Abstract
Melatonin has emerged as an important signaling molecule that regulates plant responses to environmental stresses. In this research, melatonin was used to alleviate the adverse effects of oxidative stress induced by water deficit in Moldavian balm (Dracocephalum moldavica) plants and morpho-physiological traits were investigated. This experiment was conducted as a factorial arrangement based on completely randomized design with four replications. Treatments included foliar melatonin application at four levels 0 (distilled water), 50, 100 and 150 μM and drought stress 100 (control), 80, 60 and 40% of field capacity (FC). Higher levels of drought stress at 60% and 40% FC, caused the reduction of plant height, shoot fresh and dry weight, root length, root fresh and dry weight, photosynthetic pigments and protein content. Increased amount of soluble sugar content, malondialdehyde content and lipoxygenase activity, non-enzyme antioxidants (including flavonoid, polyphenol compounds and anthocyanin), phenylalanine ammonia-lyase and polyphenol oxidase enzymes activities were also observed at 60% and 40% FC. Melatonin at 100 µM improved morphological parameters, photosynthetic pigments and protein content under moderate and severe drought stress. The obtained results suggested that foliar application of 100 μM melatonin also alleviated oxidative burst and malondialdehyde production in Moldavian balm plant under moderate and severe drought stress probably through regulation of secondary metabolism and the enzymes activity of phenylalanine ammonia-lyase and polyphenol oxidase.
Keywords: Lipid peroxidation, Melatonin, Moldavian balm, Oxidative damage, Phenylalanine ammonia-lyase, Photosynthetic pigments
Introduction
Water stress is one of the most important factors in reducing plant productivity. If drought stress did not occur, the actual yields would be equal to the potential yields of the plants (Rai et al. 2012). Drought stress disturbs plant photosynthesis, reduces chlorophyll content and cause damage to photosynthetic apparatus, which ultimately leads to oxidative stress and the formation of reactive oxygen species (ROS). Furthermore, defects in electron transport system within both mitochondria and chloroplast result in excessive ROS generation. Water deficit adaptation and tolerance is the result of physiological and biochemical responses which result in tissue water conservation, maintaining chloroplasts and homeostasis of ions (Rai et al. 2012); so, understanding these responses that alleviate the adverse effects of water loss is clearly important (Guo et al. 2006). If toxic trace radicals are not quickly removed or inactivated by antioxidant defense system, they can diminish plant growth and yields as a result of intensified MDA production, protein degradation and DNA breakdown and perturbation of cell metabolism (Polle 2001). To cope with drought-mediated oxidative stress, plants use either enzymatic anti-oxidative system such as superoxide dismutase, ascorbate peroxidase, catalase and guaiacol peroxidase or non-enzymatic antioxidant compounds like glutathione, ascorbic acid, α-tocopherol, flavonoids, polyphenol compounds, anthocyanin and carotenoids (Shi et al. 2007). Flavonoids, polyphenol compounds, anthocyanins and carotenoids are the most important non-enzymatic antioxidants that scavenge both free radicals and their excessive production. Flavonoids, due to a number of hydroxyl groups in their structural chain, scavenge ROS (Ma et al. 2014). Application of drought stress raised flavonoids contents and polyphenol compounds as an antioxidant in Solanum tuberosum (Watkinson et al. 2006), Cistus clusii (Hernandez et al. 2004) and anthocyanins and carotenoids protect the cell membrane and prevent chlorophyll degradation in Arabidopsis thaliana (Jung 2004) and Brassica napus (Sangtarash et al. 2009) under drought stress.
The regulation of antioxidant compounds by exogenous substances could mediate plant tolerance to drought stress (Arnao and Hernandez-Ruiz 2014). Melatonin (Mel) is one of the compounds whose accumulation in plants, may be indicative of a mechanism for protection of the tissues from oxidative damage arising from drought stress. Melatonin (N-acetyl-5-methoxy-tryptamine) is an indole compound that is naturally synthesized in mitochondria and chloroplasts of plants (Zheng et al. 2017). The results of previous researches indicated the protective role of Mel against biotic and abiotic stresses such as drought, salinity, extreme temperature, excess copper, pathogen attack and senescence (Manchester et al. 2000; Wang et al. 2013a). Among all plant growth regulators, Mel has the highest antioxidant capacity and is recognized as the strongest molecule with antioxidant properties (Zhang et al. 2014; Arnao and Hernandez-Ruiz 2014). The antioxidant activity of Mel is related to directly scavenging of free radicals, stimulating the synthesis of enzymatic and non-enzymatic antioxidants and increasing the capacity of mitochondria electron transfer chains, hence reducing the production of free radicals and ion leakage (Zhang et al. 2014). Exogenous application of Mel caused the reduction of water deficit in Cucumis sativus (Zhang et al. 2013), apple (Wang et al. 2013a), Malus species (Li et al. 2015), Glycine max (Wei et al. 2015) and Lupinus albus (Arnao and Hernandez-Ruiz 2007). Tan et al. (1993) claimed that Mel acts as a forefront molecule to overcome the adverse effect of oxidative stress and other antioxidants acted as a back-up after Mel.
Dragonhead or Moldavian balm (Dracocephalum moldavica) is a herb from Lamiaceae family (Hussein et al. 2006). The effective substances of its vegetative organs are sedative and appetizing. Its essence is antibacterial and is consumed in curing stomachache, liver disorders, headache and flatulence as well as in food industries, soda manufacturing and health and make-up industries (Hussein et al. 2006). Although the effects of water stress on crops have been comprehensively studied, but there are limited investigations on the behavior of aromatic and medicinal herbs under drought stress condition. Various environmental factors such as water stress affect growth of pharmaceutical plants (Letchamo and Gosselin 1996). Moldavian balm with high susceptibility to drought stress (Alaei et al. 2013), is an important medicinal and economic plant. Considering lack of rainfall and occurrence of drought stress in most regions of Iran, the application of plant growth promoters (such as melatonin) for production of optimal yields could be a promising approach which allows the cultivation of medicinal plants in arid and low water areas. The main objective of the present experiment was to investigate the effect of melatonin as a plant growth regulator on antioxidant defense system and secondary metabolism of Moldavian balm plant under drought stress. For completion of the study, some morpho-physiological traits were also analyzed.
Materials and methods
Plant material and experimental condition
This study was carried out at the research greenhouse of the Bardsir College of Agriculture, Kerman (29°55′39″N, 56°34′20″E), Iran in 2016. The experiment was performed as a completely randomized design in a factorial arrangement with 16 treatments and four replications. Seeds of Dracocephalum moldavica (Isfahan Seedlings and Seeds Co.) were grown in plastic pots containing loamy-sandy soil. During this experiment, greenhouse had the temperature of 25/22 °C (day/night), light/dark period of 14/10 h and relative humidity of 60%. Plant were irrigated every day for 5 weeks. After 5 weeks of growth under normal conditions, three healthy and uniform plants per pot were selected for foliar application of melatonin and four watering regimes. After optimizing melatonin concentrations, experimental treatments including 0, 50, 100 and 150 μM of melatonin in distilled water and drought stress at four levels of 100, 80, 60 and 40% of field capacity (FC) were applied on plants and Tween-20 was used as a surfactant. Approximately 30 cc melatonin was sprayed on each plant. Melatonin was applied to belonging treatments two times per week. To reach the desired watering regimes, pots weight measured every 2 days until the water content dropped to 80, 60 and 40% of field capacity. When 15% of plants reached to flowering stage, morphological and physiological traits were measured. Plant height, shoot fresh and dry weight, root length, root fresh and dry weight were measured. To analyze physiological traits, leaf samples were frozen in liquid nitrogen and stored at − 80 °C until laboratory experiments.
Estimation of photosynthetic pigments and carotenoids
The Lichtentaler method (1987) was used to measure chlorophylls and carotenoids content. 100 mg fresh leaves of Moldavian balm were extracted in the 80% acetone. After filtration, its absorption was read by UV–Visible Spectrophotometer (SPEKOL-2000, Germany) at wavelengths of 646.8, 663.2 and 470 nm.
Estimation of flavonoid content
Flavonoid was measured spectrophotometrically using Nogues and Baker (2000) method. 100 mg of dragonhead leaf tissues were homogenized in 10 ml of acidified methanol [ethanol: acetic acid, 99:1 (v/v)]. The absorption intensity was read at 300 nm. Result was reported in terms of mg/g DW.
Determination of polyphenol contents
The polyphenol compounds was measured by Gao et al. (2000) method and using folin’s reagent. 100 mg of plant tissue was homogenized in 1 ml of 80% ethanol. The extract was stored at room temperature for 24 h (samples were preferably stored in darkness). Then, the samples were centrifuged for 10 min in 2000×g and the supernatants were used to measure the polyphenols at 765 nm wavelength and their contents were expressed as mg/g DW.
Determination of anthocyanin content
The Wanger (1979) method was applied to measure the anthocyanin content. 100 mg of samples were dissolved in 10 mL of acidified methanol (methanol: HCl 99:1 (v/v)). The extracts were placed in dark room at 25 °C for 24 h; centrifuged at 2000×g for 10 min, the absorption of supernatant was read at 550 nm. The extinction coefficient of 33,000 M−1Cm−1 was used for the calculation of anthocyanin content.
Determination of soluble sugar content
The soluble sugar content of samples was determined using an anthrone reagent based on Roe (1955) method. 100 mg fresh leaf materials were kept in 2.5 ml of alcohol at 95 °C in incubator for 60 min. After filtration, final volume was made up to 2.5 ml by adding water. Then 200 μl of each sample was poured into a test tube and added 5 ml of anthrone reagent. Afterwards, it was placed in water bath, at 90 °C for 17 min, and after cooling, the absorbance of samples was read at 625 nm. Results are expressed as mg soluble sugar per g DW−1.
Thiobarbituric acid reactive substance (TBARS)
The amount of lipid peroxidation products was measured according to the procedure of Heath and Packer (1969). 100 mg of the Moldavian balm leaf tissues were homogenized in 0.1% TCA (W/V), and centrifuged at 10,000×g for 15 min. 1 ml of supernatant was added to 5 ml of 20% TCA (W/V) solution containing 0.5% 2-thiobarbituric acid (TBA) (W/V), and the mixture was heated for 30 min at 90 °C. Samples were quickly immersed in ice for 5 min and then re-centrifuged at 10,000×g for 10 min. For MDA measurement, the absorbance of the supernatant was read at 532 nm and correction for unspecific pigments was performed by deducting the absorbance of the same samples at 600 nm. The extinction coefficient (ε) of 155 mM−1cm−1 was used for determination of MDA concentration.
Enzyme extraction and activity determination
500 mg leaf samples were homogenized in 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM ethylene diamine tetra acetic acid (EDTA), 1% soluble polyvinyl pyrrolidone (PVP) and 1 mM phenylmethylsulfonyl fluoride (PMSF). All extraction steps were carried out on ice at 4 °C. The mixture was centrifuged at 20,000×g for 20 min, and the supernatant applied for assay of the activity of antioxidant enzymes and protein content.
Total soluble proteins
Protein content was calculated following the method of Bradford (1976) in which Bovine serum albumin was used as standard. 5 ml of Bio-Rad reagent was added to the test tubes containing 100 μl of protein extract, then the absorption intensity was read at 595 nm.
Lipoxygenase (LOX) activity (EC 1.13.11.12)
The activity of LOX was carried out according to the method of Minguez-Mosquera et al. (1993). The reaction mixture contained 100 mM linoleic acid as a substrate, 100 mM acetic acid buffer (pH 6.5) and an enzyme extract. The absorbance of the reaction was measured at 234 nm and the activity of LOX was calculated by using the extinction coefficient of 25,000 M−1Cm−1 and LOX activity was calculated as U/mg protein.
Phenylalanine ammonia-lyase (PAL) activity (EC 4.3.1.5)
PAL activity was measured according to the amount of phenylalanine conversion to cinnamic acid based on the method of D’cünha et al. (1996). The reaction mixture contained 100 mM Tris–HCl buffer (pH 8.5), 1 mM 2-mercaptoethanol, 50 mM l-Phenylalanine and enzyme extract. The reaction was terminated by the addition of 6 M HCl and absorbance of the supernatant was read at 290 nm.
Polyphenol oxidase (PPO) activity assay (EC1.14.18.1)
Haplin and Lee (1987) procedure was used. The reaction mixture consisted of 0.2 M Tris buffer (pH 7.6), 0.02 M pyrogallol and 100 μl of the enzyme extract. PPO activity was expressed as change in absorbance at 412 nm.
Statistical analysis
Statistical analysis were accomplished via one-way ANOVA followed by LSD test to compare whether the means were significantly different, taking p value of < 0.05 as significant. Computations and statistical analysis were done using SAS and MSTATC.
Results
Foliar application of 100 μM Mel was more effective than the other concentrations of Mel (50 and 150 μM) especially under moderate and severe drought stress conditions. According to the results, we focused on the best concentration of Mel (100 µM).
Plant height
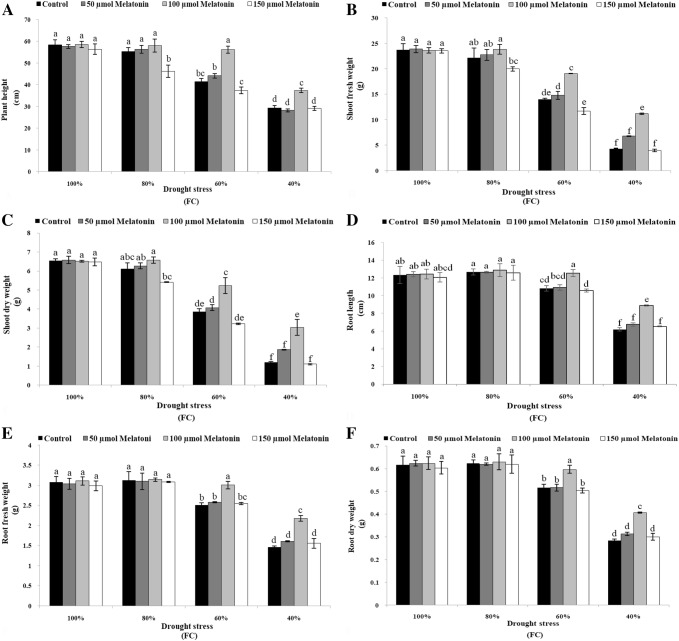
The results of analysis of variance showed that Mel, drought stress and the interaction of Mel treatments and drought levels caused a significant (p ≤ 0.01) effect on plant height (Table 1). There was no significant difference between control and all concentrations of Mel under normal condition (Fig. 1a). Exogenous application of 100 μM Mel markedly alleviated the adverse effects of drought stress on plant height (Fig. 1a). The concentration of 100 μM Mel had significant difference compared with non-treated Moldavian balm plants and other doses of Mel under moderate (60% FC) and severe (40% FC) drought stress conditions (Fig. 1a).
Table 1.
Mean squares (MS) for morphological parameters containing plant height, shoot fresh weight (SFW), shoot dry weight (SDW), root length (RL), root fresh weight (RFW) and root dry weight (RDW) of Dracocephalum moldavica
| Sources of variance | Degree of freedom | MS | |||||
|---|---|---|---|---|---|---|---|
| Plant height | SFW | SDW | RL | RFW | RDW | ||
| Melatonin | 3 | 217.4** | 46.07** | 3.51** | 4.07** | 0.279** | 0.008* |
| Drought stress | 3 | 1698.5** | 738.2** | 56.04** | 79.21** | 5.1** | 0.231** |
| Melatonin × drought stress | 9 | 40.85** | 7.75* | 0.567ns | 0.995ns | 0.068ns | 0.002ns |
| Error | 48 | 12.28 | 3.3 | 0.337 | 0.838 | 0.051 | 0.002 |
| CV% | 7.47 | 10.78 | 12.54 | 8.44 | 8.57 | 8.59 | |
**, * and ns denote significant differences at 0.01, 0.05% levels, and not significant respectively
Fig. 1.
Effect of melatonin foliar application on growth characteristics [plant height (a), shoot fresh weight (b), shoot dry weight (c), root length (d), root fresh weight (e) and root dry weight (f)] of Dracocephalum moldavica under different levels of drought stress. The mean comparisons were performed using LSD method at p ≤ 0.05 significant level. Means (± SE) followed by the same letter(s) are not significantly different
Shoot fresh and dry weight
The response of shoot fresh weight (SFW) to the interaction of Mel concentrations and drought levels was significant (p ≤ 0.01) (Table 1). Water deficit had a significant effect on shoot dry weight (SDW) of Dracocephalum moldavica (Table 1). The highest level of drought stress (40% FC) caused a reduction of 81.6% and 80% in SFW and SDW as compared to control respectively (Fig. 1b, c), but the concentration of 100 μM Mel increased SFW and SDW approximately 61.1% and 60.5% compared with non-treated plants under mentioned drought level (Fig. 1b, c).
Root length (RL), root fresh weight (RFW) and root dry weight (RDW)
RL, RFW and RDW reduced by increasing of drought stress and as shown in Table 1, the effect of water deficit on these mentioned traits was significant (p ≤ 0.01). RL, RFW and RDW reduced from 12.35 cm, 3.08 g and 0.617 g under normal condition to 6.2 cm, 1.47 g and 0.283 g at the highest drought level respectively (Fig. 1d–f). Interaction between drought stress and Mel treatment indicated that foliar application of 100 μM Mel statistically showed the highest RL, RFW and RDW in contrast with control and other concentrations of Mel under drought stress of 60% and 40% FC (Fig. 1d–f).
Estimation of photosynthetic pigments and secondary metabolites
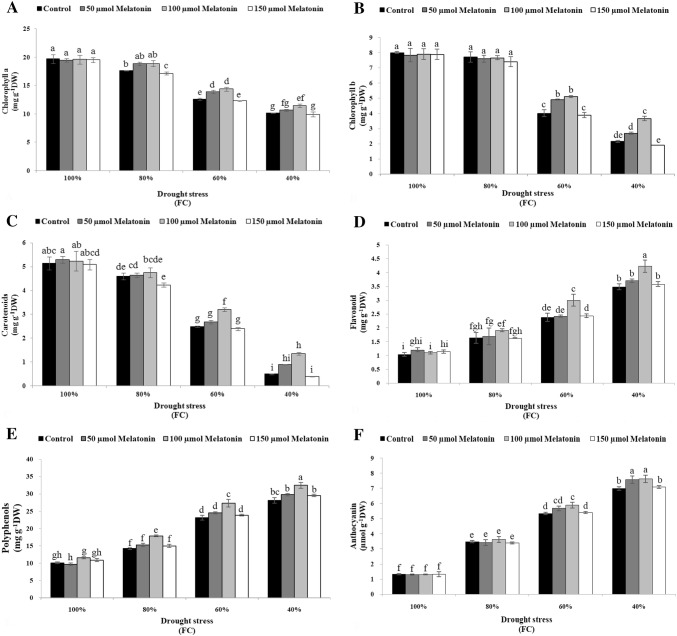
Drought stress had a significant effect on chlorophyll a (Chl a), chlorophyll b (Chl b) and carotenoids (Car), flavonoid, polyphenols and anthocyanin contents of Moldavian balm plants (Table 2). Water stress at the level of 40% FC decreased Chl a, Chl b and Car by approximately 48.4, 73.15 and 90.2% compared to non-treated plants respectively (Fig. 2a–c). Exogenous application of 100 μM Mel was more effective on photosynthetic pigments under severe drought stress condition (Fig. 2a–c). The highest Flavonoid content was recorded for 100 μM Mel compared with the other concentrations of Mel and plants which were sprayed with distilled water under 60% and 40% FC (Fig. 2d). The application of 100 μM Mel caused an increment of 17.68% and 15.8% in polyphenol compounds compared with non-treated Moldavian balm plants under drought stress of 60% and 40% FC, respectively (Fig. 2e). The difference in anthocyanin content was not statistically significant between 50 and 100 μM Mel, while both were significantly higher than control and 150 μM Mel under 60% and 40% FC (Fig. 2f). The greatest increase in anthocyanin content was observed in the concentrations of 50 μM and 100 μM Mel as 7.9% and 8.5% compared to non-treated plants under severe drought stress respectively (Fig. 2f).
Table 2.
Mean squares (MS) for photosynthetic pigments including chlorophyll a (Chl a), chlorophyll b (Chl b), carotenoids (Car), and secondary metabolites consisting of flavonoid, polyphenols, and anthocyanin of Dracocephalum moldavica
| Sources of variance | Degree of freedom | MS | |||||
|---|---|---|---|---|---|---|---|
| Chl a | Chl b | Car | Flavonoid | Polyphenols | Anthocyanin | ||
| Melatonin | 3 | 4.68** | 1.51** | 0.831** | 0.431** | 25.69** | 0.3* |
| Drought stress | 3 | 211.1** | 78.31** | 47.43** | 15.56** | 922.5** | 81.38** |
| Melatonin × drought stress | 9 | 0.696ns | 0.514* | 0.105ns | 0.079ns | 1.31ns | 0.084ns |
| Error | 48 | 0.624 | 0.215 | 0.101 | 0.092 | 1.15 | 0.078 |
| CV% | 5.1 | 8.2 | 9.6 | 13.2 | 5.3 | 6.3 | |
**, * and ns denote significant differences at 0.01, 0.05% levels, and not significant respectively
Fig. 2.
Effect of melatonin foliar application on photosynthetic pigments chlorophyll a (a), chlorophyll b (b) and carotenoids (c)) and secondary metabolites; flavonoid (d), polyphenols (e), and anthocyanin (f)] of Dracocephalum moldavica under different levels of drought stress. The mean comparisons were performed using LSD method at p ≤ 0.05 significant level. Means (± SE) followed by the same letter(s) are not significantly different
Soluble sugar content
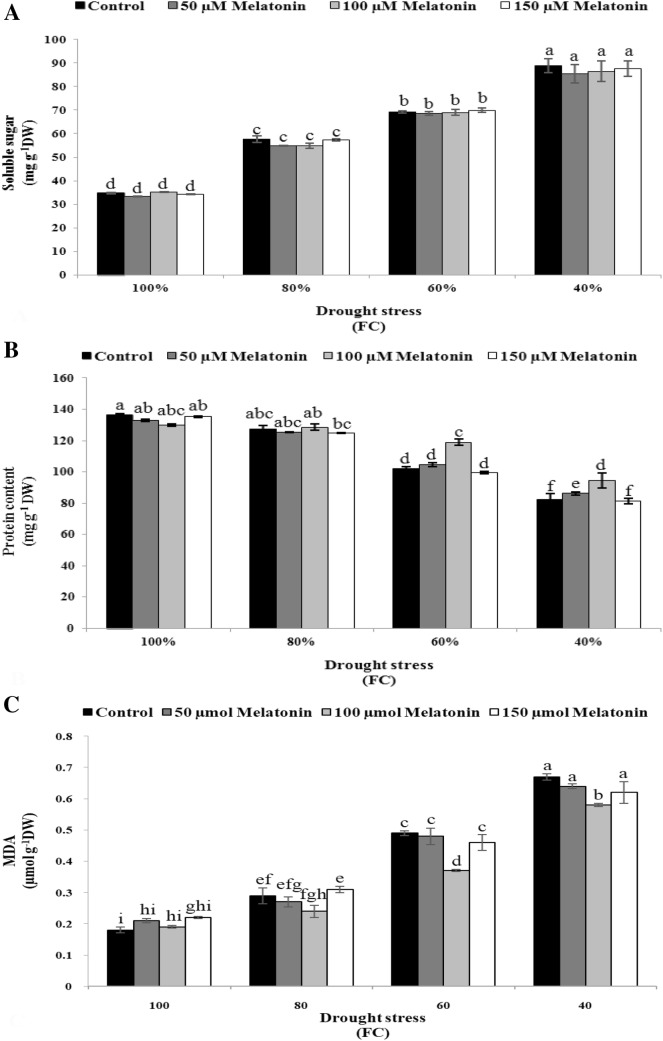
The effect of different levels of drought stress on soluble sugar content was significant (Table 3). Increment of drought stress from 100 to 40% FC led to increase of soluble sugar (Fig. 3a). Highest level of drought stress caused an increase of 60.73% in soluble sugar content in comparison with control (Fig. 3a). The difference in soluble sugar wasn’t statistically significant between control and Mel treatment at any concentrations under both normal and stress conditions (Fig. 3a).
Table 3.
Mean squares for physio-biochemical traits comprising soluble sugar, protein, MDA and LOX activity and antioxidant enzymes including PAL and PPO activities of Dracocephalum moldavica
| Sources of variance | Degree of freedom | MS | |||||
|---|---|---|---|---|---|---|---|
| Soluble sugar | Protein | MDA | LOX activity | PAL activity | PPO activity | ||
| Melatonin | 3 | 10.73ns | 134.9* | 0.011** | 126.7** | 1.72** | 0.307** |
| Drought stress | 3 | 5892.6** | 5502.2** | 0.435** | 1.701** | 159.7** | 96.81** |
| Melatonin × drought stress | 9 | 1.99ns | 76.55ns | 0.002ns | 0.267** | 0.082* | 0.142* |
| Error | 48 | 23.34 | 44.51 | 0.001 | 0.083 | 0.034 | 0.066 |
| CV% | 7.8 | 5.9 | 8.4 | 8 | 2.9 | 8.4 | |
**, * and ns denote significant differences at 0.01, 0.05% levels, and not significant respectively
Fig. 3.
Effect of melatonin foliar application on physio-biochemical characteristics [soluble sugar (a), protein content (b) and MDA (c)] of Dracocephalum moldavica under different levels of drought stress. The mean comparisons were performed using LSD method at p ≤ 0.05 significant level. Means (± SE) followed by the same letter(s) are not significantly different
Protein content
Implication of Mel and drought stress had significant effect on protein content (p ≤ 0.05 and p ≤ 0.01, respectively) (Table 3). Increased drought stress level alleviated the adverse effects of water stress on protein content in Dracocephalum moldavica plants which were treated with 100 µM Mel concentration (Fig. 3b). In comparison with control, exogenous application of 100 μM Mel increased protein content under moderate and severe drought stress (14.18% and 12.68%, respectively) (Fig. 3b).
Malondialdehyde (MDA) and lipoxygenase activity (LOX)
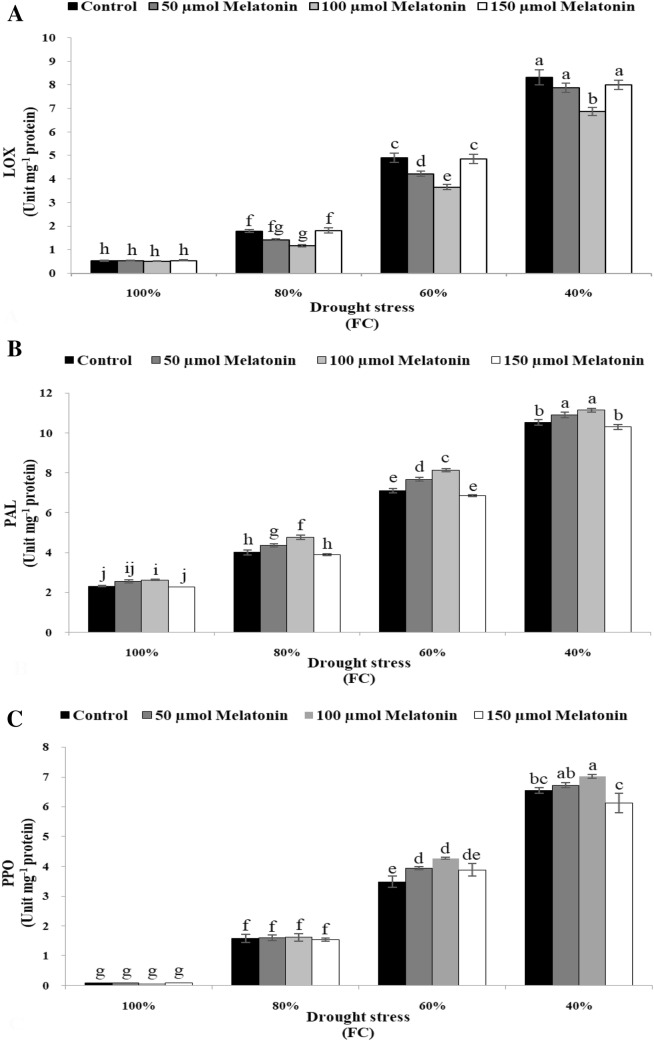
MDA was measured as an indicator of lipid peroxidation and LOX is an oxidation enzyme that contributes to oxidation of polyunsaturated fatty acids. Drought stress and Mel significantly affected MDA content and LOX activity (p ≤ 0.01) (Table 3). The response of LOX activity to the interaction of Mel concentrations and drought levels was significant (Table 3). The data showed that water stress increased MDA content (Fig. 3c) and LOX activity (Fig. 4a). The highest and lowest MDA content (Fig. 3c) and LOX activity (Fig. 4a) was recorded for drought stress of 40% FC and control, respectively. The concentration of 100 μM Mel applied through foliar spray significantly reduced MDA content (Fig. 3c) and LOX activity (Fig. 4a) especially under moderate and severe drought stress.
Fig. 4.
Effect of melatonin foliar application on LOX activity (a) and antioxidant enzymes including PAL (b) and PPO (c) activities of Dracocephalum moldavica under different levels of drought stress. The mean comparisons were performed using LSD method at p ≤ 0.05 significant level. Means (± SE) followed by the same letter(s) are not significantly different. Means (± SE) followed by the same letter(s) in each column are not significantly different at the 5% level
Estimation of phenylalanine ammonia-lyase (PAL) activity and polyphenol oxidase (PPO) activity
As shown in Table 3, the effect of Mel and drought stress on the activities of PAL and PPO was significant. The interaction of Mel concentrations with drought stress significantly affected PAL activity and PPO activity (Table 3). Results showed that drought stress caused an increase of both mentioned traits (Fig. 4b, c). The difference in PAL activity wasn’t statistically significant between the concentrations of 50 and 100 μM Mel, while both were significantly higher than control and the other concentration of Mel under 40% FC drought stress (Fig. 4b). PPO activity increased from 0.088 U/mg protein at normal condition to 6.56 U/mg protein at the highest level of drought stress (Fig. 4c). No significant difference was observed between control and all concentrations of Mel in the activity of PPO under normal and mild drought stress (Fig. 4c), while foliar application of 100 μM Mel caused an increase of 18.5% and 6.7% in PPO activity as compared to control under moderate and severe water stress conditions respectively (Fig. 4c).
Discussion
Several environmental factors affect growth, development and ultimately yield in plants. Water deficit is one of the environmental stresses that causes adverse effects on plant growth stages, organs structure and activity; eventually, causing oxidative stress by disturbing the balance between the production of ROS and the plant’s antioxidant defense system (Gholami et al. 2010). In this regard, increasing plant tolerance to drought stress as an effective strategy can be the most feasible and economical approach to reduce the damaging effects of stress. In recent years, researchers have found that exogenous application of Mel (Mel is known as an inexpensive and safe substance for the environment) can enhance plant tolerance to drought stress (Li et al. 2015; Zhang et al. 2014). Results showed that plant height (Fig. 1a) and RL (Fig. 1d) reduced with increasing of drought stress level. One of the reasons for reduction of these traits under drought stress conditions could be related to less water uptake, photosynthesis disruption, decreased production of hormones and the activity of enzymes. It has been reported that plants which grow in stressful environments have shorter stems and roots (Farooq et al. 2009; Katerji et al. 1994). In this research, different levels of drought stress in Moldavian balm plants caused a significant decrement in root and shoot length. Similar results were obtained by Agnihotri et al. (2007), Haq et al. (2010) and Kaydan and Yagmur (2008) under drought stress conditions. SFW (Fig. 1b), SDW (Fig. 1c), RFW (Fig. 1e) and RDW (Fig. 1f) were other parameters which were reduced under moderate and severe drought stress. Lower SFW, SDW, FRW and RDW due to limited water uptake was reported by Yucel et al. (2010) and Radhouane (2007).
Among different strategies which were applied to cope with drought stress, foliar application has proven to be an excellent technique and this approach has recently been used to overcome drought stress condition (Farooq et al. 2009). So that, foliar spraying of Mel especially 100 µM concentration could mitigate the adverse effects of drought stress. Our previous study clarified that the maintenance of high turgor potential and relative water content in plants which were treated with melatonin compared with untreated plants prevented the reduction of shoot and root growth under drought stress. In plants, Mel affected plants metabolites and stimulated biosynthesis of phytohormones, facilitated nutrients absorption, stimulating root and shoot growth and finally lead to enhancement of the quality and quantity of production. Mel regulated rooting through induction of auxin and caused an increasing of shoot growth via the increment of cytokinin production (Arnao 2014). Our results confirm the report that Mel in low-dose promoted root elongation, vegetative growth and development of Glycyrrhiza uralensis (Afreen et al. 2006) and Prunus avium (Sarropoulou et al. 2012).
In this research, water stress caused the reduction of photosynthetic pigments and carotenoids, while 100 μM Mel treatment increased Chl (as the main part of photosynthetic structure) and carotenoids in stress condition (Fig. 2a–c). A possible reason for the reduction of photosynthetic pigments under drought stress condition could be related to the destruction of chloroplast and photosynthetic apparatus, chlorophyll photo-oxidation, degradation of chlorophyll synthesis precursors, inhibition of new chlorophyll biosynthesis, activation of chlorophyll degrading enzymes including chlorophyllase and hormonal disturbance (Wang et al. 2013a). On the other hand, Costa et al. (2005) reported that LOX was one of the enzymes involved in chlorophyll degradation. The reduction of carotenoids in drought stress may be related to beta-carotene decomposition and the formation of zeaxanthin in the xanthophyll cycle (Sultana et al. 1999). Melatonin at different concentrations caused the delaying of chlorophyll loss and leaf senescence compared to control plants (Arnao and Hernandez-Ruiz 2009). Long-term exogenous application of Mel to one-year-old apple trees under water stress caused delaying leaf senescence, with a significant reduction in chlorophyll degradation, through the enhancement of ROS-scavenging enzyme activities (Wang et al. 2013a, b). Similar data was also obtained in the researches of Zhang et al. (2013) and Sarropoulou et al. (2012). The induction of carotenoid biosynthesis under drought stress condition by foliar application of Mel could be due to their protective role in photosynthetic apparatus, because these pigments were responsible for scavenging ROS, preventing lipid peroxidation and ultimately reducing oxidative stress (Wang et al. 2013a, b; Zhang et al. 2013).
It has been reported that soluble sugars accumulation during salinity (Juan et al. 2005) and drought (Pinheiro et al. 2001) stresses is a plant resistance response to protect macromolecules structures and the stability of DNA in the cell (Juan et al. 2005). In this study, increasing soluble sugars content was observed under drought stress (Fig. 3a), which was consistent with the results of some researchers in other plants (Pinheiro et al. 2001; Sato et al. 2004). Exogenous application of Mel caused an increment of soluble sugars in Prunus cerasus (Sarropoulou et al. 2012). Water deficit caused the reduction of water potential gradient between roots and their surrounding media. Plants recruit different mechanisms such as osmotic adjustment and compatible osmolytes (soluble sugars and proline) production to enhance their water potential and continuous water absorption (Rai et al. 2012). The data of this research and our previous results well illuminated that osmolyte compounds of Moldavian balm plant which was sprayed with different concentrations of Mel are not able to mitigate the adverse effects of drought stress which causes the reduction of plant growth. So that, it seems that the protective role of melatonin on dragonhead plant was attained through enzymatic and non-enzymatic antioxidant defense system.
Data presented in Fig. 3b, demonstrated that protein content decreased under drought stress. The reduction of protein content was the prevalent phenomenon in drought stress, because water deficit had a main effect on the nitrogen metabolism. The reasons for the reduction of protein content could be related to ROS generation causing the induction of amino acids oxidation (Hsu and Kao 2003), increased degradation of protein structure (Iturbe-Ormaexte et al. 1998), inhibition of protein biosynthesis through the influence of polyribosomes (Hsiao 1970), reduced efficiency of mechanisms involved in regeneration of proteins (Rahnama and Ebrahimzadeh 2004) and decreased activity of nitrate reductase enzyme (Shibli et al. 2007). However, melatonin prevented from excessive protein degradation (Fig. 3b). The effect of Mel on increase of nitrate contents and nitrate reductase activity could be the reason for increment of protein content in Mel treated plants (Lazar et al. 2013). Mel caused the increment of protein content in Chara australis (Lazar et al. 2013), Malus domestica (Wang et al. 2013a) and Vitis vinifera (Meng et al. 2014).
The results in Moldavian balm plants showed that drought stress caused significant increase in MDA content (Fig. 3c) and LOX activity (Fig. 4a). Water stress induced lipid peroxidation via ROS generation and thus, led to cell injury and increased electrolyte leakage (Shi et al. 2007). These results were in agreement with the findings of Sharma and Shanker (2005) in Oryza sativa and Turkan et al. (2005) in Phaseolus acutifolia. Reducing relative water content and induction of water stress in plant raised the transcription of LOX-related genes, which results in a higher amount of this enzyme (Turkan et al. 2005). Increased LOX activity in barley has been reported under drought stress (Kubis 2006). Mel alleviated the adverse effect of MDA content (Fig. 3c) and LOX activity (Fig. 4a) under drought stress in Dracocephalum moldavica plants. Several studies have been revealed that the role of Mel as an inhibitor of lipid peroxidation can be related to its ability to react with lipid alcoxyl (LO•) and lipid peroxyl (LOO•) radicals and interrupt the chain of peroxidation (Li et al. 2012; Sarropoulou et al. 2012; Tan et al. 1993; Zhang et al. 2013). A protective effect of Mel on the reduction of MDA content and LOX activity has been reported under drought stress (Meng et al. 2014; Zhang et al. 2013; Wang et al. 2013a). Our research findings showed that Mel through the induction of enzymatic and non-enzymatic antioxidant mechanisms, made it possible to reduce electrolyte leakage and protected the plant from oxidative damage.
Upon drought stress imposition, flavonoid, polyphenol compounds, anthocyanin content (Fig. 2d–f), PAL activity (Fig. 4b) were increased on both control treatment and plants which were sprayed with different concentrations of Mel. Foliar application of 100 μM Mel showed a higher increasing in secondary metabolites (Fig. 2d–f) and PAL activity (Fig. 4b). Polyphenol compounds, anthocyanin and flavonoid are synthesized from the phenylpropanoid pathway. These secondary metabolites act as antioxidants and can scavenge free radicals and other oxidative species (Syvacy and Sokmen 2004). In plant cells, polyphenol compounds act as backup of ascorbate–glutathione cycle to scavenge hydrogen peroxide (Syvacy and Sokmen 2004). Flavonoids are also potent antioxidants that can decompose H2O2 in vacuole or cell wall, furthermore, flavonoids directly inhibit oxidative stress by entering into redox reactions and indirectly by iron chelating (Kreft et al. 2002). Anthocyanins are made at the last point of flavonoids biosynthetic pathway and are involved in scavenging of ROS in environmental stresses (Kreft et al. 2002). Phenylalanine ammonia-lyase (PAL) is the key enzyme of the phenylpropanoid pathway, which converts phenylalanine into a trans-cinnamic acid by L-deamination reaction. Cinnamic acid is the first substance for synthesis of secondary metabolites (Wen et al. 2005). An increasing of PAL activity and consequently the accumulation of polyphenol compounds, flavonoids and anthocyanin content has been reported under biotic and abiotic stresses (Ma et al. 2014; Wen et al. 2005). PPO activity increased in Moldavian balm plants which were subjected to drought stress (Fig. 4c). PPO activity enhanced in Sesamum indicum under drought stress (Fazeli et al. 2007). The concentration of 100 µM Mel-applied plants had significantly higher levels of secondary metabolites by induction of PAL and PPO activities compared to only drought-stressed ones (Fig. 4b, c). Our findings were identical with the results of Szafranska et al. (2012) in Vigna radiata. It has been reported that PPO in chloroplasts of mesophilic cells plays a crucial role in regulating the Mehler reaction and oxygen level on thylakoids membrane in plastids. Therefore, it can be expected that PPO reduces the risk of ROS generation by oxidizing oxygen (Vaughn et al. 1988). Thipyapony et al. (2004) reported that enhanced PPO activity caused to protect the Chlorophyll contents and polyphenol compounds. The results of Kostopoulou et al. (2015) showed that application of Mel increased phenolic compounds, anthocyanin and PPO activity along with reducing oxidative stress because PPO could act as non-destructive sink for extra light energy.
Conclusion
Drought stress triggered oxidative damage in Moldavian balm plant through excessive generation of ROS and exogenous Mel, greatly improves the dehydration tolerance through elevated activities of antioxidant systems under drought stress. Under drought stress conditions, direct function of melatonin as an antioxidant caused chemical detoxification, membrane stabilization and repaired membrane fluidity. Indirect action of melatonin as a stimulator of enzymatic and non-enzymatic antioxidant defense system and the growth response of Mel as plant growth regulator depended on its concentration which in low dose incited shoot and root growth. Also, Mel as a bio-stimulator prevented chlorophyll degradation as well as elevated photosynthetic pigments, protein content and biomass. It seems that the concentration of 100 μM Mel caused an increase of morphological traits, photosynthetic pigments and protein content, while reduction of MDA content and LOX activity. Finally, in contrast with control, 100 μM Mel decreased the adverse effects of oxidative damage via enhancement of non-enzyme antioxidants (polyphenol compounds, flavonoid and anthocyanin) and the enzyme activities of PAL and PPO. All different concentrations of melatonin had no significant effect on soluble sugar content (as a osmoregulator). Based on our results, it seems that melatonin alleviates drought stress mostly through activating antioxidant defense system in Moldavian balm plant rather than other regulatory pathways such as osmoprotection and soluble sugar content. Further researches concerning details of the method and focus on elucidating the influence of exogenous melatonin on changes in the specific molecular and biochemical pathways will provide new information about the direct and indirect mechanisms of melatonin activity in plants.
Abbreviations
- Car
carotenoids
- Chl
chlorophyll
- llDW
dry weight
- FC
field capacity
- LOX
lipoxygenase
- MDA
malondialdehyde
- Mel
melatonin
- PAL
Phenylalanine ammonia-lyase
- PPO
Polyphenol oxidase
- RDW
root dry weight
- RFW
root fresh weight
- RL
root length
- ROS
reactive oxygen species
- SDW
shoot dry weight
- SFW
shoot fresh weight
- TBARS
thiobarbituric acid reactive substance
Authors’ contributions
A.H., H.O. and F.N. equally design experiments; M.N. provided greenhouse, laboratory and experimental materials; H.O. and M.N. supervised the laboratory work; R.K. performed analytical measurement, accomplished statistical analyses and contributed to manuscript writing; Z.T. helped in statistical analyses; H.O. revised the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Afreen F, Zobayed SMA, Kozai T. Melatonin in Glycyrrhiza uralensis: response of plant roots to spectral quality of light and UV-B radiation. J Pineal Res. 2006;41:108–115. doi: 10.1111/j.1600-079X.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- Agnihotri RK, Palni LMS, Pandey DK. Germination and seeding growth under moisture stress. Screening of landraces of Rice (Oryza sativa L.) from Kumaun region of Indian central Himalaya. J Plant Biol. 2007;34:21–27. [Google Scholar]
- Alaei Sh, Melikyan A, Kobraee S, Mahna N. Effect of different soil moisture levels on morphological and physiological characteristics of Dracocephalum moldavica. Agric Commun. 2013;1:23–26. [Google Scholar]
- Arnao MB. Phytomelatonin: discovery, content, and role in plants. Adv Bot. 2014;2014:e815769. [Google Scholar]
- Arnao MB, Hernandez-Ruiz J. Melatonin promotes adventitious and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J Pineal Res. 2007;42:147–152. doi: 10.1111/j.1600-079X.2006.00396.x. [DOI] [PubMed] [Google Scholar]
- Arnao MB, Hernandez-Ruiz J. Chemical stress by different agents affects the melatonin content of barley roots. J Pineal Res. 2009;46:295–299. doi: 10.1111/j.1600-079X.2008.00660.x. [DOI] [PubMed] [Google Scholar]
- Arnao MB, Hernandez-Ruiz J. Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014;19:789–797. doi: 10.1016/j.tplants.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantites of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Costa M, Civell PM, Chaves AR, Martinez GA. Effects of ethephon and 6- benzylaminopurine on chlorophyll degrading enzymes and peroxidase-linked chlorophyll bleaching during post-harvest senescence of broccoli (Brassica oleracea L.) at 20 C. Postharvest Biol Technol. 2005;35:191–199. doi: 10.1016/j.postharvbio.2004.07.007. [DOI] [Google Scholar]
- D’cünha GB, Satyanarayan V, Nair PM. Purification of phenylalanine ammonialyase from Rhodotorula glutinis. Phytochem. 1996;42:17–20. doi: 10.1016/0031-9422(95)00914-0. [DOI] [Google Scholar]
- Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA. Plant drought stress: effects, mechanisms and management. Agron Sustain Dev. 2009;29:185–212. doi: 10.1051/agro:2008021. [DOI] [Google Scholar]
- Fazeli F, Ghorbanli M, Niknam V. Effects of drought on biomass, protein content, lipid peroxidation and antioxidant enzymes in two sesame cultivars. Biol Plant. 2007;51:98–103. doi: 10.1007/s10535-007-0020-1. [DOI] [Google Scholar]
- Gao X, Ohlander M, Jeppsson N, Bjork L, Trajkovski V. Changes in antioxidant effects and their relationship to phytonutrients in fruit of sea buckthourn (Hippophae rhamnoides L.) during maturation. J Agric Food Chem. 2000;48:1458–1490. doi: 10.1021/jf991072g. [DOI] [PubMed] [Google Scholar]
- Gholami M, Rahemi M, Kholdebarin B. Effect of drought stress induced by polyethylene glycol on seed germination of four wild Almond species. Aust J Basic Appl Sci. 2010;4:785–791. [Google Scholar]
- Guo Z, Ou W, Lu S, Zhong Q. Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiol Biochem. 2006;44:828–836. doi: 10.1016/j.plaphy.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Haplin BE, Lee CY. Effect of blanching on enzyme activity and quality changes in green peas. J Food Sci. 1987;52:1002–1005. doi: 10.1111/j.1365-2621.1987.tb14261.x. [DOI] [Google Scholar]
- Haq A, Rashmi V, Agnihotri RK. Effect of osmotic stress (PEG) on germination and seedling survival of Lentil (Lens culinaris MEDIK.) Res J Agric Sci. 2010;1:201–204. [Google Scholar]
- Heath RL, packer L. Photoperoxidation in isolated chloroplast: I. kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophy. 1969;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hernandez I, Alegre L, Munne-Bosch S. Drought-induced changes in flavonoids and other low molecular weight antioxidants in Cistus clusii grown under Mediterranean field conditions J. Tree Physiol. 2004;24:1303–1311. doi: 10.1093/treephys/24.11.1303. [DOI] [PubMed] [Google Scholar]
- Hsiao TC. Rapid changes in levels of polyribosome in Zea mays in response to water stress. Plant Physiol. 1970;46:281–285. doi: 10.1104/pp.46.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SY, Kao CH. The protective effect of free radical scavengers and metal chelators on polyethylene glycol-treated rice leaves. Biol Plant. 2003;46:617–619. doi: 10.1023/A:1024888217021. [DOI] [Google Scholar]
- Hussein MS, El-Sherheny SE, Khalil MY, Naguib NY, Aly SM. Growth characters and chemical constituents of Dracocephalum moldavica L. plants in relation to compost fertilizer and planting distance. Sci Hortic. 2006;108:322–331. doi: 10.1016/j.scienta.2006.01.035. [DOI] [Google Scholar]
- Iturbe-Ormaexte I, Escordeo P, Arrese-Igor C, Becana M. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol. 1998;116:173–181. doi: 10.1104/pp.116.1.173. [DOI] [Google Scholar]
- Juan M, Rivero RM, Romero L, Ruiz JM. Evaluation of some nutritional and biochemical indicators in selecting salt-resistant tomato cultivars. Environ Exp Bot. 2005;54:193–201. doi: 10.1016/j.envexpbot.2004.07.004. [DOI] [Google Scholar]
- Jung S. Variation in antioxidant metabolism of young and mature leaves of Arabiodopsis thaliana subjected to drought. Plant Sci. 2004;166:459–466. doi: 10.1016/j.plantsci.2003.10.012. [DOI] [Google Scholar]
- Katerji N, Van Hoorn JW, Hamdy A, Karam F, Mastrorilli M. Effect of salinity on emergence and on water stress and early seedling growth of sunflower and maize. Agric Water Manag. 1994;26:81–91. doi: 10.1016/0378-3774(94)90026-4. [DOI] [Google Scholar]
- Kaydan D, Yagmur M. Germination, seedling growth and relative water content of shoot in different seed sizes of triticale under osmotic stress of water and NaCl. Afr J Biotechnol. 2008;7:2862–2868. [Google Scholar]
- Kostopoulou Z, Therios I, Roumeliotis E, Kanellis AK, Molassiotis A. Melatonin combined with ascorbic acid provides salt adaptation in Citrus aurantium L. seedlings. Plant Physiol Biochem. 2015;86:155–165. doi: 10.1016/j.plaphy.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Kreft S, Strukel JB, Gaberski A, Kreft I. Rutin in buckwheat herbs grown at different UV-B radiation levels: comparison of two UV spectrophotometric and HPLC method. J Exp Bot. 2002;53:1801–1804. doi: 10.1093/jxb/erf032. [DOI] [PubMed] [Google Scholar]
- Kubis J. Exogenous spermidine alters in different way membrane permeability and lipid peroxidation in water stressed barley leaves. Acta Physiol Plant. 2006;28:27–33. doi: 10.1007/s11738-006-0065-3. [DOI] [Google Scholar]
- Lazar D, Murch SJ, Beilby MJ, et al. Exogenous melatonin affects photosynthesis in characeae Chara australis. Plant Signal Behav. 2013;8(3):e23279. doi: 10.4161/psb.23279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchamo W, Gosselin A. Transpiration, essential oil glands, epicuticular wax and morphology of Thymus Vulgaris are influenced by light intensity and water supply. J Hortic Sci. 1996;71:123–134. doi: 10.1080/14620316.1996.11515388. [DOI] [Google Scholar]
- Li C, Wang P, Wei Z, Liang D, Liu C, Yin L, Jia D, Fu M, Ma F. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. Journal of Pineal Research. 2012;53(3):298–306. doi: 10.1111/j.1600-079X.2012.00999.x. [DOI] [PubMed] [Google Scholar]
- Li C, Tan DX, Liang D, Chang C, Jia DF, Ma FW. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging and Stomatal behaviour in two Malus species under drought stress. J Exp Bot. 2015;66:669–680. doi: 10.1093/jxb/eru476. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. doi: 10.1016/0076-6879(87)48036-1. [DOI] [Google Scholar]
- Ma D, Sun D, Wang C, Li Y, Guo T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol Biochem. 2014;80:60–66. doi: 10.1016/j.plaphy.2014.03.024. [DOI] [PubMed] [Google Scholar]
- Manchester LC, Tan DX, Reiter RJ, Park W, Monis K, Qi W. High levels of melatonin in the seeds of edible plants possible function in germ tissue protection. Life Sci. 2000;67:3023–3029. doi: 10.1016/S0024-3205(00)00896-1. [DOI] [PubMed] [Google Scholar]
- Meng JF, Xu TF, Wang ZZ, Fang YL, Xi ZM, Zhang ZW. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: antioxidant metabolites, leaf anatomy, and chloroplast morphology. J Pineal Res. 2014;57:200–212. doi: 10.1111/jpi.12159. [DOI] [PubMed] [Google Scholar]
- Minguez-Mosquera MI, Jaren-Galen M, Garrido-Fernandez J. Lipoxygenase activity during pepper ripening and processing of paprika. Phytochemistry. 1993;32:1103–1108. doi: 10.1016/S0031-9422(00)95073-8. [DOI] [Google Scholar]
- Nogues S, Baker NR. Effects of drought on photosynthesis in Mediterranean plants growth under enhanced UV-B radiation. J Exp Bot. 2000;51:1309–1317. doi: 10.1093/jxb/51.348.1309. [DOI] [PubMed] [Google Scholar]
- Pinheiro C, Chaves M, Ricardo C. Alteration in carbon and nitrogen metabolism induced by water deficit in the stems and leaves of Lupinus albus. J Exp Bot. 2001;52:1063–1070. doi: 10.1093/jexbot/52.358.1063. [DOI] [PubMed] [Google Scholar]
- Polle A. Dissecting the superoxide dismutase-ascorbate glutathione pathway in chloroplasts by metabolic modeling. Computer simulations as a step towards flux analysis. Plant Physiol. 2001;126:445–462. doi: 10.1104/pp.126.1.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhouane L. Response of Tunisian autochthonous pearl millet (Pennisetum glaucum L.) to drought stress induced by polyethylene glycol (PEG) 6000. Afr J Biotechnol. 2007;6:1102–1105. [Google Scholar]
- Rahnama H, Ebrahimzadeh H. The effect of NaCl on proline accumulation in potato seedlings and calli. Acta Physiol Plant. 2004;26:263–270. doi: 10.1007/s11738-004-0016-9. [DOI] [Google Scholar]
- Rai AC, Singh M, Shah K. Effect of water withdrawal on formation of free radical, proline accumulation and activities of antioxidant enzymes in ZAT12-transformed transgenic tomato plants. Plant Physiol Biochem. 2012;61:108–114. doi: 10.1016/j.plaphy.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Roe JH. The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem. 1955;212:335–343. [PubMed] [Google Scholar]
- Sangtarash MH, Qaderi MM, Chinnappa CC, Reid DM. Carotenoid differential sensitivity of canola (Brassica napus) seedlings to ultraviolet-B radiation, water stress and abscisic acid. Environ Exp Bot. 2009;66:212–219. doi: 10.1016/j.envexpbot.2009.03.004. [DOI] [Google Scholar]
- Sarropoulou VN, Dimassi-Theriou K, Therios I, Koukourikou-Petridou M. Melatonin enhances root regeneration, photosynthetic pigments, biomass, total carbohydrates and proline content in the cherry rootstock PHL-C (Prunus avium × Prunus cerasus) Plant Physiol Biochem. 2012;61:162–168. doi: 10.1016/j.plaphy.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Sato F, Yoshioka T, Fujiwara H, Higashio A, Uragami A, Tokuda S. Physiological responses of cabbage plug seedlings to water stress during low-temperature storage in darkness. Sci Hortic. 2004;101:349–357. doi: 10.1016/j.scienta.2003.11.018. [DOI] [Google Scholar]
- Sharma P, Shanker R. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. J Plant Growth Regul. 2005;46:209–221. doi: 10.1007/s10725-005-0002-2. [DOI] [Google Scholar]
- Shi Q, Ding F, Wang X, Wei M. Exogenous nitric oxides protect cucumber roots against oxidative stress induced by salt stress. Plant Physiol Biochem. 2007;45:542–550. doi: 10.1016/j.plaphy.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Shibli RA, Kushad M, Yousef GG, Lina MA. Physiological and biochemical responses of tomato micro-shoots to induced salinity with associated ethylene accumulation. Plant Growth Regul. 2007;51:159–169. doi: 10.1007/s10725-006-9158-7. [DOI] [Google Scholar]
- Sultana N, Ikeda T, Itoh R. Effect of NaCl salinity on photosynthesis and dry matter accumulation in developing rice grains. Environ Exp Bot. 1999;42:211–220. doi: 10.1016/S0098-8472(99)00035-0. [DOI] [Google Scholar]
- Syvacy A, Sokmen M. Seasonal changes in antioxidant activity, total phenolic and anthocyanin constituent of the stems of two Morus species (Morus alba L. and Morus nigra L.) Plant Growth Regul. 2004;44:251–256. doi: 10.1007/s10725-004-4500-4. [DOI] [Google Scholar]
- Szafranska K, Glinska S, Janas KM. Changes in the nature of phenolic deposits after re-warming as a result of melatonin pre-sowing treatment of Vigna radiata seeds. J Plant Physiol. 2012;169:34–40. doi: 10.1016/j.jplph.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr J. 1993;1:57–60. [Google Scholar]
- Thipyapony P, Melkonia J, Wolfe DW, Steffens JC. Suppression of polyphenol oxidase increases stress tolerance in tomato. Plant Sci. 2004;167:693–703. doi: 10.1016/j.plantsci.2004.04.008. [DOI] [Google Scholar]
- Turkan I, Bor M, Ozdemir F, Koca H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolia Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci. 2005;168:223–231. doi: 10.1016/j.plantsci.2004.07.032. [DOI] [Google Scholar]
- Vaughn KC, Lax AR, Duke SO. Polyphenol oxidase: the chloroplast oxidase with no established function. Physiol Plant. 1988;72:659–665. doi: 10.1111/j.1399-3054.1988.tb09180.x. [DOI] [Google Scholar]
- Wang P, Sun X, Chang C, Feng FJ, Liang D, Cheng LL, Ma FW. Delay in leaf senescence of Malus hupehensis by long-term melatonin application is associated with its regulation of metabolic status and protein degradation. J Pineal Res. 2013;55:424–434. doi: 10.1111/jpi.12069. [DOI] [PubMed] [Google Scholar]
- Wang P, Sun X, Li C, Wei ZW, Liang D, Ma FW. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J Pineal Res. 2013;54:292–302. doi: 10.1111/jpi.12017. [DOI] [PubMed] [Google Scholar]
- Wanger GJ. Content and vacuole/extra vacuole distribution of neutral sugars, free amino acids, and anthocyanins in protoplast. Plant Physiol. 1979;64:88–93. doi: 10.1104/pp.64.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkinson J, Hendricks I, Sioson L, et al. Accessions of Solanum tuberosum spp. andigena show differences in photosynthetic recovery after drought stress as reflected in gene expression profiles. Plant Sci. 2006;171:754–758. doi: 10.1016/j.plantsci.2006.07.010. [DOI] [Google Scholar]
- Wei W, Li QT, Chu YN, Reiter RJ, Yu XM, Zhu DH, Zhang WK, Ma B, Lin Q, Zhang JS, Chen SY. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J Exp Bot. 2015;66:695–707. doi: 10.1093/jxb/eru392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen PF, Chen JY, Wan SB, Kong WF, Zhang P, Wang W, Zhan J, Pan QH, Hung WD. Salicylic acid activates phenylalanine ammonia-lyase in grape berry in response to high temperature stress. Plant Growth Regul. 2005;55:1–10. doi: 10.1007/s10725-007-9250-7. [DOI] [Google Scholar]
- Yucel DO, Anlarsal AE, Mart D, Yucel C. Effects of drought stress on early seedling growth of Chickpea (Cicer arietinum L.) genotypes. World Appl Sci J. 2010;11:478–485. [Google Scholar]
- Zhang N, Zhao B, Zhang HJ, Weeda S, Yang C, Yang ZC, Ren S, Guo YD. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.) J Pineal Res. 2013;54:15–23. doi: 10.1111/j.1600-079X.2012.01015.x. [DOI] [PubMed] [Google Scholar]
- Zhang HJ, Zhang N, Yang RC, Wang L, Sun QQ, Li DB, et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.) J Pineal Res. 2014;57:269–279. doi: 10.1111/jpi.12167. [DOI] [PubMed] [Google Scholar]
- Zheng X, Tan DX, Allan AC, Zuo B, Zhao Y, Reiter RJ, Wang L, Wang Z, Guo Y, Zhou J, Shan D, Li Q, Han Z, Kong J. Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress. Sci Rep. 2017;7:1–12. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]