Abstract
Quercetin is a plant flavonoid that has anti-oxidant, anti-inflammatory, anti-cancer, and anti-ischemic properties. Moreover, quercetin exerts neuroprotective effects against focal cerebral ischemia. Protein phosphatase 2A (PP2A) is a form of serine/threonine phosphatase that modulates various biological functions. Among PP2A subunit types, subunit B exists abundantly in brain tissue and plays an essential function in nervous system. We previously reported the decrease of PP2A subunit B in focal cerebral animal model. This study explored the change of PP2A subunit B expression by quercetin treatment in cerebral ischemic animal model and glutamate-treated hippocampal-derived (HT22) cell culture. Quercetin (10 mg/kg) or vehicle was injected intraperitoneally into male rats before 30 min of middle cerebral artery occlusion (MCAO), and cerebral cortices were isolated 24 hr after MCAO. MCAO induced the neurological behavioral deficit and increased infarct volume. However, quercetin treatment attenuated the increase of neurological deficit and infarction. We detected the alleviation of MCAO-induced the decrease in PP2A subunit B by quercetin treatment using a proteomic approach. Reverse-transcription PCR and Western blot analyses confirmed lower PP2A subunit B expression levels in MCAO group with vehicle. However, quercetin treatment attenuated MCAO-induced this reduction. We also observed the neuroprotective effect of quercetin and the change of PP2A subunit B expression in glutamate-exposed HT22 cells. Glutamate exposure dramatically reduced cell viability and PP2A subunit B expression, and quercetin treatment significantly improved these decreases. We clearly showed that quercetin performs a neuroprotective function and modulates down-regulation of PP2A subunit B against MCAO injury and glutamate toxicity. Thus, our finding suggests that the regulation of PP2A subunit B by quercetin contributes to neuroprotective function in ischemic brain injury.
Keywords: cerebral ischemia, neuroprotection, protein phosphatase 2A, quercetin
Cerebral ischemia is the third leading cause of death and senile dementia. It is the most common cause of disability. Cerebral ischemia causes the reduction of oxygen and the deprivation of glucose. It consequently leads to serious cell damage and results in biogenetic failures through oxidative stress, inflammatory responses and blood-brain barrier (BBB) disruption [4]. Moreover, reactive oxygen species (ROS) increase dramatically after cerebral ischemia. This fact indicates that oxidative stress plays a key role in causing ischemic-related injuries because the brain is susceptible to oxidative damage [21, 29, 31].
Flavonoids are well-known free radical scavengers and are considered as potential antioxidants [1, 24]. Quercetin is a polyphenolic flavonoid found in fruits, vegetables oils, red wine, and tea [6, 36]. Quercetin has anti-oxidant, anti-inflammatory, anti-blood coagulation, anti-ischemic, and anti-cancer effects [25, 27, 28, 39, 40]. It maintains BBB integrity by scavenging ROS, attenuates cell apoptosis, and improves functional outcome in focal cerebral ischemia in rat brain [18, 38].
Protein phosphatase 2A (PP2A) is a protein ubiquitously found in all mammalian cells as a form of serine/threonine phosphatase. PP2A structure contains a structural subunit A, a regulatory subunit B, and a catalytic subunit C. For the formation of the core enzyme, subunits A and C combine to form the AC complex. This core enzyme binds to subunit B to form a complete holoenzyme [15]. Subunits A and C are found in various tissues, but subunit B is mainly expressed in brain tissues. Especially, subunit B modulates axonal outgrowth and neurogenesis in the nervous system [34]. PP2A is a crucial enzyme that regulates important cellular activities, such as cell division, development, and apoptosis [22, 32, 41]. We regard that PP2A subunit B acts as a critical factor for maintenance of neurobiological function and quercetin regulates PP2A subunit B expression in cerebral ischemic injury. However, neuroprotective mechanisms of quercetin are very complex, and not completely elucidated. Although the importance of quercetin has been suggested, little information is available regarding the regulation of PP2A subunit B by quercetin. Thus, the aim of this study was to elucidate the change of PP2A subunit B expression by quercetin treatment in a middle cerebral occlusion (MCAO) animal model and glutamate-exposed hippocampal-derived (HT22) cells.
MATERIALS AND METHODS
Animal preparation
Male Sprague-Dawley rats (220–230 g, n=40) were provided by Samtako (Animal Breeding Center, Osan, Korea). All experimental procedures were carried out according to the guidelines provided by the Institutional Animal Care and Use Committee of Gyeongsang National University. Rats were housed under controlled temperature (25°C) and light conditions (12-hr light/12-hr dark cycle), and had free access to feed and water. Before the experimental procedures, rats were kept in the animal house for 1 week to get acclimate to the environment. Rats were divided into randomly by following groups: vehicle + middle cerebral artery occlusion (MCAO) and quercetin + MCAO, vehicle + sham, quercetin + sham. Quercetin (10 mg/kg, Sigma, St. Louis, MO, U.S.A.) was dissolved in 0.1% dimethyl sulfoxide with phosphate-buffered saline. Quercetin or vehicle was administered by intraperitoneal injection 30 min before MCAO surgical operation. Vehicle group was treated only solvent solution without quercetin.
Middle cerebral artery occlusion
MCAO was surgically operated through the intraluminal filament technique to induce focal cerebral ischemia [20]. Rats were anesthetized with Zoletil (50 mg/kg, Virbac, Carros, France) and incision was given in the ventral side of neck skin to reveal the blood vessels. The common carotid artery (CCA) and external carotid artery (ECA) were exposed. The CCA was ligated temporarily and the ECA was ligated permanently. An incision was given to the ECA and flame-blunted 4/0 nylon suture was inserted toward the internal carotid artery (ICA) and finally occluded the middle cerebral artery. Rats were sacrificed 24 hr after MCAO and brain tissues were selected for further procedures. Sham-operated animals were performed as a same surgical procedure except nylon insertion.
Neurological function
Neurological deficits in experimental rats were measured 24 hr after MCAO. Evaluation was performed with modified 5-points Bederson’s neurological scoring scale [2]. Scoring criteria is as follows: 0, no deficit observed; 1, contralateral side of forelimb flexion (mild neurological deficit); 2, circling toward to the contralateral side (moderate neurological deficit); 3, fall down toward to the contralateral side (severe neurological deficit); 4, depressed consciousness and rare spontaneous movement. (very serious neurological deficit).
Triphenyl tetrazolium chloride (TTC) staining
Brains were removed from skull and placed on steel coronal brain matrix (Ted Pella, Redding, CA, U.S.A.). Brains were sliced into 2 mm thickness and sliced tissues were stained with 1% TTC solution. Damaged tissue remains white color that is unstained. Stained sections were fixed in 10% formalin for 24 hr. Infarct area was analyzed by Image-ProPlus 4.0 software (Media Cybernetics, Silver Spring, MD, U.S.A.) and infarct volumes were calculated as percentages of total hemispheric volumes.
Two-dimensional gel electrophoresis
Two-dimensional gel electrophoresis was carried out as a previously described method [16]. Briefly, the right cerebral cortex from each animal was homogenized in lysis buffer (8 M urea, 4% CHAPS, ampholytes, and 40 mM Tris-HCl) and centrifuged at 16,000 g for 20 min at 4°C. Supernatant was collected and protein concentration was evaluated by Bradford assay according to the manufacturer’s manuals (Bio-Rad, Hercules, CA, U.S.A.). The immobilized pH gradient (IPG) gel strips (pH 4–7, 17 cm, Bio-Rad) was treated with rehydration buffer (8 M urea, 2% CHAPS, 20 mM DTT, 0.5% IPG buffer, and bromophenol blue) for 13 hr at room temperature. Protein samples were loaded on the IPG gel strip and the first-dimensional isoelectric focusing (IEF) was carried out using Ettan IPGphor 3 System (GE Healthcare, Little Chalfont, Buckinghamshire, U.K.). IEF was performed as follow conditions: 250 V for 15 min, 10,000 V for 3 hr, and followed by an increase from 10,000 V to 50,000 V. IPG strips were reacted with equilibration buffer [6 M urea, 30% glycerol, 2% sodium dodecyl sulfate (SDS), 50 mM Tris-HCl, bromophenol blue] with DTT and iodoacetamide for 10 min. Strips were loaded into gradient gels (7.5–17.5%) for second-dimensional electrophoresis and gels were installed in Protein-II XI electrophoresis equipment (Bio-Rad). The gels were electrophoresed until the dye reached to the bottom of gel at 10 mA at 10°C.
Silver staining, image analysis, and protein identification
Silver staining was performed to detect and visualize protein spots. Gels were fixed with a fixation solution (12% acetic acid and 50% methanol) for 2 hr and stained with a sliver solution (0.2% silver nitrate and 0.03% formaldehyde). After staining, gels were developed in a 0.2% sodium carbonate solution and scanned by Agfar ARCUS 1200™ (Agfar-Gevaert, Mortsel, Belgium). Images of gels were analyzed by PDQuest software (Bio-Rad). Specific protein spots with intensity changes more than 2.5 times between vehicle + MCAO and quercetin + MCAO groups were selected for protein identification. Selected protein spots were separated from the gels and silver stained gel particles were destained. After destaining, gel particle were digested by trypsin containing buffer and peptides were collected for matrix-assisted laser desorption ionization time of flight (MALDI-TOF). Peptides were analyzed using a Voyager System DE™ STR biospectrometry workstation (Applied Biosystem, Forster city, CA, U.S.A.) and protein was detected by MS-Fit (University of California, San Francisco, CA, U.S.A.) and ProFound programs. Identification of protein sequences was performed by databases SWISS-PROT and National Center for Biotechnology Information (NCBI) databases.
Reverse transcription-polymerase chain reaction
Total RNA of cerebral cortex tissues were extracted using Trizol Reagent (Life Technologies, Rockville, MD, U.S.A.) according to the manufacturer’s instructions. Single-stranded complementary DNA was synthesized from RNA samples (1 µg) using the Superscript III first strand system (Invitrogen, Carlsbad, CA, U.S.A.) according to the manufacturer’s instructions. Respective target genes were amplified by polymerase chain reaction (PCR) with specific primers. Primers sequences of PP2A subunit B and actin were 5′-CCTGGTATGCCAAACTCGAT-3′ (forward primer), 5′- ACAATAGCCACCTGGTCGTC-3′ (reverse primer) and primers sequences of actin were 5′-GGGTCAGAAGGACTCCTACG-3′ (forward primer), and 5′-GGTCTCAAACATGATCTGGG-3′ (reverse primer). PCR reaction was performed as following steps: an initial polymerase activation and DNA denaturation for 5 min at 94°C; 30 cycles of denaturation step at 94°C for 30 sec, annealing step at 54°C for 30 sec, and elongation step at 72°C for 1 min; and a final extension for 10 min at 72°C. PCR products were loaded on 1% agarose gel, electrophoresed, and visualized under an ultraviolet light.
Western blot analysis
Western blot analysis was performed according to a previously described method [16]. Brain tissues were homogenized in lysis buffer [1% Triton X-100, 1 mM EDTA in PBS (pH 7.4)] and centrifuged at 15,000 g for 20 min at 4°C, and supernatant was collected. HT22 cells were grinded with lysis buffer and cell lysates were centrifuged at 5,000 g for 5 min at 4°C and then supernatant was collected. Protein concentration was analyzed using bichinchominic acid (BCA) protein assay kit (Pierce, CA, U.S.A.). Protein samples (30 µg) were electrophoresed on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred to polyvinyl difluoride (PVDF) membrane (Millipore, Billerica, MA, U.S.A.). Membranes were incubated with 5% skim milk in Tris-buffer saline containing 0.1% Tween-20 (TBST) for 1 hr to block non-specific reaction and washed with TBST. Membranes were incubated with anti-PP2A subunit B (1:1,000, Cell signaling Technology, Beverly, MA, U.S.A.) or anti-actin (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) at 4°C for overnight and were washed with TBST. Membranes were incubated with horseradish peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG (1:5,000, Cell signaling Technology) for 2 hr and were washed with TBST. Membranes were reacted with enhanced chemiluminescence detection reagents (Millipore, Billerica, MA, U.S.A.) according to the manufacturer’s protocol and immune-reactive protein signals were detected on X-ray films (FujiFilm Co., Tokyo, Japan).
Immunohistochemical staining
Brain tissues were fixed by 4% neutral buffered paraformaldehyde (PBS, pH 7.4) for 24 hr, washed with tap water, dehydrated with gradient ethyl alcohol (70 to 100%), and then cleared with xylene. Brain tissues were embedded with paraffin and sliced coronally into 4 µm thickness using a rotary microtome (Leica, Wetzlar, Germany). Sliced sections were deparaffinized by xylene and rehydrated by gradient ethyl alcohol (100 to 70%). Rehydrated brain sections were transferred to 10 mM sodium citrate buffer (pH 6.0) and heated using a microwave for antigen retrieval. Subsequently, they were washed with PBS and immersed with 1% hydrogen peroxide for 10 min to quench the endogenous peroxidase activity. To block the non-specific reaction, sections were incubated with 1% normal goat serum diluted for 1 hr in room temperature and incubated with anti-PP2A subunit B antibody (diluted 1:100, Cell Signaling Technology) at 4°C for overnight. After primary antibody incubation, sections were washed with PBS and reacted for 1 hr. Sections were washed with PBS and incubated with avidin-biotin-peroxidase complex with biotinylated goat anti-rabbit IgG (1:200 in PBS) for 1 hr using a Vector ABC Elite kit (Vector Laboratories Inc., Burlingame, CA, U.S.A.). Sections were washed with PBS and stained with 3, 3′-diaminobenzidine tetrahydrochloride (Sigma) solution with 0.03% hydrogen peroxidase for 3 min. They were counterstained with hematoxylin, dehydrated with gradient ethyl alcohol (70 to 100%), cleared with xylene, and mounted with mount media (Thermo Fischer Scientific, Walthem, MA, U.S.A.). Sections were observed and photographed using an Olympus microscope (Olympus, Tokyo, Japan).
Cell culture and treatment
The mouse hippocampal neuronal HT22 cells were cultured as a previously described method [17]. HT22 cells were cultured in L-glutamate-free Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco BRL, Gaithersburg, MD, U.S.A.) containing 10% fetal bovine serum, streptomycin (100 µg/ml), and penicillin (100 unit/ml). Cells were cultured in a humidified incubator with proper conditions (37°C, 5% CO2 atmosphere) for 24 hr. Cells were treated with quercetin (1, 3, 5 µM) 1 hr prior to treatment of glutamate, and then treated with glutamate (5 mM). Cells were incubated for 24 hr after glutamate treatment and collected by scraping for further experiments.
Cell viability
Cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. HT22 cells (5 × 103 cells/100 µl of DMEM media) were cultured on 96 well plate for 24 hr. Cells were treated with quercetin (1, 3, 5 µM) 1 hr prior to glutamate (5 mM) treatment and incubated at 37°C for 24 hr. Media was removed and MTT solution (5 mg/ml) was added to each well. Cells were subsequently incubated for 4 hr and MTT solution was discarded. Formazan dye was dissolved into solvent that containing 20% sodium dodecyl sulfate (pH 4.8) and 50% dimethylformamide, and added to the medium. Optical density (OD) was measured at 570 nm wavelength. Cell viability (%) was calculated as follows: mean OD value of treated cells/ mean OD value of control cells ×100.
Statistical analysis
All data from the experiment was expressed as mean ± S.E.M. The intensity analysis was measured with SigmaGel 1.0 (Jandel Scientific, San Rafael, CA, U.S.A.) and SigmaPlot 4.0 (SPSS Inc., Point Richmond, CA, U.S.A.). The comparison of experiment results in each group was performed by two-way analysis of variance (ANOVA) with post-hoc Scheffe’s test. P<0.05 was regarded to represent statistical significance.
RESULTS
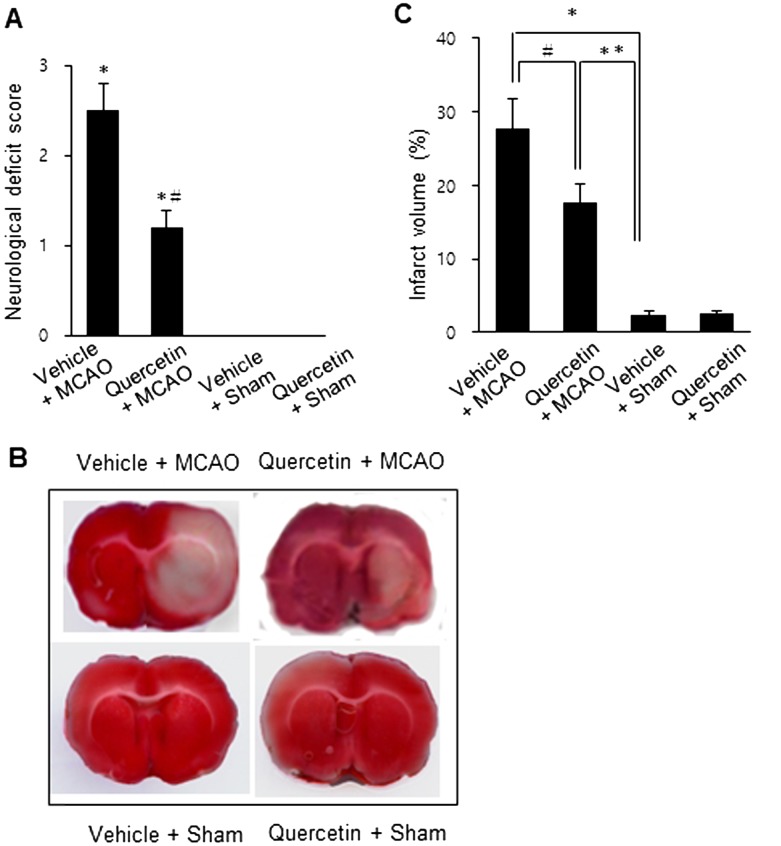
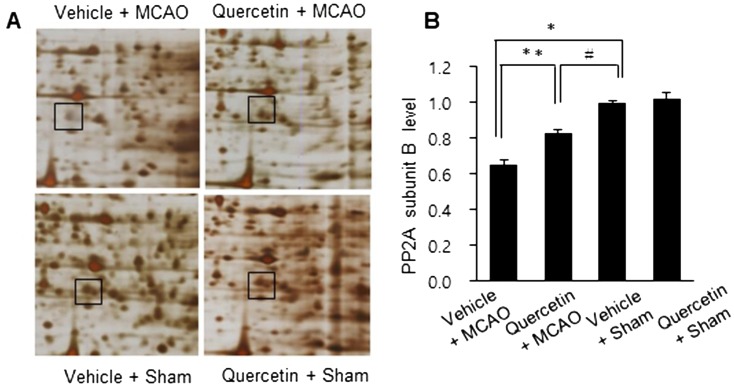
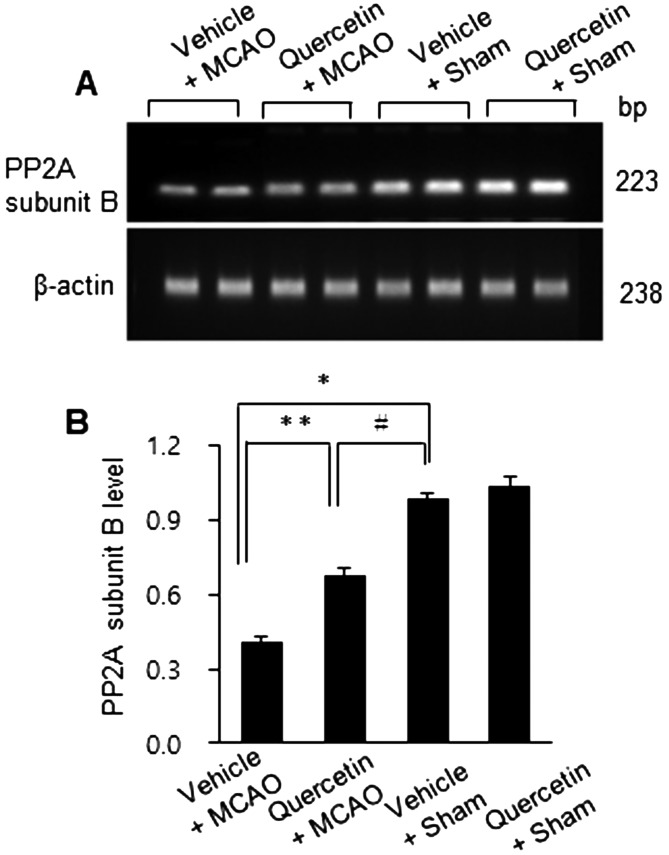
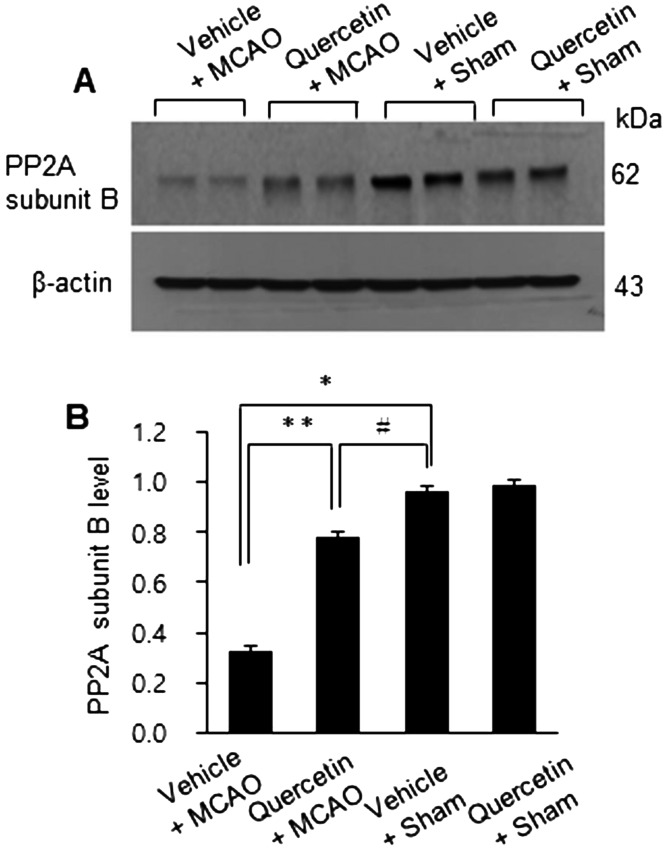
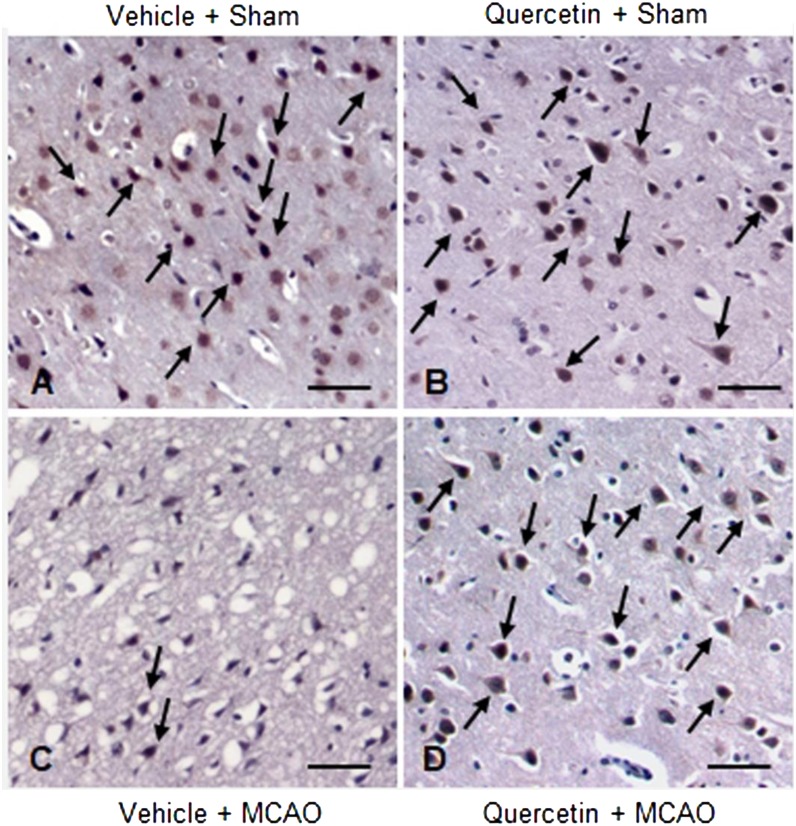
MCAO injury leads to neurological deficits and neuronal cells damage. We previously reported that quercetin attenuates the severe neurological deficits and significant infarction caused by MCAO [23]. As a supplementary data, Fig. 1 showed the neuroprotective effect of quercetin through neurological behavioral test (Fig. 1A) and measurement of infarct volume (Fig. 1B and 1C). MCAO led to severe neurological behavioral deficits and increased infarct volume, and quercetin treatment improved both the MCAO-induced neurological deficits and brain infarction. Moreover, we observed a significant change in PP2A subunit B expression between the vehicle + MCAO and quercetin + MCAO groups (Fig. 2). PP2A subunit B expression levels decreased drastically in the cerebral cortex tissues of the vehicle + MCAO group and quercetin treatment alleviated this decrease (Fig. 2A). PP2A subunit B expression levels were almost equal in the vehicle + sham and quercetin + sham groups. Mass matched peptide of PP2A subunit B is 9/56 and sequence coverage is 29%. PP2A subunit B expression levels were 0.65 ± 0.03 and 0.83 ± 0.02 in the vehicle + MCAO and quercetin + MCAO groups, respectively (Fig. 2B). Reverse transcription-PCR and Western blot analyses showed a change in PP2A subunit B expression following quercetin treatment in the MCAO group. Transcript level of PP2A subunit B was lower in the vehicle + MCAO group than in the sham-operated groups. This decrease was recovered in the quercetin + MCAO group (Fig. 3A). PP2A subunit B transcript levels were 0.41 ± 0.01 and 0.68 ± 0.03 in the vehicle + MCAO and quercetin + MCAO groups, respectively (Fig. 3B). We also obtained similar results from the Western blot analysis. PP2A subunit B protein level decreased in the vehicle + MCAO group, and quercetin treatment reversed this decline (Fig. 4A). PP2A subunit B protein levels were 0.32 ± 0.02 and 0.78 ± 0.01 in the vehicle + MCAO and quercetin + MCAO groups, respectively (Fig. 4B). In results of immunohistochemical staining, we observed positive cells of PP2A subunit B in pyramidal neurons of cerebral cortex (Fig. 5A and 5B). The number of PP2A subunit B positive cells was decreased in vehicle + MCAO animals relative to sham-operated animals regardless of quercetin treatment (Fig. 5C). However, quercetin attenuated the injury-induced these decreases (Fig. 5D).
Fig. 1.
Neurobehavioral scores (A), representative photos of TTC staining (B), and infarct volume (C) in vehicle + middle cerebral artery occlusion (MCAO), quercetin + MCAO, vehicle + sham, and quercetin + sham animals. Quercetin improved MCAO-induced the neurological deficits and infarction. Data (n=4) are represented as the mean ± S.E.M. *P<0.01, **P<0.05 vs. vehicle + sham animals, #P<0.05 vs. vehicle + MCAO animals.
Fig. 2.
Protein phosphatase 2A (PP2A) subunit B protein spots identified by MALDI-TOF in the cerebral cortices of vehicle + middle cerebral artery occlusion (MCAO), quercetin + MCAO, vehicle + sham, and quercetin + sham animals. Squares indicate protein phosphatase 2A (PP2A) subunit B spot (A). Spot intensities were measured by PDQuest software. The spot intensities are reported as a ratio relative to vehicle + sham (B). Data (n=4) are represented as the mean ± S.E.M. *P<0.01, **P<0.05 vs. vehicle + sham animals, #P<0.05 vs. vehicle + MCAO animals.
Fig. 3.
Reverse transcription-PCR analysis of protein phosphatase 2A (PP2A) subunit B protein in the cerebral cortices of vehicle + middle cerebral artery occlusion (MCAO), quercetin + MCAO, vehicle + sham, and quercetin + sham animals. Each lane represents an individual animal (A). The band intensity of each RT-PCR product was normalized to that of the actin product (B). Data (n=4) are represented as the mean ± S.E.M. *P<0.01, **P<0.05 vs. vehicle + sham animals, #P<0.05 vs. vehicle + MCAO animals.
Fig. 4.
Western blot analysis of protein phosphatase 2A (PP2A) subunit B protein in the cerebral cortices of vehicle + middle cerebral artery occlusion (MCAO), quercetin + MCAO, vehicle + sham, and quercetin + sham animals. Each lane represents an individual animal (A). Densitometric analysis is represented as a ratio of PP2A subunit B protein intensity to actin protein intensity (B). Data (n=4) are represented as the mean ± S.E.M. *P<0.01, **P<0.05 vs. vehicle + sham animals, #P<0.05 vs. vehicle + MCAO animals.
Fig. 5.
Representative photos of immunohistochemical staining of protein phosphatase 2A (PP2A) subunit B in the cerebral cortices of vehicle + sham (A), quercetin + sham (B), vehicle + middle cerebral artery occlusion (MCAO) (C) and quercetin + MCAO (D) animals. Arrows indicate positive cells of PP2A subunit B. Scale bar=100 µm.
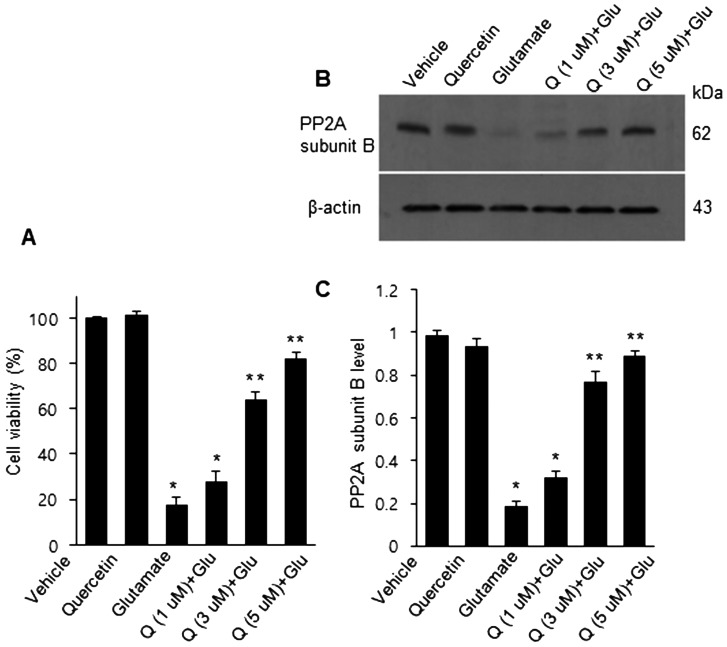
Figure 6A demonstrated the change of cell viability in glutamate-exposed HT22 cells following quercetin treatment. Glutamate treatment induced a significant decline in cell viability, and quercetin treatment recovered decrease of cell viability in a dose-dependent manner. Cell viabilities were 17.3 ± 3.5 in the glutamate-only group and 28.2 ± 4.3, 64.5 ± 3.5, and 82.1 ± 3.2 in glutamate group with 1, 3, and 5 µM of quercetin, respectively (Fig. 6A). Moreover, the glutamate-treated HT22 cells showed a decrease of PP2A subunit B expression, and quercetin treatment attenuated this decrease (Fig. 6B). PP2A subunit B protein levels were 0.18 ± 0.02 in the glutamate only group and 0.32 ± 0.02, 0.73 ± 0.03, and 0.89 ± 0.02 in glutamate group with 1, 3, and 5 µM of quercetin, respectively (Fig. 6C).
Fig. 6.
Cell viability (A) and Western blot (B and C) analyses of protein phosphatase 2A (PP2A) subunit B in HT22 cells. Cellular viability was assessed using the MTT assay. Cell survival was expressed as a percentage of neuroprotection vs. vehicle set at 100 (A). Densitometric analysis is represented as the intensity of PP2A subunit B to intensity of actin (C). Data (n=5) are expressed as mean ± S.E.M. *P<0.01, **P<0.05 vs. vehicle.
DISCUSSION
Quercetin exhibits anti-oxidant, anti-inflammatory, and exhibits protective effects against cerebral ischemia [8, 18, 38]. Quercetin improves histopathological changes and functional outcomes in brain injury models [7, 23]. We previously reported that quercetin alleviates brain edema and severe neurological behavioral deficits in focal cerebral ischemia caused by MCAO [23]. Quercetin decreases infarct volume in MCAO animal model and attenuates MCAO-induced degeneration of neuronal cells [23]. In this study, we demonstrated that quercetin exerts neuroprotective effect and regulates PP2A subunit B expression in a MCAO animal model. We screened a decrease of PP2A subunit B in MCAO-induced focal cerebral ischemia using a proteomic technique. In addition, we found that quercetin treatment attenuates a decrease of PP2A subunit B in MCAO-operated animal. Reverse transcription-PCR and Western blot analyses also elucidated that quercetin treatment mitigates this reduction. Moreover, the results of immunohistochemical staining revealed that MCAO decreases the expression of PP2A subunit B in neuronal cell of cerebral cortex, whereas quercetin treatment prevents the injury-induced this reduction. Excessive exposure of glutamate can cause increased ROS production, and consequently induces neuronal cell damage [11, 12, 26]. Quercetin protects HT22 cells from glutamate-exposured cell death by inhibiting the over-production of intracellular ROS [37]. Quercetin is a potent neuroprotectant against oxidative neuronal injury in cortical cell [9]. Our results clearly showed the dose-dependent neuroprotective effect of quercetin in glutamate-exposed HT22 cells. Moreover, glutamate treatment in HT-22 cells induced a decrease in PP2A subunit B expression, and quercetin treatment attenuated this decrease dose-dependently.
PP2A can modulate neuronal functions including cell activity, cell development, and apoptosis [14, 41]. Moreover, PP2A subunit B is present in nerve tissues and regulates various functions of PP2A [34]. It is accepted that a decrease in PP2A expression leads to neuronal disorders, and a decrease in PP2A activity results in neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease [10, 19]. PP2A also modulates the phosphorylation of the microtubule-associated protein tau [13, 33]. Phosphorylation of tau causes the aggregation of microtubules and the formation of neurofibrillary tangles, which lead to neuronal degeneration, cognitive dysfunction, and synaptic dysfunction [5]. Quercetin alleviates inflammation by controlling the phosphorylation of neutrophil proteins [3]. Quercetin protects neurons from neurodegenerative damage by inhibiting the hyper-phosphorylation of tau protein [30]. It has been reported that flavonoids regulate the apoptotic pathway by modulating the PP2A expression [35]. We previously reported a decrease in PP2A subunit B expression in both MCAO-induced focal cerebral ischemia and glutamate-exposed neurons [17]. These results suggest that a decrease in PP2A subunit B can mediate neuronal cell death. Quercetin treatment restores the injury-induced reduction in PP2A subunit B expression. We further confirmed that quercetin improves reduction of cell viability and decrease of PP2A subunit B expression in glutamate-treated HT22 cell lines. Quercetin treatment prevents neuronal cell death by restoring PP2A subunit B. Although further studies are needed to elucidate the relationship between quercetin and PP2A subunit B expression, we clearly demonstrated that quercetin restores the ischemic injury-induced a reduction of PP2A subunit B. Taken together, our results demonstrate in both in vivo and in vitro studies that quercetin exerts neuroprotective effect and prevents down-regulation of PP2A subunit B against cerebral ischemia and glutamate toxicity. Therefore, we suggest that quercetin contributes to neuroprotection mechanism through the modulation of PP2A subunit B in ischemic brain injury.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (NRF-2018R1D1A1B07044074).
REFERENCES
- 1.Andarwulan N., Batari R., Sandrasari D. A., Bolling B., Wijaya H.2010. Flavonoid content and antioxidant activity of vegetables from Indonesia. Food Chem. 121: 1231–1235. doi: 10.1016/j.foodchem.2010.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bederson J. B., Pitts L. H., Tsuji M., Nishimura M. C., Davis R. L., Bartkowski H.1986. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 17: 472–476. doi: 10.1161/01.STR.17.3.472 [DOI] [PubMed] [Google Scholar]
- 3.Blackburn W. D., Jr., Heck L. W., Wallace R. W.1987. The bioflavonoid quercetin inhibits neutrophil degranulation, superoxide production, and the phosphorylation of specific neutrophil proteins. Biochem. Biophys. Res. Commun. 144: 1229–1236. doi: 10.1016/0006-291X(87)91442-2 [DOI] [PubMed] [Google Scholar]
- 4.Brouns R., De Deyn P. P.2009. The complexity of neurobiological processes in acute ischemic stroke. Clin. Neurol. Neurosurg. 111: 483–495. doi: 10.1016/j.clineuro.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 5.Cancino G. I., Toledo E. M., Leal N. R., Hernandez D. E., Yévenes L. F., Inestrosa N. C., Alvarez A. R.2008. STI571 prevents apoptosis, tau phosphorylation and behavioural impairments induced by Alzheimer’s beta-amyloid deposits. Brain 131: 2425–2442. doi: 10.1093/brain/awn125 [DOI] [PubMed] [Google Scholar]
- 6.Careri M., Corradini C., Elviri L., Nicoletti I., Zagnoni I.2003. Direct HPLC analysis of quercetin and trans-resveratrol in red wine, grape, and winemaking byproducts. J. Agric. Food Chem. 51: 5226–5231. doi: 10.1021/jf034149g [DOI] [PubMed] [Google Scholar]
- 7.Cho J. Y., Kim I. S., Jang Y. H., Kim A. R., Lee S. R.2006. Protective effect of quercetin, a natural flavonoid against neuronal damage after transient global cerebral ischemia. Neurosci. Lett. 404: 330–335. doi: 10.1016/j.neulet.2006.06.010 [DOI] [PubMed] [Google Scholar]
- 8.Comalada M., Camuesco D., Sierra S., Ballester I., Xaus J., Gálvez J., Zarzuelo A.2005. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur. J. Immunol. 35: 584–592. doi: 10.1002/eji.200425778 [DOI] [PubMed] [Google Scholar]
- 9.Dok-Go H., Lee K. H., Kim H. J., Lee E. H., Lee J., Song Y. S., Lee Y. H., Jin C., Lee Y. S., Cho J.2003. Neuroprotective effects of antioxidative flavonoids, quercetin, (+)-dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntia ficus-indica var. saboten. Brain Res. 965: 130–136. doi: 10.1016/S0006-8993(02)04150-1 [DOI] [PubMed] [Google Scholar]
- 10.Du T. T., Chen Y. C., Lu Y. Q., Meng F. G., Yang H., Zhang J. G.2018. Subthalamic nucleus deep brain stimulation protects neurons by activating autophagy via PP2A inactivation in a rat model of Parkinson’s disease. Exp. Neurol. 306: 232–242. doi: 10.1016/j.expneurol.2018.05.017 [DOI] [PubMed] [Google Scholar]
- 11.Duan Y., Gross R. A., Sheu S. S.2007. Ca2+-dependent generation of mitochondrial reactive oxygen species serves as a signal for poly(ADP-ribose) polymerase-1 activation during glutamate excitotoxicity. J. Physiol. 585: 741–758. doi: 10.1113/jphysiol.2007.145409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekinci F. J., Linsley M. D., Shea T. B.2000. Beta-amyloid-induced calcium influx induces apoptosis in culture by oxidative stress rather than tau phosphorylation. Brain Res. Mol. Brain Res. 76: 389–395. doi: 10.1016/S0169-328X(00)00025-5 [DOI] [PubMed] [Google Scholar]
- 13.Gong C. X., Lidsky T., Wegiel J., Zuck L., Grundke-Iqbal I., Iqbal K.2000. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer’s disease. J. Biol. Chem. 275: 5535–5544. doi: 10.1074/jbc.275.8.5535 [DOI] [PubMed] [Google Scholar]
- 14.Janssens V., Goris J.2001. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 353: 417–439. doi: 10.1042/bj3530417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamibayashi C., Estes R., Lickteig R. L., Yang S. I., Craft C., Mumby M. C.1994. Comparison of heterotrimeric protein phosphatase 2A containing different B subunits. J. Biol. Chem. 269: 20139–20148. [PubMed] [Google Scholar]
- 16.Kang J. B., Kim D. K., Park D. J., Shah M. A., Kim M. O., Jung E. J., Lee H. S., Koh P. O.2018. Hyperglycemia aggravates decrease in alpha-synuclein expression in a middle cerebral artery occlusion model. Lab. Anim. Res. 34: 195–202. doi: 10.5625/lar.2018.34.4.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koh P. O.2011. Focal cerebral ischemia reduces protein phosphatase 2A subunit B expression in brain tissue and HT22 cells. Lab. Anim. Res. 27: 73–76. doi: 10.5625/lar.2011.27.1.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J. K., Kwak H. J., Piao M. S., Jang J. W., Kim S. H., Kim H. S.2011. Quercetin reduces the elevated matrix metalloproteinases-9 level and improves functional outcome after cerebral focal ischemia in rats. Acta Neurochir. (Wien) 153: 1321–1329, discussion 1329. doi: 10.1007/s00701-010-0889-x [DOI] [PubMed] [Google Scholar]
- 19.Liu R., Wang J. Z.2009. Protein phosphatase 2A in Alzheimer’s disease. Pathophysiology 16: 273–277. doi: 10.1016/j.pathophys.2009.02.008 [DOI] [PubMed] [Google Scholar]
- 20.Longa E. Z., Weinstein P. R., Carlson S., Cummins R.1989. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20: 84–91. doi: 10.1161/01.STR.20.1.84 [DOI] [PubMed] [Google Scholar]
- 21.Love S.1999. Oxidative stress in brain ischemia. Brain Pathol. 9: 119–131. doi: 10.1111/j.1750-3639.1999.tb00214.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millward T. A., Zolnierowicz S., Hemmings B. A.1999. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem. Sci. 24: 186–191. doi: 10.1016/S0968-0004(99)01375-4 [DOI] [PubMed] [Google Scholar]
- 23.Park D. J., Shah F. A., Koh P. O.2018. Quercetin attenuates neuronal cells damage in a middle cerebral artery occlusion animal model. J. Vet. Med. Sci. 80: 676–683. doi: 10.1292/jvms.17-0693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Procházková D., Boušová I., Wilhelmová N.2011. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 82: 513–523. doi: 10.1016/j.fitote.2011.01.018 [DOI] [PubMed] [Google Scholar]
- 25.Pu F., Mishima K., Irie K., Motohashi K., Tanaka Y., Orito K., Egawa T., Kitamura Y., Egashira N., Iwasaki K., Fujiwara M.2007. Neuroprotective effects of quercetin and rutin on spatial memory impairment in an 8-arm radial maze task and neuronal death induced by repeated cerebral ischemia in rats. J. Pharmacol. Sci. 104: 329–334. doi: 10.1254/jphs.FP0070247 [DOI] [PubMed] [Google Scholar]
- 26.Reynolds I. J., Hastings T. G.1995. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J. Neurosci. 15: 3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rezaei-Sadabady R., Eidi A., Zarghami N., Barzegar A.2016. Intracellular ROS protection efficiency and free radical-scavenging activity of quercetin and quercetin-encapsulated liposomes. Artif. Cells Nanomed. Biotechnol. 44: 128–134. doi: 10.3109/21691401.2014.926456 [DOI] [PubMed] [Google Scholar]
- 28.Rogerio A. P., Kanashiro A., Fontanari C., da Silva E. V., Lucisano-Valim Y. M., Soares E. G., Faccioli L. H.2007. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm. Res. 56: 402–408. doi: 10.1007/s00011-007-7005-6 [DOI] [PubMed] [Google Scholar]
- 29.Saito A., Maier C. M., Narasimhan P., Nishi T., Song Y. S., Yu F., Liu J., Lee Y. S., Nito C., Kamada H., Dodd R. L., Hsieh L. B., Hassid B., Kim E. E., González M., Chan P. H.2005. Oxidative stress and neuronal death/survival signaling in cerebral ischemia. Mol. Neurobiol. 31: 105–116. doi: 10.1385/MN:31:1-3:105 [DOI] [PubMed] [Google Scholar]
- 30.Shen X. Y., Luo T., Li S., Ting O. Y., He F., Xu J., Wang H. Q.2018. Quercetin inhibits okadaic acid-induced tau protein hyperphosphorylation through the Ca2+‑calpain‑p25‑CDK5 pathway in HT22 cells. Int. J. Mol. Med. 41: 1138–1146. [DOI] [PubMed] [Google Scholar]
- 31.Song Y. S., Narasimhan P., Kim G. S., Jung J. E., Park E. H., Chan P. H.2008. The role of Akt signaling in oxidative stress mediates NF-kappaB activation in mild transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. 28: 1917–1926. doi: 10.1038/jcbfm.2008.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sontag E.2001. Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell. Signal. 13: 7–16. doi: 10.1016/S0898-6568(00)00123-6 [DOI] [PubMed] [Google Scholar]
- 33.Sontag E., Nunbhakdi-Craig V., Lee G., Bloom G. S., Mumby M. C.1996. Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron 17: 1201–1207. doi: 10.1016/S0896-6273(00)80250-0 [DOI] [PubMed] [Google Scholar]
- 34.Strack S., Zaucha J. A., Ebner F. F., Colbran R. J., Wadzinski B. E.1998. Brain protein phosphatase 2A: developmental regulation and distinct cellular and subcellular localization by B subunits. J. Comp. Neurol. 392: 515–527. doi: [DOI] [PubMed] [Google Scholar]
- 35.Vauzour D., Corsini S., Müller M., Spencer J. P. E.2018. Inhibition of PP2A by hesperetin may contribute to Akt and ERK1/2 activation status in cortical neurons. Arch. Biochem. Biophys. 650: 14–21. doi: 10.1016/j.abb.2018.04.020 [DOI] [PubMed] [Google Scholar]
- 36.Wach A., Pyrzyńska K., Biesaga M.2007. Quercetin content in some food and herbal samples. Food Chem. 100: 699–704. doi: 10.1016/j.foodchem.2005.10.028 [DOI] [Google Scholar]
- 37.Yang E. J., Kim G. S., Kim J. A., Song K. S.2013. Protective effects of onion-derived quercetin on glutamate-mediated hippocampal neuronal cell death. Pharmacogn. Mag. 9: 302–308. doi: 10.4103/0973-1296.117824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao R. Q., Qi D. S., Yu H. L., Liu J., Yang L. H., Wu X. X.2012. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem. Res. 37: 2777–2786. doi: 10.1007/s11064-012-0871-5 [DOI] [PubMed] [Google Scholar]
- 39.Yoshida M., Sakai T., Hosokawa N., Marui N., Matsumoto K., Fujioka A., Nishino H., Aoike A.1990. The effect of quercetin on cell cycle progression and growth of human gastric cancer cells. FEBS Lett. 260: 10–13. doi: 10.1016/0014-5793(90)80053-L [DOI] [PubMed] [Google Scholar]
- 40.Yu P. X., Zhou Q. J., Zhu W. W., Wu Y. H., Wu L. C., Lin X., Chen M. H., Qiu B. T.2013. Effects of quercetin on LPS-induced disseminated intravascular coagulation (DIC) in rabbits. Thromb. Res. 131: e270–e273. doi: 10.1016/j.thromres.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 41.Zolnierowicz S.2000. Type 2A protein phosphatase, the complex regulator of numerous signaling pathways. Biochem. Pharmacol. 60: 1225–1235. doi: 10.1016/S0006-2952(00)00424-X [DOI] [PubMed] [Google Scholar]