Abstract
Drought or water deficit is a major abiotic stress that can reduce growth and productivity in the rice crop especially in the rain-fed areas, which face long-term water shortage. The objective of this investigation was to promote the drought tolerant abilities in pigmented rice cv. ‘Hom Nil’ at booting stage using arbuscular mycorrhizal fungi (AMF)-inoculation, mixed spores of Glomus geosporum, G. etunicatum and G. mosseae in the soil before rice seedling transplantation. At booting stage, the AMF-inoculated (+AMF) and AMF-uninoculated plants (−AMF) were subjected to control (well-watering; 46.6% SWC) and water deficit condition (14 days water withholding; 13.8% SWC). Colonization percentage in the AMF-inoculated root tissues were evidently proved in both with and without water deficit conditions, leading to elevate total phosphorus in root and leaf tissues. Interestingly, sucrose and total soluble sugar concentration in the flag leaf were increased by 5.0 folds and 1.5 folds, respectively in the plants under water deficit (WD). Free proline was accumulated in flag leaf when exposure to water deficit, subsequently regulated by AMF-inoculation. Total soluble sugar and free proline enrichment in ‘Hom Nil’ was a major mode of osmotic adjustment to control osmotic potential in the cellular level when exposed to water deficit, leading to maintained photosynthetic abilities and growth performances. Concentration of chlorophyll b in AMF-inoculated plants under water deficit stress was retained, causing to improve chlorophyll fluorescence and net photosynthetic rate. Shoot height and number of tillers were significantly declined by 12.5% and 11.6%, respectively, when subjected to WD. At the harvest, grain yield, panicle dry weight and fertility percentage of AMF-inoculated rice from WD were greater than those without AMF by 1.5, 3.9 and 2.4 folds, respectively. Cyanidin-3-glucoside and peonidin-3-glucoside concentrations in pericarp were enriched in the grain derived from AMF-inoculation with water deficit stress. Overall growth characters and physiological adaptations in ‘Hom Nil’ grown under water deficit condition were retained by AMF inoculation, resulting in enhanced yield attributes and anthocyanin fortification in rice grain.
Electronic supplementary material
The online version of this article (10.1007/s12298-019-00658-4) contains supplementary material, which is available to authorized users.
Keywords: Anthocyanins, Free proline, Net photosynthetic rate, Total soluble sugars, Water deficit, Yield traits
Introduction
Rice is a major source of carbohydrate and a staple food crop feeding half of world population (3.5 billion people), especially in Asia (Kubo and Purevdorj 2004; Muthayya et al. 2014). The major challenge among the agriculturists is to double the production of the cereal grains to generate food resources for world’s population, which is estimated to reach 9 billion by 2050 (Tilman et al. 2002). In paddy field, costs are distributed variably for land preparation (25.27%), labor (19.72%), fertilizers (18.9%), pesticides (11.56%), and manures (7.31%) (Nirmala and Muthuraman 2009; Adhikari 2011). Fertilizer application either via chemical or manure supplements, is required in all the growth and developmental stages of the rice production (Peng et al. 2009). In general, the macronutrients i.e. nitrogen, phosphorus and potassium, can easily be dissolved in the irrigated water, and therefore lost along with surface runoff. This is especially common in case of phosphorus (P), leading to deficiency of P in the next crop cycle (Hart et al. 2004; Jian et al. 2016; Hua et al. 2017). Plant inoculation with Arbuscular Mycorrhizal Fungi (AMF) is one of the most common approaches to influence host P status, growth regulation and yield improvement (Gosling et al. 2006). AMF belonging to Glomeromycota phylum have been categorized into four orders including Diversisporales, Glomerales, Archaeosporales and Paraglomerales to symbiont with root of host plants, in term of hyphae, vesicle and arbuscules, regulating on photosynthetic abilities, overall growth performance and resistant to abiotic stresses, especially in rice crop (Panneerselvam et al. 2017; Basu et al. 2018; Mbodj et al. 2018). In contrast, the inorganic fertilizer application, especially P in rice crop negatively affects the AMF-activation, P availability, and P uptake/translocation, leading to slow growth and yield reduction (Hajiboland et al. 2009; Frosi et al. 2016; Zhang et al. 2016). Now-a-days, total organic agricultural land and organic foods have been continuously increasing year by year (Reganold and Wachter 2016). Low input rice production using organic farming system is a good choice to reduce the production cost (chemical fertilizers, pesticides, herbicides and fungicides) and produce premium quality and consumer friendly products with low chemical contamination (Hokazono and Hayashi 2012; Bacenetti et al. 2016).
Irrigation and water management in the paddy field with declining fresh water in each developmental stage of rice crop are the critical issues (Tuong and Bouman 2003; Tuong et al. 2005; Tao et al. 2006). Moreover, rice production in rainfed areas generally copes with water shortage, since it depends on the annual precipitation and irregularities in the rainfall may lead to yield losses up to 10–50% (Tuong and Bouman 2003; Wassman et al. 2009; Li et al. 2015). In rice crop, plant have many strategies to cope against drought conditions at morphological level (growth inhibition), molecular level (up or down regulation in drought related genes), biochemical level (osmoprotectant accumulation/antioxidants production), physiological level (chlorophyll degradation, PSII diminution, and reduction in photosynthesis, transpiration, stomatal conductance, and water use efficiency) and via inhibition of yield attributes (Cattivelli et al. 2008; Serraj et al. 2011; Pandey and Shukla 2015). Alternatively, AMF inoculation has been well reported to improve the soil physical and chemical properties, P availability in the root tissues of host plants, and water availability, and in addition to that being environmental friendly, it can be utilized in organic farming systems (Gosling et al. 2006; Maiti et al. 2013; Zhang et al. 2014). Plant–microbe crosstalk between hypha of AMF (to consume soluble sugar as carbon source) and root tissues of higher plants (to uptake P and translocate it to other organs) has been well studied, and so did the regulation of the expression of abiotic defensive responses to alleviate the drought tolerant abilities (García-Garrido and Ocampo 2002; Hause and Fester 2005; Garg and Chandel 2010; Haldar and Sengupta 2015). In case of rice crop, several strains of AMF, i.e. Glomus fasciculatum, G. etunicatum, G. mosseae, G. intraradices, Acaulospora sp. and Scutellospora sp. have been employed to cope with water deficit stress and to improve overall growth and development (Solaiman and Hirata 1997; Zhang et al. 2014). Anthocyanin, a member of flavonoids groups, common components of the human diet, presenting in many foods, fruits, vegetables and cereal grains to play a key role as antioxidant activity against chronic diseases (Martín et al. 2017; Silva et al. 2017). In lettuce cvs. Batavia Rubia Munguía and Maravilla de Verano, anthocyanins and soluble phenolic compounds in outer and inner leaves upregulated by AMF-inoculation and cyclic drought treatments (Baslam and Goicoechea 2012). In addition, total pelargonidin concentration, a member of anthocyanins in strawberry fruit cv. Selva is regulating by AMF co-cultivation (Ligua et al. 2013). In pigmented rice crop, there are a large number of research topics to mention on the anthocyanin profiles i.e. peonidin-3-glucoside (P3G) and peonidin-3-glucoside (P3G), especially black pericarp of rice grain cultivars as Suwon#415, Kilimheugmi, Suwon#425, Heugjinmi, Hom Nil, Niaw Dam, China Black Rice (Ryu et al. 1998; Sompong et al. 2011; Tisarum et al. 2018). Hom Nil rice, a premium quality for human health is non-glutenous rice with a dark-purple pericarp, which is enriched by anthocyanins and total phenolic compounds (Pitija et al. 2013; Tisarum et al. 2018; Wongsa et al. 2018) to function as antioxidant activities measuring by FRAP (Ferric reducing antioxidant power), TEAC (Trolox equivalent antioxidant activity), ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) antioxidant activity, scavenging capacity of antioxidant using DPPH (2,2-diphenyl-1-picrylhydrazyl) and antioxidant power (Sadabpod et al. 2010; Sutharut and Sudarat 2012; Daiponmak et al. 2014; Thiranusornkij et al. 2018). In the present study, we aimed to improve drought tolerant abilities in pigmented rice cv. ‘Hom Nil’ using AMF-colonization and to promote the anthocyanins in pericarp of the rice grain.
Materials and methods
Plant materials, AMF-inoculation and water deficit treatment
Seeds of pigmented rice cv. ‘Hom Nil’ were sown in the mixed soil (16% sand, 30% silk and 54% clay; EC = 2.69dS m−1; pH = 5.5; organic matter = 10.36%; total nitrogen = 0.17%; total phosphorus = 0.07%, and total potassium = 1.19%) for 4 weeks. Three leaves seedlings were transplanted into clay pots containing 2 kg of the sterilized mixed soil using autoclave equipment (121 °C and 15 lb in−2 for 20 min). Two types of soil had been prepared, (1) sterilized soil without arbuscular mycorrhizal fungi (AMF), and (2) sterilized soil with AMF (240 spores of G. geosporum, G. etunicatum and G. mosseae per pot). In present study, AMF composing of three species of G. geosporum, G. etunicatum and G. mosseae were collected from the rainfed upland rice in the Northern region of Thailand. The rice plants were grown in a net house under 500–1000 μmol m−2 s−1 photosynthetic photon flux density (PPFD) with a 10 h day−1 photoperiod, 35 ± 2 °C (day time)/28 ± 2 °C (night time) temperature and 80 ± 5%RH until booting stage. Thereafter, six groups of plants: well-watered without AMF (control; WW–AMF), well-watered with AMF treatment (WW+AMF) and water withholding for 14 days (water deficit; WD at 13.8% soil water content) with (WD+AMF) or without (WD–AMF), were set as the experimental layout (Fig. S1). Morphological characters, osmotic potential, free proline, soluble sugar, chlorophyll concentration, chlorophyll fluorescence, and net photosynthetic rate were measured in the control/treated plants. In addition, the grain yield traits i.e. grain yield per clump, panicle weight, panicle length, fertility percentage, weight per 100-grains, and anthocyanin concentration in the pericarp of the rice grain were evaluated after harvest (100 days after sowing).
AMF colonization assay
Fresh root samples (3.0 ± 0.5 cm in length) of rice crop in each treatment were collected, washed with distilled water and then cut into small pieces (~ 1.0 cm length) and kept in 60% ethanol (storage solution). Roots were washed thrice with distilled water, transferred to 10% KOH and incubated at 95 °C for 30 min for clearing. Cleaned roots were washed with distilled water and then stained using 0.05% (w/v) Trypan blue for 15 min. AMF-colonization in the roots was observed under light microscope (Zeiss, Germany), and the mycorrhizal hyphae were counted (Fig. S2), according to the method of Brundrett et al. (1996). In brief, the number and frequency of mycorrhizal hyphae in 10 possible intersects along the root tissues were count and calculated the colonization using gridline intersection method.
Phosphorus analysis
Available phosphorus was extracted and determined spectrophotometrically as blue molybdate–phosphate complexes under partial reduction with ascorbic acid (Jackson 1958). Briefly, one-hundred milligrams of dried root and flag leaf samples in each treatment were ground, transferred to 1 mL digestion mixture (0.42 g Se, 14 g LiSO4·2H2O to 350 mL H2O2, and 420 mL H2SO4) and then placed on the hot plate (gradually increased from 50 to 150 °C) until the mixture turned back. Five-hundred microliter of 72% HClO4 was added to each sample and heated until the material became colorless. After cooling, the samples were diluted with equal volume of HClO4, filtered with filter paper (Whatman #42, England) and then mixed with 0.5 mL of Barton’s reagent [25 g (NH4)6Mo7O24 (400 mL), 1.25 g NH4VO3 (350 mL) and HNO3 (250 mL)] for 10 min. Total phosphorus [mg g−1 dry weight (DW)] was measured at 420 nm by UV-spectrophotometer (HACH DR/4000; Model 48,000, HACH Company, Loveland, Colorado, USA) using KH2PO4 as a calibration standard.
Plant biochemical analysis
Free proline in the flag leaf tissues was extracted and analyzed according to the method of Bates et al. (1973). Fifty milligrams of fresh material were ground with liquid nitrogen in a mortar. The homogenate powder was mixed with 1 mL of aqueous sulfosalicylic acid (3%, w/v) and filtered through filter paper (Whatman #1). The extracted solution was reacted with an equal volume of glacial acetic acid and ninhydrin reagent (1.25 mg ninhydrin in 30 mL glacial acetic acid and 20 mL 6 M H3PO4) and incubated at 95 °C for 1 h. The reaction was terminated by placing the container in an ice bath. The reaction mixture was mixed vigorously with 2 mL of toluene. After cooling to 25 °C, the chromophore was measured at 520 nm by UV–VIS spectrophotometer using l-proline as a calibration standard.
Soluble sugars (sucrose, glucose and fructose) in the flag leaf tissues were assayed following the method of Karkacier et al. (2003). In brief, 50-mg of flag leaf sample were ground in a mortar with liquid nitrogen. One mL of nanopure water was added and centrifuged at 12,000×g for 15 min. The supernatant was collected and filtered through a 0.45 μm membrane filter (VertiPure™, Vertical®, Vertical Chromatography Co., Ltd., Thailand). Twenty micro-litres of the filtrate were injected into a Waters HPLC (Waters Associates, Milford, MA, USA) equipped with a MetaCarb 87C column and a guard column (Agilent Technologies, Santa Clara, CA, USA). Deionized water was used as the mobile phase at a flow rate of 0.5 mL min−1. The online detection was performed using a Waters 410 differential refractometer detector and the data was analyzed by Empower® software. Sucrose, glucose and fructose (Fluka, USA) were used as the standards.
Plant physiological assay
Osmotic potential in the flag leaf of ‘Hom Nil’ rice was measured, according to Lanfermeijer et al. (1991). In brief, one hundred milligrams of fresh tissue were chopped into small pieces, transferred to 1.5 mL micro tube, and then crushed using a glass rod. The 20 μL of the extracted solution was dropped directly onto a filter paper in an osmometer chamber (5520 Vapro®, Wescor, Utah, USA) and subsequently the data were collected. Then, the osmolarity (mmol kg−1) was converted to osmotic potential (MPa) using conversion factor of osmotic potential measurement.
Chlorophyll a (Chla), chlorophyll b (Chlb), total chlorophyll (TC) concentration in the flag leaf tissues were analyzed according to the method of Shabala et al. (1998), whereas total carotenoid (Cx+c) concentration was assayed following Lichtenthaler (1987) method. One hundred milligrams of leaf tissue was homogenized in glass vials using 10 mL of 99.5% acetone and blended using a homogenizer. The glass vials were sealed with Parafilm® (Sigma-Aldrich, USA) to prevent evaporation, and then stored at 4°C for 48 h. Chla and Chlb concentrations were measured at 662 nm and 644 nm, whereas Cx+c concentration was measured at 470 nm using UV–VIS spectrophotometer against acetone (99.5%) as a blank.
Chlorophyll fluorescence emission was measured from the adaxial surface of flag leaf using a fluorescence monitoring system (model FMS 2; Hansatech Instruments Ltd., Norfolk, UK) in the pulse amplitude modulation mode (Loggini et al. 1999). A leaf kept in dark for 30 min was initially exposed to the modulated measuring beam of far-red light (LED source) with typical peak at wavelength 735 nm. Initial fluorescence (F0) and maximum (Fm) fluorescence yields were measured under weakly modulated red light (< 85 μmol m−2 s−1) with 1.6 s pulses of saturating light (> 1500 μmol m−2 s−1 PPFD) and calculated using FMS software for Windows®. The variable fluorescence yield (Fv) was calculated using the equation: Fv = Fm–F0. The ratio of variable to maximum fluorescence (Fv/Fm) was calculated as the maximum quantum yield of PSII photochemistry. The photon yield of PSII (ΦPSII) in the light was calculated as: ΦPSII = (Fm′ − F)/Fm′ after 45 s of illumination, when steady state was achieved (Maxwell and Johnson 2000).
Net photosynthetic rate (Pn; μmol m−2 s−1), transpiration rate (E; mmol H2O m−2 s−1) and stomatal conductance (gs; mmol m−2 s−1) were measured using a Portable Photosynthesis System with an Infra-red Gas Analyzer (Model LI 6400, LI-COR® Inc., Lincoln, Nebraska, USA). All parameters were measured continuously by monitoring the content of the air entering and existing in the IRGA headspace chamber, according to Cha-um et al. (2007).
Plant morphological characterization and yield traits
Shoot height, number of leaves, leaf length, leaf width and number of tillers of ‘Hom Nil’ rice were measured. Grain yield per clump, panicle dry weight, panicle length, seed fertility and 100-grain weight were determined.
Anthocyanin assay
Total anthocyanins [cyanidin-3-glucoside (C3G) and peonidin-3-glucoside (P3G)] were assayed following the method of Chandra et al. (2001). Hand-dehusked seeds (2 g) were weighted and transferred in a capped glass vial and then 1.5 mL of 2% HCl in methanol was added (Fig. S4). Extracted solution was vortexed and kept in the darkness on the shaker (150 rpm) for 12 h in the cold room (8°C). Supernatant was collected and filtered through a 0.45 μm PTFE filter (VertiPure, Vertical Chromatography). Each sample was analyzed by Waters HPLC equipped with a Waters 2998 photodiode array detector. The column was an ODS C18 Hypersil column (250 mm × 4.6 mm, i.d.) with a particle size 5 μm (Thermo Fisher Scientific Inc., CA, USA). The mobile phase was (A) 0.5% aqueous phosphoric acid (v/v), and (B) water/acetonitrile/glacial acetic acid/phosphoric acid, 50:48.5:1:0.5 (v/v/v/v). A gradient: initially as 20%; 26 min, 60%; 30 min, 20%; 35 min, 20% of solvent B was used as mobile phase. Detection wavelength was set at 520 nm. Flow rate was 0.8 mL min−1. Column temperature was 30 °C and injected volume was 20 μL. Cyanidin-3-glucoside (Fig. S5) and peonidin-3-glucoside (Fig. S6) (Sigma-Aldrich, USA) were injected as standard.
Statistical analysis
The experiment was arranged as 2 × 2 factorial in Completely Randomized Design (CRD) with six replicates (n = 6). The mean values obtained from six treatments were compared using Tukey’s HSD and analyzed by SPSS software (version 11.5 for Window®).
Results
AMF colonization and phosphorus enrichments
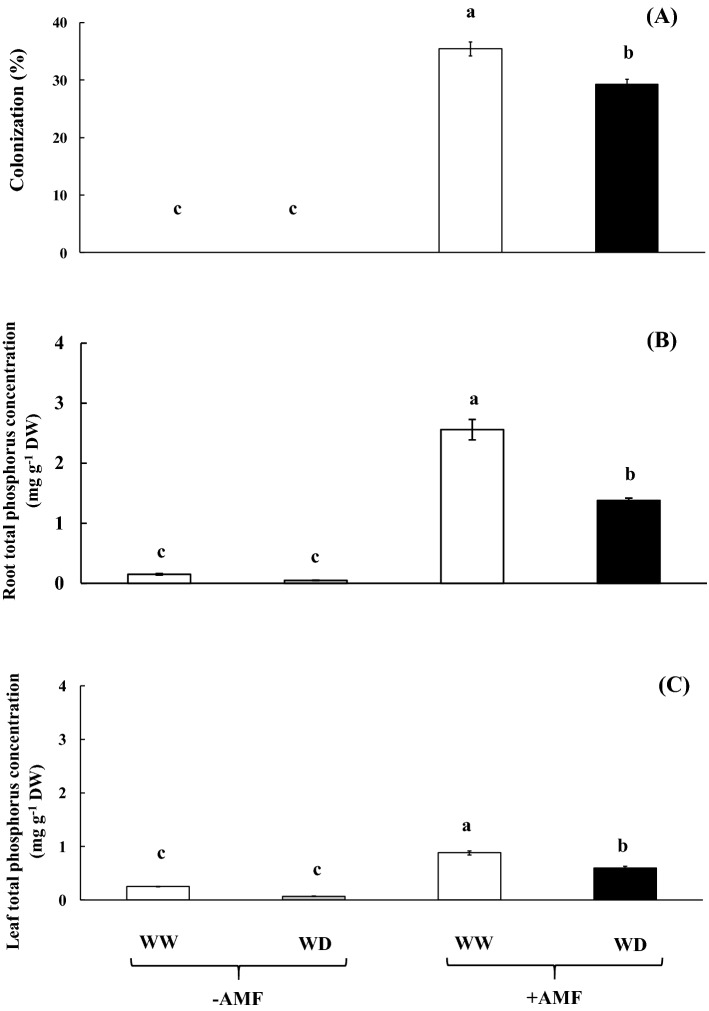
Colonization of AMF in the root tissues of ‘Hom Nil’ rice plants, inoculated with or without AMF under different water regimes was evaluated. Only AMF-inoculated roots of rice crop in both WW and WD displayed the high colonization percentage (Fig. 1a), which was confirmed by counting the hypha stained by trypan blue under light microscope (Fig. S2). Colonization percentage in the root tissues of rice grown under WW (35.42%) was significantly higher over WD (29.27%), whereas it was absent in the plants without AMF inoculation. Total phosphorus concentration in the root tissues of AMF inoculated plants was found to be enriched than that in the flag leaf tissues. In addition, the total phosphorus concentration in the root and flag leaf tissues was peaked at 2.56 and 0.88 mg g−1 DW, respectively (Fig. 1b, c) and it declined when plants were subjected to WD conditions. In contrast, total phosphorus concentrations in both root and flag leaf tissues of un-inoculated AMF plants were very low (Fig. 1b, c).
Fig. 1.
AMF colonization a in the root tissue, and total phosphorus concentration in root (b) and leaf tissues c of rice cv. ‘Hom Nil’ plants inoculated with AMF and subsequently exposed to water deficit conditions for 7 days. Error bars represent ± SE. Different letters in each bar show significant difference at ≤ 0.05 according to Tukey’s HSD
Growth characters in responses to water deficit conditions
In WW (control), shoot height, number of leaves, flag leaf length, flag leaf width and number of tillers in Hom Nil rice were normally developed either with or without AMF inoculation (Fig S3). Interestingly, flag leaf length of rice crop under AMF inoculation and water regime treatments were unaffected. Shoot height, flag leaf width and number of tillers, in the plants under WD without AMF inoculation, were sharply decreased by 12.50%, 29.17% and 11.63%, respectively (Table 1). Moreover, shoot height and number of tillers in the AMF-pretreated plants were maintained (declined only by 4.63% and 0% over well watering) better than those in without AMF inoculation (Table 1).
Table 1.
Shoot height, number of leaves, leaf length, leaf width and number of tillers in rice cv. ‘Hom Nil’ plants inoculated with AMF and subsequently exposed to well-watered (WW) and water deficit (WD) conditions at booting stage
| Water regime | AMF inoculation | Shoot height (cm) | Number of leaves | Leaf length (cm) | Leaf width (cm) | Number of tiller |
|---|---|---|---|---|---|---|
| WW (46.6% SWC) | −AMF | 86.4ab | 2.3ab | 28.8 | 1.20a | 4.3a |
| +AMF | 88.2a | 2.5a | 30.1 | 1.23a | 4.5a | |
| WD (13.8% SWC) | −AMF | 75.6c | 1.5b | 28.3 | 0.85b | 3.8b |
| +AMF | 82.4b | 2.3ab | 30.3 | 0.87b | 4.3a | |
| Significant level | ||||||
| Watering | ** | * | ns | ** | ns | |
| AMF | ** | * | ns | ns | ** | |
| Watering × AMF | * | ns | ns | ns | ns | |
Different letters in each column represent significant difference at p ≤ 0.05 applying Tukey’s HSD
ns not significant
*, **Significant difference at p ≤ 0.05 and p ≤ 0.01, respectively
Soluble sugar concentration, free proline content and osmotic potential
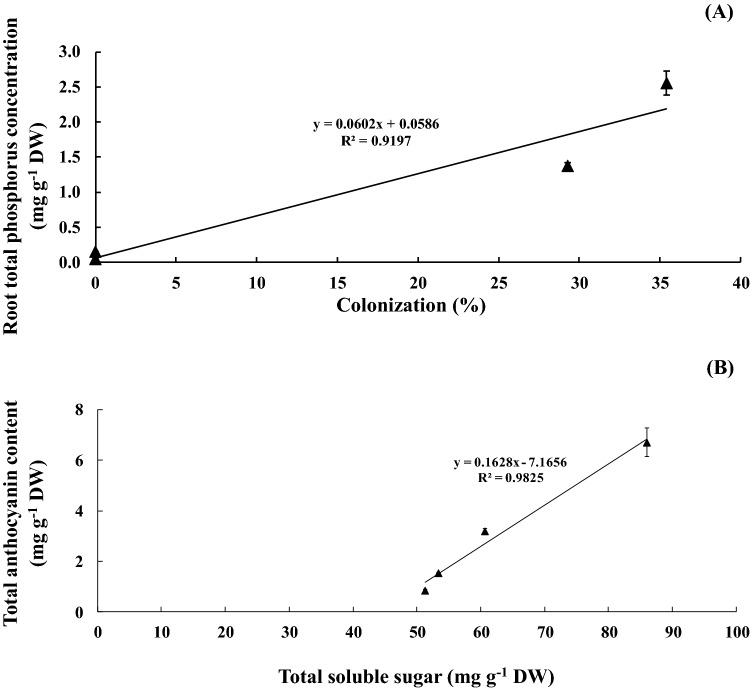
Sucrose concentration in un-inoculated AMF under WD conditions was significantly declined by 70.83% and 63.09% to that of the control (WW), respectively (Table 2). Under WD, glucose concentration in AMF inoculated plants was increased by 1.24-fold over WW without AMF. In addition, total soluble sugar was enriched in AMF-inoculated plants under WD by 1.51 folds over control (Table 2). Moreover, a positive relationship between colonization percentage and total phosphorus concentration in the root (R2 = 0.920; Fig. 2a), total soluble sugar and total anthocyanins in flag leaf tissues (R2 = 0.983; Fig. 2b).
Table 2.
Sucrose, glucose, fructose and total soluble sugar in rice cv. ‘Hom Nil’ plants inoculated with AMF and subsequently exposed to well-watered (WW) and water deficit (WD) conditions at booting stage
| Water regime | AMF inoculation | Sucrose (mg g−1 DW) | Glucose (mg g−1 DW) | Fructose (mg g−1 DW) | Total soluble sugar (mg g−1 DW) |
|---|---|---|---|---|---|
| WW (46.6% SWC) | −AMF | 4.85b | 19.36b | 23.74ns | 47.95c |
| +AMF | 6.22b | 22.29ab | 25.61 | 54.12b | |
| WD (13.8% SWC) | −AMF | 1.79c | 21.66ab | 25.99 | 49.88bc |
| +AMF | 31.13a | 24.06a | 26.32 | 81.51a | |
| Significant level | |||||
| Watering | ** | * | ns | ** | |
| AMF | ** | * | ns | ** | |
| Watering × AMF | ** | ns | ns | ** | |
Different letters in each column represent significant difference at p ≤ 0.05 applying Tukey’s HSD
ns not significant
*, **Significant difference at p ≤ 0.05 and p ≤ 0.01, respectively
Fig. 2.
Relationships between AMF colonization and phosphorus concentration in root tissues (a), total soluble sugar in flag leaf and total anthocyanins in rice grain b of rice cv. ‘Hom Nil’ plants inoculated with AMF and subsequently exposed to water deficit conditions at booting stage. Error bars represent ± SE
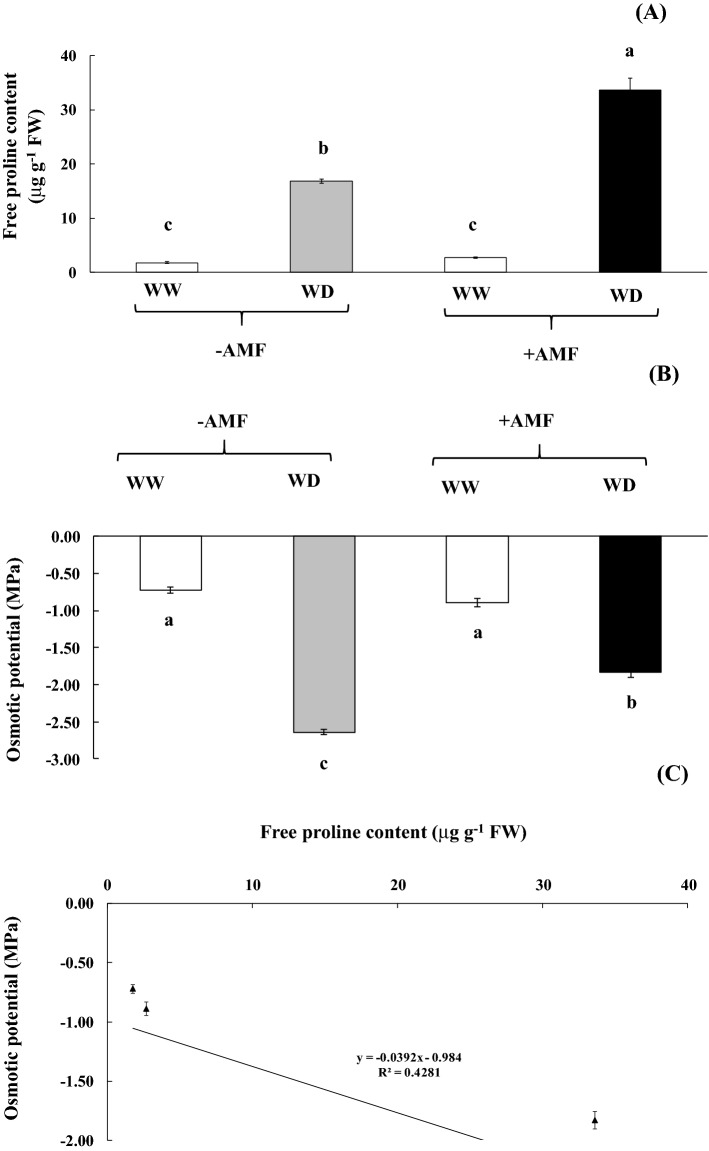
Free proline content in the plants with AMF- and without AMF-inoculation under WD was significantly increased by 12.45 and 9.48 folds over control (Fig. 3a). was displayed. Osmotic potential of flag leaf tissues declined when plants were exposed to water deficit conditions. Similarly, osmotic potential in flag leaf of plants without AMF under WD peaked to − 2.643 MPa but was reduced to − 1.833 MPa in AMF-inoculated plants (Fig. 3b). In addition, a negative relationship between free proline content and osmotic potential in plants grown under WD (R2 = 0.428; Fig. 3c) was established.
Fig. 3.
Free proline content (a), osmotic potential (b) and relationship between free proline content and osmotic potential c in the leaf tissues of rice cv. ‘Hom Nil’ plants inoculated with AMF and subsequently exposed to water deficit (14 days-water withholding; b). Error bars represent ± SE. Different letters in each bar show significant difference at p ≤ 0.05 according to Tukey’s HSD
Photosynthetic performance
Chla, TC and Cx+c in flag leaf tissues of AMF inoculated plants under WW were stimulated by 1.69, 1.35 and 1.85 folds over AMF un-inoculated plants, respectively. Moreover, Chla, Chlb, TC and Cx+c of AMF un-inoculated plants under WD were significantly dropped by 90.91%, 89.38%, 90.25% and 87.50% to that of WW, respectively (Table 3). Chlb concentration in the flag leaf of AMF inoculated plants was maintained (degraded only 15% of WW); however, in contrast, Chla, TC and Cx+c concentrations were degraded by 59.98%, 44.07% and 59.52% over WW, respectively (Table 3). Fv/Fm and ΦPSII in the flag leaf of AMF un-inoculated plants under WD were reduced by 75.62% and 83.37% to that of WW, respectively (Table 4). Similarly, Pn in the flag leaf of AMF un-inoculated plants under WD (Table 4) was sharply declined by 82.63% over the control, respectively, and it was significantly improved by inoculating the plants with AMF before subjecting to water deficit condition [reduction only by 55.42% in WD]. Stomatal conductance (gs) and transpiration rate (E) were sensitive to water deficit stress, which were largely declined (≥ 85% over well watering), especially in AMF un-inoculated plants (Table 4). In addition, gs and E in AMF-inoculated plants were significantly upgraded when exposed to water deficit stress conditions (Table 4).
Table 3.
Chlorophyll a, chlorophyll b, total chlorophyll and total carotenoids in ‘rice cv. ‘Hom Nil’ plants inoculated with AMF and subsequently exposed to well-watered (WW) and water deficit (WD) conditions at booting stage
| Water regime | AMF inoculation | Chlorophyll a (μg g−1 FW) | Chlorophyll b (μg g−1 FW) | Total chlorophyll (μg g−1 FW) | Total carotenoids (μg g−1 FW) |
|---|---|---|---|---|---|
| WW (46.6% SWC) | −AMF | 475.0b | 356.8a | 831.8ab | 3.2b |
| +AMF | 611.7a | 335.9a | 947.6a | 4.2a | |
| WD (13.8% SWC) | −AMF | 43.2d | 37.9b | 81.1c | 0.4d |
| +AMF | 244.8c | 285.2a | 530.0b | 1.7c | |
| Significant level | |||||
| Watering | ** | ** | ** | ** | |
| AMF | ** | ** | ** | ** | |
| Watering × AMF | ns | ** | ** | ns | |
Different letters in each column represent significant difference at p ≤ 0.05 applying Tukey’s HSD
ns not significant
**Significant difference at p ≤ 0.05 and p ≤ 0.01, respectively
Table 4.
Maximum quantum yield of PSII (Fv/Fm), photon yield of PSII (ΦPSII), net photosynthetic rate (Pn), stomatal conductance (gs) and transpiration rate (E) in ‘rice cv. ‘Hom Nil’ plants inoculated with AMF and subsequently exposed to well-watered (WW) and water deficit (WD) conditions at booting stage
| Water regime | AMF inoculation | Fv/Fm | ΦPSII | Pn (μmol m−2 s−1) | gs (mol H2O m−2 s−1) | E (mmol m−2 s−1) |
|---|---|---|---|---|---|---|
| WW (46.6% SWC) | −AMF | 0.841a | 0.782a | 8.81b | 0.122ab | 3.38a |
| +AMF | 0.864a | 0.806a | 12.83a | 0.158a | 3.76a | |
| WD (13.8% SWC) | −AMF | 0.205c | 0.130c | 1.53d | 0.015c | 0.41c |
| +AMF | 0.491b | 0.339b | 5.72c | 0.076b | 2.29b | |
| Significant level | ||||||
| Watering | ** | ** | ** | ** | ** | |
| AMF | ** | ** | ** | ** | ** | |
| Watering × AMF | ** | ** | ** | ns | ** | |
Different letters in each column represent significant difference at p ≤ 0.05 applying Tukey’s HSD
ns not significant
**Significant difference at p ≤ 0.05 and p ≤ 0.01, respectively
Yield traits and anthocyanins enrichment
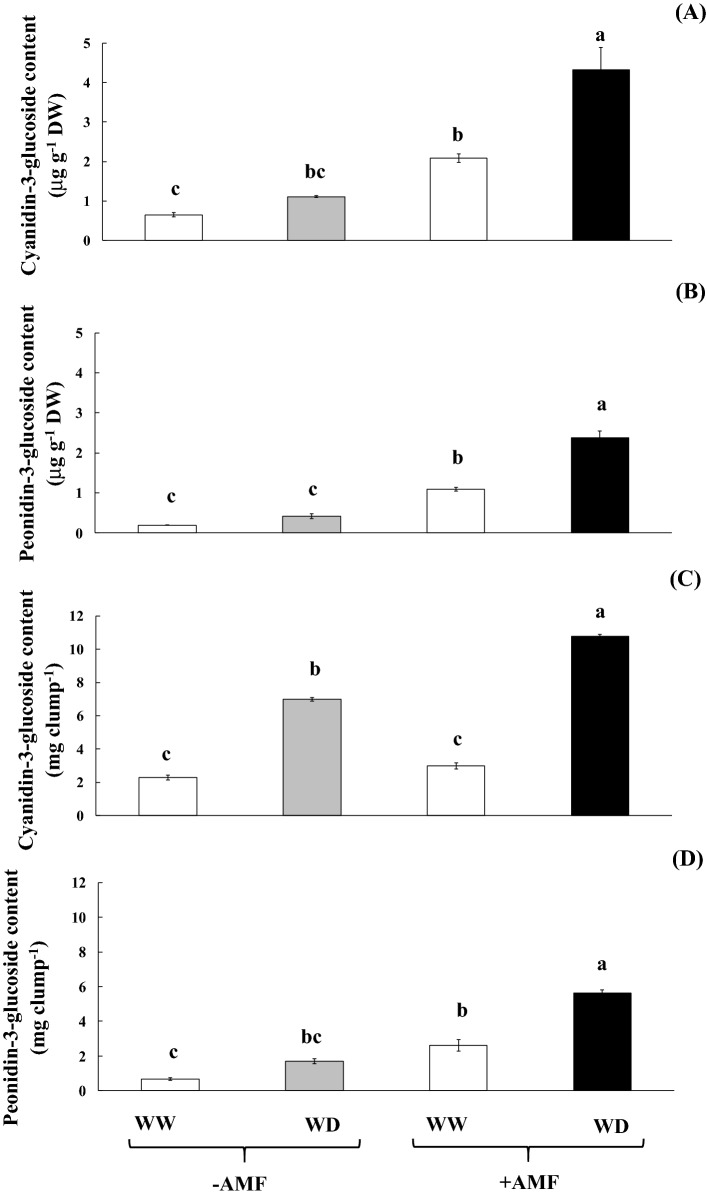
Grain yield and panicle dry weight observed during the harvesting process of AMF inoculated plants under WW were enhanced over un-inoculated plants by 1.79 and 1.59 folds, respectively. Under water deficit conditions, grain yield, panicle dry weight, panicle length, fertility percentage and weight of 100 grains of AMF un-inoculated plants were declined by 56.22%, 73.25%, 19.98%, 57.42% and 16.93% to that of AMF inoculated plants (Table 5). Moreover, panicle dry weight, panicle length, fertility percentage and weight of 100 grains were maintained upon AMF inoculation, whereas the grain yield showed a little improvement when subjected to water deficit stress (Table 5). Cyanidin-3-glucoside (C3G) and peonidin-3-glucoside (P3G) in the harvested grains of WD plants were enriched by 1.71 and 2.21 folds over well watering (Fig. 4a, b). The C3G (4.33 mg g−1) and P3G (2.38 mg g−1) in the pericarp of rice grain were strongly promoted with the interaction of water deficit conditions and AMF-inoculation (Fig. 4a, b). A profile of anthocyanins, C3G and P3G in rice grain harvesting from WW + AMF (a) and WD + AMF (b) was presented (Fig. S7). In addition, the total concentrations of C3G and P3G per clump in AMF-inoculated plants under water deficit conditions were peaked at 10.77 and 5.63 mg clump−1, respectively (Fig. 4c, d).
Table 5.
Grain yield per clump, panicle dry weight, panicle length, fertility, 100-grain weight in ‘rice cv. ‘Hom Nil’ plants inoculated with AMF and subsequently exposed to well-watered (WW) and water deficit (WD) conditions at booting stage prior to harvest
| Water regime | AMF inoculation | Grain yield (g) | Panicle DW (g) | Panicle length (cm) | Fertility (%) | 100-grain weight (g) |
|---|---|---|---|---|---|---|
| WW (46.6% SWC) | −AMF | 3.54b | 1.57b | 24.65a | 46.99a | 2.54a |
| +AMF | 6.33a | 2.50a | 25.63a | 67.60a | 2.41ab | |
| WD (13.8% SWC) | −AMF | 1.55d | 0.42c | 19.75b | 20.01b | 2.11b |
| +AMF | 2.39c | 1.67b | 23.55a | 47.11a | 2.27ab | |
| Significant level | ||||||
| Watering | ** | ** | ** | ** | ** | |
| AMF | ** | ** | ** | ** | ns | |
| Watering × AMF | * | ns | * | ns | * | |
Different letters in each column represent significant difference at p ≤ 0.05 applying Tukey’s HSD
ns not significant
*, **Significant difference at p ≤ 0.05 and p ≤ 0.01, respectively
Fig. 4.
Cyanidin-3-glucoside (a) and peonidin-3-glucoside b concentration in the rice grain of rice cv. ‘Hom Nil’ plants inoculated with AMF and subsequently exposed to water deficit conditions. Cyanidin-3-glucoside (c) and peonidin-3-glucoside d yield per clump in rice cv. ‘Hom Nil’ plants inoculated with AMF and subsequently exposed to water deficit conditions. Error bars represent ± SE. Different letters in each bar show significant difference ≤ 0.05 according to Tukey’s HSD
Discussion
In the present study, colonization percentage in the root tissues of rice grown under WW was greater than that under WD and it was absent in treatments without AMF inoculation. In ryegrass, root colonization by Glomus intraradices under well watering (200 mL daily irrigation) was recorded to be 51.4%, whereas it declined by 20.1% during the initial period when exposed to drought stress (20 mL daily irrigation) for 28 days (Lee et al. 2012). In addition, AMF (G. etunicatum and G. mosseae) root colonization in well-watered conditions of wheat cvs. TAM-105 and Steardy was better than that in water-stressed plants at both heading and grain filling developmental stages (Al-Karaki et al. 2004). In rice cv. INCA LP-5, AMF (G. intraradices) root colonization in both well-watered (100% water holding capacity) and drought stressed conditions (50% water holding capacity for 2 weeks and then 25% water holding capacity for 2 weeks) exhibited no significant differences, and it was undetected in plants inoculated without AMF (Ruíz-Sánchez et al. 2011). In contrast, AMF root colonization in rice cv. INCA LP-5 was significantly improved by reducing the rate of water supply to 5–10 mL per day from 25 mL per day (Ruíz-Sánchez et al. 2010). Moreover, total phosphorus in the roots of AMF inoculated plants cv. Hom Nil at booting stage was higher than in the flag leaf tissues. In japonica rice cv. Nipponbare, total phosphorus concentration in AMF-inoculated plants at maturing stage (140/150 DAS; days after sowing) was accumulated in un-hulled grain (3.3 g kg−1), followed by root (1.7 g kg−1) and shoot (1.3 g kg−1) (Solaiman and Hirata 1995). In contrast, phosphorus concentration in shoots of rice at tillering stage (50 DAS) was greater than that in roots (Liu et al. 2013). The uptake and accumulation rate of phosphorus in different organs of rice crop may depend on variety, soil types, developmental stages and water regime situation (Hajiboland et al. 2009; Zhang et al. 2016). The phosphorus concentration in aerial parts of date palm plants after 9 months of AMF-inoculation was declined when subjected to 25% field capacity (FC) in relation to low frequency of colonization (Meddich et al. 2015). In maize, phosphorus in the shoot of AMF-inoculated plants (14.55 mg pot−1) was decreased, in relation to the degree of drought condition [moderate drought; 60% FC (11.63 mg pot−1) and severe drought; 40% FC (9.32 mg pot−1)] (Zhao et al. 2015). In Pistachio cv. Akbari, total phosphorus concentration in AMF-inoculated plants under well irrigation was peaked at 20.3 mg g−1 DW and declined by 36.31% under drought stressed conditions (12.93 mg g−1 DW), and it corresponded to % colonization along root (Abbaspour et al. 2012). In addition, phosphorus uptake rate in rice crop cv. Shafagh was promoted by non-flooded water regime, lack of phosphorus fertilizer and inoculation of highly efficient AMF strains (G. mosseae and G. intraradices), and it correlated to root biomass and AMF colonization (R2 = 0.56) (Hajiboland et al. 2009). Likewise, a positive relation was observed between AMF root colonization and phosphorus uptake (R2 = 0.545) in upland rice cv. Vandana (Maiti et al. 2013).
Under severe drought condition, shoot height of AMF-inoculated marigold plants were declined by 37.71%, whereas it was sharply decreased by 42.28% in plants without AMF-inoculation (Asrar and Elhindi 2011). Plant height and number of leaves in trifoliate orange were promoted by AMF inoculation using Funneliformis mosseae and Paraglomus occultum over the control (un-inoculated), and subsequently declined under drought stress (50% water holding capacity for 71 days) (Wu et al. 2017). In Roselle, shoot height and lateral branches in plants without AMF inoculation was significantly inhibited by 25.28% and 54.24%, respectively, when subjected to 200 mm pan evaporation, whereas it was sustained in G. versiforme (only 14.45% and 13.54% reduction) and Rhizophagus irregularis (only 18.99% and 3.84% reduction) inoculated plants (Fallahi et al. 2016). In rice crop, overall growth performances including shoot height and root length in plants under water deficit conditions were maintained by AMF inoculation (Mary et al. 2018), leading to retain the yield attributes, especially in the late season of paddy filed (Okonji et al. 2018).
Total soluble sugar, sucrose and glucose in AMF-inoculated plants under WD were enriched over the control. In Poincianella pyramidalis, soluble sugar, sucrose and fructose in AMF-inoculated plants under water deficit stress (12 days) in greenhouse conditions were significantly accumulated over the un-inoculated control plants (Frosi et al. 2016). Carbohydrates, i.e. sucrose, glucose and fructose, in trifoliate orange under well watering were significantly enhanced by AMF inoculation (Funneliformis mosseae and Paraglomus occullum) and dropped when subjected to drought stress (Wu et al. 2017). Total soluble sugar in the outer leaves of AMF-inoculated lettuce tends to increase, in relation to the degree of field capacity reduction (2/3 and 1/2 FC) (Baslam and Goicoechea 2012). Likewise, total soluble sugars in the inner leaves of AMF-inoculated commercial lettuce cvs. Betavia Rubia Munguía and Maravilla de Verano were peaked when compared to the plants without inoculation (Baslam et al. 2011). In Pistachio, accumulation of soluble sugars in drought stressed plants was greater than that of well-watered condition by 1.46 and 1.28 folds in AMF inoculated and un-inoculated plants, respectively (Abbaspour et al. 2012). Also, the free proline content in rice crop under WD was significantly enhanced irrespective of the AMF treatment. Shoot proline content in AMF-inoculated rice cv. INCA LP-5 under drought conditions was significantly increased over well-watered conditions (Ruíz-Sánchez et al. 2011). Similarly, proline content in AMF-inoculated plants of maize cv. Potro under drought stress was enriched over the control (well watering) (Bárzana et al. 2015). In tomato cv. San Marzano nano, proline accumulation may maintain leaf water potential, depending on AMF species (Rhizophagus intraradices < F. mosseae) and water stress conditions (Chitarra et al. 2016). Flag leaf osmotic potential was declined when exposed to drought conditions, especially in case of plants without AMF inoculation. Similarly, the water potential in the leaf of maize and tomato grown under drought stress was significantly declined when compared with those plants under well-watered conditions, and it was strongly improved in maize plants using AMF-inoculation (Glomus intrarradices strain EEX 58) (Bárzana et al. 2012). In endemic conifer (Cupressus atlantica), needle leaf water potential in mild- (50% field capacity) and severe-drought stresses (25% field capacity) was significantly improved by AMF-inoculation (mixture of Rhizophagus manihotis, R. aggregatus, R. fasciculatus and Acaulospora sp.) (Zarik et al. 2016). In addition, leaf water potential of perennial ryegrass (Lolium perenne) under drought condition for 28d was considerably upgraded by AMF-inoculation (Glomus intraradices) (Lee et al. 2012). In rice cv. INCA LP-5, shoot water potential in AMF-inoculated plants (only Glomus intraradices isolate EEZ 01) under drought conditions was declined in relation to the shoot proline enrichment (major osmotic adjustment), whereas it was unchanged in Azospirillum brasilense strain AZ-39 inoculated plants as well as uninoculated plants (Ruíz-Sánchez et al. 2011). In contrast, proline enrichment in AMF-inoculated maize plants under drought stress was unrelated to leaf water potential reduction (Bárzana et al. 2015).
Photosynthetic pigments, Chla, TC and Cx+c in flag leaf tissues of AMF inoculated rice plants under WW were stimulated by 1.69, 1.35 and 1.85 folds over AMF un-inoculated plants, respectively. Chla, Chlb and total flavonoids in AMF-colonized (Funneliformis mosseae) Bhringraj (Eclipta prostrata) were accumulated in high levels over un-inoculated plants and the concentration was maintained when exposed to water stress (7 days-irrigation interval) (Sinha and Raghuwanshi 2016). TC (Chla + Chlb) and Cx+c in inner leaves of lettuce cvs. Batavia Munguía and Maravilla de Verano under well-watered conditions were improved by AMF-inoculation (mixture of Glomus intraradices and G. mosseae) as well as retained when subjected to water deficit conditions at WD (50% field capacity) (Baslam and Goicoechea 2012). In basil plant, TC (Chla + Chlb) in the leaf tissues of AMF un-inoculated plants were sharply degraded when exposed to water stress, whereas it was maintained upon AMF-inoculation (Hazzoumi et al. 2015). In Poincianella pyramidalis, Chla, Chlb and Cx+c in young leaves of AMF-inoculated plants (mixture of Acaulospora longula strain URM FMA 07 and Claroideoglomus etunicatum strain URM FMA 03) were increased over those in un-inoculated plants, thereby strengthening the plants to withstand in the water deficit conditions (Frosi et al. 2016). TC and flavonoids in Pistachio seedlings pretreated by AMF (G. etunicatum) were enhanced and maintained at high levels even when subjected to drought stress (Abbaspour et al. 2012). Moreover, Cx+c in both outer and inner leaves of lettuce cvs. Batavia Rubia Munguía (BRM) and Maravilla de Verano (MV) under AMF inoculation (G. fasciculatum and commercial inoculum) was enriched over un-inoculated plants (Baslam et al. 2011). The photosynthetic efficiency (Fv/Fm) in AMF-inoculated plants of rice (Ruíz-Sánchez et al. 2010), wheat (Mathur et al. 2018) and tomato (Bárzana et al. 2012) was unaffected over un-inoculated plants under water limited situations. CO2 assimilation rate (Pn), transpiration rate (E) and stomatal conductance (gs) are good indicators of stomatal closure under water deficit conditions. In rice cv. INCA LP-5, gs in AMF inoculated plants (G. intraradices) was higher than un-inoculated plants and tend to decline in relation to the degree of drought stress (Ruíz-Sánchez et al. 2010). Similarly, gs in AMF-inoculated tomato plants was peaked over un-inoculated plants and tend to decrease when subjected to drought stress (Bárzana et al. 2012). Pn in AMF-colonized perennial ryegrass during water withholding period (28 days) was maintained over plants without AMF inoculation (Lee et al. 2012). Moreover, Pn in AMF-inoculated (Rhizophagus intraradices) tomato plants under well-watered conditions was improved over un-inoculated plants but was significantly reduced under water stress (Chitarra et al. 2016).
Rice yield traits i.e. grain yield, panicle dry weight, panicle length, fertility percentage and 100-grain weight, were strongly improved by AMF-inoculation, especially in the water deficit conditions. In wetland rice, grain yield of AMF-inoculated plants increased over un-inoculated plants, especially under low nitrogen supplies (Zhang et al. 2014). In Nipponbare japonica rice, the grain yield in pot culture (53.7 g pot−1) and field trial (8.49 t ha−1) was maximized under dry nursery with AMF-inoculation (Solaiman and Hirata 1997). Grain yield of winter wheat cv. Steardy in AMF-inoculated (Glomus mosseae) plants grown under water deficit stress was highly improved (with only 57.49% reduction to that of well-watering) (Al-Karaki et al. 2004). Biological yield and economic yield (kg ha−1) of AMF inoculated (Glomus versiforme) Roselle plants was retained over control when subjected to deficit irrigation (Fallahi et al. 2016). Flower fresh weight, dry weight and diameter in AMF-inoculated marigold plants under moderate drought conditions were maintained (Asrar and Elhindi 2011). Anthocyanins, C3G and P3G, in pericarp of rice grain, harvested during WD were enriched over well watering and these were regulated by AMF-inoculation. This is the first report suggesting the regulation of anthocyanins species in the pericarp of rice grain cv. Hom Nil using AMF colonization under water deficit conditions. Previously, we reported the regulation of P3G in dark purple pericarp of rice cv. Hom Nil using Mg spray and low temperature incubation (Tisarum et al. 2018). In lettuce cvs. CT (Cogollos de Tudela), BRM and MV, total anthocyanins in both inner and outer leaves of AMF-inoculated plants were enriched over the control (without AMF) (Baslam et al. 2011), and these were further promoted by water deficit conditions (Baslam and Goicoechea 2012). In addition, anthocyanins in AMF-inoculated pomegranate shoots were accumulated in high levels and retained even when subjected to 23% field capacity (Bompadre et al. 2015).
Conclusion
In conclusion, root colonization by AMF in rice cv. Hom Nil regulated the phosphorus availability and enrichment in host plants, leading to improved plant growth and development, particularly in the reproductive stages. It also enhanced the plant defense against drought condition, as exhibited by greater accumulation of free proline and maintenance of other physiological characters. Overall yield attributes in AMF-inoculated plants were also improved in addition to the anthocyanin species, C3G and P3G, in the pericarp of rice grain.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The research was funded by The Thailand Research Fund (TRF) for funding support (Grant Number SRI5920201) and National Science and Technology Development Agency (NSTDA) for partial support to CT as post-doctoral scholarship.
Abbreviations
- AMF
Arbuscular mycorrhizal fungi
- C3G
Cyanidin-3-glucoside
- E
Transpiration rate
- Gs
Stomatal conductance
- Fv/Fm
Maximum quantum yield of PSII
- ΦPSII
Photon yield of PSII
- Pn
Net photosynthetic rate
- P3G
Peonidin-3-glucoside
- WD
Water deficit
Authors contribution
RT conducted the research project, report to funding agency, anthocyanin assay, analyzed the data and wrote a first draft of manuscript, CT and TS performed the experiment layout, free proline assay, sugar analysis and osmotic potential assay, MP consulted on the AMF colonization and phosphorus assay, HPS conducted critical revision of the data and performed the experiment as well as grammatical proofing, and SC performed the secondary data analysis and finished the final version of manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbaspour H, Saeidi-Sar S, Afshari H, Abdel-Wahhab MA. Tolerance of mycorrhiza infected Pistachio (Pistacia vera L.) seedling to drought stress under glasshouse conditions. J Plant Physiol. 2012;169:704–709. doi: 10.1016/j.jplph.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Adhikari RK. Economics of organic rice production. J Agric Environ. 2011;12:97–103. doi: 10.3126/aej.v12i0.7569. [DOI] [Google Scholar]
- Al-Karaki G, McMichael B, Zak J. Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza. 2004;14:263–269. doi: 10.1007/s00572-003-0265-2. [DOI] [PubMed] [Google Scholar]
- Asrar AW, Elhindi KM. Alleviation of drought stress of marigold (Tagetes erecta) plants by using arbuscular mycorrhizal fungi. Saudi J Biol Sci. 2011;18:93–98. doi: 10.1016/j.sjbs.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacenetti J, Fusi A, Negri M, Bocchi S, Fiala M. Organic production systems: sustainability assessment of rice in Italy. Agric Ecosyst Environ. 2016;225:33–44. doi: 10.1016/j.agee.2016.03.046. [DOI] [Google Scholar]
- Bárzana G, Aroca R, Paz KA, Chaumont F, Martinez-Ballesta MC, Carvajal M, Ruiz-Lozano JM. Arbuscular mycorrhizal symbiosis increases relative apoplastic water flow in roots of the host plant under both well-watered and drought stress conditions. Ann Bot. 2012;109:1009–1017. doi: 10.1093/aob/mcs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárzana G, Aroca R, Ruiz-Lozano JM. Localized and non-localized effects of arbuscular mycorrhizal symbiosis on accumulation of osmolytes and aquaporins and on antioxidant systems in maize plants subjected to total or partial root drying. Plant, Cell Environ. 2015;38:1613–1627. doi: 10.1111/pce.12507. [DOI] [PubMed] [Google Scholar]
- Baslam M, Goicoechea N. Water deficit improved the capacity of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of antioxidant compounds in lettuce leaves. Mycorrhiza. 2012;22:347–359. doi: 10.1007/s00572-011-0408-9. [DOI] [PubMed] [Google Scholar]
- Baslam M, Garmendia I, Goicoechea N. Arbuscular mycorrhizal fungi (AMF) improved growth and nutritional quality of greenhouse-grown lettuce. J Agric Food Chem. 2011;59:5504–5515. doi: 10.1021/jf200501c. [DOI] [PubMed] [Google Scholar]
- Basu S, Rabara RC, Negi S. AMF: the future prospect for sustainable agriculture. Physiol Mol Plant Pathol. 2018;102:36–45. doi: 10.1016/j.pmpp.2017.11.007. [DOI] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bompadre MJ, Bidondo LF, Silvani VA, Colombo RP, Pérgola M, Pardo AG, Godeas AM. Combined effects of arbuscular mycorrhizal fungi and exogenous cytokinins on pomegranate (Punica granatum) under contrasting water availability conditions. Symbiosis. 2015;65:55–63. doi: 10.1007/s13199-015-0318-2. [DOI] [Google Scholar]
- Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N. Working with mycorrhizas in forestry and agriculture. Canberra: Australian Centre for International Agricultural Research; 1996. p. 373. [Google Scholar]
- Cattivelli L, Rizza F, Badeck FW, Mazzucotelli E, Mastrangelo AM, Francia E, Maré C, Tondelli A, Stanca AM. Drought tolerance improvement in crop plants: an integrated view from breeding to genomics. Field Crop Res. 2008;105:1–14. doi: 10.1016/j.fcr.2007.07.004. [DOI] [Google Scholar]
- Chandra A, Rana J, Li Y. Separation, identification, quantification, and method validation of anthocyanins in botanical supplement raw materials by HPLC and HPLC − MS. J Agric Food Chem. 2001;49:3515–3521. doi: 10.1021/jf010389p. [DOI] [PubMed] [Google Scholar]
- Cha-um S, Supaibulwatana K, Kirdmanee C. Glycinebetaine accumulation, physiological characterizations and growth efficiency in salt-tolerant and salt-sensitive lines of indica rice (Oryza sativa L. ssp. indica) in response to salt stress. J Agron Crop Sci. 2007;193:157–166. doi: 10.1111/j.1439-037X.2007.00251.x. [DOI] [Google Scholar]
- Chitarra W, Pagliarani C, Maserti B, Lumini E, Siciliano I, Cascone P, Schubert A, Gambino G, Balestrini R, Guerrieri E. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 2016;171:1009–1023. doi: 10.1104/pp.16.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiponmak W, Senekun C, Siriamornpun S. Antiglycation capacity and antioxidant activities of different pigmented Thai rice. Int J Food Sci Technol. 2014;49:1805–1810. doi: 10.1111/ijfs.12487. [DOI] [Google Scholar]
- Fallahi HR, Ghorbany M, Samadzadeh A, Aghhavani-Shajari M, Asadian AH. Influence of arbuscular mycorrhizal inoculation and humic acid application on growth and yield of Roselle (Hibiscus sabdariffa L.) and its mycorrhizal colonization index under deficit irrigation. Int J Hortic Sci Technol. 2016;3:113–128. [Google Scholar]
- Frosi G, Barros VA, Oliveira MT, Santos M, Ramos DG, Maia LC, Santos MG. Symbiosis with AMF and leaf Pi supply increases water deficit tolerance of wood species from seasonal dry tropical forest. J Plant Physiol. 2016;207:84–93. doi: 10.1016/j.jplph.2016.11.002. [DOI] [PubMed] [Google Scholar]
- García-Garrido JM, Ocampo JA. Regulation of the plant defense response in arbuscular mycorrhizal symbiosis. J Exp Bot. 2002;53:1377–1386. doi: 10.1093/jexbot/53.373.1377. [DOI] [PubMed] [Google Scholar]
- Garg N, Chandel S. Arbuscular mycorrhizal networks: process and functions. A review. Agron Sustain Dev. 2010;30:581–599. doi: 10.1051/agro/2009054. [DOI] [Google Scholar]
- Gosling P, Hodge A, Goodlass G, Bending GD. Arbuscular mycorrhizal fungi and organic farming. Agric Ecosyst Environ. 2006;113:17–35. doi: 10.1016/j.agee.2005.09.009. [DOI] [Google Scholar]
- Hajiboland R, Aliasgharzad N, Barzeghar R. Phosphorus mobilization and uptake in mycorrhizal rice (Oryza sativa L.) plants under flooded and non-flooded conditions. Acta Agric Slovenic. 2009;93:153–161. [Google Scholar]
- Haldar S, Sengupta S. Plant-microbe crosstalk in the rhizosphere: insight and biotechnological potential. Open Microbiol J. 2015;9:1–7. doi: 10.2174/1874285801509010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart MR, Quin BF, Nguyen ML. Phosphorus runoff from agricultural land and direct fertilizer effects: a review. J Environ Qual. 2004;33:1954–1973. doi: 10.2134/jeq2004.1954. [DOI] [PubMed] [Google Scholar]
- Hause B, Fester T. Molecular and cell biology of arbuscular mycorrhizal symbiosis. Planta. 2005;221:184–196. doi: 10.1007/s00425-004-1436-x. [DOI] [PubMed] [Google Scholar]
- Hazzoumi Z, Moustakime Y, Elharchli E, Joutei KA. Effect of arbuscular mycorrhizal fungi (AMF) and water stress on growth, phenolic compounds, glandular hairs, and yield of essential oil in basil (Ocimum gratissimum L) Chem Biol Technol Agric. 2015;2:10. doi: 10.1186/s40538-015-0035-3. [DOI] [Google Scholar]
- Hokazono S, Hayashi K. Variability in environmental impacts during conversion from conventional to organic farming: a comparison among three rice production systems in Japan. J Clean Prod. 2012;28:102–112. doi: 10.1016/j.jclepro.2011.12.005. [DOI] [Google Scholar]
- Hua L, Liu J, Zhai L, Xi B, Zhang F, Wang H, Liu H, Chen A, Fu B. Risks of phosphorus runoff losses from five Chinese paddy soils under conventional management practices. Agric Ecosyst Environ. 2017;245:112–123. doi: 10.1016/j.agee.2017.05.015. [DOI] [Google Scholar]
- Jackson ML. Soil chemical analysis. Englewood Cliffs: Prentice Hall; 1958. [Google Scholar]
- Jian L, Qiang Z, Li-mei L, Hong-bin L, Hong-yuan W, Shen L, Guo-yuan Z, Tian-zhi R. Phosphorus losses via surface runoff in rice-wheat cropping systems as impacted by rainfall regimes and fertilizer applications. J Integr Agric. 2016;15:667–677. doi: 10.1016/S2095-3119(15)61087-5. [DOI] [Google Scholar]
- Karkacier M, Ebras M, Uslu MK, Aksu M. Comparison of different extraction and detection methods for sugars using amino-bonded phase HPLC. J Chromatogr Sci. 2003;41:331–333. doi: 10.1093/chromsci/41.6.331. [DOI] [PubMed] [Google Scholar]
- Kubo M, Purevdorj M. The future of rice production and consumption. J Food Distrib Res. 2004;35:128–142. [Google Scholar]
- Lanfermeijer FC, Koerselman-Kooij JW, Borstlap AC. Osmosensitivity of sucrose uptake by immature pea cotyledons disappears during development. Plant Physiol. 1991;95:832–838. doi: 10.1104/pp.95.3.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Muneer S, Jung WJ, Avice JC, Ourry A, Kim TH. Mycorrhizal colonization alleviates drought-induced oxidative damage and lignification in the leaves of drought-stressed perennial ryegrass (Lolium perenne) Physiol Plant. 2012;145:440–449. doi: 10.1111/j.1399-3054.2012.01586.x. [DOI] [PubMed] [Google Scholar]
- Li T, Angeles O, Radanielson A, Iii MM, Manalo E. Drought stress impacts of climate change on rainfed rice in South Asia. Clim Change. 2015;133:709–720. doi: 10.1007/s10584-015-1487-y. [DOI] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol. 1987;148:350–380. doi: 10.1016/0076-6879(87)48036-1. [DOI] [Google Scholar]
- Ligua G, Bona E, Manassero F, Todeschini V, Cantemessa S, Copetta A, D’Agostino G, Gamalero E, Berta G. Pseudomonads increases anthocyanin concentration in strawberry fruits (Fragaria x annanassa var. Selva) in conditions of reduced fertilization. Int J Mol Sci. 2013;14:16207–16225. doi: 10.3390/ijms140816207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZL, Li YJ, Hou HY, Zhu XC, Rai V, He XY. Difference in the arbuscular mycorrhizal fungi-improved rice resistance to low temperature at two N levels: aspects of N and C metabolism on the plant side. Plant Physiol Biochem. 2013;71:87–95. doi: 10.1016/j.plaphy.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Loggini B, Scartazza A, Brugnoli E, Navari-Izzo F. Antioxidant defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiol. 1999;119:1091–1100. doi: 10.1104/pp.119.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti D, Singh CV, Variar M, Mandal NP, Anantha MS. Impact of rainfall pattern on native arbuscular-mycorrhizal activity influencing phosphorus utilization by direct seeded rainfed upland rice (Oryza sativa L.) Proc Natl Acad Sci. 2013;83:159–162. [Google Scholar]
- Martín J, Navas MJ, Jiménez-Moreno AM, Asuero AG. Anthocyanin pigments: Importance, sample preparation and extraction. In: Soto-Hernández M, editor. Phenolic compounds. Rijeka: Intech Open; 2017. pp. 117–152. [Google Scholar]
- Mary KJ, Marimuthu P, Kumutha K, Sivakumar U. Seed priming of arbuscular mycorrhizal fungi against induced drought in rice. J Pharmacogn Phystochem. 2018;7:1742–1746. [Google Scholar]
- Mathur S, Tomar RS, Jajoo A. Arbuscular mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynt Res. 2018 doi: 10.1007/s11120-018-0538-4. [DOI] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence-a practical guide. J Exp Bot. 2000;51:659–668. doi: 10.1093/jexbot/51.345.659. [DOI] [PubMed] [Google Scholar]
- Mbodj D, Effa-Effa B, Kane A, Manneh B, Gantet P, Lapaze L, Diedhiou AG, Grondin A. Arbuscular mycorrhizal symbiosis in rice: establishment, environmental control and impact on plant growth and resistance to abiotic stresses. Rhizophere. 2018;8:12–26. doi: 10.1016/j.rhisph.2018.08.003. [DOI] [Google Scholar]
- Meddich A, Jaiti F, Bourzik W, Asli AE, Hafidi M. Use of mycorrhizal fungi as a strategy for improving the drought tolerance in date palm (Phoenix dactylifera) Sci Hortic. 2015;192:468–474. doi: 10.1016/j.scienta.2015.06.024. [DOI] [Google Scholar]
- Muthayya S, Sugimoto JD, Montgomery S, Maberly GF. An overview of global rice production, supply, trade, and consumption. Ann NY Acad Sci. 2014;1324:7–14. doi: 10.1111/nyas.12540. [DOI] [PubMed] [Google Scholar]
- Nirmala B, Muthuraman P. Economics and constraint analysis of rice cultivation in Kaithal District of Haryanan. Indian Res J Ext Educ. 2009;9:47–49. [Google Scholar]
- Okonji CJ, Sakariyawo OS, Okeleye KA, Osunbiyi AG, Ajayi EO. Effects of arbuscular mycchorhizal fungi inoculation on soil properties and yield of selected rice varieties. J Agric Sci. 2018;63:153–170. [Google Scholar]
- Pandey V, Shukla A. Acclimation and tolerance strategies of rice under drought stress. Rice Sci. 2015;22:147–161. doi: 10.1016/j.rsci.2015.04.001. [DOI] [Google Scholar]
- Panneerselvam P, Kumar U, Sugitha TCK, Parameswaran C, Sahoo S, Binodh AK, Jahan A, Anandan A, et al. Arbuscular mycorrhizal fungi (AMF) for sustainable rice production. In: Adhya T, et al., editors. Advances in soil microbiology: recent trends and future prospects, microorganisms for sustainable. Singapore: Springer; 2017. pp. 99–126. [Google Scholar]
- Peng S, Tang Q, Zou Y. Current status and challenges of rice production in China. Plant Prod Sci. 2009;12:3–8. doi: 10.1626/pps.12.3. [DOI] [Google Scholar]
- Pitija K, Nakornriab M, Sriseadka T, Vanavichit A, Wongpornchai S. Anthocyanin content and antioxidant capacity in bran extracts of some Thai black rice varieties. Int J Food Sci Technol. 2013;48:300–308. doi: 10.1111/j.1365-2621.2012.03187.x. [DOI] [Google Scholar]
- Reganold JP, Wachter JM. Organic agriculture in the twenty-first century. Nat Plants. 2016;2:1–5. doi: 10.1038/nplants.2015.221. [DOI] [PubMed] [Google Scholar]
- Ruíz-Sánchez M, Arora R, Muñoz Y, Polón R, Ruiz-Lozano JM. The arbuscular mycorrhizal symbiosis enhances the photosynthetic efficiency and the antioxidative response of rice plants subjected to drought stress. J Plant Physiol. 2010;167:862–869. doi: 10.1016/j.jplph.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Ruíz-Sánchez M, Armada E, Muñoz Y, de Salamone IEG, Arora R, Ruiz-Lozano JM, Azcón R. Azospirillum and arbuscular mycorrhizal colonization enhance rice growth and physiological traits under well-watered and drought conditions. J Plant Physiol. 2011;168:1031–1037. doi: 10.1016/j.jplph.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Ryu SN, Park SZ, Ho CT. High performance liquid chromatographic determination of anthocyanin pigments in some varieties of black rice. J Food Drug Anal. 1998;6:729–736. [Google Scholar]
- Sadabpod K, Kangsadalampai K, Tongyonk L. Antioxidant activity and antimutagenicity of Hom Nil rice and black glutenous rice. J Health Res. 2010;24:49–54. [Google Scholar]
- Serraj R, McNally KL, Slamet-Loedin I, Kohli A, Haefele SM, Atlin G, Kumar A. Drought resistance improvement in rice: an integrated genetic and resource management strategy. Plant Prod Sci. 2011;14:1–14. doi: 10.1626/pps.14.1. [DOI] [Google Scholar]
- Shabala SN, Shabala SI, Martynenko AI, Babourina O, Newman IA. Salinity effect on bioelectric activity growth, Na+ accumulation and chlorophyll fluorescence of maize leaves: a comparative survey and prospects for screening. Aust J Plant Physiol. 1998;25:609–616. [Google Scholar]
- Silva S, Costa EM, Calhau C, Morais RM, Pintado D. Anthocyanin extraction from plant tissues: a review. Crit Rev Food Sci. 2017;57:3072–3083. doi: 10.1080/10408398.2015.1087963. [DOI] [PubMed] [Google Scholar]
- Sinha S, Raghuwanshi R. Synergistic effect of arbuscular mycorrhizal fungi and mycorrhizal helper bacteria on physiological mechanism to tolerance of drought in Eclipta prostrata (L.) L. J Pure Appl Microbiol. 2016;10:1117–1130. [Google Scholar]
- Solaiman MZ, Hirata H. Effects of indigenous arbuscular mycorrhizal fungi in paddy field on rice growth and N, P, K nutrition under different water regimes. Soil Sci Plant Nutr. 1995;41:505–514. doi: 10.1080/00380768.1995.10419612. [DOI] [Google Scholar]
- Solaiman MZ, Hirata H. Effect of arbuscular mycorrhizal fungi inoculation of rice seedlings at the nursery stage upon performance in the paddy field and greenhouse. Plant Soil. 1997;191:1–12. doi: 10.1023/A:1004238028617. [DOI] [Google Scholar]
- Sompong R, Siebenhandl-Ehn S, Linsberger-Martin G, Berghofer E. Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chem. 2011;124:132–140. doi: 10.1016/j.foodchem.2010.05.115. [DOI] [Google Scholar]
- Sutharut J, Sudarat J. Total anthocyanin content and antioxidant activity of germinated colored rice. Int Food Res J. 2012;19:215–221. [Google Scholar]
- Tao H, Brueck H, Dittert K, Kreye C, Lin S, Sattelmacher B. Growth and yield formation of rice (Oryza sativa L.) in the water-saving ground cover rice production system (GCRPS) Field Crop Res. 2006;95:1–12. doi: 10.1016/j.fcr.2005.01.019. [DOI] [Google Scholar]
- Thiranusornkij L, Thamnarathip P, Chandrachai A, Kuakpetoon D, Adisakwattana S. Physicochemical properties of Hom Nil (Oryza sativa) rice flour as gluten free ingredient in bread. Foods. 2018;7:159. doi: 10.3390/foods7100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418:671–677. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- Tisarum R, Theerawitaya C, Samphumphuang T, Cha-um S. Regulation of anthocyanin accumulation in rice (Oryza sativa L. subsp. indica) using MgSO4 spraying and low temperature. Arch Agron Soil Sci. 2018;64:1663–1677. doi: 10.1080/03650340.2018.1450501. [DOI] [Google Scholar]
- Tuong TP, Bouman BAM. Rice production in water-scarce environments. In: Kijne JW, Barker R, Molden D, editors. Water productivity in agriculture: limits and opportunities for improvement. Wallingford: CAB International; 2003. [Google Scholar]
- Tuong TP, Bouman BAM, Mortimer M. More rice, less water–integrated approaches for increasing water productivity in irrigated rice-based systems in Asia. Plant Prod Sci. 2005;8:231–241. doi: 10.1626/pps.8.231. [DOI] [Google Scholar]
- Wassman R, Jagadish SVK, Sumfleth K, Pathak H, Howell G, Ismail A, Serraj R, Redona E, Singh PK, Heuer S. Regional vulnerability of climate change impacts on Asian rice production and scope for adaptation. Adv Agron. 2009;102:91–133. doi: 10.1016/S0065-2113(09)01003-7. [DOI] [Google Scholar]
- Wongsa P, Landberg R, Rattanapanone N. Chemical compositions and metabolite profiling of rice varieties from Chiang Rai province Thailand. Chiang Mai J Sci. 2018;45:2703–2714. [Google Scholar]
- Wu HH, Zou YN, Rahman MM, Ni QD, Wu QS. Mycorrhizas alter sucrose and proline metabolism in trifoliate orange exposed to drought stress. Sci Rep. 2017;7:42389. doi: 10.1038/srep42389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarik L, Meddich A, Hijri M, Hafidi M, Ouhammou A, Ouahmane L, Duponnois R, Boumezzough A. Use of arbuscular mycorrhizal fungi to improve the drought tolerance of Cupressus atlantica G. C R Biol. 2016;339:185–196. doi: 10.1016/j.crvi.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang L, Ma F, Bloomfield KJ, Yang J, Atkin OK. Is resource allocation and grain yield of rice altered by inoculation with arbuscular mycorrhizal fungi? J Plant Ecol. 2014;8:436–448. doi: 10.1093/jpe/rtu025. [DOI] [Google Scholar]
- Zhang S, Wang L, Ma F, Zhang X, Fu D. Arbuscular mycorrhiza improved phosphorus efficiency in paddy fields. Ecol Eng. 2016;95:64–72. doi: 10.1016/j.ecoleng.2016.06.029. [DOI] [Google Scholar]
- Zhao R, Guo W, Bi N, Guo J, Wang L, Zhao J, Zhang J. Arbuscular mycorrhizal fungi affect the growth, nutrient uptake and water status of maize (Zea mays L.) grown in two types of coal mine spoils under drought stress. Appl Soil Ecol. 2015;88:41–49. doi: 10.1016/j.apsoil.2014.11.016. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.