Abstract
A case of laryngeal squamous cell carcinoma (SCC) and squamous papilloma in a 19-year-old Thoroughbred stallion is described. The animal exhibited severe wheezing caused by laryngopharyngeal stenosis. Histological examination identified laryngeal, laryngotracheal, and guttural pouch tumor masses consisting of areas of SCC. In the epiglottic lesion, the overlying epithelium was replaced by papilloma cells, and superficial cells frequently had nuclear inclusion bodies that expressed oncoprotein E6, which is characteristic of high risk human papillomaviruses. The papillomatous epithelium was continuous with epithelium composed of SCC cells. Equus caballus papillomavirus 2 (EcPV2) DNA was detected in the guttural pouch tumor. These findings suggest that laryngeal SCC and papilloma are a continuum of EcPV2-induced neoplastic lesions in horses.
Keywords: Equus caballus papillomavirus 2, horse, larynx, papilloma, squamous cell carcinoma
Squamous cell carcinoma (SCC) is the most common malignant skin neoplasm in horses and other equids, and accounts for 7–37% of equine skin lesions [21]. In the initial stage, the lesions present as whitish plaques or papillomas that can progress to carcinoma in situ and, ultimately, to invasive SCC [20]. SCC and related precursor lesions can arise at any site on the skin and mucosa, but preferentially develop on non-pigmented skin and at mucocutaneous junctions, such as the ocular and oral regions and the external genitalia [20, 23]. Papillomaviruses (PVs) are the causative agents of certain types of SCC in horses, but the biological mechanisms underlying tumor development and progression are still unclear [15].
Eight equine papillomaviruses, Equus caballus papillomavirus 1–8 (EcPV1–8), have been identified to date [13]. Each virus type is associated with a different form of skin disorder such as classic epithelial papillomatosis (EcPV1, 8), genital papillomas (EcPV2, 7), and aural plaques (EcPV3–6) [3, 6, 11, 13, 17]. One of the viruses, EcPV2, is believed to cause genital papillomas and carcinomas [10, 11, 19] as viral DNA has been detected in penile SCC but not in periocular or oral SCCs [15, 16]. Although absent from ocular SCCs, EcPV2 DNA is present in metastatic lesions formed in the retropharyngeal regions and the left jugular groove [9].
Human papillomavirus (HPV) is implicated in the development of oropharyngeal SCC [4]. In particular, HPV16 contributes significantly to carcinomas in the oropharynx [24]. High risk HPV types for cervical carcinomas such as HPV16 and 18, express E6 and E7 oncoproteins that can regulate the cell cycle and induce apoptosis in cells; both oncoproteins play pivotal roles in malignant transformation [14]. Oropharyngeal SCCs arise from squamous intraepithelial lesions (epithelial dysplasia) [24]. SCCs can also occur in recurrent respiratory papillomatosis in relation to infection with low risk HPV types, HPV6 or 11 [2, 7, 18]; these virus types are known to be associated with condyloma acuminatum [22]. Immunohistochemical assessment of cell cycle-related proteins such as cyclin D1 and Ki-67 can help to distinguish benign and malignant conditions of the cervix from squamous intraepithelial lesions [1]. In this paper, we report a case of laryngeal SCC and papilloma, in which EcPV2 DNA was demonstrated to be present.
A 19-year-old Thoroughbred stallion was examined because of anorexia, a fever of 39°C, and the occurrence of abnormal sounds by cervical auscultation. The condition did not improve, and nosebleed and severe nasal discharge occurred 2 and 3 days later, respectively. Endoscopy revealed the presence of laryngopharyngeal stenosis with reddening of the mucosa. The epiglottis and arytenoid cartilage were deformed due to swelling. A tumor was also detected on the left guttural pouch. Despite antibiotic treatment, the condition deteriorated and the horse showed severe wheezing and died 11 days after initial examination, presumably due to suffocation. At necropsy, there was an 8 × 4 × 4 cm elevated tumor mass on and around the epiglottis; a raised tumor mass of 5 cm diameter was observed in the area between the larynx and trachea, and another of 2 cm diameter on the guttural pouch. No abnormal findings were obtained in other organs. Animal housing and sampling in this study conformed to the institutional guidelines approved by the ethics committee of National Institute of Animal Health.
Formalin-fixed, paraffin-embedded tissues from the tumor masses were stained with hematoxylin and eosin (HE). Histological sections were immunostained using the streptavidin-biotin complex/horseradish peroxidase (SAB) method with a Histofine SAB kit (Nichirei, Tokyo, Japan). Mouse primary monoclonal antibodies against HPV16 E6 and HPV18 E6, prediluted (clone C1P5, Abcam, Cambridge, U.K.), HPV, 1:160 (clones bovine papilloma virus type 1 [BPV-1]/1H8 and CAMVIR, Abcam), proliferating cell nuclear antigen (PCNA), prediluted (clone PC10, BioGenex Laboratories, San Ramon, CA, U.S.A.), and cytokeratin 5 (CK5), prediluted (clone XM26, Lab Vision, Fremont, CA, U.S.A.) were used. Rabbit polyclonal antibodies against cyclin D1, prediluted (Lab Vision) and human Ki-67, 1:200 (Abcam) were also utilized. For immunohistochemical comparison, tissues from a mare with SCC showing mucinosis in the gingiva were treated in the same manner.
Total DNA was extracted from paraffin sections using the phenol-chloroform-isoamyl alcohol method [8] and used as template for polymerase chain reaction (PCR) amplification. Primer information (target specificity, nucleotide sequence, and expected amplicon size) is shown in Table 1. Ex Taq DNA polymerase (Takara Bio, Kusatsu, Japan) was used for the amplification using the following PCR conditions: 98°C for 10 sec; 35 cycles at 98°C for 10 sec, 50°C for 30 sec and 72°C for 45 sec; final extension at 72°C for 1 min. The nucleotide sequence of both strands of the PCR products were determined by cycle sequencing using an ABI 3130 genetic analyzer (Applied Biosystems). Positive and negative strands were aligned and a consensus sequence was generated. The sequences were compared with the NCBI database (BLASTX) and homology with closely related sequences was determined.
Table 1. List of primer pairs for detection of each target used in this study.
| Target specificity | Primer names | Nucleotide sequences | References |
|---|---|---|---|
| EcPV1 L1 | EPV-F | 5′-TGCGTTCGCCCCAATAGTCATCTT-3′ | [17] |
| EPV-R | 5′-ACCGCCCGCCTCACCCTTGTC-3′ | ||
| EcPV2 L1 | EcPV2 f | 5′-CAGACTTGTCTGGGCTCTCC-3′ | [11] |
| EcPV2 r | 5′-TCCCGCCTAGCATAGAAGAA-3′ | ||
| EcPV3 L1 | EcPV3-For | 5′-CGTTAAGCGGCCTTCCCCCTC-3′ | [6] |
| EcPV3-Rev | 5′-GCTTTAGCACGCAAGCCCGC-3′ | ||
| EcPV4 L1 | EcPV4-For | 5′-AGCTGACGTCCCATTAGACTTGGT-3′ | [6] |
| EcPV4-Rev | 5′-GCAGGTGCGCCACTGTCTCA-3′ | ||
| consensus EcPV L1 | EcPVF | 5′-CCDGCHHTBGGNGAGTAYTGG-3′ | [3] |
| EcPVR | 5′-TGCCAGCANARNCCATTGTT-3′ | ||
| animal and human PV L1 | FAP59 | 5′-TAACWGTIGGICAYCCWTATT-3′ | [5] |
| FAP64 | 5′-CCWATATCWVHCATITCICCATC-3′ | ||
| equine β-actin | β-actin For | 5′-CATTGTCCACCTTCCAGCAGATGT-3′ | [6] |
| β-actin Rev | 5′-CTAGAAGCATTTGCGGTGGACGAT-3′ |
EcPV: Equus caballus papillomavirus 2. For, forward; Rev, reverse.
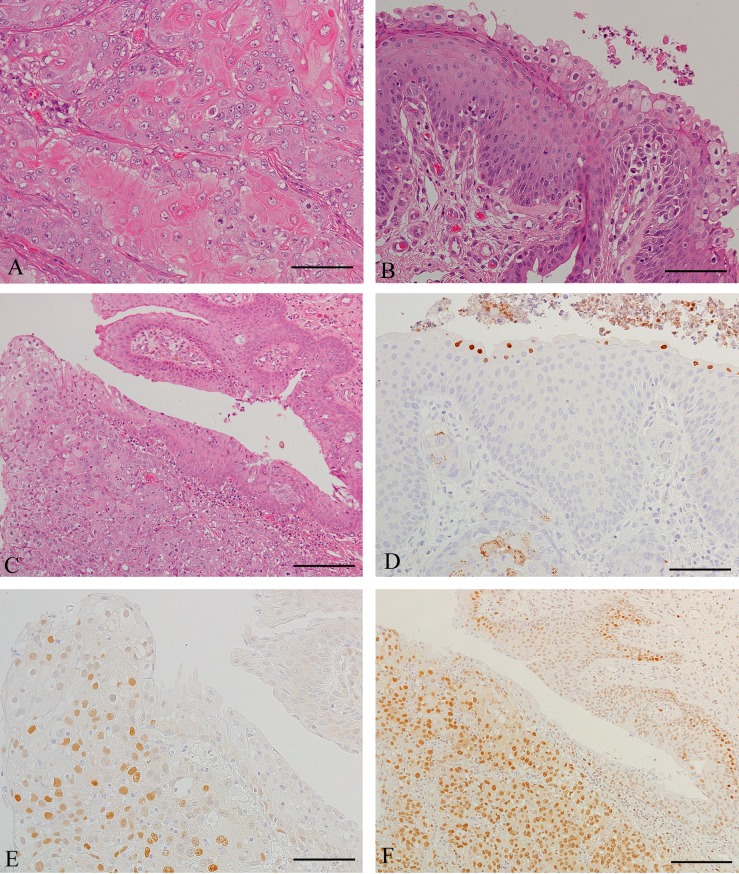
Histologically, most areas of the macroscopically visible tumors were composed of solid nests or lobules of abnormal squamous cells, separated by thin fibrovascular septa (Fig. 1A). The cells were divided into two types: well-differentiated and moderately-differentiated cells. Well-differentiated cells were centrally located in each group of cells, and had oval, or irregular and vesicular nuclei with small to moderately prominent nucleoli. The cytoplasm was generally abundant and clear, but occasionally eosinophilic and keratinized; distinct intercellular bridges were formed. Peripherally located moderately-differentiated cells were smaller in size and contained nuclei with slightly condensed chromatin; nucleoli were mostly inconspicuous; the cytoplasm was weakly basophilic; intercellular bridges were unclear. Mitotic figures were rare in well-differentiated cells but plentiful in moderately-differentiated cells. There was widespread central necrosis in all of the tumor masses. Only a few metastatic tumor cells (indubitable evidence of malignancy) were present in a lymph node adjacent to the laryngotracheal lesion and in two lymph nodes of the guttural pouch. However, another node of the pouch was nearly completely replaced by SCC cells.
Fig. 1.
Histology or immunohistochemistry of neoplastic tissues. (A) Epiglottis. This tissue consists of tightly packed nests of well-differentiated and less-differentiated tumor cells. The cells are stained using HE. Scale bar=50 µm. (B) Epiglottis. In papillae of squamous epithelium with central fibrovascular cores, superficial epithelial cells contain basophilic intranuclear inclusion bodies and occasionally show koilocyte-like features. HE staining. Scale bar=50 µm. (C) Epiglottis. The layer of papilloma cells (upper right) is continuous with the SCC lesion (lower left). HE staining. Scale bar=100 µm. (D) Epiglottis. Some inclusion bodies stain positively with an E6 antibody. Additionally, well-differentiated SCC cells have dot-like positive deposits in the cytoplasm (lower left). The cells were stained using the SAB method. Scale bar=50 µm. (E) Epiglottis. Transition from papilloma to carcinoma is observed in this field. SCC cells with nuclear positivity for cyclin D1 predominate, whereas nearly all papilloma cells are negative (right) after SAB staining. Scale bar=50 µm. (F) Epiglottis. Most SCC cells are intensely labeled for PCNA. Papilloma cells reveal intense staining in deeper layers of papillomatous tissue (upper right) after SAB staining. Scale bar=100 µm.
On the surface of the epiglottic tissue, normal-appearing epithelium and broad papilliform processes of stratified squamous epithelium were observed; surface cell flattening and keratinization were absent in the latter (Fig. 1B). The papilloma cells had round to oval nuclei with finely dispersed chromatin and small nucleoli. The cytoplasm was abundant and pale staining, except in deep layers. Intercellular bridges were present. Mitotic figures were infrequent and seen mainly in the deep layers. Cells with basophilic intranuclear inclusion bodies were often present in surface layers, and sometimes showed koilocytic changes (Fig. 1B). The papillomatous epithelium was continuous with areas of SCC (Fig. 1C).
Immunohistochemical analysis of papilloma cells indicated that intranuclear inclusion bodies were negative for HPV, but sometimes stained positively with antibodies against HPV16 E6 and HPV18 E6 (Fig. 1D). Moreover, some SCC cells, which were well differentiated and showed occasional koilocytosis, had a positive reaction mainly in the cytoplasm (Fig. 1D). No positive cells were observed in the control in which areas of well-differentiated neoplastic squamous cells were present. Many SCC cells expressed cyclin D1; few positive cells were present in the papilloma (Fig. 1E), while none were observed in the normal surface epithelium, laryngeal glands, and ducts. Most of the moderately-differentiated SCC cells showed intense staining for PCNA (Fig. 1F), but weak or negative staining was observed in well-differentiated cells. Papilloma cells were strongly PCNA positive in deep layers, and showed less intense staining in other layers (Fig. 1F). The staining pattern in the normal surface epithelium was similar to that in the papilloma, but cells in the stratum pavimentosum were PCNA negative. Intense Ki-67 staining was observed in almost all carcinoma and papilloma cells, and in the basal layer of the normal surface epithelium. CK5 was expressed in normal squamous and papillomatous cells, and in most SCC cells except for well-differentiated cells with clear cytoplasm.
Papillomavirus-specific DNA (474 bp) was amplified from the guttural pouch tumor tissue using primers for EcPV2 L1 (Table 1). No amplicons were generated from the same tissue using primers for EcPV1 L1, EcPV3 L1, EcPV4 L1, consensus EcPV L1, animal and human PV L1 (data not shown). Sequence analysis of PCR amplicons (DDBJ accession number LC46661) revealed 99.6% identity to the British EcPV-2 sequence (DDBJ accession number EU503122).
In the present case, we found transition from papilloma to carcinoma in the epiglottic lesion. These benign and malignant neoplasms are considered to be associated with the presence of EcPV2, because this virus is capable of inducing papilloma leading to SCC [20]. EcPV2 is not usually demonstrable by immunostaining for HPV or BPV [15]. In the current study, however, inclusion bodies in the surface layers of squamous papilloma stained positively with an anti-HPV E6 antibody. In addition, well-differentiated neoplastic squamous cells also showed positivity in the cytoplasm. Similar cytoplasmic staining has been observed in human breast carcinoma and may be associated with HPV [12]. In humans, laryngopharyngeal SCCs with low risk HPVs arise from juvenile-onset recurrent respiratory papillomatosis; this type of malignant transformation takes one to two decades after the onset of papillomatosis [2, 7]. In the present case, the disease occurred when the horse had reached an advanced age, suggesting that a similarly lengthy period of time may be required for progression from papilloma to carcinoma in equids. However, the frequency of malignant transformation may be higher in horses than in humans, because E6 typical of high risk HPVs was expressed in the current case.
In humans, immunohistochemical assessment of cell cycle proteins may help to distinguish normal and benign conditions of the cervix from precursor lesions of cervical carcinoma. Positive expression of p16, Ki-67, and ProEx C are correlated with the severity of dysplasia [1]. Cyclin D1 is expressed mostly in cervical carcinomas, but similar expression is also observed in some cases of normal or benign cervical epithelium [1]. In the present case, cyclin D1 proved of use for distinguishing benign and malignant lesions. PCNA was less helpful as it was was highly expressed in SCC cells and also in deeper layers of normal epithelium and papilloma. Ki-67 showed similar patterns of expression in benign and malignant lesions, but was positive only in the basal layer of normal epithelium. Thus, Ki-67 may be a useful marker for distinguishing normal squamous epithelium and papilloma.
The presence of EcPV2 DNA was demonstrated in the tissue sample from the guttural pouch tumor, but not in the samples from the laryngeal and laryngotracheal tumors, although the histological features of these three tumors were similar. There are two possibilities for the negative results from laryngeal and laryngotracheal tumors: first, the tumors do not contain EcPV2: second, that DNA extraction was insufficient. To test these possibilities, we performed a genomic PCR for β-actin. Horse genomic sequences were amplified only from the guttural pouch tumor samples. Therefore, we conclude that the negative results from the other samples are due to insufficient recovery of DNA from the tissue sections or fragmentation of DNA by the formalin fixation. Taken together with the histological and immunohistochemical results, there is consistent evidence for EcPV2 being involved in the development of equine laryngeal papillomatosis. Furthermore, it is important to note that EcPV2-associated papillomas are at high risk of malignant transformation.
Acknowledgments
The authors appreciate the technical assistance of Ms. R. Ishihara and Mr. Y. Ishikawa, Hokkaido Research Station, National Institute of Animal Health, in molecular biology and histopathology.
REFERENCES
- 1.Conesa-Zamora P., Doménech-Peris A., Orantes-Casado F. J., Ortiz-Reina S., Sahuquillo-Frías L., Acosta-Ortega J., García-Solano J., Pérez-Guillermo M.2009. Effect of human papillomavirus on cell cycle-related proteins p16, Ki-67, Cyclin D1, p53, and ProEx C in precursor lesions of cervical carcinoma: a tissue microarray study. Am. J. Clin. Pathol. 132: 378–390. doi: 10.1309/AJCPO0WY1VIFCYDC [DOI] [PubMed] [Google Scholar]
- 2.Cook J. R., Hill D. A., Humphrey P. A., Pfeifer J. D., El-Mofty S. K.2000. Squamous cell carcinoma arising in recurrent respiratory papillomatosis with pulmonary involvement: emerging common pattern of clinical features and human papillomavirus serotype association. Mod. Pathol. 13: 914–918. doi: 10.1038/modpathol.3880164 [DOI] [PubMed] [Google Scholar]
- 3.Dong J., Zhu W., Yamashita N., Chambers J. K., Uchida K., Kuwano A., Haga T.2017. Isolation of equine papillomavirus type 1 from racing horse in Japan. J. Vet. Med. Sci. 79: 1957–1959. doi: 10.1292/jvms.17-0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ducatman B. S.2018. The role of human papillomavirus in oropharyngeal squamous cell carcinoma. Arch. Pathol. Lab. Med. 142: 715–718. doi: 10.5858/arpa.2018-0083-RA [DOI] [PubMed] [Google Scholar]
- 5.Forslund O., Antonsson A., Nordin P., Stenquist B., Hansson B. G.1999. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J. Gen. Virol. 80: 2437–2443. doi: 10.1099/0022-1317-80-9-2437 [DOI] [PubMed] [Google Scholar]
- 6.Gorino A. C., Oliveira-Filho J. P., Taniwaki S. A., Basso R. M., Zakia L. S., Araujo J. P., Jr., Borges A. S.2013. Use of PCR to estimate the prevalence of Equus caballus papillomavirus in aural plaques in horses. Vet. J. 197: 903–904. doi: 10.1016/j.tvjl.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 7.Hammoud D., Haddad B. E.2010. Squamous cell carcinoma of the lungs arising in recurrent respiratory papillomatosis. Respir. Med. CME 3: 270–272. doi: 10.1016/j.rmedc.2009.09.021 [DOI] [Google Scholar]
- 8.Imai K., Mase M., Yamaguchi S., Yuasa N., Nakamura K.1998. Detection of chicken anaemia virus DNA from formalin-fixed tissues by polymerase chain reaction. Res. Vet. Sci. 64: 205–208. doi: 10.1016/S0034-5288(98)90126-6 [DOI] [PubMed] [Google Scholar]
- 9.Kainzbauer C., Rushton J., Tober R., Scase T., Nell B., Sykora S., Brandt S.2012. Bovine papillomavirus type 1 and Equus caballus papillomavirus 2 in equine squamous cell carcinoma of the head and neck in a Connemara mare. Equine Vet. J. 44: 112–115. doi: 10.1111/j.2042-3306.2010.00358.x [DOI] [PubMed] [Google Scholar]
- 10.Knight C. G., Munday J. S., Rosa B. V., Kiupel M.2011. Persistent, widespread papilloma formation on the penis of a horse: a novel presentation of equine papillomavirus type 2 infection. Vet. Dermatol. 22: 570–574. doi: 10.1111/j.1365-3164.2011.00987.x [DOI] [PubMed] [Google Scholar]
- 11.Lange C. E., Tobler K., Lehner A., Grest P., Welle M. M., Schwarzwald C. C., Favrot C.2013. EcPV2 DNA in equine papillomas and in situ and invasive squamous cell carcinomas supports papillomavirus etiology. Vet. Pathol. 50: 686–692. doi: 10.1177/0300985812463403 [DOI] [PubMed] [Google Scholar]
- 12.Lawson J. S., Glenn W. K., Heng B., Ye Y., Tran B., Lutze-Mann L., Whitaker N. J.2009. Koilocytes indicate a role for human papilloma virus in breast cancer. Br. J. Cancer 101: 1351–1356. doi: 10.1038/sj.bjc.6605328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linder K. E., Bizikova P., Luff J., Zhou D., Yuan H., Breuhaus B., Nelson E., Mackay R.2018. Generalized papillomatosis in three horses associated with a novel equine papillomavirus (EcPV8). Vet. Dermatol. 29: 72–e30. doi: 10.1111/vde.12481 [DOI] [PubMed] [Google Scholar]
- 14.Münger K., Phelps W. C., Bubb V., Howley P. M., Schlegel R.1989. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J. Virol. 63: 4417–4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasir L., Brandt S.2013. Papillomavirus associated diseases of the horse. Vet. Microbiol. 167: 159–167. doi: 10.1016/j.vetmic.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 16.Newkirk K. M., Hendrix D. V., Anis E. A., Rohrbach B. W., Ehrhart E. J., Lyons J. A., Kania S. A.2014. Detection of papillomavirus in equine periocular and penile squamous cell carcinoma. J. Vet. Diagn. Invest. 26: 131–135. doi: 10.1177/1040638713511618 [DOI] [PubMed] [Google Scholar]
- 17.Postey R. C., Appleyard G. D., Kidney B. A.2007. Evaluation of equine papillomas, aural plaques, and sarcoids for the presence of equine papillomavirus DNA and papillomavirus antigen. Can. J. Vet. Res. 71: 28–33. [PMC free article] [PubMed] [Google Scholar]
- 18.Reidy P. M., Dedo H. H., Rabah R., Field J. B., Mathog R. H., Gregoire L., Lancaster W. D.2004. Integration of human papillomavirus type 11 in recurrent respiratory papilloma-associated cancer. Laryngoscope 114: 1906–1909. doi: 10.1097/01.mlg.0000147918.81733.49 [DOI] [PubMed] [Google Scholar]
- 19.Scase T., Brandt S., Kainzbauer C., Sykora S., Bijmholt S., Hughes K., Sharpe S., Foote A.2010. Equus caballus papillomavirus-2 (EcPV-2): an infectious cause for equine genital cancer? Equine Vet. J. 42: 738–745. doi: 10.1111/j.2042-3306.2010.00311.x [DOI] [PubMed] [Google Scholar]
- 20.Scott D. W., Miller W. H. J.2003. Squamous cell carcinoma. pp. 707–712. In: Equine Dermatology, 1st ed., W. B. Saunders, St. Louis. [Google Scholar]
- 21.Sykora S., Brandt S.2017. Papillomavirus infection and squamous cell carcinoma in horses. Vet. J. 223: 48–54. doi: 10.1016/j.tvjl.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 22.Tominaga S., Fukushima K., Nishizaki K., Watanabe S., Masuda Y., Ogura H.1996. Presence of human papillomavirus type 6f in tonsillar condyloma acuminatum and clinically normal tonsillar mucosa. Jpn. J. Clin. Oncol. 26: 393–397. doi: 10.1093/oxfordjournals.jjco.a023254 [DOI] [PubMed] [Google Scholar]
- 23.van den Top J. G., de Heer N., Klein W. R., Ensink J. M.2008. Penile and preputial squamous cell carcinoma in the horse: a retrospective study of treatment of 77 affected horses. Equine Vet. J. 40: 533–537. doi: 10.2746/042516408X281171 [DOI] [PubMed] [Google Scholar]
- 24.Zaravinos A.2014. An updated overview of HPV-associated head and neck carcinomas. Oncotarget 5: 3956–3969. doi: 10.18632/oncotarget.1934 [DOI] [PMC free article] [PubMed] [Google Scholar]