Abstract
This study aimed to assess the effects of mosapride at various dosages on ruminal motility in cattle and the absorption kinetics of mosapride in cattle. Mosapride was rapidly absorbed after oral administration in cattle. Oral administration at dosages of 1, 3, and 10 mg/kg resulted in a dose-dependent increase in the detection time. At the 1 mg/kg dose, the motility index of rumen in cattle significantly increased at 60, 70, and 80 min (134 ± 20, 168 ± 37, 173 ± 45%, respectively), compared with that of the control. The administration of mosapride in cattle did not cause any subsequent adverse clinical signs or blood test abnormalities. It was suggested that mosapride enhanced ruminal motility without adverse effects in cattle.
Keywords: cattle, mosapride, oral administration, ruminal motility
Prokinetic agents promote gastrointestinal motor activity and are used for symptomatic treatment of gastrointestinal motility disorders. Several prokinetic agents have been developed in humans and some of them are utilized in animals such as dogs or cattle [2, 9]. Mosapride selectively acts on the 5-hydroxytryptamine-4 (5-HT4) receptor, and promotes the release of acetylcholine in enteric nerves by activating the 5-HT4 receptor, thereby enhancing gastrointestinal motility [4, 13, 14]. In humans, mosapride citrate has been used as a prokinetic agent for the treatment of dyspeptic symptoms of gastrointestinal disorders such as chronic gastritis, functional dyspepsia, irritable bowel syndrome, and gastric esophageal reflux disease [4]. Mosapride also enhances upper gastrointestinal motility in dogs [7, 12, 13] and has been used for the control of upper gastrointestinal symptom, such as anorexia and vomiting that are associated with gastrointestinal motility disorders. In horses, the oral administration of mosapride can increase the electrical activity of the small intestine and caecum, which suggests that mosapride promotes motility in the small intestine and caecum of horses [11]. However, the prokinetic effect of mosapride on ruminal motility in cattle is unknown, and its pharmacokinetics in cattle has not been clarified.
The purposes of this study were to assess the effects of mosapride administered orally at various dosages on ruminal motility in cattle as measured by a strain gauge force transducer, and to examine the absorption kinetics of mosapride in cattle.
Eight healthy Holstein cows weighing 408 to 620 kg were used in this study. We divided these cattle into two groups. Four cattle (body weight: 522 ± 76 kg) were used in experiment 1, and another four cattle (body weight: 524 ± 60 kg) were used in experiment 2. All cattle were housed in individual stanchion stalls, and were given commercial cattle concentrate, hay and free access to water. Food was offered twice daily (9:00 and 16:00). The handling of all animals used in this study was approved by the Institutional Care and Use Committee for Laboratory Animals of the National Institute of Animal Health.
In experiment 1, we examined the changes in the serum concentration of mosapride following its oral administration to cattle. Mosapride citrate (Gasmotin Powder 1%; Sumitomo Dainippon Pharma Co., Ltd., Osaka, Japan) was dissolved in 500 ml of water. Mosapride at various dosages (0, 1, 3, and 10 mg/kg) was administered to cattle through a gastric tube once at 12:00. This study was carried out with a Latin-square design. There was a 1-week washout period between subsequent treatments. Blood samples were collected from the jugular vein both before (Pre) and at 0.5, 1, 2, 4, 8, and 24 hr following the oral single administration of mosapride. Blood samples were collected into EDTA-2K and plain tubes. White blood cell counts, red blood cell counts, hematocrit, hemoglobin concentration and platelet counts were measured using an automatic blood cell counter (MEK-6450, Nihon Koden, Tokyo, Japan). Serum was stored at −20°C until it was used for analysis. The serum concentration of mosapride was quantified using the API-3200 LC-MS/MS system (Shimadzu Corp., Kyoto, Japan) equipped with an electrospray ionization interface to generate positive ions. This assay system was detectable to 0.001 µg/ml. Recovery rate of mosapride in this system was 95–100%. The blood chemistry of serum such as aspartate aminotransferase, lactate dehydrogenase, total protein, total cholesterol, triglyceride, creatinine, total bilirubin, and calcium levels was assessed using an automatic analyzer (7020, Hitachi High-Technologies Corp., Tokyo, Japan). The clinical assessments were performed by a veterinarian.
In experiment 2, we examined the effects of mosapride administered at various dosages on the motility of rumen in cattle. Ruminal motility in cattle could be assessed physiologically using strain gauge force transducers [5, 6, 13]. The size of the force transducer used in this study was 18 × 35 mm and it contained a strain gauge (Foil Strain Gauge; NEC San-ei Instruments, Ltd., Tokyo, Japan). It was covered with silicon. Four cattle were sedated intramuscularly with xylazine hydrochloride (0.05 mg/kg; Bayer, Leverkusen, Germany). After 10 min, local anesthesia was applied to the left paralumbar fossa with an intramuscular infusion of procaine hydrochloride (80–100 ml/body, Kyoritsu Seiyaku Co., Tokyo, Japan). The force transducer was sutured onto the serosa of the dorsal sac of the rumen. The lead wires of the force transducer were exteriorized through the abdominal wall. Then the wires from the force transducer were subcutaneously tunneled and pulled out through the skin between the paralumbar fossa. The cattle were injected intramuscularly with antibiotics (10 mg/kg, ampicillin sodium; Meiji Seika Pharma Co., Ltd., Tokyo, Japan) after the surgery.
Ruminal motility was recorded on a polygraph (MP100A, BIOPAC Systems Inc., Santa Barbara, CA, U.S.A.) by connecting the lead wires of the transducers to the connecting cables from the amplifiers (DA100, BIOPAC Systems Inc.), was digitized by an analog-to-digital converter, and continuously recorded on a computer for analysis. To assess the amplitude of ruminal motility quantitatively, the motility index [7, 13] was determined. The motility index given by the processing system corresponded to the measurements of the area surrounded by the contraction wave and base line. The motility index during the 10-min interval before the test drugs were administered was designated as 100%, and the percentage change in the motility index induced by the test drug was calculated every 10 min.
Experiment 2 was carried out with cattle at 7 days after surgery. Mosapride citrate was dissolved in 500 ml of water. Mosapride at various dosages (0, 1, and 3 mg/kg) was administered to the cattle through a gastric tube once at 12:00. This study was carried out with a Latin-square design. There was a 1-week washout period between subsequent treatments. Blood samples were collected from the jugular vein both before (Pre) and at 30 min following the oral single administration of mosapride. The serum concentration of mosapride was quantified using an API-3200 LC-MS/MS system equipped with an electrospray ionization interface to generate positive ions. To assess the mosapride citrate effect, we compared the motility index of the rumen for 180 min after the oral single administration of either mosapride (1 and 3 mg/kg) or a control (0 mg/kg).
Means for quantitative data are expressed as means ± standard deviation. The statistical analysis was performed by one-way repeated measures of ANOVA. When significant, multiple comparisons were performed using Dunn’s procedure. A P-value of less than 0.05 was regarded as significant.
In experiment 1, mosapride was rapidly absorbed after oral administration to the cattle. Mosapride administered orally at dosages of 1, 3, and 10 mg/kg resulted in a dose-dependent increase in the detection time (Table 1). The serum concentration of mosapride at a dosage of 10 mg/kg was significantly higher than at 1 and 3 mg/kg (Table 1). The maximum serum concentration of mosapride was at 0.5 hr after the oral administration at all doses, and the serum concentration decreased gradually. The administration of mosapride in cattle did not cause any subsequent adverse clinical signs or blood test abnormalities (data not shown).
Table 1. Changes in serum concentrations of mosapride following oral administration to cattle.
| Time (hr) after administration | Dose of mosapride (mg/kg) |
|||
|---|---|---|---|---|
| 0 | 1 | 3 | 10 | |
| Pre | ND | ND | ND | ND |
| 0.5 | ND | 0.009 ± 0.004 | 0.006 ± 0.001a) | 0.016 ± 0.003b) |
| 1 | ND | 0.003 ± 0.001a) | 0.003 ± 0.001a) | 0.009 ± 0.002b) |
| 2 | ND | 0.001 ± 0 | 0.001 ± 0a) | 0.005 ± 0.001b) |
| 4 | ND | ND | 0.001 ± 0 | 0.004 ± 0.001 |
| 8 | ND | ND | ND | 0.003 ± 0.001 |
| 24 | ND | ND | ND | 0.002 ± 0 |
Data are expressed as means ± SD (µg/ml). a, b) P<0.05. ND: Not detected (<0.001 µg/ml).
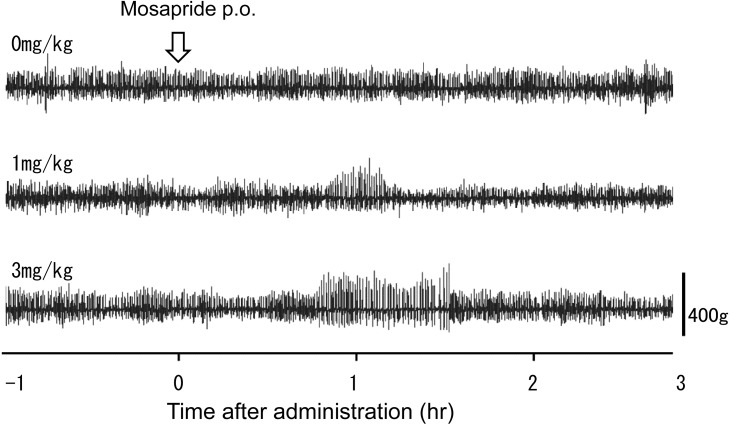
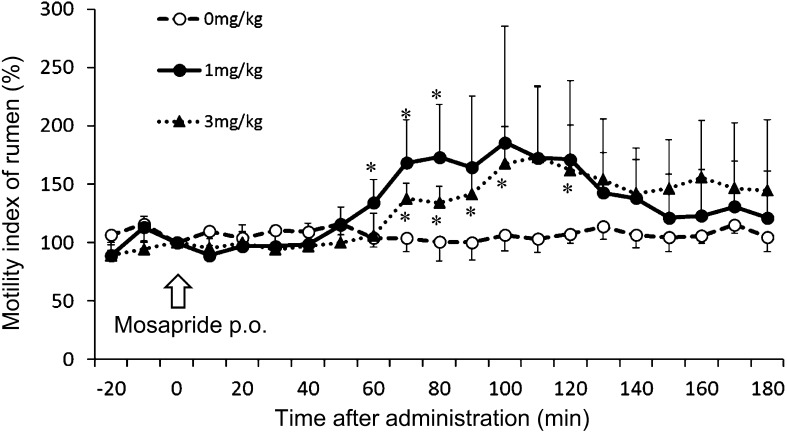
In experiment 2, the serum concentration of mosapride at a dosage of 1 mg/kg was 0.011 ± 0.005 µg/ml, and at a dosage of 3 mg/kg was 0.014 ± 0.005 µg/ml at 30 min after administration. There was no significant difference in the serum concentration of mosapride between the two groups (1 and 3 mg/kg). The effect of mosapride on ruminal motility in cattle after oral administration at several doses is shown in Fig. 1. Mosapride (1 and 3 mg/kg p.o.) enhanced contraction in the rumen (Figs. 1 and 2). The motility index at a dose of 1 mg/kg significantly increased at 60, 70, and 80 min (134 ± 20, 168 ± 37, 173 ± 45%, respectively), compared with that of the control (0 mg/kg) as shown in Fig. 2. The motility index at a dose of 3 mg/kg significantly increased at 70, 80, 90, 100, and 120 min (138 ± 13, 134 ± 14, 141 ± 21, 168 ± 32, 162 ± 39%, respectively), compared with that of the control (0 mg/kg) as shown in Fig. 2. There was no significant difference in the motility index between the two groups (1 and 3 mg/kg), but prolonged the duration time of its enhancement dose-dependently (Fig. 2).
Fig. 1.
Changes in ruminal motility after the oral administration of mosapride at several doses in cattle (cattle No.1).
Fig. 2.
Effect of mosapride on ruminal motility in cattle. The motility index for the 10-min period after the oral administration of mosapride is expressed as a percentage of that for the 10-min period before the administration. Each point represents the mean ± SD in four cattle. *Value differs significantly (P<0.05) from the value for 0 mg/kg. Motility index: percentage mean amplitude of ruminal motility in cattle.
The pharmacokinetics of mosapride have been investigated in human and other species, such as rats, dogs, and monkeys [3, 4, 8]. In the present study, mosapride was rapidly absorbed after oral administration in cattle. After the oral administration of single doses of mosapride of 1–10 mg/kg in cattle, peak mosapride concentrations (Cmax) were reached after 0.5 hr. In humans, the time to maximum concentration (Tmax) of a single dose of mosapride of 10 mg was 0.6 hr [4], and we observed similar results in cattle. A previous report indicated that after oral administration, carbonyl-14C mosapride radioactivity was rapidly absorbed through the intestinal tract [8]. In rats, the concentration of plasma radioactivity reached the maximum (Cmax; 1.41 µg eq/ml) 1 hr after administration (10 mg/kg p.o.) and decreased biphasically with half-lives of about 2 hr in the alpha-phase and of about 8 hr in the beta-phase [8]. In the present study, The Cmax of the serum concentration of mosapride at a dosage of 10 mg/kg was 0.016 µg/ml. The plasma Cmax in humans after oral administration (10 mg/kg) was 0.064 µg/ml, and that in dogs after oral administration (5 mg/kg) was 2.76 µg/ml [3, 4]. The serum Cmax of mosapride in cattle was lower than that in other species, such as humans, rats and dogs [3, 4, 8]. This Cmax difference may be associated with dissimilar bioavailability or metabolism in animals [8]. Food intake had a marked effect on the pharmacokinetics of mosapride in dogs. The plasma Cmax of mosapride was four times higher in fasted dogs than fed dogs [3]. In the present study, cattle were given diet twice daily (9:00 and 16:00), therefore food intake might impact serum concentration of mosapride.
In the present study, we evaluated the prokinetic action of mosapride by assessing the motility index of rumen (the percentage mean amplitude of ruminal motility) in cattle. Mosapride enhanced the ruminal motility in cattle, and prolonged the duration time of its enhancement dose-dependently. Mosapride selectively acts on 5-HT4 receptors and increases the level of acetylcholine released from cholinergic nerve endings in the digestive tract [13, 14]. Acetylcholine binds to muscarinic receptors on the smooth muscle and induces contractions. The present study showed that the oral administration of mosapride at a dose of 1 mg/kg significantly increased the motility index (ex. 168% at 70 min) of rumen in cattle. In dogs, about 1 mg/kg (0.75 to 2 mg/kg, p.o.) of mosapride promoted motility in the gastric antrum without adverse effects [12]. The recommended dose of mosapride in cattle and dogs may be about 1 mg/kg (p.o.). On the other hand, in humans, mosapride is usually prescribed at 5 mg three times a day for the treatment of gastrointestinal discomfort [4]. The dose of mosapride appears to be higher in cattle and dogs than in humans. This dose difference may be associated with dissimilar bioavailability or metabolism [8, 10]. In horses, the oral administration of mosapride at a dosage of 1 mg/kg induced a significant promotion of motility in the small intestine but not in the cecum [11]. This difference may have been attributable to a difference in the capacity for mosapride in the proximal and distal portions of the gastrointestinal tract. Therefore, it is necessary to take into account the difference in the optimum activity for 5-HT4 receptor agonists among various parts of the gastrointestinal tract [11].
In the present study, the administration of mosapride in cattle did not cause any subsequent adverse clinical signs or blood test abnormalities. Some gastroprokinetic agents, such as metoclopramide, may cause sedation through dopamine receptors in the central nervous system [1, 2]. Mosapride has a high affinity for the 5-HT4 receptor in the gastrointestinal tract and does not have an affinity for 5-HT1, 5-HT2, adrenalineα1, adrenalineα2, or dopamine-2 (D2) receptors [4, 14]. The advantage of mosapride over several prokinetic agents in humans is its safety [4].
In conclusion, mosapride was rapidly absorbed after oral administration in cattle, and it enhanced the ruminal motility and dose-dependently prolonged the duration time of enhancement of ruminal motility in cattle. Therefore, we suggest that mosapride may be useful for the treatment of cattle with gastrointestinal tract dysfunctions such as ruminal atony and anorexia.
Acknowledgments
This work was supported by the research fund of DS Pharma Animal Health., Co., Ltd.
REFERENCES
- 1.Agostinucci W. A., Gannon R. H., Schauer P. K., Walters J. K.1986. Continuous infusion of metoclopramide for prevention of chemotherapy-induced emesis. Clin. Pharm. 5: 150–153. [PubMed] [Google Scholar]
- 2.Briejer M. R., Akkermans L. M., Schuurkes J. A. J.1995. Gastrointestinal prokinetic benzamides: the pharmacology underlying stimulation of motility. Pharmacol. Rev. 47: 631–651. [PubMed] [Google Scholar]
- 3.Chae J. W., Song B. J., Baek I. H., Yun H. Y., Ma J. Y., Kwon K. I.2015. Effects of food intake on pharmacokinetics of mosapride in beagle dogs. J. Vet. Pharmacol. Ther. 38: 497–499. doi: 10.1111/jvp.12200 [DOI] [PubMed] [Google Scholar]
- 4.Curran M. P., Robinson D. M.2008. Mosapride in gastrointestinal disorders. Drugs 68: 981–991. doi: 10.2165/00003495-200868070-00007 [DOI] [PubMed] [Google Scholar]
- 5.Daniel R. C. W.1983. Motility of the rumen and abomasum during hypocalcaemia. Can. J. Comp. Med. 47: 276–280. [PMC free article] [PubMed] [Google Scholar]
- 6.Jacoby H. I., Bass P., Bennett D. R.1963. In vivo extraluminal contractile force transducer for gastrointestinal muscle. J. Appl. Physiol. 18: 658–664. doi: 10.1152/jappl.1963.18.3.658 [DOI] [PubMed] [Google Scholar]
- 7.Kubota K., Tatsutomi Y., Kitajima M., Mafune K., Ohta K., Yoshida M., Suwa T., Kuroda J., Hiki N., Seto Y., Kaminishi M.2011. Physiological evaluation of residual stomach motility after local resection in conscious dogs. Surg. Today 41: 680–687. doi: 10.1007/s00595-010-4329-6 [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto S., Tagawa M., Amejima H., Nakao M., Kagemoto A., Fujii T., Miyazaki H., Sekine Y.1993. Absorption, distribution and excretion of [carbonyl-14C]mosapride citrate after a single oral administration in rats, dogs and monkeys. Arzneimittelforschung 43: 1084–1094. [PubMed] [Google Scholar]
- 9.Roussel A. J., Brumbaugh G. W., Waldron R. C., Baird A. N.1994. Abomasal and duodenal motility in yearling cattle after administration of prokinetic drugs. Am. J. Vet. Res. 55: 111–115. [PubMed] [Google Scholar]
- 10.Sakashita M., Mizuki Y., Yamaguchi T., Miyazaki H., Sekine Y.1993. Pharmacokinetics of the gastrokinetic agent mosapride citrate after intravenous and oral administrations in dogs and monkeys. Arzneimittelforschung 43: 864–866. [PubMed] [Google Scholar]
- 11.Sasaki N., Okamura K., Yamada H.2005. Effects of mosapride, a 5-hydroxytryptamine 4 receptor agonist, on electrical activity of the small intestine and cecum in horses. Am. J. Vet. Res. 66: 1321–1323. doi: 10.2460/ajvr.2005.66.1321 [DOI] [PubMed] [Google Scholar]
- 12.Tsukamoto A., Ohno K., Maeda S., Nakashima K., Fukushima K., Fujino Y., Tsujimoto H.2011. Prokinetic effect of the 5-HT4R agonist mosapride on canine gastric motility. J. Vet. Med. Sci. 73: 1635–1637. doi: 10.1292/jvms.11-0270 [DOI] [PubMed] [Google Scholar]
- 13.Yoshida N., Ito T., Karasawa T., Itoh Z.1991. AS-4370, a new gastrokinetic agent, enhances upper gastrointestinal motor activity in conscious dogs. J. Pharmacol. Exp. Ther. 257: 781–787. [PubMed] [Google Scholar]
- 14.Yoshida N., Omoya H., Oka M., Furukawa K., Ito T., Karasawa T.1989. AS-4370, a novel gastrokinetic agent free of dopamine D2 receptor antagonist properties. Arch. Int. Pharmacodyn. Ther. 300: 51–67. [PubMed] [Google Scholar]