Abstract
Drought is the most perilous abiotic stress that affects finger millet growth and productivity worldwide. For the successful production of finger millet, selection of drought tolerant varieties is necessary and critical stages under drought stress, germination and early seedling growth, ought to be fully understood. This study investigated the physiological and biochemical responses of six finger millet varieties (GBK043137, GBK043128, GBK043124, GBK043122, GBK043094 and GBK043050) under mannitol-induced drought stress. Seeds were germinated in sterile soil and irrigated with various concentrations of mannitol (200, 400 and 600 mM) for 2 weeks. In a comparative analysis relative water content (RWC), chlorophyll, proline and malondialdehyde (MDA) contents were measured to obtain the physiological and biochemical characteristics of drought stress. The results showed that increased levels of drought stress seriously decreased germination and early seedling growth of finger millet varieties. However, root growth was increased. In addition, exposition to drought stress triggered a significant decrease in relative water content and chlorophyll content reduction, and the biochemical parameters assay showed less reduction in RWC. Furthermore, oxidative damage indicating parameters, such as proline concentration and MDA content, increased. Varieties GBK043137 and GBK043094 were less affected by drought than the other varieties as shown by significant changes in their physiological parameters. Our findings reveal the differences between the physiological and biochemical responses of finger millet to drought and are vital for breeding and selecting drought tolerant varieties of finger millet. Further, genomic and molecular investigations need to be undertaken to gain a deeper insight into the detailed mechanisms of drought tolerance in finger millet.
Keywords: Drought stress, Finger millet, Germination, Lipid peroxidation, Mannitol, Oxidative stress
Introduction
Drought stress is the most severe environmental stress which is responsible for poor agricultural productivity and yield decline (Zougmoré 2018). Due to global climate change, it is predicted that drought episodes will increase in frequency, be longer and more severe, exacerbating its negative effects on crops and compromise food security particularly in developing countries. Over time, plants have evolved a range of drought tolerance adaptative mechanisms to counteract the detrimental effects of drought. When grown under desiccation stress, plants exhibit a sequence series of morphological, physiological, biochemical, cellular and molecular changes that severely compromise plant’s growth, development and productivity (Li and Liu 2016). Plants under water deficit conditions decrease net photosynthesis and transpiration rates. These two physiological responses, which vary depending on the species, are often seen in regions with very high evaporative demand (Anjum et al. 2011). Protection systems against excess reducing power are therefore a vital approach for plants under desiccation stress (Chaves and Oliveira 2004). Drought stress in plants is physiologically complex, and it encompasses osmotic stress and specific ion toxicity (Todaka et al. 2015). Drought stress in plants is associated with nutritional imbalance, adjustment in metabolic fluxes, distortion and disorganization of cell and chloroplast membranes, as well as reduction in division and expansion of cells and overproduction of reactive oxygen species (ROS) (Forni et al. 2017). Toxicity accruing from overproduction of ROS triggers cascades of oxidative reactions, which consequently causes inactivation of enzymes and an increase of lipid peroxidation, whose final product is malondialdehyde (MDA), and its quantification is used as a marker for oxidative damage (Møller et al. 2007). To abate the effects of oxidative stress, plants have evolved complex enzymatic and non-enzymatic systems. When exposed to water deficit stress conditions, many plant species enhance the activities of antioxidant enzyme which are associated with increased proline concentration (Ashraf and Foolad 2007). Proline plays a significant role in the osmoregulation, allowing cells to retain more water. Moreover, the amino acid also displays plant defense properties as a ROS scavenger (Szabados and Savoure 2010) and as a regulator of the cellular redox status (Sharma et al. 2011). Proline accumulation in plants is therefore considered as a positive indicator for their tolerance to water stress (Verslues et al. 2014). Plants’ capability to retain water during desiccation is a vital strategy for plant tolerance to stress caused by water deficit stress. Accordingly, evaluation of relative water content change is the best representation and a fast approach for evaluating genetic differences in cellular hydration, plant water deficit and physiological water status after water deficit stress treatments (Sánchez-Rodríguez et al. 2010). The best effective approach of mitigating drought is development of the tolerant crop varieties. Accordingly, it is important to identify the genetic resources with high tolerance and to understand the physiological and biochemical response mechanisms of drought tolerance in important cereal crops such as finger millet.
Finger millet [Eleusine coracana (L.) Gaertn.] is a cereal crop that is cultivated in semi-arid and arid regions of the world under rain fed conditions (Thilakarathna and Raizada 2015). The crop plays a significant role in food security in arid and semi-arid regions of sub-Saharan Africa and South Asia. Finger millet is therefore an ideal crop for reshaping food propensity of people due to its nutritional richness, high photosynthetic efficiency and better tolerance to biotic and abiotic stresses than other crops (Kumar et al. 2016). As a member of the Panicoideae subfamily, finger millet acts as a model cereal crop for investigating basic biological processes. Although most of the finger millet varieties are considered to be drought tolerant when compared with other cereal crops, such as sorghum, maize, rice, barley and wheat, the crop is drought sensitive especially at early stages. Genetic variations in response to drought stress have been shown in many plant relatives and among accessions within the same species. To our knowledge, there is no literature available that reports morphological, physiological and biochemical responses of finger millet to water deficit stress. We therefore investigated the physiological and biochemical mechanisms involved in six finger millet varieties from distinct geographical zones in Kenya, under mannitol induced drought stress. Physiological and biochemical parameters were measured; these parameters included germination rate, shoot growth and root growth, relative water content, chlorophyll content, proline accumulation and lipid peroxidation.
Materials and methods
Plant material, growth conditions and germination assay
Finger millet varieties GBK043137, GBK043128, GBK043124, GBK043122, GBK043094 and GBK043050 obtained from Kenya Agricultural and Livestock Research Organization, Gene Bank, Muguga, Kenya were used in this study. Seeds were sorted by handpicking the healthy ones, which were used for subsequent experiments. Selected seeds were washed with distilled water to remove dust and other particles. Germination assay was performed by using 10 seeds of each variety. Seeds were planted in germination trays containing sterile soil to a depth of approximately 1 cm and irrigated with different concentrations of mannitol (200, 400 and 600 mM). The controls were irrigated with distilled water. Drought stress was imposed on treatment groups by irrigating the seeds with various concentrations of mannitol at an interval of 3 days for 2 weeks. Observations on the rate of germination were scored on the 17th day of treatment.
Growth conditions and drought treatment
Germinated finger millet seedlings were grown for 2 weeks under greenhouse conditions of 25 ± 2 °C and 60–70% humidity, with a 16/8-h photoperiod provided by natural sunlight. The seedlings were subjected to osmotic stress by irrigating with mannitol (200, 400 and 600 mM) for 21 days at an interval of 3 days. Control plants were watered with distilled water. Shoot length and root length were measured after the experiment.
Determination of relative water content
A leaf was excised from each plant on the 21st day of water deficit stress. Immediately, the fresh weight (FW) of each leaflet was determined. Thereafter, the leaflet was immersed in double distilled water and incubated under normal room temperature for 4 h. Afterwards, the leaflet was taken out, thoroughly wiped to remove the water on the blade surface and its weight measured to obtain turgid weight (TW). The leaflet was afterwards dried in an oven for 24 h and its dry weight (DW) was measured. The relative water content (RWC %) was calculated using the formula: RWC = [(FW − DW)/(TW − DW)] × 100.
Total chlorophyll content
Total chlorophyll (TC) content was determined using the method of described by Arnon (1949). Fresh leaves (0.2 g) of plants were crushed in 80% acetone. The extract was centrifuged at 5000 g for 3 min. The absorbance of the obtained supernatants was measured at 645 and 663 nm using 1240 UV–Vis Spectrophotometer (Shimadzu, Kyoto, Japan). The total chlorophyll content in each sample, expressed in mg/g fresh mass (FM) was calculated using the formula: TC = 20.2(A645)+ 8.02(A663) × V/1000 × W where V corresponds to the volume of total extract per litre, W is the mass of the fresh material and A is the absorbance as 645 and 663 nm.
Estimation of proline content
The amount of free proline in fresh plant leaves was determined as reported by Bates et al. (1973). Fresh leaf tissues (50 mg) from each variety and treatment was homogenized in 10 ml of 3% w/v sulphosalicylic acid and the homogenate was filtrated. The resulting solution was mixed with acidic ninhydrin solution [40% (w/v) acidic ninhydrin (8.8 µM ninhydrin, 10.5 M glacial acetic acid, 2.4 M orthophosphoric acid), 40% (v/v) glacial acetic acid and 20% (v/v) of 3% (v/v) sulphosalicylic acid]. Thereafter, the reaction mixtures were put in a water bath at 100 °C for 60 min to develop colors. The reaction was terminated by incubating the mixtures in ice for 5 min. Toluene was added to separate chromophores. The optical density was measured at 520 nm using 1240 UV–Vis Spectrophotometer. Free proline content [µmol/g fresh weight (F. WT)] in leaf tissues was calculated from a standard curve made by using 0–100 µg l-proline (Bates et al. 1973).
Lipid peroxidation assay
Fresh upper second fully expanded leaves (0.3 g) were harvested and homogenized in 0.1% (w/v) trichloroacetic acid and the homogenates were centrifuged at 10,000 g for 15 min at 4 °C. The supernatant was mixed with 0.5 ml of 1.5 ml 0.5% thiobarbituric acid diluted in 20% trichloroacetic acid and the resulting mixture was heated to 95 °C for 25 min in a water bath before incubating it on ice for 10 min. The absorbance was measured at 532 and 600 nm using UVmini-1240 UV–Vis Spectrophotometer with 1% thiobarbituric acid in 20% trichloroacetic acid as control. The amount of malondialdehyde (µmol/g FW), calculated as a measure of lipid peroxidation, was determined according to Heath and Packer (1968).
Statistics data analysis
The experiment was completely randomized block design with five replications of 10 plants. For germination and physiological assays, 10 seeds per replication were employed. Data collected was subjected to one-way analysis of variance (ANOVA) followed by a Fisher’s protected LSD test to compare the means. A confidence level was set at of 95% (P ≤ 0.05). All statistical procedures were performed using Minitab statistical computer software version 17.
Results
Effects of drought stress on seed germination
The results demonstrated that the gemination rate of the tested finger millet varieties was influenced by seed variety and mannitol concentration (Table 1). Under untreated conditions, results showed that the highest gemination rate was recorded after 5 days in GBK043137 (83.75%) followed by varieties GBK043124, GBK043128, GBK043122 and GBK043050 whose rates ranged from 65.0 to 72.5%. in comparison, GBK043094 recorded the lowest one at 51.25%. Seeds sprout in absence of stress treatment recorded superior percentages. Imposition of increasing concentration of mannitol resulted in a decrease in germination percentage. The decline was significantly pronounced at 400 mM mannitol, where a 0% rate for GBK043137, GBK043122, GBK043094 and GBK043050 was recorded while, GBK043124 and GBK043128 recorded 16.25% and 1.25%, respectively (Table 1). Under moderate drought stress of 200 mM mannitol, variety GBK043137 recorded the highest germination rate of 41.25% compared to the other varieties, whose rates ranged from 3.75 to 16.25% (Table 1). In severe osmotic pressure of 600 mM mannitol concentration, none of the planted seeds sprout. The average germination period under 0 mM mannitol concentration was 5.2 to 7.4 days for all varieties, while under 200 mM mannitol, the germination interval was longer, ranging from 7.5 to 13.6 days.
Table 1.
Effects of mannitol on germination of six finger millet varieties
| Variety | 0 mM | 200 mM | 400 mM | 600 mM |
|---|---|---|---|---|
| GBK043137 | 83.75 ± 4.7a | 41.25 ± 8.75a | 0.00 ± 0.00b | 0.00 ± 0.00a |
| GBK043128 | 65.0 ± 14.00ab | 3.75 ± 2.39b | 1.25 ± 1.25b | 0.00 ± 0.00a |
| GBK043124 | 72.50 ± 4.33ab | 16.25 ± 3.75b | 16.25 ± 8.26a | 0.00 ± 0.00a |
| GBK043122 | 65.00 ± 7.36ab | 3.75 ± 2.39b | 0.00 ± 0.00b | 0.00 ± 0.00a |
| GBK043094 | 51.25 ± 5.91b | 8.75 ± 7.18b | 0.00 ± 0.00b | 0.00 ± 0.00a |
| GBK043050 | 66.25 ± 9.66ab | 6.25 ± 3.15b | 0.00 ± 0.00b | 0.00 ± 0.00a |
Means (± SE) followed by different alphabets in each column are significantly different (P ≤ 0.05) using Fishers LSD
Effects of drought stress on growth
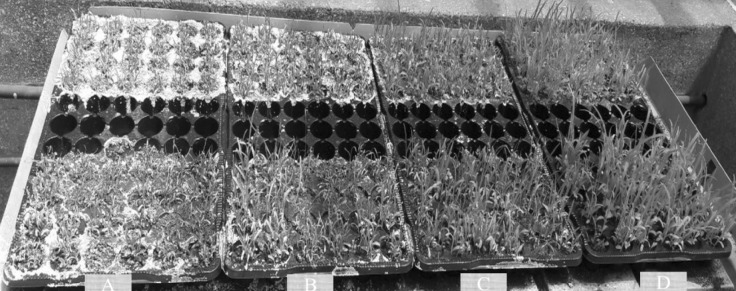
We also investigated the changes in the growth parameters (shoot and root growth) under mannitol induced drought conditions in the six selected finger millet varieties. In all studied varieties, mannitol-induced drought stress treatment reduced shoot growth (Fig. 1). Moreover, the shoot length decreased progressively with the increase in mannitol concentration (Table 2). Under mannitol stress conditions, higher growth responses were recorded at 200 mM mannitol as witnessed by long shoot length, while the least pronounced responses were recorded at 600 mM mannitol (Table 2). The greatest shoot length (3.00 cm) was recorded in the GBK043128, while the shortest (1.20 cm) was noted for GBK043137 and GBK043094 (Table 2). Statistically significant differences in the effect of mannitol on shoot length among the six varieties were observed only at 200 mM mannitol concentration (Table 2).
Fig. 1.
Effect of drought stress on growth of finger millet. Seedling growth on a 600 mM mannitol. b 400 mM mannitol; c 200 mM mannitol; d 0 mM mannitol
Table 2.
Effect of mannitol on shoot length
| Variety | 0 mM | 200 mM | 400 mM | 600 mM |
|---|---|---|---|---|
| GBK043137 | 7.80 ± 0.86a | 2.30 ± 0.20b | 1.80 ± 0.27a | 1.20 ± 0.20a |
| GBK043128 | 7.60 ± 1.33a | 3.00 ± 0.27a | 2.20 ± 0.26a | 1.30 ± 0.20a |
| GBK043124 | 4.40 ± 0.40b | 2.20 ± 0.20b | 2.00 ± 0.27a | 1.30 ± 0.20a |
| GBK043122 | 4.00 ± 0.45b | 2.40 ± 0.29ab | 1.70 ± 0.20a | 1.30 ± 0.20a |
| GBK043094 | 3.00 ± 0.00b | 2.40 ± 0.19ab | 1.60 ± 0.19a | 1.20 ± 0.20a |
| GBK043050 | 3.70 ± 0.62b | 2.10 ± 0.10b | 1.60 ± 0.19a | 1.30 ± 0.20a |
Means (± SE) followed by different alphabets in each column are significantly different (P ≤ 0.05) using Fishers LSD
Contrary to shoot growth under mannitol osmotic stress conditions, the six finger millet varieties recorded an increase in root growth with increase in drought severity. The mannitol stressed plants recorded relatively higher responses when compared to control plants (Table 3). Variety GBK043094 recorded the highest root length under drought of 6.00 cm at 600 mM mannitol while GBK043050 and GBK043137 showed the least response with 2.30 cm and 2.60 cm respectively, at 200 mM mannitol treatment level (Table 3). The observed increase of root length across different drought stress levels was variety dependent.
Table 3.
Effect of mannitol on root growth
| Variety | 0 mM | 200 mM | 400 mM | 600 mM |
|---|---|---|---|---|
| GBK043137 | 3.10 ± 0.75a | 2.60 ± 0.73b | 2.70 ± 0.62a | 3.20 ± 0.68c |
| GBK043128 | 3.20 ± 0.37a | 4.30 ± 0.49a | 4.60 ± 0.93a | 5.00 ± 0.45ab |
| GBK043124 | 2.60 ± 0.40a | 3.20 ± 0.37ab | 3.60 ± 0.40a | 3.60 ± 0.68bc |
| GBK043122 | 2.70 ± 0.62a | 3.40 ± 0.25ab | 3.60 ± 0.68a | 5.00 ± 0.45ab |
| GBK043094 | 2.0 ± 0.57a | 3.50 ± 0.78ab | 3.60 ± 0.68a | 6.00 ± 0.84a |
| GBK043050 | 2.00 ± 0.61a | 2.30 ± 0.30b | 2.90 ± 0.56a | 3.90 ± 0.25bc |
Means (± SE) followed by different alphabets in each column are significantly different (P ≤ 0.05) using Fishers LSD
Effects of drought stress on relative water content
Table 4 presents the RWC changes in finger millet leaves as a result of increasing water-deficit stress. Under irrigated conditions, all varieties maintained high RWC levels. However, when exposed to progressively greater mannitol concentrations, all varieties exhibited reduction in RWC values. The greatest percentage reduction in RWC was noted in GBK043122, which also exhibited the lowest RWC values relative to other varieties under water-deficit stress at all applied mannitol concentrations. On the other hand, GBK043128 sustained relatively high RWC values, and also showed a lower percentage reduction when compared to other varieties under the same mannitol stress conditions. Plants under moderate water stress treatment induced by 200 mM mannitol application displayed the highest diversity in the RWC values. The leaves exhibited wilting symptoms and leaf rolling when the plants were subjected to severe drought stress treatments.
Table 4.
Effects of mannitol on relative water content (%)
| Variety | 0 mM | 200 mM | 400 mM | 600 mM |
|---|---|---|---|---|
| GBK043137 | 85.56 ± 4.12a | 68.60 ± 5.27c | 64.96 ± 4.62ab | 49.76 ± 3.78ab |
| GBK043128 | 85.84 ± 3.05a | 74.24 ± 2.33b | 65.24 ± 2.68ab | 54.76 ± 4.23a |
| GBK043124 | 77.20 ± 5.03ab | 67.14 ± 3.02c | 60.78 ± 4.88bc | 49.38 ± 4.85b |
| GBK043122 | 74.16 ± 2.94c | 66.92 ± 3.05c | 57.98 ± 4.06c | 40.18 ± 1.96c |
| GBK042094 | 85.92 ± 3.76a | 75.50 ± 4.12b | 68.84 ± 2.71a | 46.82 ± 3.55b |
| GBK043050 | 81.94 ± 7.91ab | 83.44 ± 5.92a | 66.14 ± 6.32ab | 48.74 ± 5.28b |
Means (± SE) followed by different alphabets in each column are significantly different (P ≤ 0.05) using Fishers LSD
Effects of drought stress on total chlorophyll content
The results yielded by the present study show an inverse relationship between mannitol-induced drought stress responses and total chlorophyll content values for all examined finger millet varieties. Differences in chlorophyll content values were also observed among varieties. At the beginning of the experiment, total chlorophyll content was similar in all six varieties, ranging from 15.35 to 21.74 mg/g FW (Table 5). Imposition of moderate drought stress conditions via 200 mM mannitol application caused a slight decrease in chlorophyll content, ranging from 5.08 for GBK043094 to 14.2% for GBK043128. On the other hand, when 600 mM mannitol was applied, it induced a significant decrease in chlorophyll content, ranging from 33.04 to 45.59%. Among all examined varieties, GBK043137 and GBK042094 retained relatively high chlorophyll content when exposed to severe water stress, while drought-sensitive varieties (GBK043050, GBK043128, GBK043122 and GBK043124) recorded a higher decline in chlorophyll content, ranging from 42.4 to 45.59% under mannitol-induced drought stress (Table 5). The high drought-induced decrease in the total chlorophyll content signifies that drought stresses induced a significant loss of photosynthetic reaction centers.
Table 5.
Effects of mannitol on chlorophyll content (mg/g FW)
| Variety | 0 mM | 200 mM | 400 mM | 600 mM |
|---|---|---|---|---|
| GBK043137 | 15.35 ± 1.12b | 14.51 ± 1.23c | 11.81 ± 0.68b | 10.27 ± 0.61abc |
| GBK043128 | 21.74 ± 2.26a | 18.65 ± 1.90a | 14.23 ± 1.49a | 12.30 ± 1.29a |
| GBK043124 | 17.33 ± 1.47b | 15.16 ± 1.78bc | 11.40 ± 1.02b | 9.99 ± 1.00bc |
| GBK043122 | 16.56 ± 1.12b | 15.06 ± 0.91bc | 10.96 ± 1.03b | 9.76 ± 1.58c |
| GBK042094 | 18.26 ± 2.57b | 17.33 ± 2.35ab | 14.32 ± 2.15a | 12.14 ± 1.78ab |
| GBK043050 | 16.78 ± 0.07b | 14.86 ± 0.06bc | 10.55 ± 0.06b | 9.13 ± 0.23c |
Means (± SE) followed by different alphabets in each column are significantly different (P ≤ 0.05) using Fishers LSD
Effect of mannitol on proline content
Analyses further revealed that the variations among the varieties in proline content under control conditions were significantly different, but did not follow any discernible pattern (Table 6). In response to drought stress, all six studied finger millet varieties exhibited a steep increase in leaf proline content and the rate of increase escalated with the severity of water stress. The highest proline accumulation was noted for variety GBK042094, while the lowest proline concentration was recorded for GBK043128 in all mannitol treatments. Varietal differences in drought stress-induced proline were clearly observed in finger millet, signifying a correlation between proline accumulation and differential mannitol-induced water deficit stress tolerance response among the six finger millet varieties studied.
Table 6.
Effects of mannitol on proline content (µmol/g FW)
| Variety | 0 mM | 200 mM | 400 mM | 600 mM |
|---|---|---|---|---|
| GBK043137 | 1.76 ± 0.09a | 2.12 ± 0.19ab | 3.22 ± 0.26a | 4.28 ± 0.29a |
| GBK043128 | 1.76 ± 0.27a | 1.90 ± 0.16c | 2.76 ± 0.21b | 3.76 ± 0.18c |
| GBK043124 | 1.74 ± 0.27a | 1.98 ± 0.19abc | 2.84 ± 0.17b | 3.50 ± 0.14c |
| GBK043122 | 1.86 ± 0.34a | 1.92 ± 0.23bc | 2.86 ± 0.21b | 3.80 ± 0.17b |
| GBK042094 | 1.70 ± 0.21a | 2.16 ± 0.19a | 3.28 ± 0.18a | 4.52 ± 0.22a |
| GBK043050 | 1.74 ± 0.27a | 1.98 ± 0.15abc | 2.82 ± 0.19b | 3.60 ± 0.24bc |
Means (± SE) followed by different alphabets in each column are significantly different (P ≤ 0.05) using Fishers LSD
MDA content
Lipid peroxidation was determined by measuring the accumulation of MDA, which is a product of oxidation of polyunsaturated fatty acids present in the membrane caused by accumulation of peroxyl radicals (Kotchoni et al. 2006). Our results revealed that the MDA levels in finger millet leaves was significantly influenced by the severity of mannitol-induced osmotic stress and variety. At the beginning of the experiment, no significant difference was registered in MDA values for all varieties tested (Table 7). The MDA content was lower in control plants ranging from 2.1 to 2.79 µmol/g FW compared to plants subjected to mannitol-induced osmotic stress which ranged from 2.77 to 7.23 µmol/g FW. A progressive increase in the level of lipid peroxidation was observed with concomitant increase of mannitol concentration. The maximum MDA content under severe osmotic drought conditions (600 mM mannitol) was observed in GBK043128 followed by GBK043050 and GBK043122 varieties while varieties GBK042094 and GBK043137 had the least MDA accumulation at similar conditions (Table 7).
Table 7.
Effects of mannitol on malondialdehyde content (µmol/g FW)
| Variety | 0 mM | 200 mM | 400 mM | 600 mM |
|---|---|---|---|---|
| GBK043137 | 2.03 ± 0.55c | 2.77 ± 0.39c | 4.29 ± 0.62d | 5.26 ± 0.34c |
| GBK043128 | 2.27 ± 0.46abc | 3.43 ± 0.49b | 5.75 ± 0.36a | 7.23 ± 0.36a |
| GBK043124 | 2.58 ± 0.33abc | 3.91 ± 0.37ab | 5.00 ± 0.45bc | 6.17 ± 0.47b |
| GBK043122 | 2.66 ± 0.38ab | 4.21 ± 0.33a | 5.72 ± 0.35a | 7.03 ± 0.53a |
| GBK042094 | 2.79 ± 0.63a | 3.74 ± 0.67ab | 4.41 ± 0.77 cd | 5.39 ± 0.51c |
| GBK043050 | 2.10 ± 0.15bc | 3.63 ± 0.27b | 5.67 ± 0.60ab | 7.62 ± 0.97a |
Means (± SE) followed by different alphabets in each column are significantly different (P ≤ 0.05) using Fishers LSD
Discussion
Drought stress induces different physiological, genetic and metabolic responses among several species of plant and varieties. These responses are also influenced by edaphic, climatic and agronomic factors (Caliz et al. 2015). Vulnerability of plants to drought stress differentially varies depending on stress severity, interactions among stressors, plant species and stage of their development (Demirevska et al. 2009). This natural allelic difference may provide valuable information into the mechanisms that underline the differential responses to agriculturally important traits and search of the crops that can survive such harsh environments may assist to ensure stable and sustainable food production (Budak et al. 2013). As a dry-land crop, finger millet growth and productivity is highly affected by drought stress. In current adverse climate change era, drought stress is projected to increase in severity and frequency. In order to overcome the effects associated with desiccation stress on plants, there is need to develop new finger millet varieties with strong drought tolerance traits as an effective way to achieve high and stable yields. For this to be successful, precise identification of stress tolerance of finger millet varieties forms the basis of developing resistant finger millet varieties. Therefore, dissecting the natural differences of finger millet varieties could be viable to explore the complex mechanisms of its response to various stresses. This study was done to investigate the differential responses of finger millet to seed germination, growth, physiological and biochemical responses after exposure to different concentrations of mannitol, which causes osmotic stress and is commonly used as a drought simulator (Ullah et al. 2014; Kaya et al. 2013; Karakas et al. 1997).
In plants’ life cycle, seed germination is the most critical and sensitive stage. The process of seed germination is constrained or even completely prevented by drought stress (Hubbard et al. 2012). Germination potential is therefore an ideal index to assess the seed germination rate and germination uniformity. The germination rate under simulated drought stress showed the tolerance, though the responses were variety dependent. In absence of stress treatment, the six finger millet varieties recorded better germination percentages. However, the rate declined gradually with the increase in mannitol concentration treatment. Similar results have been reported in other plant species such, as maize (Liu et al. (2015), wheat (Yang et al. 2016) and sunflower (Ahmad et al. 2009). Seed germination process is divided into three successive stages: inhibition, metabolism that leads initiation of radicle growth, and radicle growth which primes radicle emergence. A threshold level of hydration is essential for the ensuing radicle elongation (Ramagopal 1990). In normal seed germination process, a threshold of the embryo hydration level needs to be attained, which is a critical pre-condition for the successive initiation of cell elongation and radicle development (Hegarty 1978). In our study, the presence of mannitol could have severely reduced the internal osmotic potential of the germinating seeds, therefore permitting the water uptake and leading to germination initiation processes.
Plants’ capability to retain high water status during desiccation stress is a vital strategy for plant tolerance during drought stress. Accordingly, evaluation of relative water content change is the best representation and a fast approach for evaluating genetic differences in cellular hydration, plant water deficit and physiological water status after water deficit stress treatments (Sánchez-Rodríguez et al. 2010). Normally, high relative water content values are treated as index of drought stress tolerance, as demonstrated by Pandey et al. (2016) on rice genotypes, tolerant or sensitive to drought. The differences in relative water content in all varieties observed in our study could be correlated with their different ability of water absorption from soil. The decline in RWC was a main factor that caused decreased growth, responding to osmotic stress in the finger millet plants. Under desiccation stress, sensitive finger millet varieties were more affected by the decrease in relative water content than tolerant varieties. This suggested that the six finger millet varieties had different sensitivity when subjected to mannitol-induced water-deficit stress. The enhanced water retention capacity observed in some of finger millet even when challenged by drought could play a vital role in plant survival under water deficit conditions.
Plants’ chlorophyll content heavily depends on the species’ physiological responses and their ability to resist environmental stresses (Anjum et al. 2011). Evaluation of leaf chlorophyll concentration is one of the most effective diagnostic tool for studying of drought tolerance identification, genotypic variation, altitudinal variation, and it has been employed in many crops, including cereals such as sorghum (Qadir et al. 2014) and foxtail millet (Wang et al. 2016). Plants can overcome this assault by increasing the accumulation of chlorophyll which protects the plants by getting rid of excessive energy by thermal dissipation (Reddy et al. 2004). Consequently, decline of chlorophyll concentration in response to drought stress is a common phenomenon, occasioned by disordering chlorophyll synthesis and plant chlorosis. Additionally, when plants are subjected to environmental stresses, leaf chloroplasts are injured, which leads to disrupted photosynthesis. At higher mannitol concentrations above 200 mM, chlorosis was observed in all the varieties, and the leaves turned to pale yellow which often leads to plant death.
Proline plays significant role in the osmoregulation, allowing cells to retain more water. Moreover, the amino acid also displays plant defense properties as a ROS scavenger (Szabados and Savoure 2010) and as a regulator of the cellular redox status (Sharma et al. 2011). Proline accumulation therefore has a positive connection with plants’ tolerance to various environmental stresses (Szabados and Savoure 2010). In our study, the mannitol stressed plants showed significantly higher proline concentration than control plants, especially in GBK042094. Our results revealed that free proline accumulation in the leaf tissues of drought susceptible finger millet varieties was significantly lower than the tolerant ones. These findings are corroborated by the data reported in previous research work, which indicates that total free proline in the leaves of water deficit tolerant genotypes are higher than in drought susceptible lines of maize (Efeoğlu et al. 2009), sweetpotato (Mbinda et al. 2018), and rice (Pandey et al. 2016). The responses across the plant lines were concomitantly increased with progressive increment of mannitol dosage. Our results suggest that higher proline content in drought tolerant finger millet lines could be due to altered expression of drought responsive genes, which potentially improve the hydration status of the plants. Our findings also reinforce a close association between increased proline concentration and plant relative water content in drought tolerance mechanisms.
It is vital for antioxidative systems of plants to scavenge excess ROS in order to maintain a balanced equilibrium of cellular reactions when they are challenged by various stresses either singly or in combination (van Breusegem et al. 2018). The toxicity of ROS is due to their reactions with numerous cell components, which causes lipid peroxidation among other cascades of oxidative reactions (Wang et al. 2016). Cellular lipid peroxidation damages the plasma membrane, leading to leakage of contents, swift desiccation and cellular death (Demidchik 2015). The final product of lipid peroxidation is malondialdehyde and this solute is one of the best physiological biomarkers of drought tolerance in plants (Anjum et al. 2011). In this work, we found that GBK043137 and GBK043094 with the least amounts of MDA when challenged by drought stress (Table 7). Low MDA levels have been correlated with desiccation stress tolerance and the ensuing lipid peroxidation could induce the activity of antioxidant enzymes (Wang et al. 2016). Accumulation of MDA when challenged by environmental stresses has also been found to be a good drought tolerance index in other plant species pitanga (Toscano et al. 2016), melon (Sarabi et al. 2017), desi chickpea (Farooq et al. 2018) and wheat (Mickky and Aldesuquy 2017). From all the physiological responses examined, it evident that finger millet responses to drought stress largely depends on the genotype/cultivar used, the length and severity of water deficit stress and the stage of development of the plant.
Conclusion
In conclusion, our study provided a broad analysis of the physiological features of several finger millet plants to drought stress. The results reported here demonstrate the impact of drought stress on the analysed parameters with a wide range of variability among the studied varieties. Finger millet varieties GBK042094 and GBK043137 could tolerate water deficit better than the other four varieties, as indicated by significant decreases in germination rate, shoot length, root growth, relative water content, leaf total chlorophyll content, proline accumulation and lipid peroxidation. These finger millet varieties showed considerable level of tolerance to drought stress, and they could be used for further evaluations and breeding programs. Further investigations on genomic and molecular mechanisms of drought tolerance in finger millet needs to be explored.
Acknowledgements
This work was supported financially by a grant from The World Academy of Sciences (Ref. No. 17-357 RG/BIO/AF/AC_I – FR3240297745) through the generous contribution of the Swedish International Development Cooperation Agency. The authors gratefully acknowledge Kenya Agricultural and Livestock Research Organization, Gene Bank, for providing the finger millet seeds used. The authors also thank the Department of Biochemistry and Biotechnology, Kenyatta University for providing facilities for the research.
Author Contributions
AM and AN carried all the experiments. AM helped with draft the manuscript. CM, RO, MM and WMM supervised the study, WMM contributed in statistical analysis and writing the manuscript, conceived the idea, obtained of funding, contributed with experimental design, coordination and manuscript writing. All authors agreed on the final appearance of the manuscript after careful review.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Asunta Mukami, Phone: +254713551848, Email: amukami@seku.ac.ke.
Wilton Mwema Mbinda, Phone: +254722723950, Email: wilton.mbinda@gmail.com.
References
- Ahmad S, Ahmad R, Ashraf MY, Ashraf M, Waraich EA. Sunflower (Helianthus annuus L.) response to drought stress at germination and seedling growth stages. Pak J Bot. 2009;41(2):647–654. [Google Scholar]
- Anjum SA, Xie XY, Wang LC, Saleem MF, Man C, Lei W. Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agric Res. 2011;6(9):2026–2032. [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24(1):1. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf MFMR, Foolad M. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot. 2007;59(2):206–216. doi: 10.1016/j.envexpbot.2005.12.006. [DOI] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Budak H, Kantar M, Kurtoglu KY. Drought tolerance in modern and wild wheat. Sci World J. 2013;2013:1–16. doi: 10.1155/2013/548246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliz J, Montes-Borrego M, Triadó-Margarit X, Metsis M, Landa BB, Casamayor EO. Influence of edaphic, climatic, and agronomic factors on the composition and abundance of nitrifying microorganisms in the rhizosphere of commercial olive crops. PLoS ONE. 2015;10(5):e0125787. doi: 10.1371/journal.pone.0125787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves MM, Oliveira MM. Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot. 2004;55(407):2365–2384. doi: 10.1093/jxb/erh269. [DOI] [PubMed] [Google Scholar]
- Demidchik V. Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environ Exp Bot. 2015;109:212–228. doi: 10.1016/j.envexpbot.2014.06.021. [DOI] [Google Scholar]
- Demirevska K, Zasheva D, Dimitrov R, Simova-Stoilova L, Stamenova M, Feller U. Drought stress effects on Rubisco in wheat: changes in the Rubisco large subunit. Acta Physiol Plant. 2009;31(6):1129. doi: 10.1007/s11738-009-0331-2. [DOI] [Google Scholar]
- Efeoğlu B, Ekmekci Y, Cicek N. Physiological responses of three maize cultivars to drought stress and recovery. South Afr J Bot. 2009;75(1):34–42. doi: 10.1016/j.sajb.2008.06.005. [DOI] [Google Scholar]
- Farooq M, Ullah A, Lee DJ, Alghamdi SS, Siddique KH. Desi chickpea genotypes tolerate drought stress better than kabuli types by modulating germination metabolism, trehalose accumulation, and carbon assimilation. Plant Physiol Biochem. 2018;126:47–54. doi: 10.1016/j.plaphy.2018.02.020. [DOI] [PubMed] [Google Scholar]
- Forni C, Duca D, Glick BR. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil. 2017;410(1–2):335–356. doi: 10.1007/s11104-016-3007-x. [DOI] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125(1):189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hegarty TW. The physiology of seed hydration and dehydration, and the relation between water stress and the control of germination: a review. Plant Cell Environ. 1978;1(2):101–119. doi: 10.1111/j.1365-3040.1978.tb00752.x. [DOI] [Google Scholar]
- Hubbard M, Germida J, Vujanovic V. Fungal endophytes improve wheat seed germination under heat and drought stress. Botany. 2012;90(2):137–149. doi: 10.1139/b11-091. [DOI] [Google Scholar]
- Karakas B, Ozias-Akins P, Stushnoff C, Suefferheld M, Rieger M. Salinity and drought tolerance of mannitol-accumulating transgenic tobacco. Plant Cell Environ. 1997;20(5):609–616. doi: 10.1111/j.1365-3040.1997.00132.x. [DOI] [Google Scholar]
- Kaya C, Sonmez O, Aydemir S, Ashraf M, Dikilitas M. Exogenous application of mannitol and thiourea regulates plant growth and oxidative stress responses in salt-stressed maize (Zea mays L.) J Plant Interact. 2013;8(3):234–241. doi: 10.1080/17429145.2012.725480. [DOI] [Google Scholar]
- Kotchoni SO, Kuhns C, Ditzer A, Kirch HH, Bartels D. Over-expression of different aldehyde dehydrogenase genes in Arabidopsis thaliana confers tolerance to abiotic stress and protects plants against lipid peroxidation and oxidative stress. Plant Cell Environ. 2006;29(6):1033–1048. doi: 10.1111/j.1365-3040.2005.01458.x. [DOI] [PubMed] [Google Scholar]
- Kumar A, Metwal M, Kaur S, Gupta AK, Puranik S, Singh S, Singh M, Gupta S, Babu BK, Sood S, Yadav R. Nutraceutical value of finger millet [Eleusine coracana (L.) Gaertn.], and their improvement using omics approaches. Front Plant Sci. 2016;7:934. doi: 10.3389/fpls.2016.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu F. Drought stress memory and drought stress tolerance in plants: biochemical and molecular basis. In: Hossain M, Wani S, Bhattacharjee S, Burritt D, Tran LS, editors. Drought stress tolerance in plants. Cham: Springer; 2016. [Google Scholar]
- Liu M, Li M, Liu K, Sui N. Effects of drought stress on seed germination and seedling growth of different maize varieties. J Agric Sci. 2015;7(5):231. [Google Scholar]
- Mbinda W, Ombori O, Dixelius C, Oduor R. Xerophyta viscosa aldose reductase, XvAld1, enhances drought tolerance in transgenic sweetpotato. Mol Biotechnol. 2018;60(3):203–214. doi: 10.1007/s12033-018-0063-x. [DOI] [PubMed] [Google Scholar]
- Mickky BM, Aldesuquy HS. Impact of osmotic stress on seedling growth observations, membrane characteristics and antioxidant defense system of different wheat genotypes. Egypt J Basic Appl Sci. 2017;4(1):47–54. doi: 10.1016/j.ejbas.2016.10.001. [DOI] [Google Scholar]
- Møller IM, Jensen PE, Hansson A. Oxidative modifications to cellular components in plants. Annu Rev Plant Biol. 2007;58:459–481. doi: 10.1146/annurev.arplant.58.032806.103946. [DOI] [PubMed] [Google Scholar]
- Pandey V, Ansari MW, Tula S, Yadav S, Sahoo RK, Shukla N, Bains G, Badal S, Chandra S, Gaur AK, Kumar A. Dose-dependent response of Trichoderma harzianum in improving drought tolerance in rice genotypes. Planta. 2016;243(5):1251–1264. doi: 10.1007/s00425-016-2482-x. [DOI] [PubMed] [Google Scholar]
- Qadir M, Quillérou E, Nangia V, Murtaza G, Singh M, Thomas RJ, Drechsel P, Noble AD. Economics of salt-induced land degradation and restoration. Natural Resour Forum. 2014;38(4):282–295. doi: 10.1111/1477-8947.12054. [DOI] [Google Scholar]
- Ramagopal S. Inhibition of seed germination by salt and its subsequent effect on embryonic protein synthesis in barley. J Plant Physiol. 1990;136(5):621–625. doi: 10.1016/S0176-1617(11)80224-5. [DOI] [Google Scholar]
- Reddy AR, Chaitanya KV, Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol. 2004;161(11):1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Sánchez-Rodríguez E, Rubio-Wilhelmi M, Cervilla LM, Blasco B, Rios JJ, Rosales MA, Ruiz JM. Genotypic differences in some physiological parameters symptomatic for oxidative stress under moderate drought in tomato plants. Plant Sci. 2010;178(1):30–40. doi: 10.1016/j.plantsci.2009.10.001. [DOI] [Google Scholar]
- Sarabi B, Bolandnazar S, Ghaderi N, Ghashghaie J. Genotypic differences in physiological and biochemical responses to salinity stress in melon (Cucumis melo L.) plants: prospects for selection of salt tolerant landraces. Plant Physiol Biochem. 2017;119:294–311. doi: 10.1016/j.plaphy.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Sharma S, Villamor JGC, Verslues PE. Essential role of tissue specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiol. 2011;157:111. doi: 10.1104/pp.111.183210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabados L, Savoure A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15(2):89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Thilakarathna MS, Raizada MN. A review of nutrient management studies involving finger millet in the semi-arid tropics of Asia and Africa. Agronomy. 2015;5(3):262–290. doi: 10.3390/agronomy5030262. [DOI] [Google Scholar]
- Todaka D, Shinozaki K, Yamaguchi-Shinozaki K. Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants. Front Plant Sci. 2015;6:84. doi: 10.3389/fpls.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano S, Farieri E, Ferrante A, Romano D. Physiological and biochemical responses in two ornamental shrubs to drought stress. Front Plant Sci. 2016;7:645. doi: 10.3389/fpls.2016.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah I, Akhtar N, Mehmood N, Shah IA, Noor M. Effect of mannitol induced drought stress on seedling traits and protein profile of two wheat cultivars. J Animal Plant Sci. 2014;24:1246–1251. [Google Scholar]
- Van Breusegem F, Foyer C, Mann G. Reactive oxygen species are crucial “pro-life “survival signals in plants. Free Radic Biol Med. 2018;122:1–3. doi: 10.1016/j.freeradbiomed.2018.04.582. [DOI] [PubMed] [Google Scholar]
- Verslues PE, Lasky JR, Juenger TE, Liu TW, Kumar MN. Genome-wide association mapping combined with reverse genetics identifies new effectors of low water potential-induced proline accumulation in Arabidopsis. Plant Physiol. 2014;164(1):144–159. doi: 10.1104/pp.113.224014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li L, Tang S, Liu J, Zhang H, Zhi H, Jia G, Diao X. Combined small RNA and degradome sequencing to identify miRNAs and their targets in response to drought in foxtail millet. BMC Genet. 2016;17(1):57. doi: 10.1186/s12863-016-0364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Feng J, Zhai S, Dai Y, Xu M, Wu J, Shen M, Bian X, Koide RT, Liu J. Long-term ditch-buried straw return alters soil water potential, temperature, and microbial communities in a rice-wheat rotation system. Soil Tillage Res. 2016;163:21–31. doi: 10.1016/j.still.2016.05.003. [DOI] [Google Scholar]
- Zougmoré R. Promoting climate-smart agriculture through water and nutrient interactions options in semi-arid West Africa: a review of evidence and empirical analysis. In: Bationo A, Ngaradoum D, Youl S, Lompo F, Fening J, editors. Improving the profitability, sustainability and efficiency of nutrients through site specific fertilizer recommendations in West Africa agro-ecosystems. Cham: Springer; 2018. [Google Scholar]