of recurrent and complex anorectal fistulas is challenging. Surgical therapy for fistulas in patients with inflammatory bowel disease (IBD) is associated with high recurrence rates. Fistulas in patients with IBD result from chronic relapsing inflammation with subsequent intestinal fibrosis and stricture formation, and could be triggered by the epithelial–mesenchymal transition [1–3]. The etiology of the fistula reflects the existing comorbidities, which should also be addressed intraoperatively (e.g., perineal defects and scar tissue with compromised local vascularization as a result of previous colorectal procedures). Depending on the location of the fistula, different approaches can be used, including transabdominal, transperitoneal, or local techniques. A number of local surgical methods exist, such as excision, advancement flaps, and fibrin glue or biomesh application. Autologous muscle flaps—such as the Martius, sartorius, and gracilis flaps—are muscles flaps—such as the Martius, sartorius, and gracilis flaps—are further methods for fistula closure. However, no consensus regarding the preferred method of choice has been reached. Combining different procedures after an insufficient fistula closure may be useful to improve the efficacy of a single method. Larger recent studies [4–10] on GMT, including those of Ulrich et al. [6] and Wexner et al. [9], have demonstrated some excellent results.
Materials and methods
Study design and patients
A two-center retrospective study was performed from January 2000 to May 2018 in patients diagnosed with IBD. All patients underwent GMT at the surgery departments of the University Hospital Regensburg and the St. Josef Hospital Regensburg, Germany, between January 2000 and May 2018 to treat anovaginal, rectovaginal (RVF), rectourethral (RUF), and pouch–vaginal fistulas. All gracilis transpositions were performed by a single surgeon (AF). IBD patients who underwent GMT strictly due to fecal incontinence and not for fistula treatment were excluded. Follow-up procedures in cases of fistula recurrence were performed by other surgeons. Prior to GMT, all patients had undergone at least one failed attempt at fistula repair using various other procedures such as fistulotomy, fistulectomy, mucosal advancement flap, fistula plug, fibrin glue application.
Surgical technique
GMT was performed according to a standardized procedure [11]. We combined the GMT in all cases but one with a fecal diversion performing a loop ileostomy if no stoma existed. We prefer performing a GMT when a stoma is already established to reduce the local infection beforehand. Antibiotic prophylaxe with cefuroxime und metronidazole was given perioperatively. The fistula tract was identified by probing (Fig. 1) and excised completely. The defect on the rectal side was minimized with interrupted sutures. The gracilis muscle was identified and an incision measuring approximately 10–12 cm was made on the medial side of the thigh (Fig. 2). The distal muscle tendon was then identified by palpation near the pes anserinus and divided after a small incision medial to the knee joint. The muscle was mobilized and the small collateral blood vessels in the distal part of the muscle were ligated using ties for the bigger blood vessels and coagulation for the smaller ones. During mobilization, great care was taken to find a tension-free position of the neurovascular bundle on the lateral proximal side of the gracilis muscle. A subcutaneous tunnel between the ipsilateral proximal medial side of the thigh and the perineal region was created by blunt preparation. The muscle then was pulled through the tunnel and placed exactly underneath the suture line closing the rectal side of the fistula (Fig. 3), covering the rectum, placing well-vascularized tissue in the defect area (Fig. 4). Rotation of the muscle around its axis was carefully avoided. The distal tendon of the muscle was then fixed with Prolene sutures (Ethicon, Bridgewater, NJ and Cincinnati, OH, USA) to the contralateral pubic or ischial periosteum after transposition through an additional subcutaneous tunnel and a small incision on this side. Finally, the gracilis muscle was covered by a soft tissue flap (Fig. 5). If the medical history of the patient indicated fecal incontinence, the distal muscle tendon was transposed further to encircle the anal canal, and fixed at the ipsilateral pubic or ischial periosteum, thus creating a loop (gamma-loop; Fig. 6). This procedure potentially increases anal continence by contributing additional muscle tension. A secondary dynamization of the gracilis muscle remains an additional option for optimization of anal sphincter function. If no fecal diversion existed preoperatively, a protective stoma was added simultaneously. Only 1 patient opted against a protective stoma.
Fig. 1.
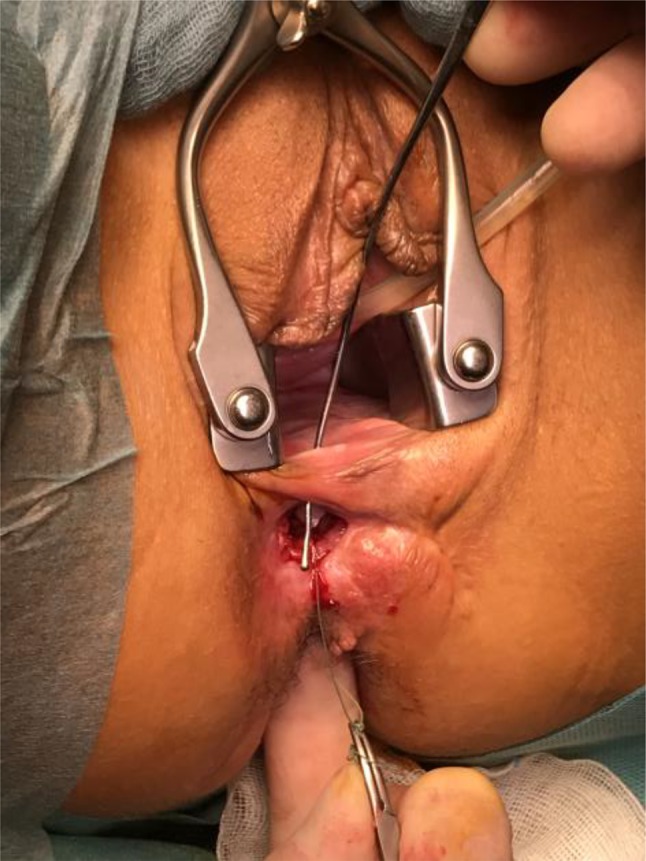
Complex rectovaginal and perineal fistula combined with a perineal defect
Fig. 2.
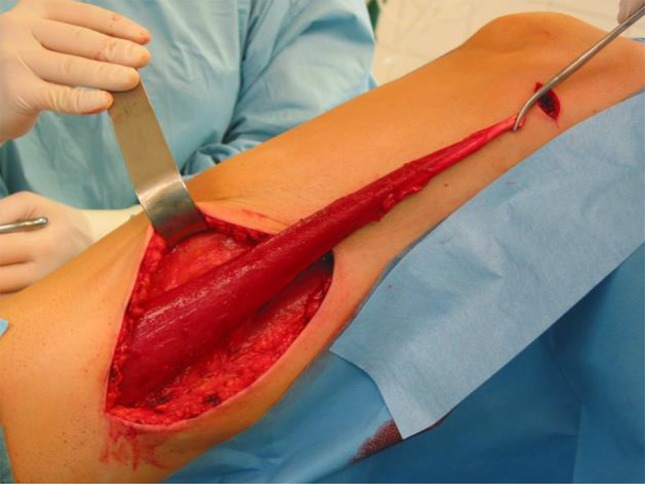
Mobilization of the gracilis muscle
Fig. 3.
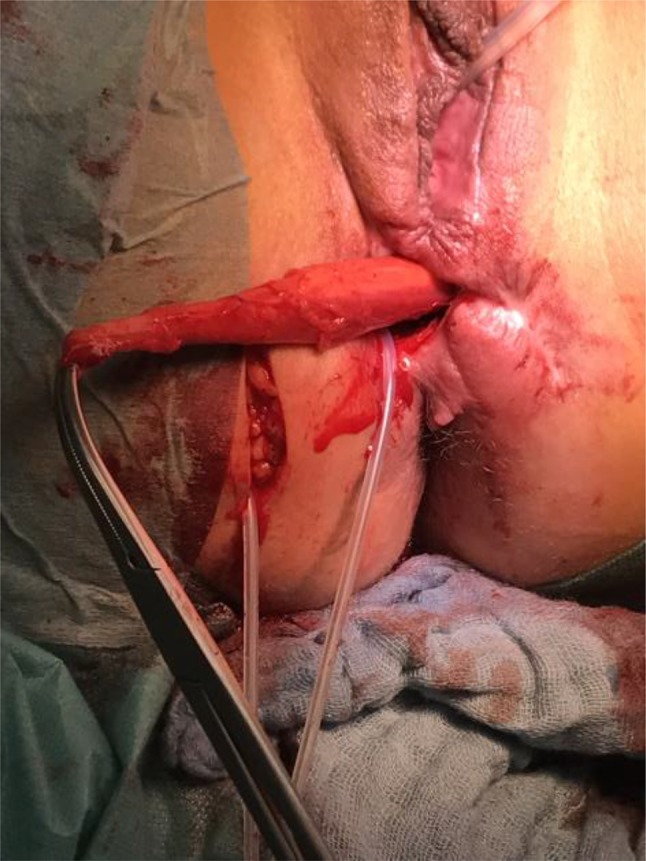
Transposition of the gracilis muscle
Fig. 4.
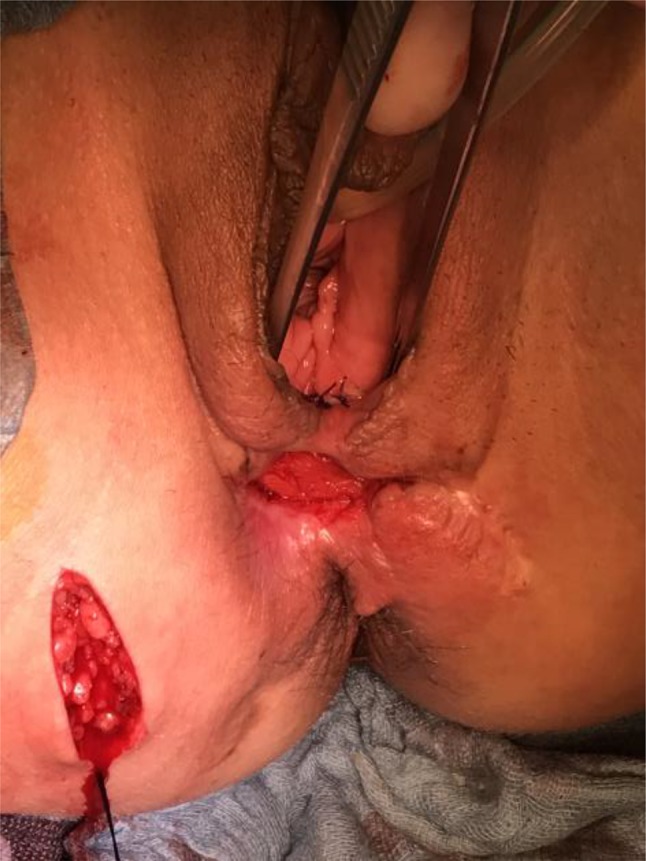
Fixation of the gracilis muscle on the contralateral pubic periost (tunneling technique)
Fig. 5.
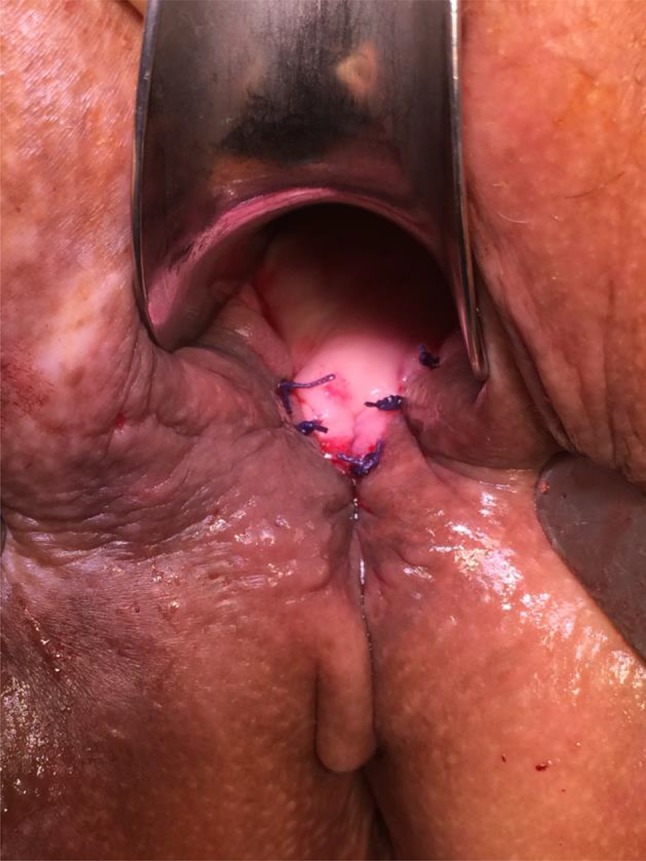
Vaginal flap to cover the gracilis muscle
Fig. 6.
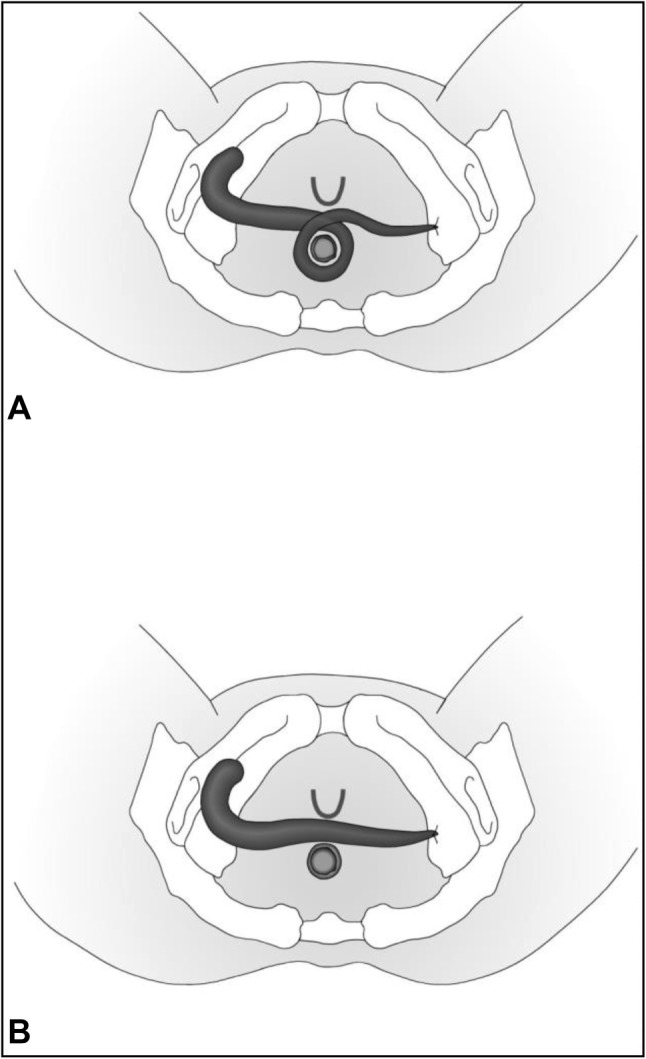
Types of gracilis muscle transposition (GMT) [10]. a Circular GMT, b transverse GMT
Outcome assessment
The primary outcome measure was complete closure of the fistula by the first follow-up (approximately 3 months postoperatively) without additional follow-up operations. In patients with anovaginal, rectovaginal, and pouch–vaginal fistulas, the initial follow-up was performed 3 months postoperatively. A sensitive sign of the presence of a residual fistula is a feeling of air-trapping and vaginal discharge. All patients had clinical examination, including anoscopy, air insufflation, and fistula probing (in selected cases under general anesthesia). In case of uncertainty regarding a residual fistula, a water-soluble contrast enema, anorectal endosonography, or pelvic magnetic resonance imaging was performed. Stoma reversal was performed upon definitive closure of a fistula tract.
Secondary outcomes included fistula closure after follow-up procedures in cases of fistula persistence and stoma closure rate. Fistula-free time in cases of late recurrences was also documented.
Data analysis
Information regarding patients’ demographic characteristics, number of previous operations, types of fistula, fistula closure, complications, follow-up procedures, and stoma closure was collected retrospectively from medical records. Data were gathered in a Microsoft Excel Sheet Version 2010 (Microsoft Corporation, Redmond, WA, USA). Data are presented using summary statistics in the form of means and median values for quantitative data and percentages for qualitative data.
Ethical considerations
Due to the retrospective nature of the study, formal consent was not required. The study was approved by the local ethics committee of the authors’ study center.
Results
A total of 32 patients (2 male, 30 female; mean age 39 years, range 24–55 years) with IBD underwent gracilis transposition or re-transposition for a recurrent fistula at the authors’ institutions between January 2000 and March 2018 and attended at least the first follow-up visit at 3 months. Two patients with less than 3 months of follow-up were also included (one because of graft ischemia and the other because of a persistent fistula) to minimize selection bias and present a more realistic recurrence and complication rate. All patients had undergone diverse previous surgical procedures for fistula closure (mean 2 procedures, range 1–25 procedures) before the gracilis transposition. Mean follow-up time after GMT was 47 months (range 1–144 months). After GMT, some patients suffered a recurrence or had a smaller residual fistula, prompting different follow-up procedures to achieve definitive closure (Tables 1, 2 and 3).
Table 1.
Additional procedures after GMT for patients with recurrence
| Patient | Date of GMT | Follow-up procedures | Last follow-up |
|---|---|---|---|
| I | 09/2002 |
12/2002 stoma closure 12/2005 recurrence 03/2006 abscess excision and fistulectomy 05/2006 fistulectomy and double barrel ileostomy |
09/2013 fistula not closed |
| II | 04/2005 |
07/2005 stoma closure 02/2010 recurrence/ new fistula 02/2010 fistulectomy 04/2010 fistulectomy, rectal muscle flap and fistula plug 08/2010 vaginal advancement flap 09/2010 loop sigmoidostomy 11/2010 fistulectomy and vaginal flap 03/2011 stoma closure |
03/2011 fistula closed |
| III | 04/2013 |
07/2013 stoma closure 10/2013 recurrence 11/2013 biomesh 12/2013 seton 06/2015 vaginal flap 03/2018 seton |
03/2018 fistula not closed |
| IV | 06/2009 |
09/2009 stoma closure 08/2014 recurrence after crohn relapse 10/2014 rectal flap 11/2014 fistulectomy and rectal flap 02/2015 rectal flap and double barrel colostomy 03/2015 rectal and vaginal flap 05/2015 rectal flap 09/2017 fistulectomy and rectal flap 02/2018 martius flap |
03/2018 fistula not closed |
| V | 12/2015 |
02/2016 stoma closure 04/2017 abscess excision 07/2017 fistulectomy |
08/2017 fistula closed |
| VI | 02/2016 |
09/2016 stoma closure 11/2016 fistulectomy |
12/2017 fistula closed |
| VII | 10/2005 |
07/2006 stoma closure 03/2007 recurrence 03/2007 abscess excision 12/2007 seton and double barrel ileostomy |
12/2007 house ban, fistula not closed |
| VIII | 05/2016 |
12/2016 recurrence 12/2016 abscess excision 02/2017 fistulectomy and rectal flap 03/2017 abscess excision 03/2018 stoma closure |
03/2018 fistula closed |
| IX | 05/2006 |
10/2006 recurrence 11/2006 fistulectomy and rectal advancement flap |
11/2009 fistula closed |
Table 2.
Additional procedures after GMT for patients with fistula persistence
| Patient | Date of GMT | Follow-up procedures | Last follow-up |
|---|---|---|---|
| I | 03/2016 |
Persistence 05/2016 fistulectomy 04/2017 fistulectomy and rectal flap 03/2018 fistulectomy and rectal flap |
03/2018 fistula not closed |
| II | 05/2014 |
Persistence 04/2015 rectal flap 11/2016 stoma closure |
11/2016 fistula closed |
| III | 03/2015 |
Persistence 06/2015 rectal flap 10/2015 stoma closure |
10/2015 fistula closed |
| IV | 01/2000 |
Persistence 03/2000 vaginal flap 09/2007 proctocolectomy and converting loop ileostomy in double barrel ileostomy (not because of fistula) 05/2010 abscess excision |
01/2012 fistula closed |
| V | 07/2014 |
Persistence 09/2014 vaginal flap 02/2015 rectal flap 05/2015 vaginal flap 12/2015 vaginal flap 03/2017 seton |
11/2017 fistula not closed |
| VI | 02/2016 |
Persistence 04/2016 vaginal flap |
04/2016 fistula not closed |
| VII | 05/2012 |
Persistence 10/2012 fistulectomy, vaginal and rectal flap and fistula plug 02/2013 fistulectomy and vaginal flap |
04/2013 fistula not closed |
| VIII | 07/2015 | 08/2015 wound infection with necrosis and excision of the gracilis flap | 10/2015 fistula not closed |
Table 3.
Fistula closure rate after follow-up procedures depending on gender
| Female IBD patients | Male IBD patients |
|---|---|
| n = 21/30 | n = 2/2 |
| 70% | 100% |
IBD inflammatory bowel disease
Of the 32 patients with IBD, 1 developed a wound infection with necrosis of the gracilis flap and 7 had a residual fistula at the first postoperative follow-up and required additional procedures. During the subsequent follow-up period, 9 further patients had a recurrent or new fistula and one patient had an abscess without a verification of a fistula (median time after GMT among these 9 patients before recurrence: 17 months; range 5–62 months). In 7 of those cases a recurrence took place after stoma closure (median time after stoma closure until recurrence 17 months, range 2–59). It was difficult to distinguish whether the patient had a recurrence or a new spontaneous fistula due to Crohn’s disease (CD). In 1 patient, a recurrence was documented directly after a CD relapse. The primary fistula closing rate where the fistula stayed closed during the follow-up and no further procedures were needed was 47% (15/32).
In total, an additional 43 operations were required for the 17 patients with recurrences and persistence of a fistula after GMT to achieve a definitive fistula closure rate after recurrences of 71% (23/32) among all 32 patients (Table 1). Of these operations, 17 were performed to close a recurrent or persistent fistula in 8 patients (2.1 follow-up procedures per patient per closed fistula after recurrence or persistence). Despite performing a total of 26 other follow-up procedures on 9 other patients, those 9 other patients had a persistent fistula and in one another case there is a suspicion of a recurrence due to an abscess without a clinical proof of a fistula.
At the time of GMT, 15 patients were not taking any specific CD medication (Table 4). In six of those cases the fistula healed without follow procedures. Only in 4 of those 15 patients (healing rate 73%) without specific CD medication at the time of GMT a fistula remained after follow procedures. In 5 of the 17 cases (healing rate 71%) where patients took a specific CD medication a residual fistula remained after follow procedures. However, the p value for comparing the healing rate with or without specific CD medication is 0.589 and thus not of statistical significance.
Table 4.
CD medication at the time of GMT
| Patient no. | Gender | CD onset (year) | GMT (year) | Fistula closed | Perioperative IBD medication | IBD Medication before GMT | IBD medication after GMT |
|---|---|---|---|---|---|---|---|
| 1 | f | 1984 | 2000 | y | Short chain fatty acid enema | 1997 steroids; 1998 azathioprine | 2002 and 2005 mesalazine foam, steroids |
| 2 | f | 1985 | 2001 | y | Azathioprine, steroids, colifoam | Unknown | Unknown |
| 3 | f | 1989 | 2004 | y | None | Unknown | Unknown |
| 4 | f | 1988 | 2004 | y | Azathioprine and steroids | 2001 azathioprine, steroids | 2007 azathioprine, steroids |
| 5 | f | 1984 | 2010 | y | None | Unknown | Unknown |
| 6 | f | 2003 | 2010 | y | Mercaptopurine | 02/2010 adalimumab, mercaptopurine | Unknown |
| 7 | m | 2000 | 2005 | y | None | Unknown | 09/2005 azathioprine |
| 8 | m | 1989 | 2012 | y | Azathioprine | 11/2011 azathioprine | 12/2012 azathioprine |
| 9 | f | 2002 | 2014 | y | adalimumab (pause) | 12/2013 adalimumab | 03/2015 adalimumab |
| 10 | f | 1994 | 2010 | y | Adalimumab, azathioprine, steroids | Unknown | Unknown |
| 11 | f | 2006 | 2005/2006 | y | None | Unknown | Unknown |
| 12 | f | 1988 | 2002 | n | Azathioprine, steroids, mesalazine foam | Unknown | 2005, 2006, 2013 azathioprine |
| 13 | f | 2001 | 2005 | y | None | Unknown | 2010 none; 2011 none; 2014 steroids |
| 14 | f | 1984 | 2006 | y | None | Unknown | 2016 azathioprine |
| 15 | f | 1997 | 2005 | n | None | Infliximab | 2007 azathioprine, steroids |
| 16 | f | 2012 | 2012 | n | None | None | None |
| 17 | f | 1995 | 2012 | n | Steroids | Unknown | Unknown |
| 18 | f | 2005 | 2013 | n | None | Unknown | 07/2013 sulfasalazine supp |
| 19 | f | 2007 | 2009 | n | Methotrexate (MTX) | Unknown | 09/2009 MTX; 2014–2015 MTX, steroids; 10/2015–2016 steroids, golimumab; 09/2016 adalimumab |
| 20 | f | 1999 | 2014 | y | Sulfasalazine | 02/2012 sulfasalazine | 2014 sulfasalazine; 2015 sulfasalazine, steroids, azathioprine |
| 21 | f | 2002 | 2014 | y | None | Unknown | 11/2016 none |
| 22 | f | 2006 | 2015 | y | None | 10/2014 steroids, adalimumab | 10/2015 vedolizumab |
| 23 | f | 1988 | 2000 | y | Azathioprine, steroids, sulfasalazine | Unknown | 10/2002 steroids, azathioprine; 2006 azathioprine |
| 24 | f | 2010 | 2015 | y | Azathioprine | Azathioprine, mesalazine | 10/2015 azathioprine |
| 25 | f | 2005 | 2016 | y | None | Unknown | None |
| 26 | f | 1998 | 2015 | y | None | Unknown | None |
| 27 | f | 1995 | 2015 | y | Sulfasalazine | Unknown | 07/2017 steroid foam |
| 28 | f | 2005 | 2016 | y | None | Infliximab | Unknown |
| 29 | f | 2007 | 2016 | y | Vedolizumab (pause) | Vedolizumab | 02/2017 vedolizumab; 03/2018 ustekinumab |
| 30 | f | 1992 | 2015 | n | None | Unknown | None |
| 31 | f | 1998 | 2016 | n | Mercaptopurine | Mercaptopurine | 2016 mercaptopurine |
| 32 | f | 2008 | 2016 | n | Azathioprine, steroids | Azathioprine, steroids | 05/2016 azathioprine, steroids; 04/2017 azathioprine |
f female, m male, y yes, n no, GMT gracilis muscle transposition, CD Crohn’s disease, IBD inflammatory bowel disease
GMT was performed without fecal diversion in 1 patient and the fistula healed anyway. In 19 patients, the stoma could be reversed after fistula closure and in 2 patients, stoma reversal is intended after fistula healing. A late recurrence after stoma reversal was suffered by 7 patients, 4 of whom received a new stoma with follow-up surgical procedures thereafter. As a result, in 3 of the 7 latter cases, fistula closure was achieved. In summary, a stoma reversal after follow-up procedures due to fistula persistence or recurrence was achieved in 18 of 31 (58%) of the stoma patients and in two other cases where a fistula closure has been achieved a stoma closure is being planned.
Mean age was 42 years (median 43 years, range 24–54 years, 95% CI 33–51 years) in the patients with complete remission after GMT and 40 years (median 41 years, range 27–55 years, 95% CI 33–49 years) in the patients who suffered a recurrence (p = ns).
The mean number of operations before GMT was 4.3 (median3, range 1–20) and 4.4 (median2, range 1–25) in the groups who did not and did suffer a recurrence, respectively (p = ns).
There was no difference in fistula closure rates between patients who received a gamma-loop of gracilis muscle to the ipsilateral pubic ramus (7/10, 70%) and those who underwent GMT on the contralateral pubic ramus (15/22, 68,1%) (p = ns).
Data were collected on fistula closure and the closure rate by fistula etiology was calculated (Table 5).
Table 5.
Fistula closure rate among patients with IBD
| Fistula types | Fistula closure rate | Stoma closure rate |
|---|---|---|
| Rectovaginal fistula n = 21 | 71% (incl. one patient with an abscess after GMT without fistula proof) | 55% (1 patient operated without stoma and 1 patient opting against stoma closure after fistula closure) |
| Anovaginal fistula n = 2 | 50% | 50% |
| Anal fistula n = 4 | 100% | 100% |
| Pouch fistula n = 3 | 67% | 67% |
| Complex fistula n = 1 | 0% | 0% |
| Rectourethral fistula n = 1 | 100% | 0%a |
| Total n = 32 | 71% | 58% |
IBD inflammatory bowel disease
aStoma closure being planned
Complications
Aside from 1 case of wound infection with ischemia of the gracilis muscle, 1 case of postoperative hemorrhage in the thigh 2 cases of suture granuloma and complaints about scar tissue and some numbness on the operated thigh, no other major complications following harvesting and inserting of the gracilis muscle were documented.
When a new fecal diversion was performed during the same session, some patients needed postoperative intravenous fluid and laxative agents due to ongoing paralytic ileus, but no surgical interventions were needed (Table 6).
Table 6.
Complications
| Complications | Number of patients |
|---|---|
| Postoperative hemorrhage | 1 |
| Thigh swelling | 1 |
| Lymphoedema | 1 |
| Suture granuloma | 2 |
| Knee pain | 1 |
| Thigh hypoesthesia | 2 |
| Necrosis of the gracilis muscle | 1 |
Discussion
Muscle grafts, particularly gracilis muscle grafts, have been in use since the 1930s. It should be noted that the gracilis muscle does not close the existing fistulas per se, but is used to reduce dead space in the perineum and improve vascularization and healing in areas with compromised blood supply due to soft tissue and muscle loss as a result of previous operations. GMT can be combined with other surgical interventions and, thus, improve the rate of fistula closure.
Alternative approaches include transperineal, endorectal, endovaginal, and transabdominal procedures. Fistula closure can be achieved directly using sutures or glue, with a mucosal flap or biomaterials, via partial bowel resection with anastomosis, or through muscle flap transposition. Fecal diversion can be performed as a stand-alone treatment or used perioperatively as a supplementary measure in some surgical procedures.
Biomaterials represent a relatively new method of treating fistulas. Benefits include less intraoperative trauma and a decreased impact on fecal continence, while treating trans-sphincteric fistulas with moderate–good outcomes [12–17]. Chan et al. [18] showed good results using a fistula plug in simple fistulas, but also a decrease in efficacy after multiple procedures. Other studies showed a high rate of postoperative sepsis after implantation of fistula plugs alone [19, 20]. In combination with a draining seton or an advancement flap, the success rate increases. Long-term studies of this method are necessary to judge its efficacy.
Panés et al. [21–23] describe a promising new therapeutic approach to close treatment-refractory complex perianal fistulas in CD using a single intralesional injection of allogeneic expanded adipose-derived stem cells. This method achieved a 50% remission rate (53 of 107 patients) in an intention-to-treat population. The most common treatment-related adverse events were anal abscess and proctalgia.
The fistula closing rates by sex are shown in (Table 3); however, the significantly smaller number of patients in the male group makes comparisons difficult. All in all, we achieved a definitive fistula closing rate of 71%. Our study including 32 patients is the largest study of performing GMT on IBD patients in the literature so far.
Compared to other studies on GMT, the current study group consisted entirely of patients with IBD, which is generally associated with lower fistula healing rates using different procedures [6, 9, 24–26]. Some patients with IBD show apparent late recurrences; however, some of these may be attributable to spontaneous development of new fistula due to CD activity, rather than a recurrence of a previous fistula.
No significant difference in terms of age was detected between the groups with remission and recurrence. Therefore, the authors would recommend the procedure for IBD patients of all ages. There was also no significant difference in healing rate between patients taking CD specific medications and patients not taking any CD specific medications at time of GMT.
Fistula persistence after GMT was detected no later than at the first follow-up after GMT, and was treated accordingly. Late recurrences, however, appeared unequally distributed after a mean of 24 months after GMT (median 23 months, range 5–62 months). Almost half of the recurrences took place 5–7 months after GMT and the others appeared 1.5–5 years postoperatively. Another follow-up with clinical examination and anorectal sonography 6–9 months after GMT might be advantageous for earlier detection of a recurrence and initiation of treatment.
Adequate treatment of IBD seems to have a positive impact in preventing late recurrences. Due to the small number of patients in the present cohort, it was not possible to detect whether success after GMT correlated with the different types of CD medication.
The fact that all GMTs were performed by a single surgeon using a standardized procedure reduced the possibility of the outcome being influenced by varying skill levels of different surgeons.
The study has some limitations. First, only a small number of patients were included. Further studies with larger patient numbers are needed. There are, however, only a few similar studies on fistula treatment in IBD patients in the recent literature.
Due to its retrospective design, the current study lacks a uniform follow-up period. A defined interval between the procedure and examination of the patients could improve comparison between the different patients and success rates. The follow-up at 3 months postoperatively did not apply to all patients, mainly because some suffered a persistent fistula and were examined earlier than after 3 months. The next follow-up after the 3-month follow-up was also somewhat heterogeneous, since most patients received a further follow-up only due to complaints; a large proportion of the patients who achieved complete remission and stoma closure did not attend further examinations.
Another critical point of the study is the correlation between GMT and the fistula closure rate when additional procedures were needed. It is impossible to determine the exact extent of the contribution of GMT to the fistula closure rate when additional operations were performed after GMT. The authors are convinced that the gracilis muscle is the basis for the success of additional fistula operations. Larger trials directly comparing GMT to other surgical procedures with a long follow-up period should be performed to better understand its benefits. This would contribute to determining the best way to treat recurrent fistulas in patients with IBD (Table 7).
Table 7.
Comparison of pre-existing studies
| Author | Year | Number of patients | Fistula type | Follow-up period (months) | Success rate |
|---|---|---|---|---|---|
| Zmora et al. [8] | 2003 | 11 | RUF | 4–40 (median 18, 2) | 100% |
| Fürst et al. [10] | 2008 | 12 | RVF and anovaginal | Mean 40 | 91.6% |
| Wexner et al. [9] | 2008 | 17 |
15 × RVF 2 × pouch–vaginal |
33% (Crohn’s) and 75% (non-Crohn’s) | |
| Wexner et al. [9] | 2008 | 36 | RUF | 97% | |
| Lefevre et al. [5] | 2009 | 8 | RVF | 4–55 (median 28) | 88% |
| Ulrich et al. [6] | 2009 | 35 |
9 × RVF 26 × RUF |
Mean 28 ± 15 | 94% |
| Maeda et al. [7] | 2011 | 14 | Complex fistula | 1–88 (median 10) | 64% |
| Maeda et al. [7] | 2011 | 4 | Persistent nonhealing perineal sinus | 1–88 (median 10) | 50% |
| Chen et al. [4] | 2013 | 19 | RUF and RVF | 6–35 (median 18) | 94.7% |
| Current study | 2018 | 32 | RVF, RUF, pouch–vaginal, and anovaginal | 1–144 (mean 47) | 71% |
RUF rectourethral fistula, RVF rectovaginal fistula
Conclusions
Our results are promising and suggest that GMT is a safe and very effective option for the treatment of recurrent fistulas in IBD patients. It should be noted that for complete fistula closure, follow-up procedures may be necessary. The option of dynamization of the muscle creates the possibility of improving fecal continence and, thus, quality of life. The authors suggest that in cases of recurrence after initially addressing a fistula with conventional methods, and after repeated unsuccessful procedures, GMT should be considered.
Acknowledgements
S. Korsun contributed to study design, collected and analyzed the data, and wrote the manuscript. G. Liebig-Hoerl assisted in data collection. A. Fuerst supervised data collection, data analysis, the study, and corrected the paper.
Funding
The authors did not receive any funding for this study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest in connection with this study.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Footnotes
The original version of this article was revised due to a retrospective Open Access order.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/26/2019
The article Gracilis muscle transposition for treatment
References
- 1.Scharl M, Huber N, Lang S, et al. Hallmarks of epithelial to mesenchymal transition are detectable in Crohn’s disease associated intestinal fibrosis. Clin Transl Med. 2015;4:1. doi: 10.1186/s40169-015-0046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bataille F, Rohrmeier C, Bates R, et al. Evidence for a role of epithelial mesenchymal transition during pathogenesis of fistulae in Crohn’s disease. Inflamm Bowel Dis. 2008;14(11):1514–1527. doi: 10.1002/ibd.20590. [DOI] [PubMed] [Google Scholar]
- 3.Siegmund B, Feakins RM, Barmias G, et al. Results of the fifth scientific workshop of the ECCO (II): pathophysiology of perianal fistulizing disease. J Crohn’s Colitis. 2015;10(4):377–386. doi: 10.1093/ecco-jcc/jjv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen XB, Liao DX, Luo CH, et al. [Prospective study of gracilis muscle repair of complex rectovaginal fistula and rectourethral fistula] Zhonghua Wei Chang Wai Ke Za Zhi. 2013;16(1):52–55. [PubMed] [Google Scholar]
- 5.Lefevre JH, Bretagnol F, Maggiori L, et al. Operative results and quality of life after gracilis muscle transposition for recurrent rectovaginal fistula. Dis Colon Rectum. 2009;52(7):1290–1295. doi: 10.1007/DCR.0b013e3181a74700. [DOI] [PubMed] [Google Scholar]
- 6.Ulrich D, Roos J, Jakse G, Pallua N. Gracilis muscle interposition for the treatment of recto-urethral and rectovaginal fistulas: a retrospective analysis of 35 cases. J Plast Reconstr Aesthet Surg. 2009;62(3):352–356. doi: 10.1016/j.bjps.2008.11.067. [DOI] [PubMed] [Google Scholar]
- 7.Maeda Y, Heyckendorff-Diebold T, Tei TM, Lundby L, Buntzen S. Gracilis muscle transposition for complex fistula and persistent nonhealing sinus in perianal Crohn’s disease. Inflamm Bowel Dis. 2011;17(2):583–589. doi: 10.1002/ibd.21311. [DOI] [PubMed] [Google Scholar]
- 8.Zmora O, Potenti FM, Wexner SD, et al. Gracilis muscle transposition for iatrogenic rectourethral fistula. Ann Surg. 2003;237(4):483–487. doi: 10.1097/01.SLA.0000059970.82125.DB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wexner SD, Ruiz DE, Genua J, et al. Gracilis muscle interposition for the treatment of rectourethral, rectovaginal, and pouch-vaginal fistulas: results in 53 patients. Ann Surg. 2008;248(1):39–43. doi: 10.1097/SLA.0b013e31817d077d. [DOI] [PubMed] [Google Scholar]
- 10.Fuerst A, Schmidbauer C, Justyna S-B, et al. Gracilis transposition for repair of recurrent anovaginal and rectovaginal fistulas in Crohn’s disease. Int J Colorectal Dis. 2008;23(4):349–353. doi: 10.1007/s00384-007-0413-9. [DOI] [PubMed] [Google Scholar]
- 11.Fuerst A. Gracilis transposition for repair of recurrent rectovaginal fistula. Coloproctology. 2017;39(2):84. doi: 10.1007/s00053-017-0151-6. [DOI] [Google Scholar]
- 12.Borda Mederos LA, Chiroque Benites LI, Pinto Elera JO, Manzaneda Pineda AJ. [Experience with a biological plug for biological in complex anal fistula] Rev Gastroenterol Peru. 2011;31(4):345–350. [PubMed] [Google Scholar]
- 13.Cintron JR, Abcarian H, Chaudhry V, et al. Treatment of fistula-in-ano using a porcine small intestinal submucosa anal fistula plug. Tech Coloproctol. 2013;17(2):187–191. doi: 10.1007/s10151-012-0897-3. [DOI] [PubMed] [Google Scholar]
- 14.Kleif J, Hagen K, Wille-Jorgensen P. Acceptable results using plug for the treatment of complex anal fistulas. Dan Med Bull. 2011;58(3):A4254. [PubMed] [Google Scholar]
- 15.Schwandner T, Roblick MH, Kierer W, et al. Surgical treatment of complex anal fistulas with the anal fistula plug: a prospective, multicenter study. Dis Colon Rectum. 2009;52(9):1578–1583. doi: 10.1007/DCR.0b013e3181a8fbb7. [DOI] [PubMed] [Google Scholar]
- 16.Schwandner O, Fuerst A, Kunstreich K, Scherer R. Innovative technique for the closure of rectovaginal fistula using Surgisis mesh. Tech Coloproctol. 2009;13(2):135–140. doi: 10.1007/s10151-009-0470-x. [DOI] [PubMed] [Google Scholar]
- 17.Champagne BJ, O’Connor LM, Ferguson M, et al. Efficacy of anal fistula plug in closure of cryptoglandular fistulas: long-term follow-up. Dis Colon Rectum. 2006;49(12):1817–1821. doi: 10.1007/s10350-006-0755-3. [DOI] [PubMed] [Google Scholar]
- 18.Chan S, McCullough J, Schizas A, et al. Initial experience of treating anal fistula with the Surgisis anal fistula plug. Tech Coloproctol. 2012;16(3):201–206. doi: 10.1007/s10151-012-0810-0. [DOI] [PubMed] [Google Scholar]
- 19.El-Gazzaz G, Zutshi M, Hull T. A retrospective review of chronic anal fistulae treated by anal fistulae plug. Colorectal Dis. 2010;12(5):442–447. doi: 10.1111/j.1463-1318.2009.01802.x. [DOI] [PubMed] [Google Scholar]
- 20.Lawes DA, Efron JE, Abbas M, Heppell J, Young-Fadok TM. Early experience with the bioabsorbable anal fistula plug. World J Surg. 2008;32(6):1157–1159. doi: 10.1007/s00268-008-9504-1. [DOI] [PubMed] [Google Scholar]
- 21.Panes J, Garcia-Olmo D, Van AG, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388(10051):1281–1290. doi: 10.1016/S0140-6736(16)31203-X. [DOI] [PubMed] [Google Scholar]
- 22.Panes J, Rimola J. Perianal fistulizing Crohn’s disease: pathogenesis, diagnosis and therapy. Nat Rev Gastroenterol Hepatol. 2017;14(11):652–664. doi: 10.1038/nrgastro.2017.104. [DOI] [PubMed] [Google Scholar]
- 23.Panes J, Ordas I, Ricart E. Stem cell treatment for Crohn’s disease. Expert Rev Clin Immunol. 2010;6(4):597–605. doi: 10.1586/eci.10.27. [DOI] [PubMed] [Google Scholar]
- 24.Athanasiadis S, Oladeinde I, Kuprian A, Keller B. Endorectal advancement flap-plasty vs. transperineal closure in surgical treatment of rectovaginal fistuls. A prospective long-term study of 88 patients. Chirurg. 1995;66(5):493–502. [PubMed] [Google Scholar]
- 25.MacRae HM, McLeod RS, Cohen Z, Stern H, Reznick R. Treatment of rectovaginal fistulas that has failed previous repair attempts. Dis Colon Rectum. 1995;38(9):921–925. doi: 10.1007/BF02049726. [DOI] [PubMed] [Google Scholar]
- 26.Jones IT, Fazio VW, Jagelman DG. The use of transanal rectal advancement flaps in the management of fistulas involving the anorectum. Dis Colon Rectum. 1987;30(12):919–923. doi: 10.1007/BF02554276. [DOI] [PubMed] [Google Scholar]


