Abstract
This study evaluated the effects of increasing the proportion of concentrate in the diet on the rumen pH and bacterial community in Japanese Black beef cattle at different fattening stages. Six rumen-cannulated beef cattle were studied in the middle (Mid group, n=3, age 21–22 months) and late (Late group, n=3, age 31 months) fattening stages. The cattle were fed rice straw with control (CON period) or high-concentrate (HC period) diets for 14 consecutive days in each period. Rumen pH was measured continuously and the rumen fluids were collected on the last day of each period. The 24-hr mean and minimum rumen pH in the Mid group were significantly (P<0.05) lower during the HC period compared with the CON period, whereas those in the Late group were continuously low during both periods. In the Late group, the ruminal volatile fatty acid and lactic acid concentrations were significantly (P<0.05) higher during the HC period. During the HC period, the proportions of Prevotella and Caloramator were significantly (P<0.05) higher and lower, respectively, in the Mid group. From these findings, significant changes in the rumen pH and bacterial community induced by dietary changes were mainly observed in the Mid group. Therefore, the ruminal fermentative function in response to a higher concentrate diet might adapt differently in Japanese Black beef cattle at the two different fattening stages.
Keywords: beef cattle, Japanese Black cattle, ruminal bacterial community, ruminal pH subacute ruminal acidosis
To increase the yield of beef cattle, they are often fed large amounts of a concentrated diet [26], which can lead to low rumen pH and high ruminal volatile fatty acids (VFAs) [14, 21]. As a result, consistently low rumen pH, such as subacute ruminal acidosis (SARA), can induce changes in the microbial community and the release of lipopolysaccharide (LPS) in the rumen [7] because the bacterial community structure is closely related to rumen pH and diet composition [13]. For example, feeding beef cattle a high-grain diet causes declines in rumen bacterial diversity [19] and cellulolytic bacteria in the rumen fluids [3]. The rumen can adapt to repeated SARA by minimizing the changes in rumen pH, which may affect the composition and diversity of the ruminal bacterial community or vice versa [15].
Japanese Black cattle, a purebred Japanese breed, are usually fed a large amount of high concentrate diet in a unique feeding system that increases the consumption of concentrate and decreases roughage (rice straw) to maintain a low peripheral vitamin A concentration during the middle to late fattening period [6, 16]. However, feeding a high-grain diet can depress the rumen pH, causing unbalanced rumen fermentation and the release of ruminal LPS, resulting in diseases such as hepatitis, hepatic abscesses, abomasum displacement, and laminitis [1, 7, 20]. However, the rumen fermentation and bacterial responses of beef cattle, including Japanese Black beef cattle, at different fattening stages to prolonged concentrate feeding are still largely unknown. Especially, the effect of an increased concentrate rate in the diet on rumen pH, fermentation, and the bacterial community have not been studied in Japanese Black cattle until recently. Therefore, this study examined the effect of an increased concentrate diet on the rumen pH, fermentation, and bacterial community in Japanese Black beef cattle at different fattening stages.
MATERIALS AND METHODS
All animals were cared for according to protocols approved by the Iwate University Laboratory Animal Care and Use Committee (A201720; Morioka, Japan).
Animals
This study used six Japanese Black steers equipped with rumen cannulas. They were divided into two groups according to fattening stage: the middle (Mid group, age 21−22 months, n=3) and late (Late group, age 31 months, n=3) fattening stages. The body weight of the Mid and Late groups was 436 ± 7 and 685 ± 24 kg (mean ± SE), respectively. After feeding of daily diet for extended periods, the cattle were fed daily (CON period) and high-concentrate diets (HC period) for 14 consecutive days in each period, and fed rice straw as roughage during the CON and HC periods. The forage-to-concentrate ratio was 14:86 and 6:94 in the Mid group and 16:84 and 5:95 in the Late group during the CON and HC periods, respectively. The Mid group was fed the same concentrate diet in the two periods, while in the Late group, the concentrate diet during the HC period had a higher ratio of non-fiber carbohydrate (59.3%) than that of the CON period (49.7%). Table 1 shows the components and amounts fed based on the Japanese feeding standards for beef cattle. In this study, only six cattle were studied because we chose the animals best suited to our experiment that adapted to the offered feed, and due to the cost of beef cattle and limited animal management capacity.
Table 1. Components of the diet and nutrient supply fed to the Japanese Black beef cattle.
| Mid (n=3) |
Late (n=3) |
||||
|---|---|---|---|---|---|
| CON | HC | CON | HC | ||
| Components of diet | |||||
| Concentrate (kg) | 6.0 | 8.0 | 6.8 | 6.3 | |
| Rice straw (kg) | 1.0 | 0.5 | 1.3 | 0.4 | |
| Crude protein (%) | 13.3 | 14.1 | 13.3 | 14.0 | |
| Total digestible nutrients (%) | 76.8 | 80.1 | 74.8 | 81.8 | |
| Neutral detergent fiber (%) | 30.8 | 27.0 | 34.8 | 20.8 | |
| Acid detergent fiber (%) | 13.1 | 10.5 | 17.2 | 8.1 | |
| Non fibers carbohydrate (%) | 46.4 | 50.3 | 42.6 | 58.0 | |
| Nutrient supply (%) | |||||
| Dry matter | 86 | 111 | 96 | 79 | |
| Crude protein | 130 | 172 | 149 | 126 | |
| Total digestible nutrients | 109 | 142 | 97 | 87 | |
| Calcium | 99 | 127 | 53 | 21 | |
| Phosphorus | 227 | 301 | 118 | 111 | |
Mid, middle fattening stage; Late, late fattening stage; CON, control diet period; HC, high-concentrate diet period.
Sampling and pH measurements
Rumen pH was measured every 10 min throughout the experiment using a radio transmission system (YCOW-S; DKK-TOA, Yamagata, Japan), and area under the curve (pH × hour, pH<5.6, AUC) and duration of time (pH<5.6, hr/day) were analyzed by the YcowReport software (DKK-TOA, Yamagata, Japan). The pH sensor was placed in the ventral sac of the rumen, as reported previously [11]. Ruminal fluids were collected from adjacent to the pH sensor at 13:00 on the last day of each period. These samples were immediately filtered through two layers of cheesecloth, and treated for further analyzes as reported previously [5]. Briefly, the VFAs were quantified with gas chromatography (Hitachi-163, Hitachi, Tokyo, Japan), and the ruminal LPS activity was measured using a kinetic colorimetric method with Limulus amebocyte lysate (Pyrochrome; Seikagaku, Tokyo, Japan). Lactic acid was analyzed with a commercial kit (d-lactate/l-lactate F-kit; R-Biopharm, Germany) and ammonia nitrogen (NH3-N) using the steam distillation method with an NH3-N analyzer (Kjeltec Auto 1035; Actac, Tokyo, Japan).
DNA extraction
Total bacterial DNA was extracted from the rumen fluid samples as described elsewhere [10]. The concentration and purity of the refined DNA solution were measured using a spectrophotometer (BioSpec-nano 260-26300-31; Shimadzu, Kyoto, Japan). The purity (absorbance ratio of 260/280 nm) ranged from 1.8 to 2.1.
DNA pyrosequencing
DNA pyrosequencing was performed using the forward primer 357F (5′-CCATCTCATCCCTGCGTGTCCGACTCAGNNNNNNNNNNAGRGTTTGATYMTGGCTCAG-3′) and reverse primer 518R (5′-CCTATCCCCTGTGTGCCT TGGCAGTCTCAGTGCTGCCTCCCGTAGGAGT-3′) and an EmeraldAmp® PCR MaPeriod9 (TaKaRa, Kusatsu, Japan). A thermal cycler (MyCycler; Bio-Rad, Tokyo, Japan) was used for PCR, and agarose gels were used for electrophoresis of the amplified products to confirm that there was no defect in the protocol. For next-generation DNA sequencing, a 16S Metagenomic Sequencing Library Preparation (Illumina: San Diego, CA, U.S.A.) was used. The V3/V4 region of the 16S RNA gene was amplified using forward (5′-CCTACGGGNGGCWGCAG-3′) and reverse (5′-GACTACHVG GGTATCTAATCC-3′) primers. A Nextera XT Index Kit (Illumina) was used to adjust the reaction liquid for PCR. The amplified products were checked with agarose gel electrophoresis and next-generation DNA sequencing (Illumina MiSeq; Illumina) was conducted.
Pyrosequencing data analysis
QIIME Visualizations, an application of the BaseSpace® Sequence Hub, was used to analyze the bacterial community, diversity, and similarity in the ruminal fluids. To analyze bacterial diversity, the unweighted UniFrac distance method was used for principal coordinate analysis (PCoA), and the operational taxonomic units (OTUs), phylogenetic diversity whole tree (PD whole tree), Chao1, and Shannon index were calculated.
Statistical analysis
The maximum, mean, and minimum pH, and AUC and duration of time (for pH<5.6) on days 8 to 14 were averaged during the CON and HC periods in each group. AUC and duration of time (for pH<5.6) in each group were calculated to assess the occurrence and severity of SARA. The rumen pH, AUC, and duration of time were summarized as the means ± standard error of the mean (SEM). GraphPad Prism ver. 5.01 (La Jolla, CA, U.S.A.) was used for the statistical calculations, and paired t-tests were used to evaluate the differences in rumen pH and VFAs, NH3N, Lactic acid, LPS, bacterial diversity, and bacterial abundance between the CON and HC periods in each group. VFAs, NH3N, Lactic acid, LPS, bacterial diversity, and bacterial abundance were summarized as the means ± standard error (SE). Differences were considered significant at P<0.05.
RESULTS
Daily intake, Ruminal pH and VFAs
In the Mid group, no residual feed was observed in both periods. In the Late group, daily intake were evaluated by excluding the residual feed from total amount of offered feed, and dietary components and nutrient supply were calculated from consumed feed (Table 1). Clinical symptom such as anorexia, fever, and diarrhea was not observed in each period of both groups.
In the Mid group, the 24-hr mean and daily minimum pH were significantly (P<0.05) lower during the HC period simultaneously with significantly higher AUC and duration of time (pH<5.6, hr/day) during the HC period than during the CON period (Table 2). In the Late group, no significant difference was found in pH, AUC, or duration of time (pH<5.6, hr/day) between the CON and HC periods (Table 2). During the CON and HC periods, SARA was diagnosed when pH remained under 5.6 for 3.79 and 10.86 hr/day in the Mid group and for 17.43 and 16.53 hr/day in the Late group. No significant difference was found in the total VFA and NH3-N concentrations or LPS activity between the CON and HC periods in the Mid group. However, the total VFA and lactic acid concentrations in the Late group were significantly (P<0.05) higher during the HC period than during the CON period (Table 3).
Table 2. Rumen pH, area under the curve (AUC) and duration of time (pH<5.6, hr/day) of Japanese Black beef cattle.
| Item | Mid (n=3) |
Late (n=3) |
|||
|---|---|---|---|---|---|
| CON | HC | CON | HC | ||
| pH | |||||
| Maximum | 6.58 ± 0.02 | 6.64 ± 0.02 | 6.43 ± 0.14 | 5.98 ± 0.15 | |
| Mean | 6.00 ± 0.04 | 5.76 ± 0.03a) | 5.48 ± 0.01 | 5.48 ± 0.02 | |
| Minimum | 5.44 ± 0.06 | 5.14 ± 0.02a) | 4.89 ± 0.04 | 5.09 ± 0.08 | |
| AUC (pH × hour, pH<5.6) | 0.64 ± 0.20 | 3.38 ± 0.31a) | 4.10 ± 0.14 | 3.78 ± 0.78 | |
| Duration of time (pH<5.6, hr/day) | 3.79 ± 0.96 | 10.86 ± 0.91a) | 17.43 ± 0.69 | 16.53 ± 0.15 | |
Values are means ± SEM. Mid, middle fattening stage; Late, late fattening stage; CON, control diet period; HC, high-concentrate diet period. a) Indicates the significant (P<0.05) difference between the LC and HC periods in each group.
Table 3. Total volatile fatty acid (VFA) concentration and VFA proportions, ammonia nitrogen (NH3-N), lactic acid, and lipopolysaccharide (LPS) in the ruminal fluids of Japanese Black beef cattle.
| Mid (n=3) |
Late (n=3) |
||||
|---|---|---|---|---|---|
| CON | HC | CON | HC | ||
| VFA | |||||
| Total VFA (mmol/dl) | 6.86 ± 0.27 | 7.25 ± 0.24 | 12.6 ± 0.3 | 15.3 ± 0.3a) | |
| Acetate (%) | 66.9 ± 0.7 | 62.9 ± 1.8 | 57.2 ± 4.0 | 49.7 ± 3.8 | |
| Propionate (%) | 17.1 ± 0.8 | 20.8 ± 2.4 | 26.7 ± 4.5 | 36.7 ± 4.6 | |
| Butyrate (%) | 12.6 ± 0.8 | 12.0 ± 0.9 | 12.9 ± 0.6 | 9.57 ± 1.08 | |
| Others (%) | 3.21 ± 0.31 | 4.16 ± 0.16 | 2.96 ± 0.18 | 3.96 ± 0.55 | |
| Acetate: Propionate | 3.94 ± 0.21 | 3.17 ± 0.41 | 2.37 ± 0.46 | 1.47 ± 0.32 | |
| NH3-N (mg/dl) | 8.85 ± 0.81 | 9.73 ± 1.35 | 10.4 ± 2.1 | 12.9 ± 2.8 | |
| Lactic acid (g/l) | 0.013 ± 0.001 | 0.014 ± 0.002 | 0.018 ± 0.006 | 0.146 ± 0.029a) | |
| LPS (×103 EU/ml) | 10.01 ± 1.81 | 16.21 ± 4.28 | 7.63 ± 2.79 | 5.80 ± 1.67 | |
Values are mean ± SE. Mid, middle fattening stage; Late, late fattening stage; CON, control diet period; HC, high-concentrate diet period. a) indicates the significant (P<0.05) difference between the LC and HC periods in each group.
Bacterial diversity analysis
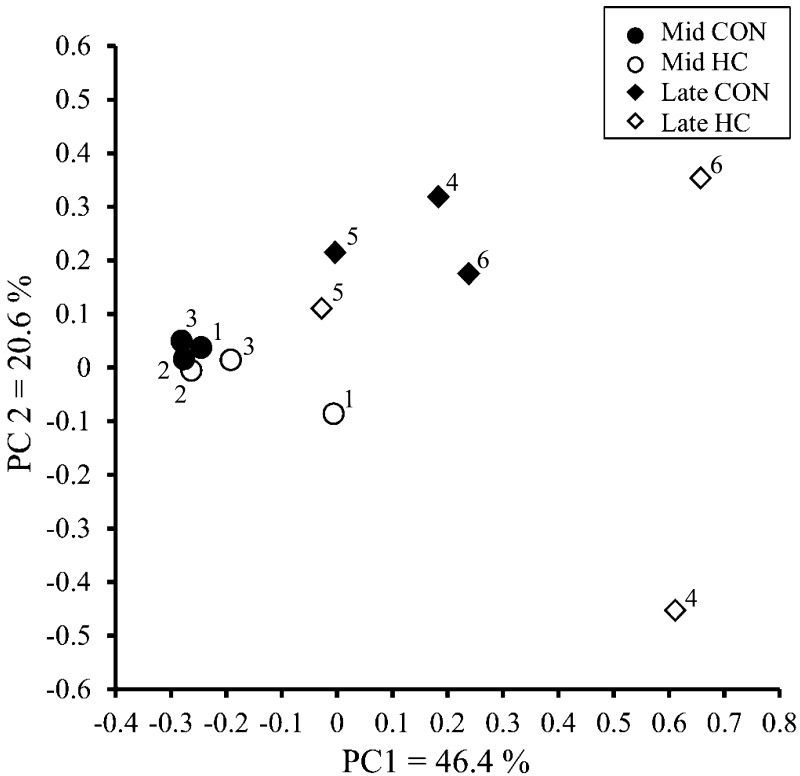
In the PCoA analysis, the plots of the Mid group showed the similarity of bacteria in the CON and HC periods, whereas those in the Late group differed in the two periods (Fig. 1). The rumen bacterial diversity estimated using OTUs, the PD whole tree, Chao1, and Shannon index did not differ between the CON and HC periods (Table 4).
Fig. 1.
Principal coordinate analysis plots generated from the QIIME visualizations of the ruminal fluids of Japanese Black beef cattle at the middle (Mid) and late (Late) fattening stage fed control (CON) and high-concentrate (HC) diets. PC1 and PC2 are principal components 1 and 2, respectively.
Table 4. Ruminal bacterial diversity in Japanese Black beef cattle.
| Item | Mid (n=3) |
Late (n=3) |
||
|---|---|---|---|---|
| CON | HC | CON | HC | |
| OTUs | 203 ± 6 | 160 ± 5 | 100 ± 7 | 81 ± 22 |
| PD whole tree | 21.5 ± 0.2 | 17.8 ± 0.2 | 13.3 ± 1.4 | 10.8 ± 1.5 |
| Chao1 | 384 ± 19 | 300 ± 11 | 179 ± 9 | 137 ± 39 |
| Shannon index | 6.66 ± 0.08 | 6.15 ± 0.06 | 4.95 ± 0.12 | 4.09 ± 0.53 |
Values are means ± SE. Mid, middle fattening stage; Late, late fattening stage; CON, control diet period; HC, high-concentrate diet period; OTUs, operational taxonomic units; PD whole tree, phylogenetic diversity whole tree, indicate the species diversity; Chao1, chao1 indicate the species richness; Shannon index, shannon index represents the species evenness.
Bacterial abundance
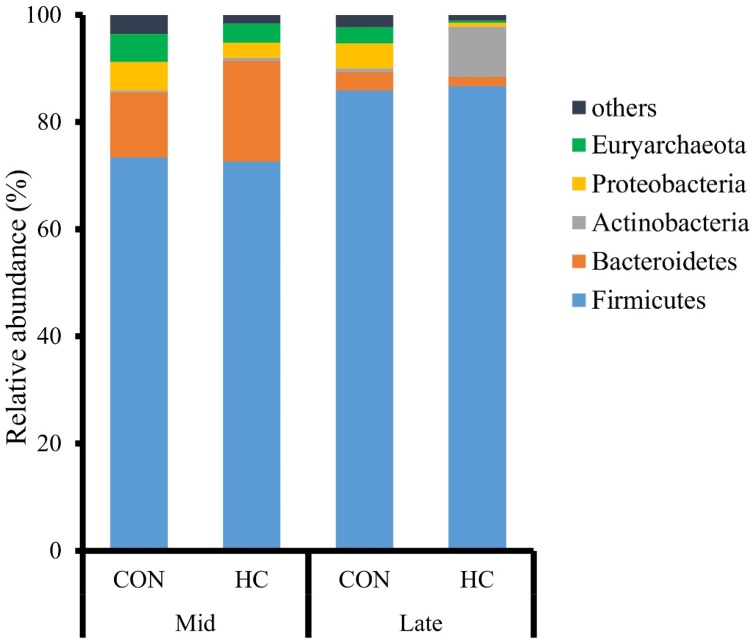
Twenty-five bacterial phyla were identified in the ruminal fluids. Of the major phyla, Firmicutes (73.0%), Bacteroidetes (12.2%), Proteobacteria (5.3%), and Euryarchaeota (4.4%) were most abundant in the Mid group, while Firmicutes (86.3%), Actinobacteria (5.0%), Proteobacteria (2.7%), and Bacteroidetes (2.6%) were most abundant in the Late group (Fig. 2). In total, 289 bacterial genera and 686 bacterial species were identified. Of the major genera, Ruminococcus (13.1%), Butyrivibrio (9.0%), Caloramator (8.1%), and Prevotella (7.9%) were most abundant in the Mid group, while Butyrivibrio (20.0%), Olsenella (11.9%), Sharpea (8.9%), and Caloramator (7.4%) were most abundant in the Late group (Table 5). In the Mid group, the relative abundance of Caloramator was significantly lower (P<0.05) and that of Succiniclasticum was significantly higher (P<0.05) during the HC period than those during the CON period.
Fig. 2.
Relative abundances of the major bacterial phyla in the ruminal fluids of Japanese Black beef cattle in the middle (Mid, n=3) and late (Late, n=3) fattening stages fed control (CON) and high-concentrate (HC) diets.
Table 5. Mean relative abundances (>1% of total sequences) of the major bacterial genera and species in Japanese Black beef cattle.
| Item | Mid (n=3) |
Late (n=3) |
|||
|---|---|---|---|---|---|
| CON | HC | CON | HC | ||
| Genus | Butyribivrio | 6.25 ± 0.50 | 11.7 ± 1.4 | 21.8 ± 2.2 | 18.1 ± 5.0 |
| Ruminococcus | 14.2 ± 1.7 | 11.9 ± 2.8 | 4.44 ± 0.84 | 7.05 ± 3.90 | |
| Caloramator | 10.6 ± 1.0 | 5.52 ± 1.26a) | 12.4 ± 3.8 | 2.34 ± 1.43 | |
| Olsenella | 0.08 ± 0.04 | 1.01 ± 0.69 | 11.0 ± 2.7 | 12.7 ± 5.9 | |
| Sharpea | 0.80 ± 0.02 | 0.82 ± 0.66 | 0.61 ± 0.47 | 17.2 ± 13.2 | |
| Prevotella | 5.60 ± 0.96 | 10.1 ± 2.1 | 2.72 ± 1.92 | 0.4 ± 0.28 | |
| Oscillospira | 6.08 ± 0.62 | 6.13 ± 1.16 | 3.38 ± 0.37 | 2.48 ± 1.54 | |
| Mogibacterium | 4.18 ± 0.59 | 3.02 ± 0.42 | 8.39 ± 2.15 | 2.49 ± 1.27 | |
| Succiniclasticum | 4.25 ± 0.95 | 7.20 ± 1.15a) | 0.25 ± 0.14 | 0.86 ± 0.22 | |
| Methanobrevibacter | 5.19 ± 0.95 | 3.61 ± 1.10 | 3.01 ± 1.09 | 0.43 ± 0.21 | |
| Slackia | 1.47 ± 0.04 | 1.41 ± 0.30 | 6.46 ± 1.76 | 3.56 ± 0.90 | |
| Blautia | 2.73 ± 0.25 | 2.43 ± 0.24 | 2.15 ± 0.25 | 2.61 ± 0.86 | |
| Thermicanus | 3.97 ± 0.54 | 1.92 ± 0.28 | 1.80 ± 0.36 | 0.78 ± 0.50 | |
| Clostridium | 2.98 ± 0.40 | 2.45 ± 0.24 | 0.88 ± 0.31 | 1.33 ± 0.50 | |
| Bifidobacterium | 0.09 ± 0.06 | 0.37 ± 0.29 | 0.60 ± 0.48 | 9.11 ± 7.24 | |
| Dysgonomonas | 1.75 ± 0.48 | 3.56 ± 1.43 | 0.09 ± 0.06 | 0.29 ± 0.07 | |
| Alkaliphilus | 2.23 ± 0.46 | 0.36 ± 0.08 | 1.75 ± 0.04 | 0.89 ± 0.46 | |
| Species | Caloramator mitchellensis | 24.8 ± 3.8 | 12.2 ± 2.6 | 29.1 ± 8.2 | 7.01 ± 4.96 |
| Olsenella uli | 0.23 ± 0.11 | 2.41 ± 1.64 | 27.4 ± 4.9 | 26.7 ± 12.7 | |
| Oscillospira eae | 13.1 ± 0.8 | 13.3 ± 2.7 | 8.68 ± 1.05 | 7.33 ± 5.21 | |
| Sharpea azabuensis | 0.03 ± 0.03 | 1.62 ± 1.32 | 1.23 ± 0.96 | 25.5 ± 19.0 | |
| Butyrivibrio proteoclasticus | 6.05 ± 1.74 | 10.2 ± 2.01 | 1.12 ± 0.45 | 1.71 ± 0.49 | |
| Dysgonomonas wimpennyi | 4.46 ± 1.13 | 8.52 ± 3.36 | 0.27 ± 0.18 | 0.78 ± 0.32 | |
| Alkaliphilus crotonatoxidans | 4.00 ± 0.87 | 3.26 ± 0.34 | 0.55 ± 0.08 | 2.22 ± 1.31 | |
| Parabacteroides goldsteinii | 3.28 ± 1.43 | 4.08 ± 1.68 | 0.07 ± 0.03 | 1.53 ± 1.25 | |
| Sedimentibacter hydroxybenzoicus | 2.83 ± 0.86 | 3.77 ± 2.20 | 0.62 ± 0.19 | 0.31 ± 0.12 | |
| Prevotella ruminicola | 0.40 ± 0.16 | 6.17 ± 4.65 | 0.25 ± 0.19 | 0.22 ± 0.18 | |
| Blautia wexlerae | 1.88 ± 0.28 | 1.12 ± 0.46 | 1.94 ± 0.66 | 0.76 ± 0.53 | |
| Longilinea arvoryzae | 4.32 ± 1.85 | 0.84 ± 0.34 | 0.20 ± 0.11 | 0.31 ± 0.23 | |
| Selenomonas infelix | 1.04 ± 0.25 | 3.48 ± 2.15 | 0.45 ± 0.11 | 0.57 ± 0.46 | |
| Clostridium alkalicellulosi | 2.29 ± 0.12 | 1.63 ± 0.55 | 0.67 ± 0.17 | 0.45 ± 0.14 | |
| Slackia faecicanis | 0.82 ± 0.07 | 0.74 ± 0.21 | 2.89 ± 0.62 | 0.54 ± 0.38 | |
| Blautia coccoides | 1.75 ± 0.18 | 1.03 ± 0.29 | 0.67 ± 0.32 | 1.22 ± 0.53 | |
| Catonella morbi | 0.00 ± 0.00 | 0.02 ± 0.01 | 0.24 ± 0.19 | 4.30 ± 1.77 | |
| Eggerthella sinensis | 0.82 ± 0.08 | 0.54 ± 0.15 | 2.48 ± 0.60 | 0.62 ± 0.19 | |
Values are means ± SE. Mid, middle fattening stage; Late, late fattening stage; CON, control diet period; HC, high-concentrate diet period. a) Indicates the significant (P<0.05) difference between the LC and HC periods in the Mid group.
DISCUSSION
A continuously low rumen pH and high AUC were reported during a rapid increase in the concentrate ratio in the diet (40 to 90%, dry matter basis) [2], and feeding a high-concentrate diet decreased the rumen pH and increased the VFA concentration in steers and non-lactating Holstein cattle [4, 21], indicating a negative correlation between the rumen pH and VFA concentration. However, there are conflicting reports that SARA induced by a grain diet was naturally mitigated by the second week, and the decrease in rumen pH was minimized in non-lactating dairy cattle [22, 23]. In the present study, a significantly lower mean and minimum rumen pH and higher AUC (pH × hour, pH<5.6) and duration of time (pH<5.6, min/day) during the HC period were observed only in the Mid group, and no significant difference was identified in the Late group. These results suggest that Japanese Black cattle in the mid fattening stage can respond to the higher concentrate composition in their diet, resulting in significantly lower rumen pH and higher AUC during the HC period compared with no significant changes in the late fattening stage.
Interestingly, we found contrasting results for rumen pH and total VFA between the Mid and Late groups. Contrasting the rumen pH, significantly higher total VFA and lactic acid concentrations during the HC period were identified only in the Late group. Previously, Sato [21] suggested that feeding a high-concentrate diet can induce acidotic conditions via higher total VFA and lactic acid concentrations, which were consistent with the findings in our Late group. Although we could not fully explain the contrasting results between the middle and late fattening stages, the results suggest that the rumen is fully adapted to long-term acidic conditions during the late fattening stage, in line with a report that the rumen can adapt to SARA in Japanese Black cattle [15].
The reduction in rumen pH caused by the high-grain diet caused a reduction in bacterial diversity when compared with feeding a high-forage diet [13, 19]. However, the bacterial diversity (OTUs, PD whole tree, Chao1, and Shannon index) did not differ between the two periods or between the two groups, although the indices in both groups were lower during the HC period compared with the CON period. The reduction in rumen pH in the Mid group during the HC period was severe enough (6.00 to 5.76) to reduce bacterial diversity compared with a previous study (6.52 to 6.10 [15]). Therefore, we postulate that the rumen of the Japanese Black beef cattle adapted to long-term feeding of a high-concentrate diet to minimize the adverse changes in the bacterial diversity.
To our knowledge, this is the first study of the ruminal bacterial community in Japanese Black beef cattle at different fattening stages. At the phylum level, Firmicutes was the most abundant in both groups, followed by Bacteroidetes, Proteobacteria, and Euryarchaeota in the Mid group and Actinobacteria, Proteobacteria, and Bacteroidetes in the Late group. Previously, an increase in the relative abundance of Firmicutes in the ruminal fluids was reported in a SARA challenge model of dairy cattle [9], and Bacteroidetes comprise the first or second largest population in the ruminal fluid in Holstein cows [12]. During a SARA challenge induced by feeding alfalfa pellets or a grain diet, the lower rumen pH led to the death and lysis of gram-negative bacteria, such as Bacteroidetes [14], and a decrease in the relative abundance of Bacteroidetes in the ruminal fluids was observed in dairy cattle [9, 25]. Therefore, more severe SARA in the Late group might induce the higher and lower proportions of Firmicutes and Bacteroidetes, respectively, in the same group, whereas the increase in dietary concentrate in our study did not induce a dramatic change in the rumen bacterial composition compared with previous SARA challenge models [9, 25], likely due to prolonged high-grain feeding in Japanese Black cattle.
Similar to the phylum level, differences in the bacterial proportions were noted at the genus level. For example, Ruminococcus was the most abundant in the Mid group, followed by Butyrivibrio, Caloramator, and Prevotella, while Butyrivibrio was the most abundant in the Late group, followed by Olsenella, Sharpea, and Caloramator. Although Ruminococcus is a cellulolytic species, conflicting results on the Ruminococcus proportion were reported when feeding beef cattle a high-grain diet or SARA challenge models in Holstein steers [18, 21]. Ruminococcus was the most abundant in our Mid group despite the low proportion of roughage. Previously, Nagata et al. [15] postulated that an increase in starch-fermenting Ruminococcus species may contribute to the higher Ruminococcus prevalence on a high-grain diet in Holstein steers, which may be consistent with our Mid group. Contrasting the Mid group, Butyrivibrio was the most abundant genus in the Late group. Butyrivibrio species have various functions in rumen fermentation, such as producing butyrate [17], enabling the utilization of cellulose and starch [3], and controlling the proportion of lactate [8]. In the present study, the relative abundance of Butyrivibrio in the Mid group increased during the HC period, and it was higher in the Late group regardless of the period, although there was no statistical significance. Although Petri et al. [18] reported that the Butyrivibrio population decreased in beef cattle during a SARA challenge, we observed the opposite, i.e., the relative abundance of Butyrivibrio decreased when the cattle were fed a high-grain diet. Therefore, further studies are required to clarify the role of this genus in ruminal fermentation during long-term concentrate feeding and the different responses to dietary changes in Japanese Black cattle at differing fattening stages.
Caloramator species, members of the phylum Firmicutes, ferment carbohydrates, such as glucose, fructose, maltose, galactose, and sucrose [20]. In the present study, the relative abundance of Caloramator in the Mid group was significantly lower during the HC period compared with the CON period. Although the dynamics of Caloramator due to changes in the ruminal conditions are still unknown, it is plausible that Caloramator are representative bacteria in the middle fattening stage in Japanese Black cattle.
Succiniclasticum species is characterized by fermenting succinate and converting it quantitatively propionate [24]. In the present study, the proportion of propionate in the Mid group was higher during the HC period compared with during the CON period, which was consistent with higher relative abundance of Succiniclasticum genus in the same group and period. Therefore, these results suggest that a higher proportion of Succiniclasticum might partly contribute to produce propionate in accordance with the increased concentrate diet proportion in the middle fattening stage in Japanese Black cattle.
To conclude, different ruminal responses in pH and the bacterial community to an increase in the proportion of concentrate in the diet were identified in Japanese Black cattle at different fattening stages. The reduction in rumen pH during the HC period was mainly observed in the Mid group, while SARA was more severe in the Late group. Furthermore, the rumen bacterial community diversity and composition differed slightly between the middle and late fattening stages, although few significant changes due to the dietary changes were found. These results suggest that the ruminal fermentative function in response to a higher concentrate diet might adapt differently at the two different fattening stages with the duration of long-term high-concentrate feeding. However, we studied a limited number of animals and only two fattening stages. Therefore, further studies are needed to clarify the adaptive characteristics of rumen pH, fermentation, and the bacterial community to prolonged feeding with a high-grain diet based on more animals and longer periods in Japanese Black cattle.
REFERENCES
- 1.Attia N. E.2016. Subacute ruminal acidosis in feedlot: incidence, clinical alterations and its sequelae. Adv. Anim. Vet. Sci. 4: 513–517. doi: 10.14737/journal.aavs/2016/4.10.513.517 [DOI] [Google Scholar]
- 2.Bevans D. W., Beauchemin K. A., Schwartzkopf-Genswein K. S., McKinnon J. J., McAllister T. A.2005. Effect of rapid or gradual grain adaptation on subacute acidosis and feed intake by feedlot cattle. J. Anim. Sci. 83: 1116–1132. doi: 10.2527/2005.8351116x [DOI] [PubMed] [Google Scholar]
- 3.Fernando S. C., Purvis H. T., 2nd., Najar F. Z., Sukharnikov L. O., Krehbiel C. R., Nagaraja T. G., Roe B. A., Desilva U.2010. Rumen microbial population dynamics during adaptation to a high-grain diet. Appl. Environ. Microbiol. 76: 7482–7490. doi: 10.1128/AEM.00388-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goad D. W., Goad C. L., Nagaraja T. G.1998. Ruminal microbial and fermentative changes associated with experimentally induced subacute acidosis in steers. J. Anim. Sci. 76: 234–241. doi: 10.2527/1998.761234x [DOI] [PubMed] [Google Scholar]
- 5.Goto H., Qadis A. Q., Kim Y. H., Ikuta K., Ichijo T., Sato S.2016. Effects of a bacterial probiotic on ruminal pH and volatile fatty acids during subacute ruminal acidosis (SARA) in cattle. J. Vet. Med. Sci. 78: 1595–1600. doi: 10.1292/jvms.16-0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gotoh T., Nishimura T., Kuchida K., Mannen H.2018. The Japanese Wagyu beef industry: current situation and future prospects - A review. Asian-Australas. J. Anim. Sci. 31: 933–950. doi: 10.5713/ajas.18.0333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gozho G. N., Plaizier J. C., Krause D. O., Kennedy A. D., Wittenberg K. M.2005. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response. J. Dairy Sci. 88: 1399–1403. doi: 10.3168/jds.S0022-0302(05)72807-1 [DOI] [PubMed] [Google Scholar]
- 8.Kenney N. M., Vanzant E. S., Harmon D. L., McLeod K. R.2015. Direct-fed microbials containing lactate-producing bacteria influence ruminal fermentation but not lactate utilization in steers fed a high-concentrate diet. J. Anim. Sci. 93: 2336–2348. doi: 10.2527/jas.2014-8570 [DOI] [PubMed] [Google Scholar]
- 9.Khafipour E., Li S., Plaizier J. C., Krause D. O.2009. Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Appl. Environ. Microbiol. 75: 7115–7124. doi: 10.1128/AEM.00739-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim Y. H., Nagata R., Ohtani N., Ichijo T., Ikuta K., Sato S.2016. Effects of dietary forage and calf starter diet on ruminal pH and bacteria in Holstein calves during weaning transition. Front. Microbiol. 7: 1575. doi: 10.3389/fmicb.2016.01575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimura A., Sato S., Goto H., Yamagishi N., Okada K., Mizuguchi H., Ito K.2012. Simultaneous estimation of the pH of rumen and reticulum fluids of cows using a radio-transmission pH-measurement system. J. Vet. Med. Sci. 74: 531–535. doi: 10.1292/jvms.11-0425 [DOI] [PubMed] [Google Scholar]
- 12.Kong Y., Teather R., Forster R.2010. Composition, spatial distribution, and diversity of the bacterial communities in the rumen of cows fed different forages. FEMS Microbiol. Ecol. 74: 612–622. doi: 10.1111/j.1574-6941.2010.00977.x [DOI] [PubMed] [Google Scholar]
- 13.Liu J. H., Bian G. R., Zhu W. Y., Mao S. Y.2015. High-grain feeding causes strong shifts in ruminal epithelial bacterial community and expression of Toll-like receptor genes in goats. Front. Microbiol. 6: 167. doi: 10.3389/fmicb.2015.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagaraja T. G., Titgemeyer E. C.2007. Ruminal acidosis in beef cattle: the current microbiological and nutritional outlook. J. Dairy Sci. 90 Suppl 1: E17–E38. doi: 10.3168/jds.2006-478 [DOI] [PubMed] [Google Scholar]
- 15.Nagata R., Kim Y. H., Ohkubo A., Kushibiki S., Ichijo T., Sato S.2018. Effects of repeated subacute ruminal acidosis challenges on the adaptation of the rumen bacterial community in Holstein bulls. J. Dairy Sci. 101: 4424–4436. doi: 10.3168/jds.2017-13859 [DOI] [PubMed] [Google Scholar]
- 16.Oka A., Maruo Y., Miki T., Yamasaki T., Saito T.1998. Influence of vitamin A on the quality of beef from the Tajima strain of Japanese Black cattle. Meat Sci. 48: 159–167. doi: 10.1016/S0309-1740(97)00086-7 [DOI] [PubMed] [Google Scholar]
- 17.Paillard D., McKain N., Chaudhary L. C., Walker N. D., Pizette F., Koppova I., McEwan N. R., Kopecný J., Vercoe P. E., Louis P., Wallace R. J.2007. Relation between phylogenetic position, lipid metabolism and butyrate production by different Butyrivibrio-like bacteria from the rumen. Antonie van Leeuwenhoek 91: 417–422. doi: 10.1007/s10482-006-9121-7 [DOI] [PubMed] [Google Scholar]
- 18.Petri R. M., Schwaiger T., Penner G. B., Beauchemin K. A., Forster R. J., McKinnon J. J., McAllister T. A.2013. Changes in the rumen epimural bacterial diversity of beef cattle as affected by diet and induced ruminal acidosis. Appl. Environ. Microbiol. 79: 3744–3755. doi: 10.1128/AEM.03983-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitta D. W., Pinchak E., Dowd S. E., Osterstock J., Gontcharova V., Youn E., Dorton K., Yoon I., Min B. R., Fulford J. D., Wickersham T. A., Malinowski D. P.2010. Rumen bacterial diversity dynamics associated with changing from bermudagrass hay to grazed winter wheat diets. Microb. Ecol. 59: 511–522. doi: 10.1007/s00248-009-9609-6 [DOI] [PubMed] [Google Scholar]
- 20.Plaizier J. C., Krause D. O., Gozho G. N., McBride B. W.2008. Subacute ruminal acidosis in dairy cows: the physiological causes, incidence and consequences. Vet. J. 176: 21–31. doi: 10.1016/j.tvjl.2007.12.016 [DOI] [PubMed] [Google Scholar]
- 21.Sato S.2016. Pathophysiological evaluation of subacute ruminal acidosis (SARA) by continuous ruminal pH monitoring. Anim. Sci. J. 87: 168–177. doi: 10.1111/asj.12415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steele M. A., Croom J., Kahler M., AlZahal O., Hook S. E., Plaizier K., McBride B. W.2011. Bovine rumen epithelium undergoes rapid structural adaptations during grain-induced subacute ruminal acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300: R1515–R1523. doi: 10.1152/ajpregu.00120.2010 [DOI] [PubMed] [Google Scholar]
- 23.Steele M. A., Vandervoort G., AlZahal O., Hook S. E., Matthews J. C., McBride B. W.2011. Rumen epithelial adaptation to high-grain diets involves the coordinated regulation of genes involved in cholesterol homeostasis. Physiol. Genomics 43: 308–316. doi: 10.1152/physiolgenomics.00117.2010 [DOI] [PubMed] [Google Scholar]
- 24.van Gylswyk N. O.1995. Succiniclasticum ruminis gen. nov., sp. nov., a ruminal bacterium converting succinate to propionate as the sole energy-yielding mechanism. Int. J. Syst. Bacteriol. 45: 297–300. doi: 10.1099/00207713-45-2-297 [DOI] [PubMed] [Google Scholar]
- 25.Wang H., Pan X., Wang C., Wang M., Yu L.2015. Effects of different dietary concentrate to forage ratio and thiamine supplementation on the rumen fermentation and ruminal bacterial community in dairy cows. Anim. Prod. Sci. 55: 189–193. doi: 10.1071/AN14523 [DOI] [Google Scholar]
- 26.Yang W. Z., Li Y. L., McAllister T. A., McKinnon J. J., Beauchemin K. A.2012. Wheat distillers grains in feedlot cattle diets: feeding behavior, growth performance, carcass characteristics, and blood metabolites. J. Anim. Sci. 90: 1301–1310. doi: 10.2527/jas.2011-4372 [DOI] [PubMed] [Google Scholar]