Abstract
Manifestation of male sterility in plants is an important requirement for hybrid seed production. Tapetum cell layer of anther is a primary target for genetic manipulation for male sterility. In our previous report, the targeted expression of Arachis cysteine protease in tapetum led to premature degeneration of tapetal layer that resulted in complete male sterility in transgenic tobacco plants. To correlate cysteine protease mediated cell death of tapetum, transmission electron microscopy (TEM) and proteomic pattern of anthers of cysteine protease induced male sterile plant were compared with the untransformed control plant. TEM study revealed the abnormal growth of tapetal cells exhibiting excessive vacuolization that synchronized with irregular exine wall formation of the microspores. In anther proteome, a total 250 protein spots were detected that were reproducible and exhibited similar distribution pattern. Further, anther proteome of male sterile plant showed the significant upregulation (≥ 1.5) of 56 protein spots. Using Mass spectroscopy (MALDI TOF/TOF), we have identified 14 protein spots that were involved in several processes such as energy metabolism, protein synthesis, plastid protein, lipid metabolism, and cell wall assembly. Upregulation of patatin-like protein-2 homolog, carboxylesterase 17 and dicer like protein-4 in male sterile anthers that have been demonstrated to induce cell death, suggesting that cysteine protease mediated premature tapetal cell death might involve the lipid peroxidation pathway in coordination with gene silencing mechanism.
Keywords: Cysteine protease, Anther proteome, Cell death, Male sterility, Patatin, Dicer like protein
Introduction
Genetic engineering approach for manipulating male sterility systems has immense potential as the required breeding lines can be genetically transformed to develop the male sterile lines in the most straight forward way. The manifestation of male sterility in plants is an important prerequisite for the hybrid seed production. In last two decades, several approaches have been tried for the development of male sterile lines (Mariani et al. 1990; Nizampatnam et al. 2009; Rao et al. 2017, 2018; Shukla et al. 2017; Singh et al. 2010). In our previous report, we have also reported that the targeted expression of cysteine protease in tapetum tissue resulted in the production of transgenic tobacoo male sterile plants (Shukla et al. 2014). And subsequently, male fertility of cysteine protease induced male sterile female plants was restored using co-expression of cystatin, a well-known cysteine protease inhibitor, in same male sterile transgenic plants (Shukla et al. 2016). The proposed pollination control system can be an effective alternative to the barnase-barstar system and may face less regulatory issues for its deployment to fields as the genes used in this system are of plant origin. Both the genes viz. cysteine protease and cystatin were cloned and characterized from wild peanut genotype, Arachis diogoi. Cysteine protease and cystatin displayed differential expression in wild peanut in response to fungal pathogen Phaeoisariopsis personata that causes late leaf spot disease in peanut (Kumar and Kirti 2011, 2015).
Further, many reports documented the involvement of the cysteine protease in programmed cell death. However, the mechanism involved in cysteine protease-mediated cell death in tapetal tissue of the male sterile transgenic plants remained obscure. In addition, reports suggest that overexpression and silencing of cysteine protease in tapetum resulted in male sterility. This was argued that both the cases i.e. overexpression and silencing of cysteine protease disturbed the total protease expression that resulted in premature tapeum cell death (Lee et al. 2004; Zhang et al. 2009). Our earlier report also anticipated that the overexpression of peanut cysteine protease under the control of TA29 promoter in tapeum resulted in premature tapetum cell death that lead to the appearance of male sterility in tobacco transgenic plants (Shukla et al. 2014). Identification of proteins getting upregulated during early tapetal cell death in cysteine protease induced male sterile plant might provide a clue about the mechanism of cysteine protease mediated cell death.
Proteomic approach allows us to study the expression of total proteins profile at organ, tissue, cell and organelle levels under various conditions in an organism encoded by its genome (Anderson and Anderson 1998). The major advantage of proteomics over transcriptomics is that it deals with the actual expressed proteins rather than transcripts, which might not be translated into functional proteins always due to post-transcriptional and translational modifications, protein folding, stability and localization, protein–protein interactions. Therefore, proteomics related techniques provide a platform for identification of an array of proteins that play a crucial role in cysteine protease induced male sterility in transgenic plants.
In the present communication, the anthers from cysteine protease induced male sterile plants and untransformed control plants were used for a comparative study of TEM analysis and proteomic study in order to understand the mechanism of cysteine protease induced premature tapetal cell death in male sterile tobacco plants.
Materials and methods
Plant materials
Flower buds from control and cysteine protease induced male sterile transgenic tobacco were used to carry out the proteomic study. Flower buds of 3.0 mm to 1.0 cm size were collected and immediately frozen in liquid nitrogen. Samples were stored in a − 80 °C freezer until further use.
Transmission electron microscopy
The anther samples of stage 7 from male sterile and untransformed control plants were collected and fixed in 2.5% gluteraldehyde in 0.05 M phosphate buffer (pH 7.2) for 24 h at 4 °C and post-fixed in 2% aqueous osmium tetroxide for 2 h. Fixed anther samples were dehydrated in graded alcohol, infiltrated and embedded in Spurr’s resin (Spurr 1969). The ultrathin sectioning (50–70 nm thickness) was performed with a glass knife on a Leica Ultracut UCT-GA-D/E-1/100 ultra microtome. The sections were mounted on to copper grids, stained with saturated aqueous uranyl acetate and counterstained with 4% lead citrate and examined in an electron microscope (Hitachi H-7500) at RUSKA Labs, College of Veterinary Sciences, Hyderabad.
Protein extraction and two-dimensional electrophoresis (2-DE)
Anther protein extraction was done using a phenol extraction method (Saravanan and Rose 2004). Proteins were resuspended in 100 µL of the rehydration solution [8 M (w/v) urea, 2 M (w/v) thiourea, 4% (w/v) CHAPS, 30 mM DTT, 0.8% (v/v) IPG buffer pH range 4–7 (GE, Healthcare)] and the protein concentration was determined by the standard Bradford method. Proteins were resolved by two-dimensional electrophoresis (2-DE) as described earlier (Sengupta et al. 2011). It involves active rehydration of protein (450 µg) on immobilized pH gradient (IPG) strips (11 cm, 4–7 pH linear gradient; Amersham, GE) for 12 h at 50 V. Isoelectric focusing (IEF) was performed in Ettan IPGphor II (GE Healthcare). After IEF, the strips were equilibrated twice for 30 min with gentle rocking at room temperature in equilibration buffers (6 M urea, 50 mM Tris–HCl buffer (pH 8.8), 30% (w/v) glycerol, 2% (w/v) SDS. First equilibrium was performed in a solution containing equilibrium buffer with 2% DTT and second with 2.5% (w/v) iodoacetamide instead of DTT. Second dimension separation of proteins was done through SDS-PAGE (12% vertical polyacrylamide slab gels). The gels were stained with modified colloidal Coomassie Blue, scanned using a densitometric scanner (GE, Healthcare) and analyzed (normalization, spot matching, expression analyses, and statistics) using Image Master 2-D Platinum version 7 image analysis software (GE, Healthcare). Two-dimensional electrophoresis was performed in triplicate. Proteins that displayed one and a half fold or greater changes in the relative spot volume were considered as proteins with altered expression.
In-gel digestion and mass spectrometry (MS)
Interested protein spots were excised from three Coomassie-Blue stained replicated gels and in-gel trypsin digestion was performed as described earlier (Kumar and Kirti 2015). MALDI-TOF/TOF Mass Spectrometer matrix-assisted laser desorption/ionization time of flight mass spectrometric (MALDI-TOF MS) analysis was conducted with a MALDI-TOF/TOF Mass Spectrometer (Bruker Autoflex III smartbeam, Bruker Daltonics, Germany) according to the method described earlier (Shevchenko et al. 1996) with slight modifications.
Protein identification through peptide mass fingerprinting and MS/MS analysis
The data were analyzed by similarity search for mass values against sequence information from NCBInr and Swiss Prot database using a MASCOT program (http://www.matrixscience.com).
The MALDI-TOF/TOF data were loaded into the MASCOT program (http://www.matrixscience.com) employing Biotools software (Bruker Daltonics) and protein identification was performed against the NCBInr and Swiss-Prot databases using a combination of MS (peptide mass fingerprint approach) with MS/MS. The taxonomic category was set to Viridiplantae (Green plants). The other search parameters were: monoisotopic peptide mass (MH+); one missed cleavage per peptide; enzyme, trypsin; precursor-ion mass tolerance on an average 200 ppm; MS/MS fragment-ion mass tolerance, 0.6 Da; variable modifications like carbamidomethylation (C) for cysteine and oxidation for methionine (M) were allowed. If a protein spot matched multiple proteins under different accession numbers, the candidate protein with the maximum Mascot score was selected. The nearest experimental MW (molecular weight) and PI (isoelectric point) values to the theoretical values (having the same Mascot score) were given equal weightage in spot selection. The identified proteins were named according to the corresponding annotations in NCBI and Swiss-Prot.
RNA isolation and quantitative real time PCR
Total RNA was isolated from the anthers tissues of cysteine protease induced male sterile plants and untransformed control using Trizol reagent (Takara Bio, UK) as per the manufacturer’s instructions. First strand cDNA synthesis was performed using MMLV-Reverse transcriptase (TaKaRa, Japan) as per the manufacturer’s instructions. The cDNA was diluted to 2.5 times (1:2.5) for qRT-PCR experiments. Real-time PCR analysis was performed in Realplex PCR machine (Eppendorf, Germany) with the following cycle parameters: pre-incubation at 94 °C for 2 min following 40 cycles of denaturation at 94 °C for 30 s, 58 °C for 20 s, 72 °C for 30 s. Each reaction was repeated as three biological replicates including actin as an internal control for normalization. The relative fold change was determined using ΔΔCT method (Livak and Schmittgen 2001).
Results and discussion
Abnormal tapetal and pollen grains in cysteine protease induced male sterile plants
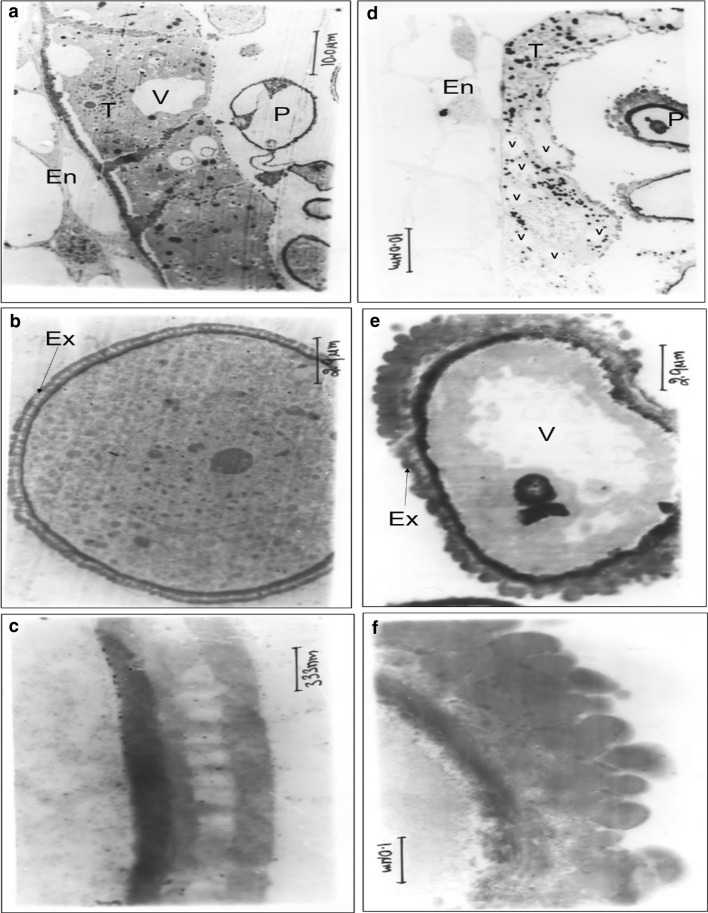
Transmission Electron Microscopy was performed to observe the tapetum development in synchronization with pollen development (Fig. 1). There was abnormal development of tapetum cell layer having increased vacuole size in male sterile plant compared to untransformed control plant. Similarly, primary structure of microspore exine was also irregular in male sterile plants compared to control plants. These observations demonstrated the occurrence of abnormal tapetal function in the male sterile tobacco transgenic plants. In line with the present results, overexpression of Brassica cysteine protease BoCysP1 exhibited swollen and excessively vacuolated tapetum, which subsequently collapsed (Konagaya et al. 2008).
Fig. 1.
Transmission electron micrographs of anther tapetum and microspore of untransfomed control plant (a, b, c) and cysteine protease induced male sterile plant (d, e, f). En endothecium, T tapetum, V vacuole, P Pollen, Ex exine
Proteomic analysis of cysteine protease induced male sterile transgenic tobacco
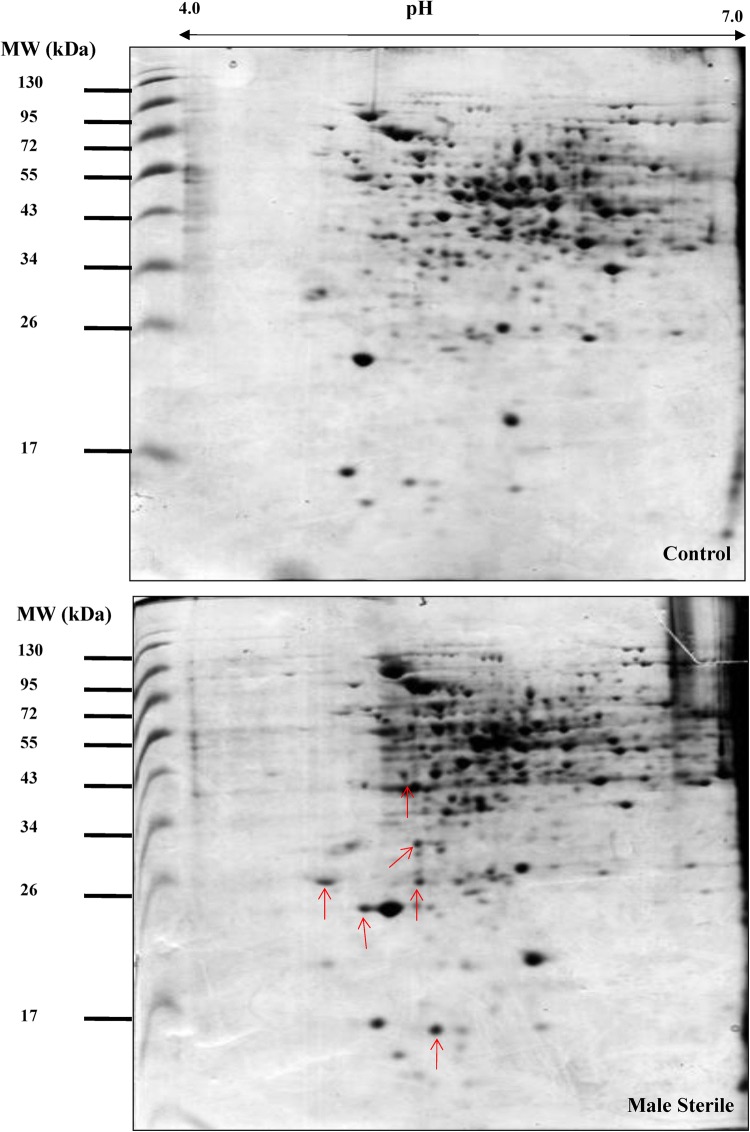
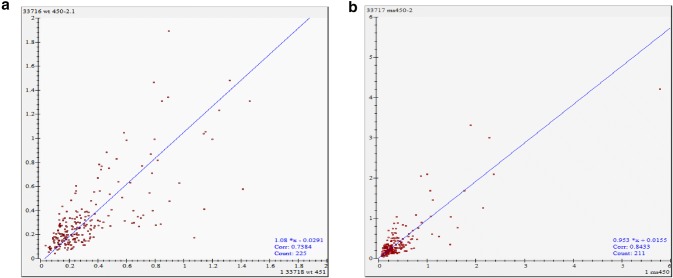
To determine the molecular mechanism of cysteine protease induced male sterility in transgenic plants, the proteome of anthers from transgenic male sterile plants and untransformed control plant were analyzed by two-dimensional electrophoresis followed by MALDI-TOF-TOF analysis (Fig. 2). Gel analyses using Image Master Platinum software suggested that more than 230 protein spots were reproducible and all gels showed similar distribution patterns in 2D images (Fig. 3). Anther proteome revealed that 56 matched spots showed significant up-regulation (≥ 1.5) (Fig. 4). We have identified 14 differentially upregulated proteins from male sterile anther proteome while rest of the spots displayed no significant hits in the database (Table 1). These proteins were related to energy metabolism such as ATP synthase subunit beta, mitochondrial and chloroplastic proteins, protein related to transcriptional and translational machinery like DNA-directed RNA polymerase subunit alpha and beta subunit, 40S ribosomal protein SA, chloroplastic protein Ycf2, protein related to anchoring and structuring of cellular events like coiled-coil domain-containing protein 103 homolog, stress inducible protein like low-temperature-induced 65 kDa protein, protein related to lipid metabolism such as probable carboxylesterase 17, patatin-like protein-2 homolog and dicer-like protein-4 of RNAi pathway. Patatin like proteins act as lipid acyl hydrolases and hydrolyze phospholipids and galactolipids (Scherer et al. 2010). Induction of tobacco Patatin-like protein genes (NtPat1-3) was reported in HR responses during tobacco mosaic virus infection (Dhondt et al. 2000). A pathogen induced patatin-like protein (PLP2) from Arabidopsis promoted cell death and negatively affected resistance against Botrytis cinerea and Pseudomonas syringae (La Camera et al. 2005). In contrast to this, it also conferred resistance against cucumber mosaic virus (CMV), which is an obligate pathogen (Camera et al. 2009). Patatin like protein expression was also reported in the anthers of potato, sweet pepper flowers and tobacco petal development (Drews et al. 1992; Vancanneyt et al. 1989). A previous report showed the up-regulation of NtPAT3 gene in a serine/arginine-rich splicing factor AdRSZ21 induced cell death on detached leaves in transient expression studies (Kumar and Kirti 2011). Further, Class 1 patatins with lipase activity act as good mobilizers of lipid reserve and the product of lipid reserve act as a fuel for pLOX mediated peroxidation pathway (May et al. 1998). For triggering the lipoxygenase signaling pathway, it is required that the reserve lipid must be mobilized to produce free linoleic (C18:2) and linolenic (C18:3) fatty acids. The necessity for lipoxygenase-mediated ROS production was previously shown to enhance plant defense by priming HR (Melan et al. 1993; Rancé et al. 1998), thereby preventing pathogen proliferation from the site of infection or accelerating cell death (Bolwell and Wojtaszek 1997).
Fig. 2.
Colloidal Coomassie stained 2D gels of protein Extract from anther of untransfomed control plant and cysteine protease induced male sterile plant. Arrow (→) indicates the protein spot getting upregulated
Fig. 3.
Scatter Plot of protein spots in 2D gels of anthers from untransformed control (a) and male sterile plants (b)
Fig. 4.
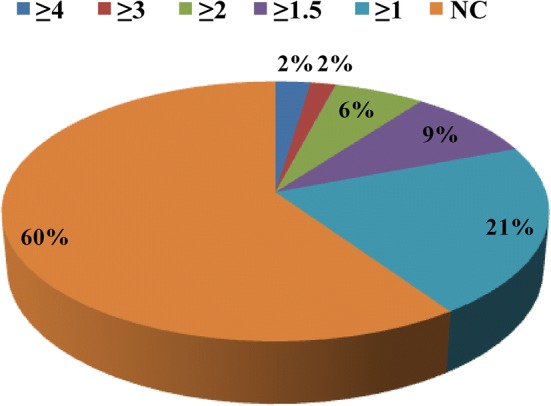
Protein expression profile pattern: fold change
Table 1.
Identification of differentially upregulated proteins by MALDI-TOF in cysteine protease male sterile anther proteome
| S. no. | Spot no. | Accession no. | Protein | Peptide sequence matched | Exp. Mr. (kD)/pI | Mascot score | No. of peptide match (NPM) | SC (%) | Related function |
|---|---|---|---|---|---|---|---|---|---|
| 1. | 1 | AAB08427 | Patatin homolog (Nicotiana tabacum) | DIVSFYFEHGPK | 28,265/5.07 | 65 | 2 | 14 | Plant defense signaling |
| GIIPATVLSFLESQLQELDNNEDAR | |||||||||
| 2. | 6 | Q3ZJ77 | DNA-directed RNA polymerase subunit alpha (Pseudendoclonium akinetum) | VQNSANPFNK | 66,769/9.64 | 40 | 1 (1) | 1 | RNA synthesis |
| 3. | 10 | Q04980; Q06737; Q42275; Q8RXF6; Q9FHC9 | Low-temperature-induced 65 kDa protein (Arabidopsis thaliana) | VTDESPDQK | 65,931/5.07 | 21 | 2 | 3 | Stress protein |
| TTATKEVEVTVEK | |||||||||
| 4. | 11 | P84634; Q3SA53 | Dicer-like protein 4 (Arabidopsis thaliana) | DIGSSLSLLPSIMHR | 193,837/6.3 | 10 | 2 | 2 | Gene silencing |
| LIGYNEDPIDVVDLVGLDVENLNILETFGGNSER | |||||||||
| 5. | 13 | Q94EY1 | Coiled-coil domain-containing protein 103 homolog (Chlamydomonas reinhardtii) | NMVLTAHLKPITAPK | 29,051/6.24 | 12 | 2 | 5 | Anchoring and structuring of cellular events |
| NMVLTAHLKPITAPK + Oxidation | |||||||||
| 6. | 14 | Q9SAA6 | Patatin homolog (Nicotiana tabacum) | GIIPATVLSFLESQLQELDNNEDAR | 28,265/5.07 | 80 | 2 | 14 | Plant defense signaling |
| IFPQGVWPPILGPK | |||||||||
| 7. | 39 | P17614 | ATP synthase subunit beta, mitochondrial (Nicotiana plumbaginifolia) | FDEGLPPILTALEVLDNQI | 59,933/5.95 | 62 | 2 | 6 | Energy metabolism |
| DAEGQDVLLFIDNIFR | |||||||||
| 8. | 45 | Q9SAA6 | Patatin homolog (Nicotiana tabacum) | NGYVKEAVEFQR | 28,265/5.07 | 249 | 3 | 19 | Plant defense signaling |
| SVSLVTFNIMINHLCKFGSYEKAK | |||||||||
| 9. | 77 | XP_009605841 | probable carboxylesterase 17 (Nicotiana tomentosiformis) | SIILSVDYR | 37,698/5.69 | 155 | 3 (1) | 17 | Plant-pathogen Interaction and HR-response |
| ADLSQVFLSGDSAGGNIVHQVAIR | |||||||||
| RVELVEAEGQVHVYHVFYPESEATR | |||||||||
| 10. | 86 | Q9ZSR8 | 40S ribosomal protein SA (Brassica napus) | VIVAIENPQDIIVQSARPYGQR | 32,238/5.14 | 47 | 3 | 16 | Protein synthesis |
| HTPGTFTNQMQTSFSEPR | |||||||||
| LLILTDPR | |||||||||
| 11. | 154 | P17614 | ATP synthase subunit beta, mitochondrial (Nicotiana plumbaginifolia) | FDEGLPPILTALEVLDNQI | 59,933/5.95 | 163 | 5 | 16 | Energy metabolism |
| IINVIGEAIDERGPITTDHFLPIHR | |||||||||
| VVDLLAPYQR | |||||||||
| DAEGQDVLLFIDNIFR | |||||||||
| IPSAVGYQPTLATDLGGLQER | |||||||||
| 12. | 169 | A8W3B5 | DNA-directed RNA polymerase subunit beta’ (Cuscuta exaltata) | NNNFSSMIDR | 80,398/9.09 | 35 | 2 | 3 | RNA synthesis |
| SSIPLFFTTQGFDTFR | |||||||||
| 13. | 210 | Q2MIC5 | Protein Ycf2 (Solanum bulbocastanum) | GVILFVVAVLIYR | 267,942/8.59 | 37 | 2 | 1 | Plastid protein |
| MNGLTVDMMPEIDR | |||||||||
| 14. | 229 | Q7YJW5 | ATP synthase subunit beta, chloroplastic (Calycanthus floridus var. glaucus) | AMNLEVESK | 53,665/5.28 | 30 | 2 | 5 | Energy metabolism |
| VALVHGQMNEPPGARMR |
Another important protein, Dicer-like protein 4 (DCL-4) was reported to act in the biogenesis of trans-acting small interfering RNAs (ta-siRNAs) (Dunoyer et al. 2005). Arabidopsis DCL-4 is reported to be involved in silencing of endogenous gene-nuclear-localized RNA binding protein (FCA). Because of this, DCL-4 promoted the expression of endogenous genes by contributing to the “tidying up” of 3′ end formation at genes where, for some reason, effective termination and polyadenylation have not occurred (Liu et al. 2012). It was reported that transcriptome analysis of NaDCL-4 silenced plant during plant–herbivore interaction (Nicotiana attenuate-Manduca sexta interaction) exhibited silencing of many genes including patatin-like protein (Ahmadovich Bozorov et al. 2012).
Carboxylesterase (EC 3.1.1.1) hydrolyzes the esters of short chain fatty acid and belong to a large family of genes (Marshall et al. 2003). Carboxylesterases role in PCD was well documented. For instance, the expression of a tobacco carboxylesterase gene, hsr203J was tightly associated with tissues undergoing programmed cell death (Marco et al. 1990). Both HR (mediated by the recognition of resistance gene encoded proteins by the avirulence proteins) and apoptosis (induced by heavy metals) resulted in increased expression of hsr203J (Pontier et al. 1998). A fungal inducible pepper carboxylesterase PepEST was shown to be involved in decomposition of fungal outer cell wall (Seo et al. 2018). Similarly, a grapevine HSR203J like protein, BIG8.1, displayed upregulation during Botrytis cinerea infection (Bézier et al. 2002). Further, PrMC3 was expressed in male cone of Pinus radiata and exhibited significant similarity with BIG8.1, Hsr203J including Arabidopsis carboxylesterases AtCXE6 and AtCXE17, thereby supporting a hypothesis for the involvement of these genes in cell death (Marshall et al. 2003; Walden et al. 1999). Increased activity of papain like cysteine proteases and Hsr203 was reported in tomato seedlings undergoing hypersensitive cell death suggesting that the cysteine protease and carboxylesterase act in a common pathway during PCD (Sueldo et al. 2014).
In addition, upregulation of proteins related to energy metabolism, transcriptional and translational pathway indicates that cell might require the energy for the synthesis of proteins related to PCD pathway.
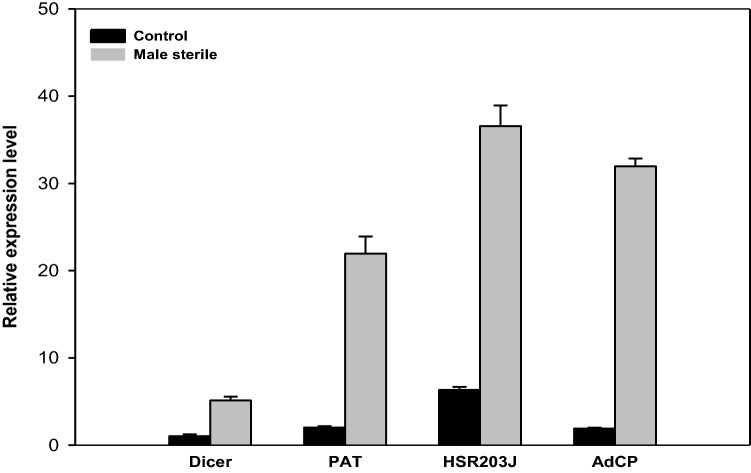
Quantitative qRT-PCR analysis for validation of proteomic results
In order to augment the proteomic results, qRT-PCR analysis was performed for selected genes like Dicer-4, patatin homolog and hsr203J in the anther tissue of cysteine protease induced male sterile plant and control plant. Significant upregulation of more than three fold of these genes was observed in the anthers of cysteine protease induced male sterile plant compared to the control plant (Fig. 5). We found that Dicer-4, patatin homolog and hsr203J genes showed consistent upregulated expression patterns at the transcriptional and translational level. The transcript levels of genes, especially patatin and HSR203J were upregulated to 21 and 36 fold levels, respectively suggesting the possible role of cysteine protease in activating the transcription of patatin and HSR203J genes in cell death.
Fig. 5.
Quantitative real-time PCR (qRT-PCR) analysis of Dicer, Patatin, HSR203J and cysteine protease (AdCP) gene expression from the anthers of cysteine protease induced male sterile and control plant
Conclusions
Studying the anther proteome is considered to be of great importance for understanding the crucial proteins getting upregulated during cysteine protease mediated cell death in transgenic tobacco male sterile plants. Proteomic study revealed the upregulation of 56 protein spots in cysteine protease induced male sterile plant. Out of 56 protein spots, some important protein spots were identified as patatin like protein, dicer-like protein 4 and carboxylesterase 17, which displayed significant upregulation in cysteine protease induced male sterile plants. These results suggest that cysteine protease, patatin-like protein, dicer-like protein and carboxylesterase enzyme act in a synergistic manner during premature degeneration of tapetal cell layer, which might lead to male sterility. However, few more investigations are required to establish a link between these proteins and male sterility.
Acknowledgements
The authors are grateful to Council of Scientific and Industrial Research, Government of India for a Research Grant [38 (1393/EMR-II)] to one of the authors (PBK), DST-FIST, UGC-SAP, Government of India, for the facilities provided to the Department of Plant Sciences, University of Hyderabad.
Abbreviations
- AdCP
Arachis cysteine protease
- TEM
Transmission electron microscopy
- DTT
Dithiothreitol
- IEF
Isoelectric focusing
- PI
Isoelectric point
- PCD
Programmed cell death
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Pawan Shukla, Phone: +914023134545, Email: shklpwn@gmail.com.
Ranjana Gautam, Email: ranjana.gautam9@gmail.com.
Naveen Kumar Singh, Email: naveenks11@gmail.com.
Israr Ahmed, Email: iahmed67@gmail.com.
Pulugurtha Bharadwaja Kirti, Email: pbkirti@gmail.com.
References
- Ahmadovich Bozorov T, Prakash Pandey S, Dinh ST, Kim SG, Heinrich M, Gase K, Baldwin IT. DICER-like proteins and their role in plant-herbivore interactions in nicotiana attenuata. J Integr Plant Biol. 2012;54:189–206. doi: 10.1111/j.1744-7909.2012.01104.x. [DOI] [PubMed] [Google Scholar]
- Anderson NL, Anderson NG. Proteome and proteomics: new technologies, new concepts, and new words. Electrophoresis. 1998;19:1853–1861. doi: 10.1002/elps.1150191103. [DOI] [PubMed] [Google Scholar]
- Bézier A, Lambert B, Baillieul F. Cloning of a grapevine Botrytis-responsive gene that has homology to the tobacco hypersensitivity-related hsr203J. J Exp Bot. 2002;53:2279–2280. doi: 10.1093/jxb/erf101. [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Wojtaszek P. Mechanisms for the generation of reactive oxygen species in plant defence: a broad perspective. Physiol Mol Plant Pathol. 1997;51:347–366. doi: 10.1006/pmpp.1997.0129. [DOI] [Google Scholar]
- Camera SL, Balagué C, Göbel C, Geoffroy P, Legrand M, Feussner I, Roby D, Heitz T. The Arabidopsis patatin-like protein 2 (PLP2) plays an essential role in cell death execution and differentially affects biosynthesis of oxylipins and resistance to pathogens. Mol Plant Microbe Interact. 2009;22:469–481. doi: 10.1094/MPMI-22-4-0469. [DOI] [PubMed] [Google Scholar]
- Dhondt S, Geoffroy P, Stelmach BA, Legrand M, Heitz T. Soluble phospholipase A2 activity is induced before oxylipin accumulation in tobacco mosaic virus-infected tobacco leaves and is contributed by patatin-like enzymes. Plant J. 2000;23:431–440. doi: 10.1046/j.1365-313x.2000.00802.x. [DOI] [PubMed] [Google Scholar]
- Drews GN, Beals TP, Bui AQ, Goldberg RB. Regional and cell-specific gene expression patterns during petal development. Plant Cell. 1992;4:1383–1404. doi: 10.1105/tpc.4.11.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunoyer P, Himber C, Voinnet O. DICER-LIKE 4 is required for RNA interference and produces the 21-nucleotide small interfering RNA component of the plant cell-to-cell silencing signal. Nat Genet. 2005;37:1356. doi: 10.1038/ng1675. [DOI] [PubMed] [Google Scholar]
- Konagaya K-I, Ando S, Kamachi S, Tsuda M, Tabei Y. Efficient production of genetically engineered, male-sterile Arabidopsis thaliana using anther-specific promoters and genes derived from Brassica oleracea and B. rapa. Plant Cell Rep. 2008;27:1741–1754. doi: 10.1007/s00299-008-0598-6. [DOI] [PubMed] [Google Scholar]
- Kumar KR, Kirti PB. Differential gene expression in Arachis diogoi upon interaction with peanut late leaf spot pathogen, Phaeoisariopsis personata and characterization of a pathogen induced cyclophilin. Plant Mol Biol. 2011;75:497–513. doi: 10.1007/s11103-011-9747-3. [DOI] [PubMed] [Google Scholar]
- Kumar D, Kirti PB. Transcriptomic and proteomic analyses of resistant host responses in Arachis diogoi challenged with late leaf spot pathogen, Phaeoisariopsis personata. PLoS ONE. 2015;10:e0117559. doi: 10.1371/journal.pone.0117559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Camera S, Geoffroy P, Samaha H, Ndiaye A, Rahim G, Legrand M, Heitz T. A pathogen-inducible patatin-like lipid acyl hydrolase facilitates fungal and bacterial host colonization in Arabidopsis. Plant J. 2005;44:810–825. doi: 10.1111/j.1365-313X.2005.02578.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Jung K-H, An G, Chung Y-Y. Isolation and characterization of a rice cysteine protease gene, OsCP1, using T-DNA gene-trap system. Plant Mol Biol. 2004;54:755–765. doi: 10.1023/B:PLAN.0000040904.15329.29. [DOI] [PubMed] [Google Scholar]
- Liu F, Bakht S, Dean C. Cotranscriptional role for Arabidopsis DICER-LIKE 4 in transcription termination. Science. 2012;335:1621–1623. doi: 10.1126/science.1214402. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Marco YJ, Ragueh F, Godiard L, Froissard D. Transcriptional activation of 2 classes of genes during the hypersensitive reaction of tobacco leaves infiltrated with an incompatible isolate of the phytopathogenic bacterium Pseudomonas solanacearum. Plant Mol Biol. 1990;15:145–154. doi: 10.1007/BF00017732. [DOI] [PubMed] [Google Scholar]
- Mariani C, De Beuckeleer M, Truettner J, Leemans J, Goldberg RB. Induction of male sterility in plants by a chimaeric ribonuclease gene. Nature. 1990;347:737–741. doi: 10.1038/347737a0. [DOI] [Google Scholar]
- Marshall SD, Putterill JJ, Plummer KM, Newcomb RD. The carboxylesterase gene family from Arabidopsis thaliana. J Mol Evol. 2003;57:487–500. doi: 10.1007/s00239-003-2492-8. [DOI] [PubMed] [Google Scholar]
- May C, Preisig-Müller R, Höhne M, Gnau P, Kindl H. A phospholipase A2 is transiently synthesized during seed germination and localized to lipid bodies1Enzymes: lipoxygenase (EC 1.13.11.12); phospholipase A2 (EC 3.1.1.4).1. Biochim Biophys Acta (BBA) Lipids Lipid Metab. 1998;1393:267–276. doi: 10.1016/S0005-2760(98)00081-2. [DOI] [PubMed] [Google Scholar]
- Melan MA, Dong X, Endara ME, Davis KR, Ausubel FM, Peterman TK. An Arabidopsis thaliana lipoxygenase gene can be induced by pathogens, abscisic acid, and methyl jasmonate. Plant Physiol. 1993;101:441–450. doi: 10.1104/pp.101.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizampatnam NR, Doodhi H, Kalinati NY, Mulpuri S, Viswanathaswamy DK. Expression of sunflower cytoplasmic male sterility-associated open reading frame, orfH522 induces male sterility in transgenic tobacco plants. Planta. 2009;229:987–1001. doi: 10.1007/s00425-009-0888-4. [DOI] [PubMed] [Google Scholar]
- Pontier D, Tronchet M, Rogowsky P, Lam E, Roby D. Activation of hsr203, a plant gene expressed during incompatible plant-pathogen interactions, is correlated with programmed cell death. Mol Plant Microbe Interact. 1998;11:544–554. doi: 10.1094/MPMI.1998.11.6.544. [DOI] [PubMed] [Google Scholar]
- Rancé I, Fournier J, Esquerré-Tugayé M-T. The incompatible interaction between Phytophthora parasitica var. nicotianae race 0 and tobacco is suppressed in transgenic plants expressing antisense lipoxygenase sequences. Proc Natl Acad Sci. 1998;95:6554–6559. doi: 10.1073/pnas.95.11.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao GS, Tyagi AK, Rao KV. Development of an inducible male-sterility system in rice through pollen-specific expression of l-ornithinase (argE) gene of E. coli. Plant Sci. 2017;256:139–147. doi: 10.1016/j.plantsci.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Rao GS, Deveshwar P, Sharma M, Kapoor S, Rao KV. Evolvement of transgenic male-sterility and fertility-restoration system in rice for production of hybrid varieties. Plant Mol Biol. 2018;96:35–51. doi: 10.1007/s11103-017-0678-5. [DOI] [PubMed] [Google Scholar]
- Saravanan RS, Rose JK. A critical evaluation of sample extraction techniques for enhanced proteomic analysis of recalcitrant plant tissues. Proteomics. 2004;4:2522–2532. doi: 10.1002/pmic.200300789. [DOI] [PubMed] [Google Scholar]
- Scherer GF, Ryu SB, Wang X, Matos AR, Heitz T. Patatin-related phospholipase A: nomenclature, subfamilies and functions in plants. Trends Plant Sci. 2010;15:693–700. doi: 10.1016/j.tplants.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Sengupta D, Kannan M, Reddy AR. A root proteomics-based insight reveals dynamic regulation of root proteins under progressive drought stress and recovery in Vigna radiata (L.) Wilczek. Planta. 2011;233:1111–1127. doi: 10.1007/s00425-011-1365-4. [DOI] [PubMed] [Google Scholar]
- Seo H-H, Park AR, Lee H-H, Park S, Han Y-J, Hoang QT, Choi GJ, Kim J-C, Kim YS, Kim J-I. A fungus-inducible pepper carboxylesterase exhibits antifungal activity by decomposing the outer layer of fungal cell walls. Mol Plant Microbe Interact. 2018;31:505–515. doi: 10.1094/MPMI-11-17-0266-R. [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Shukla P, Singh NK, Kumar D, Vijayan S, Ahmed I, Kirti PB. Expression of a pathogen-induced cysteine protease (AdCP) in tapetum results in male sterility in transgenic tobacco. Funct Integr Genom. 2014;14:307–317. doi: 10.1007/s10142-014-0367-2. [DOI] [PubMed] [Google Scholar]
- Shukla P, Subhashini M, Singh NK, Ahmed I, Trishla S, Kirti PB. Targeted expression of cystatin restores fertility in cysteine protease induced male sterile tobacco plants. Plant Sci. 2016;246:52–61. doi: 10.1016/j.plantsci.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Shukla P, Singh NK, Gautam R, Ahmed I, Yadav D, Sharma A, Kirti PB. Molecular approaches for manipulating male sterility and strategies for fertility restoration in plants. Mol Biotechnol. 2017;59:445–457. doi: 10.1007/s12033-017-0027-6. [DOI] [PubMed] [Google Scholar]
- Singh SP, Pandey T, Srivastava R, Verma PC, Singh PK, Tuli R, Sawant SV. BECLIN1 from Arabidopsis thaliana under the generic control of regulated expression systems, a strategy for developing male sterile plants. Plant Biotechnol J. 2010;8:1005–1022. doi: 10.1111/j.1467-7652.2010.00527.x. [DOI] [PubMed] [Google Scholar]
- Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31–43. doi: 10.1016/S0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Sueldo D, Ahmed A, Misas-Villamil J, Colby T, Tameling W, Joosten MH, Hoorn RA. Dynamic hydrolase activities precede hypersensitive tissue collapse in tomato seedlings. New Phytol. 2014;203:913–925. doi: 10.1111/nph.12870. [DOI] [PubMed] [Google Scholar]
- Vancanneyt G, Sonnewald U, Hofgen R, Willmitzer L. Expression of a patatin-like protein in the anthers of potato and sweet pepper flowers. Plant Cell. 1989;1:533–540. doi: 10.2307/3868974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden AR, Walter C, Gardner RC. Genes expressed in Pinus radiata male cones include homologs to anther-specific and pathogenesis response genes. Plant Physiol. 1999;121:1103–1116. doi: 10.1104/pp.121.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X-M, Wang Y, Lv X-M, Li H, Sun P, Lu H, Li F-L. NtCP56, a new cysteine protease in Nicotiana tabacum L., involved in pollen grain development. J Exp Bot. 2009;60:1569–1577. doi: 10.1093/jxb/erp022. [DOI] [PMC free article] [PubMed] [Google Scholar]