Abstract
Soil acidity causes proton (H+) rhizotoxicity, inhibits plant growth and development, and is a major yield-limiting factor for wheat production worldwide. Therefore, we investigated the physiological and biochemical responses of wheat (Triticum aestivum L.) to acidity stress in vitro. Five popular wheat cultivars developed by Bangladesh Agricultural Research Institute (BARI), namely, BARI Gom-21, BARI Gom-24, BARI Gom-25, BARI Gom-26, and BARI Gom-30, were studied in growing media under four different pH levels (3.5, 4.5, 5.5, and 6.5). We evaluated the cultivars based on their relative water content, proline (Pro) content, growth, biomass accumulation, oxidative damage, membrane stability, and mineral composition, as well as the performance of the antioxidant defense and glyoxalase systems. Although decrements of pH significantly reduced the tested morphophysiological and biochemical attributes in all the cultivars, there was high variability among the cultivars in response to the varying pH of the growing media. Acidity stress reduced growth, biomass, water content, and chlorophyll content in all the cultivars. However, BARI Gom-26 showed the least damage, with the lowest H2O2 generation, lipid peroxidation (MDA), and greater membrane stability, which indicate better tolerance against oxidative damage. In addition, the antioxidant defense components, ascorbate (AsA) and glutathione (GSH), and their redox balance were higher in this cultivar. Maximum H2O2 scavenging due to upregulation of the antioxidant enzymes [AsA peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), GSH reductase (GR), GSH peroxidase (GPX), and GSH-S-transferase (GST)] was observed in BARI Gom-26, which also illustrated significant enhancement of methylglyoxal (MG) detoxification by upregulating glyoxalase I (Gly I) and glyoxalase II (Gly II). This study also showed that balanced essential nutrient content as well as lower toxic micronutrient content was found in BARI Gom-26. Therefore, considering the physiological and biochemical attributes and growth, we conclude that BARI Gom-26 can withstand acidity stress during the early seedling stage, by regulating the coordinated action of the antioxidant defense and glyoxalase systems as well as maintaining nutrient balance.
Electronic supplementary material
The online version of this article (10.1007/s12298-019-00678-0) contains supplementary material, which is available to authorized users.
Keywords: Acidity stress, H+ rhizotoxicity, Reactive oxygen species, Antioxidant defense, Methylglyoxal
Introduction
The growing world population is projected to reach 10.9 billion by 2050, presenting a great challenge in feeding this enormous community. Moreover, climatic change is exerting abiotic stresses and reducing crop productivity, resulting in changes in agro-ecological conditions (Fraire-Velázquez and Balderas-Hernández 2013). Soil acidity is a significant abiotic stress that had been under-considered for many decades (Shavrukov and Hirai 2015). Acid soils are widespread all over the world and increasing for climatic and anthropogenic reasons (Wenzl et al. 2003). Around 26% of the ice-free land worldwide is suffering from soil acidity, and therefore, unproductive (Sumner and Noble 2003). Of that land, acid mineral soils are characterized by H+ toxicity along with metal toxicity from iron (Fe), copper (Cu), manganese (Mn), zinc (Zn), and aluminum (Al), whereas organic acid soils are characterized by H+ toxicity only (Kidd and Proctor 2001). From the agricultural point of view, pH between 6.0 and 7.3 is best, but according to Rengel (2011), almost two-third of all acidic soils in the world belongs to Ultisols (often below 5.0), Entisols (often below 3.5) and Oxisols (often below 4.6). Major limitations to crop production in acidic conditions are a combination of essential nutrient deficiency, reduction in water uptake, and metal/metalloid(s) toxicity. Furthermore, low water-holding capacity and compaction of soil might create more vulnerability for plants (Tang and Rengel 2003). Nevertheless, H+ toxicity is considered a major limiting factor for plant growth in acid soils (Kochian et al. 2015).
Plants are sessile and cannot escape acidity stress, which is initially observable in reduced root length (Angelini et al. 2005). Moreover, acidity is involved in decreased germination percentage and ion transport, altered reproductive behavior, reduced yield, and even plant survival (Van Den Berg et al. 2005; Miransari et al. 2006). The typical physiological functions in plant cells usually take place at a range of pH 7.0–7.5 (Shavrukov and Hirai 2015). When the growing media pH goes down below optimum level, it exerts a negative effect on the intracellular pH, thus hampering physiological functions that are dependent upon pH. Reports proposed that 1 unit decline in pH of the outer rooting media decrease the cytoplasmic pH to about 0.1 units (Wilkinson and Duncan 1993). To combat with the cellular pH change, the proton pumps play effective role adapting to acidity stress by pumping the proton to the vacuole in parallel with biological pH state (Yang et al. 2010; Joliot and Johnson 2011). But their performance reduced greatly and cytoplasmic pH balance disrupts, resulting in energy dissipation, which give rise to toxic reactive oxygen species (ROS) (Song et al. 2011; Zhang et al. 2015, Bhuyan et al. 2019) including singlet oxygen (1O2), superoxide anion (O·−2), hydrogen peroxide (H2O2), and hydroxyl radical (OH·). Reports have suggested that a reduction of 0.5 units in pH tends to increase ROS accumulation several folds in Hordeum vulgare L. (Song et al. 2011), Pinus sylvestris L. (Ivanov et al. 2013), and Oryza sativa L. (Zhang et al. 2015). These toxic ROS can oxidize important cellular ultrastructures (proteins, lipids, nucleic acids, etc.), leading to oxidative damage and destruction of cellular organelles (Hasanuzzaman et al. 2017), which is indicated by higher levels of malondialdehyde (MDA) in H. vulgare (Song et al. 2011) and O. sativa (Zhang et al. 2016). In addition, another cytotoxic compound, methylglyoxal (MG), a metabolic pathway byproduct, is also reported to be overproduced many fold in cytoplasm under different abiotic stress conditions, which can also damage cellular components, and result in mutations and even cell death by causing oxidative stress (Hasanuzzaman et al. 2018). To combat excess ROS and MG, plants are equipped with the antioxidant defense and glyoxalase pathways that minimize oxidative stress. Reports suggest that metabolomic modification of the antioxidant defense and glyoxalase pathways can improve abiotic stress tolerance in various crops (Hasanuzzaman et al. 2017; Mahmud et al. 2018). Hence, the physiological and molecular mechanism of acidity stress damage in plants needs further investigation.
Wheat (Triticum aestivum L.) is a major grain crop affected by acidity stress. Global wheat demand is projected to reach 1662 million tons by 2050 (CIMMYT 2017), and to fulfill this huge demand, there is no other option than to increase cultivable land. Approaches like liming acid soils have already been adopted, which increases productivity to some extent but is expensive and ecologically unsafe. Breeding tolerant cultivars to cope with soil acidity may be the most efficient way, but it is time-consuming and success depends on many factors like physiology, genetics, and gene regulatory mechanism. Hence, investigating tolerance in popular cultivars can save time and money, and at the same time, provide information regarding the morphophysiological and biochemical behavior of different cultivars under acidity stress. Therefore, we investigated five popular wheat cultivars to determine H+-induced oxidative damage and the response of the antioxidant and glyoxalase systems, along with related physiological parameters and mineral nutrient content during the early seedling stage. To the best of our knowledge, this report is the first to elucidate the negative effect of only H+ rhizotoxicity stress on wheat seedlings, in which the coordinated actions of the antioxidant defense and glyoxalase systems have been elucidated.
Materials and methods
Plant material, experimental conditions, and treatment
Seeds of different wheat cultivars (BARI Gom-21, BARI Gom-24, BARI Gom-25, BARI Gom-26, BARI Gom-30) were collected from Bangladesh Agricultural Research Institute (BARI), Bangladesh, and used as experimental material. The seeds were surface sterilized with 1% NaOCl and soaked in distilled water (DH2O) for 4 h. An equal number of seeds (35) was sown in plastic pots placed on a plastic net and incubated for 40 h, and then transferred to a growth chamber with 25 healthy seedlings in each pot. The seedlings were cultivated for 6 d according to the method of Hasanuzzaman et al. (2018) and fed with Hoagland nutrient solution (Hoagland and Arnon, 1950), which was changed every 3 d. Six-day-old seedlings were gradually exposed to a low-pH nutrient solution for 4 d at one unit every day up to the desired level, and kept fixed for the rest of the treatment period (3 d). Four different pH were used as treatments: 6.5 (Control), 5.5, 4.5, and 3.5. The nutrient solutions were checked for change in pH and adjusted every day. The seedlings were harvested at 13 d and data were taken for different morphophysiological and biochemical parameters.
Estimation of leaf relative water content and proline content
The leaf relative water content (RWC) were measured according to the method of Mahmud et al. (2018). The relative water content was calculated using the following equation:
The free proline (Pro) content was measured using the method of Nahar et al. (2017).
Determination of relative growth and biomass
The shoot and root lengths were measured using a metric scale and expressed as cm seedling−1. Afterwards, the fresh weight (FW) and dry weight (DW) of the shoots and roots were measured and expressed as mg seedling−1 (Nahar et al. 2017). The relative elongation of the seedling shoots and roots under different treatments were measured according to the procedure by Song et al. (2011).
Determination of electrolyte leakage, membrane stability index, and membrane injury
Electrolyte leakage (EL), membrane stability index (MSI), and % membrane injury (MI) of the leaves and roots were determined by observing the electrical conductivity (EC) of the leaf and root leachates in DH2O at 40 °C (EC1) and 100 °C (EC2). Thus, EL was then calculated using the following equation (Dionisio-Sese and Tobita 1998).
The MSI was then calculated using the following equation (Sairam 1994).
The leaf tissue cell MI was measured compared with the conductivity of control recorded as C1, and C2, and calculated using the following equation (Premachandra et al.1990):
Quantification of photosynthetic pigments
The photosynthetic pigment content was quantified according to the method of Wellburn (1994) using 80% acetone.
Determination of oxidative stress markers
The MDA content was estimated using the method of Mahmud et al. (2018) as thiobarbituric acid (TBA) reactive substances (TBARS). Further, H2O2 was estimated using the method of Nahar et al. (2017).
Histochemical confirmation of ROS generation
Histochemical staining was performed to confirm ROS (H2O2 and O·−2) production according to the method of Chen et al. (2010) with a slight modification. Fresh leaves were dipped in 0.1% nitrobluetetrazolium (NBT) and 0.1% 3-diaminobenzidine (DAB) solution for 48 h in the dark to confirm O·−2 and H2O2, respectively. The leaves were then bleached with 95% hot ethanol and observed for dark blue spots (due to the reaction of NBT with O·−2) and brown spots (due to the reaction of DAB and H2O2), and were then photographed.
Determination of non-enzymatic antioxidant (AsA-GSH) content
The non-enzymatic antioxidant ascorbate (AsA) and glutathione (GSH) contents were determined according to the method of Hasanuzzaman et al. (2018) by extracting the leaves with meta-phosphoric acid. Both total and reduced AsA were then determined optically at 265 nm, and dehydroascorbate (DHA) was calculated by subtracting reduced AsA from total AsA. Total GSH and GSSG were determined based on the oxidation of GSH by 5,5-dithio-bis(2-nitrobenzoic acid) (DTNB) and subsequent enzymatic recycling in the presence of GSH reductase (GR) and NADPH. The reduced portion of GSH was then calculated after subtracting GSSG from total GSH.
Estimation of protein quantity and cellular antioxidant enzyme activity
Freshly harvested wheat leaf samples (500 mg) were extracted with ice cold extraction buffer containing AsA (1 mM), K-P buffer (50 mM, pH 7.0), KCl (100 mM), β-mercaptoethanol (5 mM), and glycerol (10%, w/v). The supernatants were collected and preserved (− 60 °C), and used to determine the protein and enzyme activity.
The protein quantity was determined spectrophotometrically at 595 nm according to the method of Bradford (1976).
Lipoxygenase (LOX; EC: 1.13.11.12) activity was determined by taking linoleic acid as a substrate and observing the increase in absorbance at 234 nm (Doderer et al. 1992). Ascorbate peroxidase (APX; EC: 1.11.1.11) activity was determined by observing the decrease in absorbance at 290 nm using H2O2 as a substrate (Hasanuzzaman et al. 2018). Monodehydroascorbate reductase (MDHAR; EC: 1.6.5.4) activity was assayed through the oxidation of AsA with the enzyme AsA oxidase (AO), and subsequent reduction in the presence of NADPH. The conversion of NADPH to NADP+ was observed at 340 nm (Hossain et al. 1984). Dehydroascorbate reductase (DHAR; EC: 1.8.5.1) activity was recorded by observing the reduction in DHA at 265 nm in the presence of GSH (Hasanuzzaman et al. 2018). Measuring glutathione reductase (GR; EC: 1.6.4.2) activity involves a NADPH-dependent reduction of GSSG to GSH. The decrease in absorbance was read at 340 nm (Hasanuzzaman et al. 2018). Superoxide dismutase (SOD; EC 1.15.1.1) activity was determined based on the reduction of nitro blue tetrazolium (NBT) using the xanthine–xanthine oxidase system (El-Shabrawi et al.2010). Catalase (CAT; EC: 1.11.1.6) activity was assayed by observing the conversion of substrate H2O2 to water at 240 nm following the method of Hasanuzzaman et al. (2018). The glutathione S-transferase (GST; EC: 2.5.1.18) activity assay was based on the reaction between GSH and the substrate 1-chloro-2,4-dinitrobenzene (CDNB) to form a conjugated intermediate. The rate of conjugate production was measured spectrophotometrically at 340 nm (Hasanuzzaman et al. 2018). Glutathione peroxidase (GPX; EC: 1.11.1.9) activity was assayed through the oxidation of GSH to GSSG, to convert H2O2, and GSSG is further converted to GSH by the action of NADPH-dependent GR. The conversion of NADPH to NADP+ was observed optically at 340 nm (Elia et al. 2003).
Determination of glyoxalase activity and methylglyoxal content
Glyoxalase I (Gly I; EC: 4.4.1.5) activity was assayed based on the reaction of GSH with the substrate MG to convert into S-D-lactoylglutathione (SLG) (Hasanuzzaman et al. 2018). Glyoxalase II (Gly II; EC: 3.1.2.6) activity was assayed based on the hydrolysis of SLG to D-lactate and GSH (Principato et al. 1987). Methylglyoxal was determined spectrophotometrically using an N-acetyl-l-cysteine assay at 288 nm (Mahmud et al. 2018).
Determination of mineral content
Mineral nutrient content was determined after extracting an oven-dried plant sample (0.1 g) in an HNO3:HClO4 (5:1 v/v) acid mixture followed by inductively coupled plasma mass spectrometry (ICP-MS) (Mahmud et al. 2018).
Statistical analysis
The data were evaluated statistically using XLSTAT 2018 (Addinsoft 2018). Analysis of variance (ANOVA) was performed and the comparisons among treatments were revealed using Fisher’s LSD test.
Results
Acidity alters the osmotic balance and growth attributes
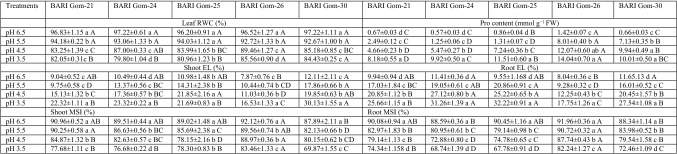
Leaf RWC of the wheat cultivar seedlings under acidic pH stress decreased in a dose-dependent manner. Compared with the control, the maximum reduction in leaf RWC (17%) was due to acidic stress (pH 3.5) in the BARI Gom-25 seedlings (Table 1). Proline content under different levels of acidity increased in a dose-dependent manner, and compared with control, a 17-fold increase in Pro content was found in the pH 3.5-stressed BARI Gom-24 seedlings. However, of all the cultivars, BARI Gom-26 had the highest Pro content under every level of acidic pH (Table 1).
Table 1.
Leaf relative water content (RWC), leaf proline (Pro) content, electrolyte leakage (EL) and membrane stability index (MSI) of root and shoot of the wheat (Triticum aestivum L.) cultivar seedlings under different levels of acidic pH
Means (± SD) were calculated from three replications (n = 3) for each treatment. Values with different letters (lower case letter in a column and upper case letter in a row) are significantly different at P ≤ 0.05 applying Fisher’s LSD test
The growth of the wheat seedling cultivars was also markedly inhibited by high H+ activity (low pH) in the nutrient medium (Supplemental Table 1, Supplemental Fig. 1). Low pH significantly decreased the shoot and root lengths and the relative elongation of the shoots and roots compared with control (pH 6.5) (Supplementary Table 1). The highest root and shoot FW and DW was observed in the seedlings in the pH 6.5 nutrient solution, and the root and shoot FW and DW decreased significantly in a dose-dependent manner with decreasing pH of the nutrient media. Therefore, the maximum reduction in shoot FW (40%) was found in BARI Gom-25, root FW (38%) in BARI Gom-24, and root FW and DW (42 and 48%, respectively) in BARI Gom-26 compared with their respective controls (Supplemental Table 1).
Acidity causes over-generated ROS and peroxidized lipids, and damages cell membranes
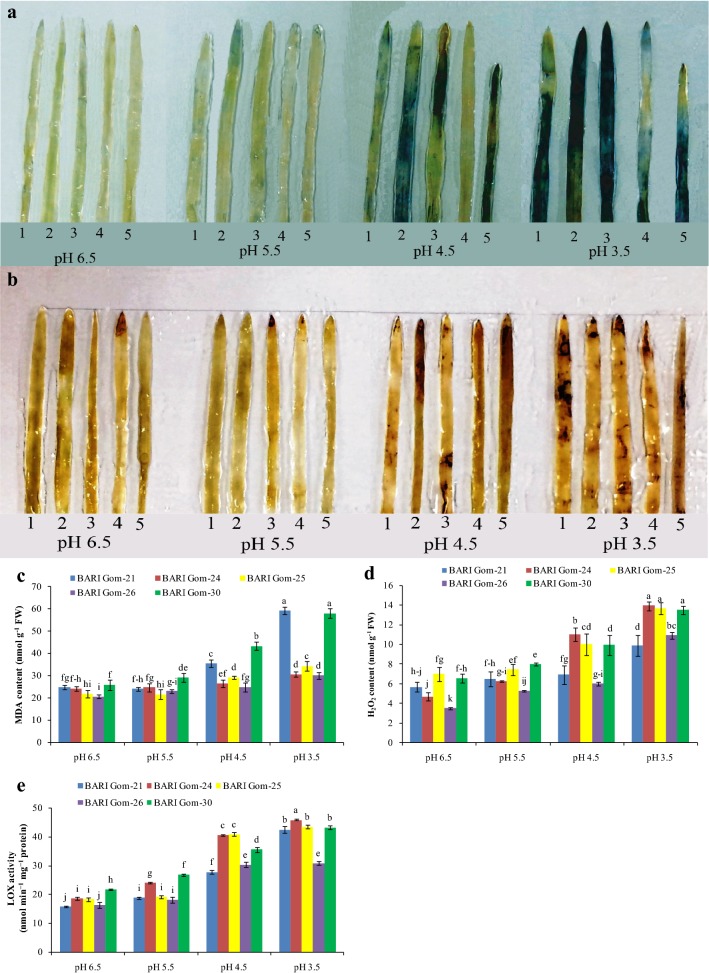
The production of ROS (O·−2 and H2O2) increased with a decrease in pH level, which was detected from the histochemical staining of the leaves. The maximum number of intense blue spots of O·−2(Fig. 1a) due to NBT staining and brown spots of H2O2 due to DAB staining (Fig. 1b) were observed in all the varieties exposed to pH 3.5 compared with control (Fig. 1a, b).
Fig. 1.
Histochemical confirmation of O·−2 (a) and H2O2 (b), and malondialdehyde (MDA) content (c), H2O2 content (d), and LOX activity (e) in the leaf tissue of wheat (Triticum aestivum L.) seedling cultivars grown under different acidic pH. (1, 2, 3, 4, and 5 represent BARI Gom-21, BARI Gom-24, BARI Gom-25, BARI Gom-26, and BARI Gom-30, respectively). Mean (± SD) was computed from three replications of each treatment. Bars with dissimilar letters are significantly different at P ≤ 0.05 from Fisher’s LSD test
The content of H2O2 was quantified and a substantial increase in H2O2 content due to dose-dependent acidic pH stress was found (Fig. 1d). Compared with control, 73, 195, 95, 209, and 106% increases in H2O2 content were observed in BARI Gom-21, 24, 25, 26, and 30, respectively. Because of over-generated ROS, lipid peroxidation (measured as MDA) also increased in a dose-dependent manner in the leaf tissue of the wheat cultivars examined, upon exposure to acidic pH. Compared with the respective controls, the maximum increase in MDA content was quantified in BARI Gom-21, followed by BARI Gom-30, in the seedlings exposed to pH 3.5 (Fig. 1c). Increased lipid peroxidation indicates an increase in LOX activity, and hence LOX was quantified and a similar increase in LOX activity was found in the wheat seedling leaf tissues (Fig. 1e).
Because of lipid peroxidation, the cell membrane bursts and the electrolytes leak out of the cell. Hence, we measured the EL of the seedling leaves and roots of each cultivar. Leaf and root EL increased as the pH decreased, and the cultivars responded differently to decreasing pH. The maximum increase in leaf EL was found in BARI Gom-30, whereas the maximum increase in root EL was found in BARI Gom-25 compared with control (Table 1). The MSI and MI measurements indicated a similar trend in the detrimental effects of low pH (Supplemental Table 1).
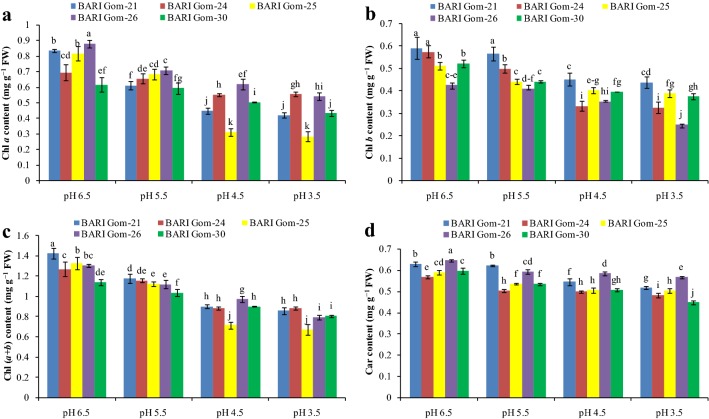
Acidity damages the photosynthetic pigments
Low rhizosphere pH destroyed photosynthetic pigments in the leaf tissues (Fig. 2 and Supplemental Fig. 1). Chlorophyll a content decreased in the low-pH-stressed wheat seedlings in a dose-dependent manner and maximum reduction was observed in BARI Gom-25 compared with the control (Fig. 2a). Chlorophyll b content decreased in a similar fashion. Compared with control, the maximum decrease in Chl b content was found in the pH 3.5-treated BARI Gom-24 seedlings (Fig. 2b). Acidic pH stress reduced Chl (a + b) content compared with the control seedlings. The maximum decrease in the value of Chl (a + b) was in BARI Gom-25 (Fig. 2c). Another important photosynthetic pigment, Car, was also reduced by acidic pH, and compared with control; the highest reduction was observed in the pH 3.5-treated BARI Gom-30 seedlings (Fig. 2d).
Fig. 2.
Chlorophyll a (a), Chl b (b), Chl (a + b) (c), and Car (d) content of the wheat cultivar leaves under different levels of acidic pH. Mean (± SD) was computed from three replications of each treatment. Bars with dissimilar letters are significantly different at P ≤ 0.05 from Fisher’s LSD test
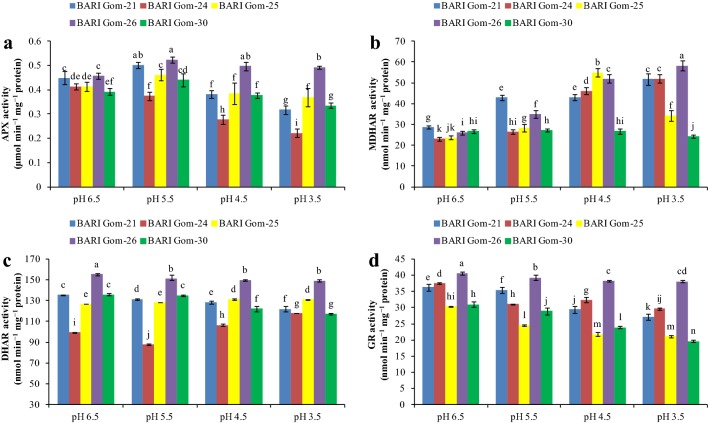
Acidity interrupts the antioxidant defense mechanism
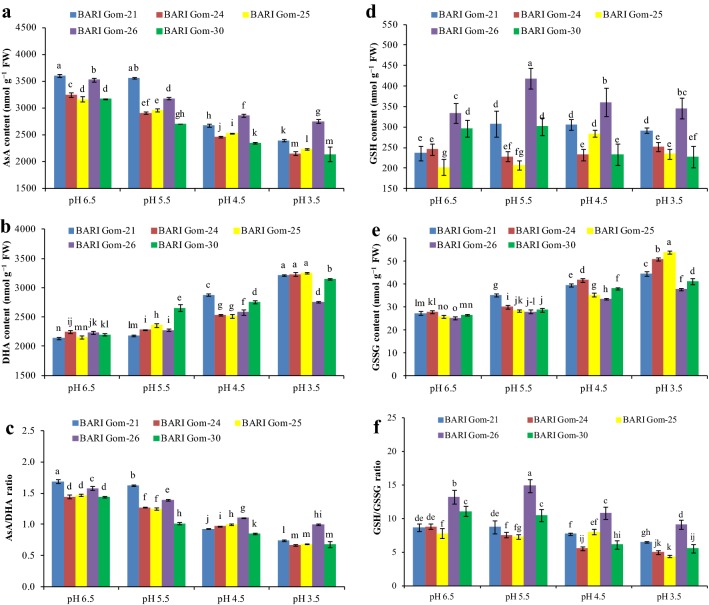
With the increase in the severity of acidity, antioxidant defense activity decreased greatly (Figs. 3, 4, 5). Ascorbate content was quantified and a substantial decrease due to dose-dependent acidic pH stress was found (Fig. 3a). In contrast, DHA increased as a result of a pH decrease in the growing media (Fig. 3b), which further reduced the ratio between AsA and DHA (Fig. 3c).
Fig. 3.
AsA (a) and DHA (b) contents, AsA/DHA ratio (c), GSH (d) and GSSG (e) content, and GSH/GSSG ratio (f) of the wheat cultivar leaves under different levels of acidic pH. Mean (± SD) was computed from three replications of each treatment. Bars with dissimilar letters are significantly different at P ≤ 0.05 from Fisher’s LSD test
Fig. 4.
APX (a), MDHAR (b), DHAR (c), and GR (d) activities of the wheat leaves under different levels of acidic pH. Mean (± SD) was computed from three replications of each treatment. Bars with dissimilar letters are significantly different at P ≤ 0.05 from Fisher’s LSD test
Fig. 5.
SOD (a), CAT (b), GPX (c), and GST (d) activities in the wheat leaves under different levels of acidic pH. Mean (± SD) was computed from three replications of each treatment. Bars with dissimilar letters are significantly different at P ≤ 0.05 from Fisher’s LSD test
Another vital component of the antioxidative defense mechanism, GSH content, also changed because of the increase in H+ toxicity. Except for BARI Gom-24, GSH content in the cultivars increased with exposure to pH 5.5 and decreased thereafter with the increasing severity of H+ toxicity. However, BARI Gom-26 maintained its GSH content (Fig. 3d). Conversely, the GSSG content increased as a result of decreased pH in the leaf tissue of the wheat cultivars, with the maximum in BARI Gom-25 (107%) compared with control (Fig. 3e). As a result, the ratio of GSH and GSSG decreased in all the cultivars, with a maximum of 50% reduction found in BARI Gom-30 treated with pH 3.5 compared with control (Fig. 3f).
Compared with pH 6.5, the APX activity of BARI Gom-26 increased with the different low-pH stress treatments, but decreased APX activity was found in BARI Gom-24. The rest of the cultivars exhibited increased APX activity upon mild stress (pH 5.5) and decreased with lower pH (Fig. 4a). Conversely, MDHAR activity increased in all the cultivars except BARI Gom-30 with exposure to extremely low pH (3.5) compared with control (Fig. 4b), while increased DHAR activity was found in BARI Gom-25 (in all low-pH stress treatments) and BARI Gom-24 (in pH 4.5 and 3.5), compared with control. The other cultivars showed a decrease in DHAR activity (Fig. 4c). Glutathione reductase activity decreased with low-pH exposure in all the cultivars (Fig. 4d). It is to be noted that BARI Gom-26 showed maximum DHAR and GR activity among the tested cultivars (Fig. 4c, d).
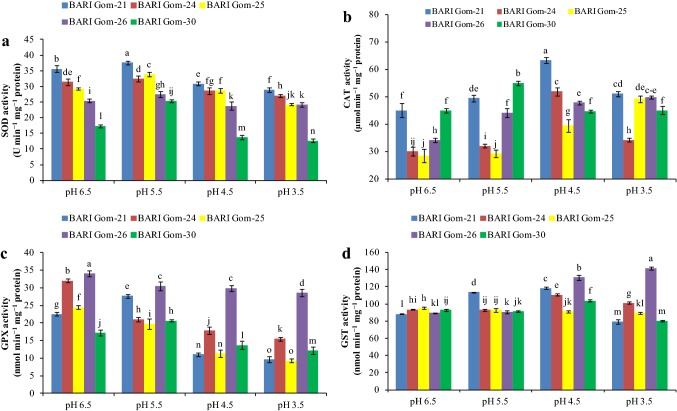
With exposure to low-pH stress, SOD activity increased slightly under mild acidity stress (pH 5.5) and decreased under lower pH in all the wheat cultivars (Fig. 5a), whereas CAT activity increased (Fig. 5b). On the other hand, GSH-dependent GPX activity decreased in a dose-dependent manner in all the cultivars except BARI Gom-30, in which GPX activity increased under pH 5.5 treatment (Fig. 5c). Another GSH-dependent enzyme, GST, responded differently with exposure to low pH. A dose-dependent reduction in GST activity was observed in BARI Gom-25, whereas a consistent increase in GST activity was found in BARI Gom-26 (Fig. 5d).
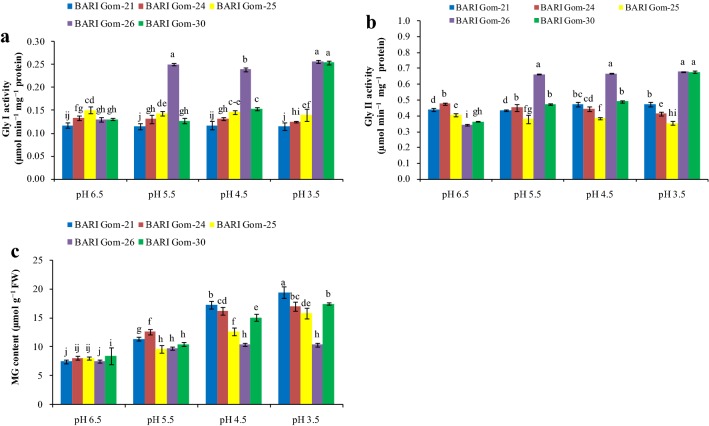
Acidity disrupts the glyoxalase pathway
Components of the MG detoxification system were also hampered because of the acidic pH of the growing media in a dose-dependent manner. The first enzyme of the MG detoxification system, Gly I, decreased sharply in all the cultivars except BARI Gom-26 (Fig. 6a). With exposure to low-pH stress, Gly II activity also declined in all the wheat cultivars except BARI Gom-26 and BARI Gom-30 (Fig. 6b). As a result, no increase in MG content was observed in BARI Gom-26 with exposure to different acidic pH, whereas a significant increase in MG content was observed in the other wheat cultivars (Fig. 6c).
Fig. 6.
Gly I (a) and Gly II (b) activities and MG content (c) in the wheat leaves under different levels of acidic pH. Mean (± SD) was computed from three replications of each treatment. Bars with dissimilar letters are significantly different at P ≤ 0.05 from Fisher’s LSD test
Acidity disrupts the nutrient balance
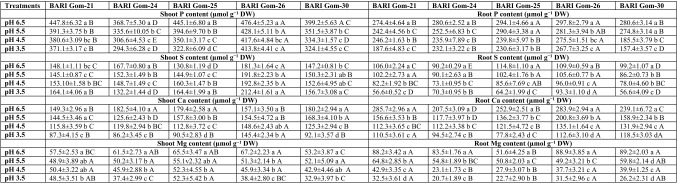
The content of P in the shoots and roots of all the studied wheat cultivars decreased in a dose-dependent manner; however, BARI Gom-26 performed better through more efficient uptake and translocation of P (Table 2). On the other hand, the content of S in the shoots increased in three cultivars (BARI Gom-25, BARI Gom-26, BARI Gom-30) and decreased in the others, whereas a significant decrease in root S content was observed in all the tested cultivars (Table 2). The content of major cations, Ca2+ and Mg2+, also decreased because of H+ toxicity in the shoots and roots of all the wheat cultivars (Table 2).
Table 2.
Shoot and root mineral content (P, S, Ca and Mg) of the wheat (Triticum aestivum L.) cultivar seedlings under different levels of acidic pH
Means (± SD) were calculated from three replications (n = 3) for each treatment. Values with different letters (lower case letter in a column and upper case letter in a row) are significantly different at P ≤ 0.05 applying Fisher’s LSD test
There were significant differences among other nutrients also. Decreased K and increased Mn, Fe, and Zn contents were found in the shoots and roots of all the tested cultivars, which indicates that acidic pH disrupts their uptake and homeostasis (Supplemental Table 2).
Discussion
Acidity altered the shoot and root growth, reduced osmotic balance, and enhanced osmolyte biosynthesis
Crop productivity can be hampered by low pH (acidity) of growing media. Acid soils, pH of 5.5 or lower, are one of the limitations of crop production (Kochian et al. 2015). In the present study, low pH (acidity) stress negatively influenced the morphophysiological attributes of wheat seedlings. After exposing the seedlings to acidic pH, a significant decrease in seedling growth in terms of height, root length, relative percent elongation of the shoots and roots, and FW and DW of both shoots and roots were observed in all the tested wheat cultivars. A significant reduction in root elongation due to higher H+ toxicity has been reported (Zhang et al. 2015; Long et al. 2017). This reduction in root growth is caused by a decrease in root cell division and enlargement (Kochian et al. 2015). Zhang et al. (2015) found that low pH inhibited the growth of O. sativa, resulting in decreased root length, root surface area, new fine root production, and root dry weight, which is in keeping with the results of our study. Long et al. (2017) also found root damage caused by acidity stress in two Citrus species.
For stable physiological and biochemical activities including photosynthesis, cell turgidity is important (Lawson and Vialet-Chabrand 2018). In our study, changes in growing media pH to an acidic condition reduced root length and subsequently caused root damage, and resulted in water unavailability in the growing shoots and consequently induced artificial drought stress in the plants, which is confirmed from the lower RWC and elevated Pro content in the seedlings exposed to acidity stress. Induction of artificial drought stress in acidity-affected Citrus plants is also reported by Long et al. (2017). Overproduced Pro may help to avoid physiological injury and maintain major physiological processes (Hasanuzzaman et al. 2014). Therefore, the higher accumulation of Pro in our study due to acidity stress might be linked to upregulated biosynthesis with a decrease in oxidation of Pro (Amist and Singh 2017). Furthermore, an elevated Pro content under acidity stress is attributed to protection against oxidative injury and maintenance of water status (Hasanuzzaman et al. 2014). Hence, a higher Pro content in BARI Gom-26 might have protected the cells from desiccation and at the same time reduced oxidative injury.
Acidity enhanced ROS production, induced oxidative stress and peroxidized lipids, increased membrane injury, and damaged the photosynthetic pigments
As a sessile organism, plants cannot avoid environmental stresses, and one obvious consequence of abiotic environmental stress is over-generated ROS (1O2, O·−2, H2O2, OH·). In line with other abiotic stress factors, low-pH stress also resulted in elevated lipid peroxidation, H2O2 generation, and LOX activity. Histochemical staining revealed signs of excess ROS generation (O·−2 and H2O2), where dark blue and brown spots were seen in the wheat cultivars leaves resulting from overgeneration of O·−2 and H2O2, respectively. The results of our study are in agreement with that of Song et al. (2011), who found oxidative damage and elevated MDA content in H. vulgare. Similarly, ROS content rises with increasing acidification toward pH 4.5 in P. sylvestris (Ivanov et al. 2013). Qiao et al. (2018) found that acidic stress hampers Arabidopsis growth by overgenerating H2O2 and MDA.
With the increase in H2O2 and MDA, the plants suffered from severe oxidative stress and consequently, membrane damage. As a result, the electrolytes leaked out of the cell. Hence, EL increased, MSI decreased, and MI was severe, resulting from the decrease in pH. This finding corroborates that of Rouphael et al. (2015), who found that acidic soil induced higher EL in Cucurbita pepo under pH 3.5. Extremely low pH accelerates cell membrane disruption of roots through high production of H2O2 (Zhang et al. 2015). In addition, Chen et al. (2013) studied some tree species that are tolerant and sensitive to low pH and found excess O·−2 and H2O2, which caused membrane lipid peroxidation and damage, and concluded that every species possesses some inherent capacity to tolerate acidity stress.
Extreme acidity not only causes oxidative stress but also destroys photosynthetic pigments (Hasanuzzaman et al. 2018). In our study, the low pH induced greater ROS generation, inhibited photosynthesis by causing leaf chlorosis and destroyed the photosynthetic pigments [Chl a, Chl b, Chl (a + b), Car]. A similar result was also reported by Long et al. (2017) in Citrus, in which lower photosynthesis resulted from an upstream accumulation of ROS. The model plant Arabidopsis also showed reduced Chl content caused by acidic stress (Qiao et al. 2018).
Acidity stress altered the antioxidant enzyme activity and non-enzyme antioxidant content, resulting in poor antioxidative defense and glyoxalase system activity
Exposure to extremely low pH disrupts the photosynthetic machinery; hence, the energy converted from sunlight cannot be consumed to produce glucose and results in increased ROS production. Therefore, plants need to detoxify extra ROS. The antioxidant defense system starts with the dismutation of O·−2 to H2O2 by using the enzyme SOD, regarded as the first line of defense (Gill et al. 2015). Reports suggest that SOD activity gradually increases under acidity (Ivanov et al. 2013). Qiao et al. (2018) also found a similar result in Arabidopsis, with a 50% increase in SOD activity. In our study, SOD activity increased under mild stress (pH 5.5) and decreased with increased severity of acidity stress. This finding may be due to overproduction of O·−2 under extreme stress, which might be beyond the scavenging ability of SOD present in the cells. Hence, our study is in keeping with that of Zhang et al. (2015), who found down-regulated SOD activity in O. sativa under low pH.
Higher CAT activity is vital for reducing H2O2 content in plants (Hasanuzzaman et al. 2018). Song et al. (2011) reported higher CAT activity in H. vulgare under acidity stress, whereas O. sativa shows a down-regulated response of CAT under low pH condition (Zhang et al. 2015). Although an upregulation of CAT activity was observed in seedlings under extremely low pH stress, the H2O2 content did not decrease, so the upregulation of CAT activity was not enough to scavenge the overproduced H2O2, which indicates the necessity of the AsA-GSH pathway in scavenging H2O2.
A decrease in AsA content with increased DHA content was observed, although the APX activity increased initially under mild stress (pH 5.5), but later decreased (pH 4.5 and 3.5). Despite lower APX activity, the decreasing AsA content is probably attributed to scavenging of O·−2 and H2O2 (Du et al. 2012; Smirnoff 2018). On the other hand, acidity stress-induced degradation of photosynthetic capacity reduced glucose production. As a result, the reduction in glucose production hampered the AsA biosynthesis, which might be one reason for the lower AsA content in our study (Smirnoff and Wheeler 2000). Conversely, increased DHA production might be attributed to higher AsA oxidation due to ROS scavenging and lower activity of DHAR to rejuvenate AsA content (Rohman et al. 2016). On the other hand, reduced APX activity under severe stress was seen, which might be due to the increase in DHA content, or conversely, the inactivity is due to a lack of coenzyme (Rohman et al. 2016). Although MDHAR activity increased under all levels of acidity, the AsA/DHA ratio could not be maintained, so the plants suffered from severe oxidative stress. The results of our study are in line with previous studies (Martins et al. 2013; Zhang et al. 2016). Hence, the insufficient functioning of the AsA-metabolism resulted from both APX and DHAR inactivation under extremely low pH stress, and consequently, the stagnation of the biochemical processes in the plants.
The S-containing potent non-enzyme antioxidant, GSH, boosts ROS scavenging together with the enzymes GPX and GST, while GST also contributes to xenobiotic detoxification (Hasanuzzaman et al. 2018). Variation in GSH content was found with the variation in genotypes, where GSH content sharply decreased in BARI Gom-30, and others showed fluctuations in a decreasing pattern with the increase in acidity. At pH 3.5, however, all the cultivars showed decreased GSH content compared with control. In contrast, GSSG content increased sharply with the increase in H+ toxicity because of decreased or a small increase in DHAR activity at the same time as the decrease in GR activity, resulting in a drop of the GSH/GSSG ratio. Glutathione reductase activity increased but failed to work proportionately to recycle GSH, and together with the GPX and GST activity increasing in the GSSG content, resulted in failure to maintain enough balance between GSH/GSSH to tackle oxidative stress. Shi et al. (2006) reported increased GR activity under acidic stress, while Qiao et al. (2018) reported increased GSH in Arabidopsis under an acidic condition, which is supported by our study to some extent. On the other hand, BARI Gom-21 and BARI Gom-30 showed increased GPX and GST activity up to certain level of pH, while it decreased at much lower pH. Shi et al. (2006) and Gabara et al. (2003) reported increased GPX and GST activities in Cucumis sativus L. and Solanum lycopersicum L., respectively, under low pH, which is partially supported by our study.
Methylglyoxal is produced normally in respiratory metabolism but overproduced under stress conditions, and becomes cytotoxic if not detoxified (Hoque et al. 2016). A sharp increase in MG content with decreasing pH was observed that corroborates the decreased activities of Gly I and Gly II, which in turn play a pivotal role in detoxifying MG. This result is consistent with previous reports, which suggested ROS induced MG production and MG induced ROS production vice versa (Kaur et al. 2015). The MG overproduced in abiotic stess is detoxified by the MG detoxification system which acts in a modulated way (Hasanuzzaman et al. 2017). However, the rate at which MG is generated under abiotic stress is usually higher than the rate of detoxification. Therefore, MG toxicity could be eliminated by overexpression or higher activity of the glyoxalase enzymes and thereby confer stress tolerance (Kaur et al. 2014), which was observed in BARI Gom-26 under low-pH stress.
Acidity stress modified the nutrient uptake ability and created a nutrient imbalance
Acidity hampers root growth, overgenerates ROS, damages the root membranes, and reduces the water and nutrient uptake ability of the seedlings (Long et al. 2017). Accordingly, a reduced amount of P, K, Ca, and Mg was found in all the tested cultivars, which indicates the inability of seedling roots to uptake these nutrient elements in the right quantities. Anugoolprasert et al. (2012) reported a decrease in P, K, Ca, and Mg in sago palm seedlings under acidic stress. In this study, high S content in the shoots of some of the varieties under low pH was found, which may be responsible for the higher GSH content in those varieties. In contrast, the S content in the roots decreased because of low pH, which might be due to the translocation of more S to the shoots to produce more GSH to confer oxidative damage tolerance. Our results are consistent with those in a previous report about increased S content in the shoots of a few crops under low pH (Islam et al. 1980). Acidity commonly increases the availability of certain micronutrients; hence, an increased accumulation of Mn, Fe, and Zn was found in the wheat seedlings, regardless of cultivar. It is thought that extremely low pH inhibits cation uptake (George et al. 2012), which was not evident in our study. Thus, plants suffer from nutrient deficiency as well as toxicity that is induced by acidity stress.
In our search for an acidity-tolerant cultivar, we found that BARI Gom-26 showed higher RWC and Pro content, lower oxidative stress, comparatively higher AsA and GSH content and their ratio, and comparatively higher activities of APX, MDHAR, DHAR, GR, GPX, and GST under an acidic condition, which lowered the H2O2 level and MDA production. Moreover, the glyoxalase system also performed better in this cultivar, compared with the others. However, reports suggest that the differential response to the same stress may happen due to the genetic makeup of the genotypes, where the tolerant genotype may show a higher antioxidative capacity, and hence have more protection against severe oxidative damage (Shah et al. 2017), which is evident from our study regarding BARI Gom-26 (Supplemental Fig. 2).
Conclusion
Our findings show that wheat growth was significantly reduced in growing media at a low pH. Different morphophysiological attributes (shoot and root length, shoot and root biomass, water and chlorophyll content in the leaves) of the wheat seedlings decreased significantly with decreasing pH. In addition, low pH disrupted the antioxidant defense system and MG detoxification pathway, causing the plants to suffer from severe oxidative stress, and subsequent lipid peroxidation and membrane damage. Furthermore, the low-pH stress caused a nutrient imbalance, which is basically due to H+ rhizotoxicity-induced root damage. The level of tolerance to low pH differed among the cultivars examined, but BARI Gom-26 exhibited better tolerance against acidity at the early seedling stages by upregulating the antioxidant defense and glyoxalase system, synthesize more osmolyte (Pro) and retain water balance to mitigate osmotic stress. Moreover, restoration of photosynthetic pigments as well as nutrient balance was also observed in BARI Gom-26 (Fig. 1). However, being sessile, plants cannot escape from a habitat to cope with acidic-pH stress, so modulating the antioxidative defense and glyoxalase systems could be an important strategy to improve the performance of wheat seedlings under acidity conditions. Thus, our findings should help researchers develop strategies to cope with acidity-stress tolerance in wheat. These results should also contribute to further research in phytoprotectant-assisted modulation of the antioxidative defense and glyoxalase systems under acidity stress in wheat and other crops.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
This research was funded by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. We thank Dr. Akinari Sonoda and Dr. Yoji Makita, Health Environment Control Research group, Health Research Institute, Advanced Industrial Science and Technology (AIST), Japan, for their kind support in analyzing the mineral contents. Thanks to Asst. Professor Khursheda Parvin and Asst. Professor Sayed Mohammad Mohsin, Sher-e-Bangla Agricultural University for their critical review and comments regarding manuscript preparation. We also acknowledge Abdul Awal Chowdhury Masud and Md. Shahadat Hossen, Laboratory of Plant Stress Response, Faculty of Agriculture, Kagawa University, Japan, for their kind assistance conducting the research.
Author Contributions
MHMBB conceived, designed, and performed the experiment and prepared the manuscript. MSH, JAM and MUA actively participated in executing the experiment. MH designed the experiment, analyzed the data and edited the manuscript. MF conceived, designed, and monitored the experiment. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mirza Hasanuzzaman, Email: mhzsauag@yahoo.com.
Masayuki Fujita, Email: fujita@ag.kagawa-u.ac.jp.
References
- Addinsoft . XLSTAT V. 2018.1.01: data analysis and statistics software for Microsoft Excel. Paris: Addinsoft; 2018. [Google Scholar]
- Amist N, Singh NB. Responses of enzymes involved in proline biosynthesis and degradation in wheat seedlings under stress. Allelopathy J. 2017;42:195–205. doi: 10.26651/allelo.j./2017-42-2-1116. [DOI] [Google Scholar]
- Angelini J, Taurian T, Morgante C, Ibáñez F, Castro S, Fabra A. Peanut nodulation kinetics in response to low pH. Plant Physiol Biochem. 2005;43:754–759. doi: 10.1016/j.plaphy.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Anugoolprasert O, Kinoshita S, Naito H, Shimizu M, Ehara H. Effect of low pH on the growth, physiological characteristics and nutrient absorption of sago palm in a hydroponic system. Plant Prod Sci. 2012;15:125–131. doi: 10.1626/pps.15.125. [DOI] [Google Scholar]
- Bhuyan MHMB, Hasanuzzaman M, Mahmud JA, Hossain M, Bhuiyan TF, Fujita M. Unraveling morphophysiological and biochemical responses of Triticum aestivum L. to extreme pH: coordinated actions of antioxidant defense and glyoxalase systems. Plants. 2019;8(1):24. doi: 10.3390/plants8010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen F, Wang F, Wu F, Mao W, Zhang G, Zhou M. Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol Biochem. 2010;48:663–672. doi: 10.1016/j.plaphy.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang WH, Liu TW, Wu FH, Zheng HL. Photosynthetic and antioxidant responses of Liquidambar formosana and Schima superba seedlings to sulfuric-rich and nitric-rich simulated acid rain. Plant Physiol Biochem. 2013;64:41–51. doi: 10.1016/j.plaphy.2012.12.012. [DOI] [PubMed] [Google Scholar]
- CIMMYT (2017) International Maize and Wheat Improvement Center. https://wheat.org/wheat-in-the-world/. Accessed 18 Oct 2018
- Dionisio-Sese ML, Tobita S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998;135:1–9. doi: 10.1016/S0168-9452(98)00025-9. [DOI] [Google Scholar]
- Doderer A, Kokkelink I, van der Veen S, Valk B, Schram A, Douma A. Purification and characterization of two lipoxygenase isoenzymes from germinating barley. Biochim Biophys Acta. 1992;112:97–104. doi: 10.1016/0167-4838(92)90429-H. [DOI] [PubMed] [Google Scholar]
- Du J, Cullen JJ, Buettner GR. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta. 2012;1826:443–457. doi: 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia AC, Galarini R, Taticchi MI, Dorr AJM, Mantilacci L. Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol Environ Saf. 2003;55:162–167. doi: 10.1016/S0147-6513(02)00123-9. [DOI] [PubMed] [Google Scholar]
- El-Shabrawi H, Kumar B, Kaul T, Reddy MK, Singla-Pareek SL, Sopory SK. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma. 2010;245:85–96. doi: 10.1007/s00709-010-0144-6. [DOI] [PubMed] [Google Scholar]
- Fraire-Velázquez S, Balderas-Hernández VE. Abiotic stress in plants and metabolic responses. In: Vahdati K, editor. Abiotic stress-plant responses and applications in agriculture. Rijeka: InTech; 2013. pp. 25–48. [Google Scholar]
- Gabara B, Sklodowska M, Wyrwicka A, Glinska S, Gapinska M. Changes in the ultrastructure of chloroplasts and mitochondria and antioxidant enzyme activity in Lycopersicon esculentum Mill. leaves sprayed with acid rain. Plant Sci. 2003;164:507–516. doi: 10.1016/S0168-9452(02)00447-8. [DOI] [Google Scholar]
- George E, Horst WJ, Neumann E. Adaptation of plants to adverse chemical soil conditions. In: Marschner P, editor. Marschner’s mineral nutrition of higher plants. 3. London: Academic Press; 2012. pp. 409–472. [Google Scholar]
- Gill SS, Anjum NA, Gill R, Yadav S, Hasanuzzaman M, Fujita M, Mishra P, Sabat SC, Tuteja N. Superoxide dismutase—mentor of abiotic stress tolerance in crop plants. Environ Sci Pollut Res. 2015;22:10375–10394. doi: 10.1007/s11356-015-4532-5. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar K, Fujita M. Alteration in chlorophylls and carotenoids in higher plants under abiotic stress condition. In: Golovko TK, Gruszecki WI, Prasad MNV, Strzałka K, editors. Photosynthetic pigments: chemical structure, biological function and ecology. Russia: Syktyvkar; 2014. pp. 218–264. [Google Scholar]
- Hasanuzzaman M, Nahar K, Hossain MS, Mahmud JA, Rahman A, Inafuku M, Oku H, Fujita M. Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int J Mol Sci. 2017;18:200. doi: 10.3390/ijms18010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar K, Alam MM, Bhuyan MHMB, Oku H, Fujita M. Exogenous nitric oxide pretreatment protects Brassica napus L. seedlings from paraquat toxicity through the modulation of antioxidant defense and glyoxalase systems. Plant Physiol Biochem. 2018;126:173–186. doi: 10.1016/j.plaphy.2018.02.021. [DOI] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil, 2nd edn. Circular. California agricultural experiment station, CA
- Hoque TS, Hossain MA, Mostofa MG, Burritt DJ, Fujita M, Tran LSP. Methylglyoxal: an emerging signaling molecule in plant abiotic stress responses and tolerance. Front Plant Sci. 2016;7:1341. doi: 10.3389/fpls.2016.01341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA, Nakano Y, Asada K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984;25:385–395. [Google Scholar]
- Islam AKMS, Edwards DG, Asher CJ. pH optima for crop growth. Results of a flowing solution culture experiment with six species. Plant Soil. 1980;54:339–357. doi: 10.1007/BF02181830. [DOI] [Google Scholar]
- Ivanov Y, Savochkin Y, Kuznetsov V. Effect of mineral composition and medium pH on Scots pine tolerance to toxic effect of zinc ions. Russ J Plant Physiol. 2013;60:260–269. doi: 10.1134/S102144371302009X. [DOI] [Google Scholar]
- Joliot P, Johnson GN. Regulation of cyclic and linear electron flow in higher plants. Proc Nat Acad Sci USA. 2011;108:13317–13322. doi: 10.1073/pnas.1110189108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur C, Singla-Pareek SL, Sopory SK. Glyoxalase and methylglyoxal as biomarkers for plant stress tolerance. Crit Rev Plant Sci. 2014;33:429–456. doi: 10.1080/07352689.2014.904147. [DOI] [Google Scholar]
- Kaur C, Kushwaha HR, Mustafiz A, Pareek A, Sopory SK, Singla-Pareek SL. Analysis of global gene expression profile of rice in response to methylglyoxal indicates its possible role as a stress signal molecule. Front Plant Sci. 2015;6:682. doi: 10.3389/fpls.2015.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd PS, Proctor J. Why plants grow poorly on very acid soils: are ecologists missing the obvious? J Exp Bot. 2001;52:791–799. doi: 10.1093/jexbot/52.357.791. [DOI] [PubMed] [Google Scholar]
- Kochian LV, Piñeros MA, Liu J, Magalhães JV. Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annu Rev Plant Biol. 2015;66:571–598. doi: 10.1146/annurev-arplant-043014-114822. [DOI] [PubMed] [Google Scholar]
- Lawson T, Vialet-Chabrand S. Speedy stomata, photosynthesis and plant water use efficiency. New Phytol. 2018;122:122. doi: 10.1111/nph.15330. [DOI] [PubMed] [Google Scholar]
- Long A, Zhang J, Yang LT, Ye X, Lai NW, Tan LL, Lin D, Chen LS. Effects of low pH on photosynthesis, related physiological parameters, and nutrient profiles of Citrus. Front Plant Sci. 2017;8:185. doi: 10.3389/fpls.2017.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmud JA, Hasanuzzaman M, Nahar K, Bhuyan MHMB, Fujita M. Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: Coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol Environ Saf. 2018;147:990–1001. doi: 10.1016/j.ecoenv.2017.09.045. [DOI] [PubMed] [Google Scholar]
- Martins N, Goncalves S, Romano A. Metabolism and aluminum accumulation in Plantago almogravensis and P. algarbiensis in response to low pH and aluminum stress. Biol Plant. 2013;57:325–331. doi: 10.1007/s10535-012-0271-3. [DOI] [Google Scholar]
- Miransari M, Balakrishnan P, Smith D, Mackenzie AF, Bahrami HA, Malakouti MJ, Rejali F. Overcoming the stressful effect of low pH on soybean root hair curling using lipochitooligosacharides. Commun Soil Sci Plant Anal. 2006;37:1103–1110. doi: 10.1080/00103620600586391. [DOI] [Google Scholar]
- Nahar K, Hasanuzzaman M, Suzuki T, Fujita M. Polyamines-induced aluminum tolerance in mung bean: a study on antioxidant defense and methylglyoxal detoxification systems. Ecotoxicology. 2017;26:58–73. doi: 10.1007/s10646-016-1740-9. [DOI] [PubMed] [Google Scholar]
- Premachandra GS, Saneoka H, Ogata S. Cell membrane stability, an indicator of drought tolerance, as affected by applied nitrogen in soyabean. J Agric Sci. 1990;115:63–66. doi: 10.1017/S0021859600073925. [DOI] [Google Scholar]
- Principato GB, Rosi G, Talesa V, Giovanni E, Uotila L. Purification and characterization of two forms of glyoxalase II from the liver and brain of Wistar rats. Biochim Biophys Acta Protein Struct Mol Enzymol. 1987;911:349–355. doi: 10.1016/0167-4838(87)90076-8. [DOI] [PubMed] [Google Scholar]
- Qiao F, Zhang XM, Liu X, Chen J, Hu WJ, Liu TW, Liu JY, Zhu CQ, Ghoto K, Zhu XY, Zheng HL. Elevated nitrogen metabolism and nitric oxide production are involved in Arabidopsis resistance to acid rain. Plant Physiol Biochem. 2018;127:238–247. doi: 10.1016/j.plaphy.2018.03.025. [DOI] [PubMed] [Google Scholar]
- Rengel Z. Soil pH, soil health and climate change. In: Singh B, Cowie A, Chan K, editors. soil health and climate change. Berlin: Springer; 2011. pp. 69–85. [Google Scholar]
- Rohman MM, Talukder MZA, Hossain MG, Uddin MS, Amiruzzaman M, Biswas A, Ahsan AFMS, Chowdhury MAZ. Saline sensitivity leads to oxidative stress and increases the antioxidants in presence of proline and betaine in maize (Zea mays L.) inbred. Plant Omics. 2016;9:35–47. doi: 10.21475/poj.16.09.04.pne31. [DOI] [Google Scholar]
- Rouphael Y, Cardarelli M, Colla G. Role of arbuscular mycorrhizal fungi in alleviating the adverse effects of acidity and aluminium toxicity in zucchini squash. Sci Hortic. 2015;188:97–105. doi: 10.1016/j.scienta.2015.03.031. [DOI] [Google Scholar]
- Sairam RK. Effects of homobrassinolide application on plant metabolism and grain yield under irrigated and moisture-stress conditions of two wheat varieties. Plant Growth Regul. 1994;14:173–181. doi: 10.1007/BF00025220. [DOI] [Google Scholar]
- Shah ZH, Rehman HM, Akhtar T, Daur I, Nawaz MA, Ahmad MQ, Rana IA, Atif RM, Yang SH, Chung G. Redox and ionic homeostasis regulations against oxidative, salinity and drought stress in wheat (a systems biology approach) Front Genet. 2017;8:141. doi: 10.3389/fgene.2017.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavrukov Y, Hirai Y. Good and bad protons: genetic aspects of acidity stress responses in plants. J Exp Bot. 2015;67:15–30. doi: 10.1093/jxb/erv437. [DOI] [PubMed] [Google Scholar]
- Shi QH, Zhu ZJ, Juan LI, Qian QQ. Combined effects of excess Mn and low pH on oxidative stress and antioxidant enzymes in cucumber roots. Agric Sci China. 2006;5(10):767–772. doi: 10.1016/S1671-2927(06)60122-3. [DOI] [Google Scholar]
- Smirnoff N. Ascorbic acid metabolism and functions: a comparison of plants and mammals. Free Radical Biol Med. 2018;122:116–129. doi: 10.1016/j.freeradbiomed.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N, Wheeler GL. Ascorbic acid in plants: biosynthesis and function. Crit Rev Plant Sci. 2000;19:267–290. doi: 10.1080/07352680091139231. [DOI] [PubMed] [Google Scholar]
- Song H, Xu X, Wang H, Tao Y. Protein carbonylation in barley seedling roots caused by aluminum and proton toxicity is suppressed by salicylic acid. Russ J Plant Physiol. 2011;58:653–659. doi: 10.1134/S1021443711040169. [DOI] [Google Scholar]
- Sumner ME, Noble AD. Soil acidification: the world story. In: Rengel Z, editor. Handbook of soil acidity. New York: Marcel Dekker; 2003. pp. 1–28. [Google Scholar]
- Tang C, Rengel Z. Role of plant cation/anion uptake ratio in soil acidification. In: Rengel Z, editor. Handbook of soil acidity. New York: Marcel Dekker; 2003. pp. 56–80. [Google Scholar]
- Van Den Berg LJ, Dorland E, Vergeer P, Hart MA, Bobbink R, Roelofs JG. Decline of acid-sensitive plant species in heathland can be attributed to ammonium toxicity in combination with low pH. New Phytol. 2005;166:551–564. doi: 10.1111/j.1469-8137.2005.01338.x. [DOI] [PubMed] [Google Scholar]
- Wellburn AR. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol. 1994;144:307–313. doi: 10.1016/S0176-1617(11)81192-2. [DOI] [Google Scholar]
- Wenzl P, Mancilla LI, Mayer JE, Albert R, Rao IM. Simulating infertile acid soils with nutrient solutions. Soil Sci Soc Am J. 2003;67:1457–1469. doi: 10.2136/sssaj2003.1457. [DOI] [Google Scholar]
- Wilkinson RE, Duncan RR. Interaction of hydrogen (H+) and manganese (Mn2+) concentrations on the shoot growth of sorghum cultivars. J Plant Nutr. 1993;16(6):983–998. doi: 10.1080/01904169309364588. [DOI] [Google Scholar]
- Yang Y, Qin Y, Xie C, Zhao F, Zhao J, Liu D, Chen S, Fuglsang AT, Palmgren MG, Schumaker KS, Deng XW. The Arabidopsis chaperone J3 regulates the plasma membrane H+-ATPase through interaction with the PKS5 kinase. Plant Cell. 2010;22:1313–1332. doi: 10.1105/tpc.109.069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YK, Zhu DF, Zhang YP, Chen HZ, Xiang J, Lin XQ. Low pH-induced changes of antioxidant enzyme and ATPase activities in the roots of rice (Oryza sativa L.) seedlings. PLoS ONE. 2015;10(2):e0116971. doi: 10.1371/journal.pone.0116971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang KX, Wen T, Dong J, Ma FW, Bai TH, Wang K, Li CY. Comprehensive evaluation of tolerance to alkali stress by 17 genotypes of apple rootstocks. J Integr Agric. 2016;15:1499–1509. doi: 10.1016/S2095-3119(15)61325-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.