Abstract
The bacteria residing in the gut environment do play a pivotal role in metabolic activities of the host. The metabolites produced by these bacteria affect the physiology and health of the host. The gut bacteria are exposed to environmental conditions where multiple factors such as lifestyle, stress, antibiotics, host genetics and infections have an influence on them. In case of pathogenesis of a disease, the gut bacterial composition is altered which leads to a diseased state. This stage is due to colonization of bacterial pathogens in the gut environment. The pathological condition can be alleviated by administering probiotic strains into the gut environment. The probiotic strains produce therapeutic molecules such as amino acids, vitamins, bacteriocins, enzymes, immunomodulatory compounds and short-chain fatty acids. This review discusses recent evidences of the impact of bioactive molecules produced by probiotic bacteria and their mechanism of action in the gut environment to maintain homeostasis and health of the host without any effect on beneficial bacteria sharing the same niche. In addition, the manufacturing challenges of probiotic products for various applications are discussed here.
Keywords: Bacteriocin, Probiotic, Short-chain fatty acids, Pathogenesis, Metabolites
Introduction
Probiotics concept was introduced in early twentieth century by Russian scientist and Nobel laureate Metchnikoff. The scientist thought that these probiotic bacteria exert a positive influence on the host by improving the intestinal microbial balance and alleviating digestive disorders (Pandey et al. 2015). The current definition of probiotics is that these are live microorganisms which, when administered in appropriate doses, confer health benefit to the host (FAO/WHO 2002). They facilitate good environment for smooth functioning of various metabolic activities in the intestine through the production of proteins, carbohydrates, vitamins and enzymes. The intestinal pathogenic organisms are suppressed by the action of acids and proteolytic activity of lactic acid bacteria (Schepper et al. 2017). The colonized probiotic bacteria serve many beneficial effects to the host cell for several reasons. The probiotic bacteria eliminate potential pathogenic organisms and help the host by increasing resistance to new colonization. They have high metabolic activity, producing useful compounds such as vitamins which maintain the health, bacteriocins that fight against various pathogens and also production of immunomodulatory compounds that regulate host immune system (Conlon and Bird 2015; Jandhyala et al. 2015). Probiotics also promote healthy environment by producing saturated fatty acids and oligosaccharides (Pandey et al. 2015). The introduced beneficial microbes alter the microbial composition and it has been associated with a therapeutic strategy which combats the pathogenic bacteria in the intestinal niche (Langella and Martín 2019; Vitetta et al. 2015). These bacteria alleviate various disorders and maintain overall health of the host (Daliri and Lee 2015; Amara and Shibl 2015; Sanchez et al. 2017). Human body harbors different microbes inside the gut environment, particularly the bacteria, fungi and viruses (Patrice 2018). This gut microbiota is influenced by several factors such as diet, human health, life style, infections, aging and host immunity; as a result, the gut environment is altered (Conlon and Bird 2015). The human gut microbiome is a potential source for novel therapeutics in the form of probiotics that produce therapeutic molecules to address the issues such as obesity, metabolic disorders, microbial infections and host immunity (Patrice 2018). Probiotics play a pivotal role in the well-being of humans which includes: (1) metabolic activity of undigested carbohydrates (Rowland et al. 2018; Kerry et al. 2018), (2) prevent multiplication of pathogenic bacteria and viruses (Zhang et al. 2015), (3) influence on host immune system (Kober and Bowe 2015; Amara and Shibl 2015), (4) synthesis of various nutrients such as vitamins, amino acids and enzymes, (5) bioavailability of nutrients (Pandey et al. 2015) and (6) creating less desirable conditions for harmful microorganisms in the intestine by changing the pH and reducing the oxygen availability (Schepper et al. 2017). As a result, the probiotic microorganisms promote beneficial effects in a host which are due to the production of bioactive compounds represented in Table 1. The bioactive compounds produced by these bacteria (Saccharomyces boulardii, Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus casei, Lactobacillus plantarum, Bifidobacterium longum, Bifidobacterium bifidium) include bacteriocins, enzymes, vitamins, amino acids, oligosaccharides, exopolysaccharides, short-chain fatty acids and immunomodulatory compounds (Vidya Prabhakar and Ramkrishna 2008). The number of viable probiotic cells present in live condition in a probiotic food must be 108–109 colony forming units (CFU) per day according to National Sanitary Surveillance Agency (ANVISA) (Costa and Miglioranza 2012). Conversely, the influences of probiotic microorganisms vary depending on species, the quantity of bacteria ingested and the physiologic conditions of the host (Campana et al. 2017; Sanders et al. 2018).
Table 1.
Bioactive compounds of probiotic bacteria and their health benefits
| Bioactive compound | Health benefits inside the gut | Probiotic microorganism | References |
|---|---|---|---|
| Bacteriocins | Helps in survival of bacteria in GI tract. Kills various intestinal pathogens. Acts as signaling molecules | Lactococcus lactis | Sturme et al. (2002), Sankar et al. (2012) |
| Enterocins | Antimicrobial activity against Pseudomonas aeruginosa | Enterococcus casseliflavus MI001 | Indira et al. (2018); Indira et al. (2019) |
| Exopolysaccharides | Antioxidant activity | B. coagulans RK-02 | Vidya Prabhakar and Ramkrishna (2008), Kodali et al. (2013) |
| SCFAs | |||
| Lactic acid |
Used as substrate for glucose, cholesterol, and lipids metabolism Lowers pH in vaginal environment |
Lactobacillus sps. | Tachedjian et al. (2017); Leblanc et al. (2017) |
| Butyric acid | The butyric acid is the energy source for colonocytes and has anti-inflammatory and anti-tumour properties | F. prausnitzii, Roseburia spp. Butyricicoccus pullicaecorum | Geirnaert et al. (2017); Miremadi and Shah (2012) |
| Propionic acid | Propionate plays a role in gluconeogenesis | P. freudenreichii. | Vorobjeva et al. (2008) |
| Acetic acid | Acetate is metabolized in muscle and used to produce adenosine-5’-triphosphate (ATP). Defense functions in host epithelial cells | Bifidobacterium sps. | Fukuda et al. (2012) |
| Fructooligosaccharides | |||
|
Inulins Levans |
Reduces fat absorption Reduces cholesterol absorption |
L. gasseri strains DSM 20604 and 20077 |
Anwar et al. (2010) |
| Amino acids | |||
| Lysine | Essential amino acid for the host | Clostridium sps. | Metges et al. (2006); Dai et al. (2015) |
| Amino acid metabolites | Essential nutrients supports the growth | Enterobacteriaceae | Dai et al. (2015) |
| Arginine | Function on both female and male reproductive system | Fusobacterium varium | Dai et al. (2015) |
| Tryptophan and Tyrosine | Function on male reproductive system | Enterococcus sps, streptococcus sps, Bacillus sps | Dai et al. (2015) |
| Vitamins | |||
| Folate |
Energy metabolism Biosynthesis of nucleic acids |
B.adolescentis DSM 18350 B. pseudocatenulatum |
Strozzi and Mogna (2008) Asrar, and O’Connor (2005) Rossi et al. (2011) |
| Vitamin B1 (Thiamin) | Role in synthesis of nucleic acids, steroids fatty acids and amino acid precursors. All these bioactive compounds are essential for functioning of brain | Lactobacillus sps. | Gu and Li (2016) |
| Vitamin B2 (Riboflavin) | Energy metabolism |
L. fermentum CECT 5716 Lactococcus lactis |
Cardenas et al. (2015) |
| Vitamin B6 (Pyridoxine) | Amino acid metabolism | Bifidobacterium sps. | Patel et al. (2013) |
| Vitamin B9 | Energy metabolism | L. fermentum CECT 5716 | Cardenas et al. (2015) |
| Vitamin B12 | Helps in red blood cell formation and makes DNA | Lactobacillus reuteri JCM1112 | Santos et al. (2008) |
| Enzymes | |||
| Amylase | Hydrolysis of starch | Lactobacillus sp G3_4_1TO2 | Padmavathi et al. (2018) |
| β-Galactosidase | Hydrolysis of β-galactosides | P. freudenreichii | Vorobjeva et al. (2008) |
| Super oxide dismutase, Catalase | Antioxidant activity | Lactobacillus fermentum E-3 and Lactobacillus fermentum E-18, Lactobacillus casei BL23 | Wang et al. (2017) |
Bioactive compounds produced by Probiotic bacteria
Bacteriocins
Bacteriocins are ribosomally synthesized antimicrobial peptides produced by one organism and kill other organisms (Zacharof and Lovitt 2012). These bacteriocins are produced by two domains such as bacteria and some members of archea. In bacterial group, the bacteriocins are produced by both gram-positive and gram-negative bacteria (Indira et al. 2015, 2018, 2019; Gillor et al. 2008). The beneficial bacteria present in the gut environment produce bacteriocins and prevent pathogenic microbial colonization. This defensive role helps probiotic bacteria to occupy a specific niche and also limits the advancement of pathogens to neighboring cells. Recent reports have been proposed for probiotic bacteria that they are mediating quorum sensing signals in bacterial biofilms (Dobson et al. 2012; Mukherjee and Ramesh 2015). Bacteriocins have a narrow spectrum of activity and they are bactericidal in nature. The bactericidal mechanism of bacteriocin action is located in the cytoplasmic membrane region of receptor binding on bacterial surfaces. Moreover, these bacteriocins are non-toxic peptides, sensitive to proteases compared to antibiotics (Yang et al. 2014; Mokoena 2017).
SCFAs (short-chain fatty acids)
The human body harbors 100 trillions of microorganisms in both small and large intestine. The host cells lack a few enzymes that play a role in digestion of carbohydrates. However, the probiotic bacteria ferment these undigested carbohydrates and produce energy which is utilized by host to carry out various functions. The probiotic bacteria co-habit with the colonocytes and maintain a symbiotic relationship between gut flora and humans (Chatterjee et al. 2017). The probiotic bacteria converts these undigested sugars into SCFAs such as butyrate, acetate, propionate and other byproducts namely heat and gases (CH4, CO2 and H2) (Leblanc et al. 2017; Thursby and Juge 2017; Yoo and Kim 2016). The short-chain fatty acids produced as end products of saccharolytic fermentation have antimicrobial activity. The organic acids are six carbon compounds that help in protection of host from microbial infections (Ciarlo et al. 2016).
The typical reaction of short-chain fatty acids production and over all stoichiometry has been summarized for a hexose sugar and is shown as follows:
59 C6H12O6 + 38 H2O → 60 CH3COOH + 18 CH3CH2CH2COOH +22 CH3CH2COOH + 96 CO2 + 268 H+ + Heat (Topping and Clifton 2001).
In humans, 10% of the daily caloric requirement is from short-chain fatty acids produced in large intestine. Among all short-chain fatty acids, 60–70% of the energy is from butyrate produced in colonocytes (den Besten et al. 2013). The conversion of butyrate to acetyl co-A, followed by ketone bodies and CO2 production was found in butyrate oxidation. Thus, butyrate acts as a fuel source for colon, muscle and brain cells (den Besten et al. 2013). Apart from production of short-chain fatty acids, the probiotic bacteria can accomplish various functions in the intestinal environment. The growth of pathogenic bacteria is prevented, regulation of immune system and physiological conditions of the gut, stimulation of epithelial cell growth by producing vitamins and hormones (Mach and Fuster-Botella 2017). SCFAs, particularly butyrate, have a therapeutic effect in various diseases such as inflammatory bowel disease, antibiotic-associated diarrhea, colon cancer and heart diseases (Sharma and Shukla 2016; Tominaga et al. 2018; Gill et al. 2018; Moss et al. 2018). In a previous study by Kimura et al. (2013), it was found that the receptors (GPR43) of short-chain fatty acids are linked with the metabolic activity of the gut microorganisms. These receptors maintained homeostasis in the host cells by controlling the energy utilization of the host. Further, they found that GPR43-deficient mice are obese on normal diet compared with over expression of GPR 43 mice.
Exopolysaccharides
Various genera of probiotic bacteria have the ability to produce exopolysaccharides (EPSs) in large quantities (Kodali et al. 2009; Indira et al. 2016). Among all, the genus lactic acid bacteria are under GRAS status (generally recognized as safe). The enzymes such as glycosyltransferases and glycantransferases convert the sugar nucleotide precursors into exopolysaccharides. Recently, the microbial exopolysaccharides (EPSs) have gained a lot of attention due to their health benefits (Ates 2015). The exopolysaccharides from lactic acid bacteria have immunostimulatory activity, antitumor effect, antioxidant activity and blood cholesterol lowering ability (Ghan et al. 2014; Tsai et al. 2014). In another study, the exopolysaccharide showed a good emulsifying activity which is an important feature to be used in food formulations (Peele et al. 2016). In addition to these, the exopolysaccharide produced from Lactic acid bacteria showed immune modulation, antiulcer and cholesterol lowering activities (Julendra et al. 2017). The antitumor activity of cell bound exopolysaccharide produced by Lactobacillus helveticus MB2-1 was tested against liver (HepG-2), gastric (BGC-823), and especially colon (HT-29) cancer cells (Li et al. 2015).
Oligosaccharides
Oligosaccharides are the non-digestible cross-linked polymers composed of monosaccharide units. The nature of the oligosaccharide is characterized by the number of units and type of its glycosyl moieties (Meyer et al. 2015). These are one of the major food sources for probiotic bacteria and gut bacteria. The non-digestible oligosaccharides enrich nutrients to the gut flora and behave as dietary fibers and prebiotics (Patel and Goyal 2012). The positive effect on the beneficial bacteria, thus, induces the host health (de Moura et al. 2015). Oligosaccharides have enormous potential for stimulating the growth of bacteria and production of bioactive compounds such as antibodies, short-chain fatty acids and organic acids. Therefore, modulation of the intestinal flora achieves homeostasis in the gut environment (Pan et al. 2009). The examples of oligosaccharides are inulin, fructooligosaccharides (FOS), glucooligosaccharides (GOS) and xylooligosaccharides (XOS) (Yoo and Kim 2016). Many clinical studies reported that the combination of probiotic bacteria and prebiotics has therapeutic effect in obesity (Cerdo et al. 2019), colon cancer (Zackular et al. 2013), irritable bowel syndrome (Dai et al. 2013), bacterial infections (Sarowska et al. 2013) and tumors (Markowiak and Slizewska 2017). The study of previous researchers reported that the glucooligosaccharide (GOS) has a protective role in development of tumors and their multiplicity (Pericleous et al. 2013; Hou et al. 2013). A different approach for nutritional therapy in case of constipation is the use of prebiotics such as fructooligosaccharides and galactooligosaccharides (Patel and Goyal 2012). The laxative effect of galactooligosaccharides reduces the symptoms of chronic constipation and irritable bowel syndrome in the elderly people (Rao et al. 2015). Oligosaccharides support the synthesis of immunoglobulins which play a role in natural defense mechanism in the host (Newburg 1996). Majority of the findings reported that the increased levels of IgA synthesis are induced by the use of fructooligosaccharides in animal models (Nakamura et al. 2004). Addition of probiotic foods to beneficial bacteria has the potential to increase the levels of secretory IgA in the body (Hardy et al. 2013). Oligosaccharides promote the absorption of minerals through the consumption of prebiotics. In a recent study by Baye et al. (2017), it was found that the prebiotics increase the absorption of iron and calcium from the colon.
Enzymes
Probiotic bacteria have the ability to carry out various metabolic activities due to the production of enzymes such as lipases, esterases and amylases (Markowiak and Slizewska 2017). Human beings lacking the enzyme lactase develop intolerance to lactose levels. The probiotic bacteria produce enzyme lactase that digests lactose present in the milk and converts it into glucose and galactose. Further, these sugars get converted into short-chain fatty acids (den Besten et al. 2013). The amount of lactose in yogurt product is reduced by hydrolytic capacity of probiotic strains (Vonk et al. 2012). Some of the species of lactic acid bacteria lessen the lactose intolerance through their enzyme β-galactosidase (Montalto et al. 2006). Amylases and peptidases produced by probiotic organisms play a role in biochemical reactions of the host metabolism. The genera producing these enzymes are lactic acid bacteria and bifidobacteria (Savaiano 2014).
Amino acids
The gut bacteria produce several amino acids by de novo process and they act as precursors for the synthesis of short-chain fatty acids (Feng et al. 2018). The produced short-chain fatty acids help in the fermentation of undigested carbohydrates. Amino acids and short-chain fatty acids alter the physiology of the host (den Besten et al. 2013). The amino acids and amino acid-derived molecules regulate the metabolism of carbohydrates and lipids and further produce metabolites and in turn regulate health of a host (den Besten et al. 2013). Mainly, the aromatic amino acids act as substrates for the production of various metabolites (Dai et al. 2015; Dodd et al. 2017). Fermentation of these amino acids by probiotic bacteria in the gut produces phenols and indole. They maintain energy balance and resistance to pathogen infections due to immune signals. Many lactic acid bacteria (LAB) produce small peptides and amino acids by proteolysis of casein molecules (Rowland et al. 2018). The biochemical conversion of amino acids results in the production of alcohols, esters, aldehydes and organic acids that gives aroma and flavor. The most potent flavor compounds are aldehydes, alcohols, esters and acids. The amino acids are methionine, threonine, phenylalanine and branched-chain amino acids. They play an important role in flavor formation for various food and dairy products (Smit et al. 2005). The amino acids produced by probiotic microorganisms play an important role in alleviating various disorders. d-tryptophan acts as an immunomodulatory substance which reduces hyperactivity in allergic reactions (Kepert et al. 2017). Some small molecules produced by gut bacteria play a role in biofilm formation and quorum sensing. These molecules are acylhomoserine lactones and autoinducer peptides derived from S-adenosyl methionine, an important intermediate from amino acid methionine (Dai et al. 2011).
Vitamins
The essential nutrients for the growth and development of a multi-cellular organism are vitamins. Humans have lost the ability to synthesize vitamins by de novo process, which leads to deficiencies, malnutrition and stunned growth starting from infant to elderly stage. These vitamins are supplied in the form of diet. All the vitamins are grouped under water-soluble and fat-soluble categories (Fitzpatrick et al. 2012; Ravisankar et al. 2015). Many bacteria produce B-group vitamins which are soluble in water and absorbed into the intestine, whereas fat soluble vitamins are absorbed with the help of lipids as micelles in the intestinal tract. The major sources of vitamins are dairy products which contain B-complex group of vitamins (Said 2011). The natural sources of vitamins are plants and animals but some vitamins are synthesized chemically (Fitzpatrick et al. 2012). The vitamins are produced by microbial fermentation processes. Vitamin is the key factor which plays an important role in growth, development, reproduction, red blood cell formation, antibody production, and lactation of the human beings. It is required by the body to carry out various metabolic activities of amino acids, fatty acids, carbohydrates and synthesis of nucleic acids. In the digestive tract, pyridoxine and iron are absorbed due to riboflavin which enhances the absorption rate and helps in the maintenance of red blood cells (LeBlanc et al. 2017). The biosynthesis of riboflavin has been studied in plants, bacteria and filamentous fungi. Extensive studies have been carried out on riboflavin production in B. subtilis and Escherichia coli, respectively (Lin et al. 2014). Another group of health promoting bacteria under the genus is Bifidobacteria which produces B-complex vitamins maintaining the overall health in the gut of a host (Markowiak and Slizewska 2017). In an experiment, rats with tamoxifen-mediated endometrial carcinoma were treated with B-complex vitamins such as Riboflavin and niacin combination with ascorbic acid (Sundravel et al. 2006). The most important vitamin under the B-complex group is the folic acid which is naturally produced by the gut microbiota in large quantities. It is one of the most important vitamins for synthesis of nucleic acids, conversion of amino acids and having antioxidative nature for removal of free radicals from the body. Various strains have been screened for folate production from the genus lactic acid bacteria; except L. plantarum, all other strains were incapable of folate production. These probiotic cultures cannot synthesize folate due to the lack of genes encoding for the folate production (Patel et al. 2013). The vitamin which plays a role in early development of the nervous system and growth of the embryo is the pyridoxine (vitamin B6). This vitamin has a functional role in the link between one carbon metabolism and antioxidative activity (Dalto and Matte 2017). The main producers for this vitamin are Bifidobacterium sps (Patel et al. 2013). The vitamin which is not synthesized by plants is Vitamin B12 and it plays an important role in the functioning of nervous system and blood formation. The vitamin production is only through bacterial fermentation. A probiotic strain of L. reuteri belonging to LAB is able to produce the cobalamin (Mohammed et al. 2014). Salmonella typhinurium consists of multiple genes for the synthesis of vitamin B12 by de novo process. Transfer of these genes to E.coli allows the production of vitamin B12 in industries (Fang et al. 2017). Another organism, Propionibacterium shermani is also capable of producing vitamin B12, propionic acid and other metabolites which are of industrial importance (Piwowarek et al. 2018). Another vitamin under B-complex group is thiamine (vitamin B1) essential for the synthesis of nucleic acids, fatty acids, aromatic amino acids and other bioactive compounds essential for brain function. Masuda et al. (2012) investigated the production levels of folate, vitamin B12 and thiamine from lactic acid bacteria and found that the thiamine level is very low compared with other two vitamins. Furthermore, the high level of thiamine production was reported in Bifidobacterium species. Vitamin K facilitates the coagulation process of blood and it is responsible for clot formation. In green plants, this vitamin exists as phylloquinone (Vitamin K1) and in intestinal bacteria it is produced as menaquinone (K2) form. For human consumption, the phylloquinone form is obtained from green plants and the menaquinone form produced by bacteria, which are used to supplement vitamin K to alleviate vitamin K deficiency (Patel et al. 2013). Through genetic engineering, a probiotic E.coli strain Nissle 1917 (EcN-BETA) was developed for the production of β-carotene. In malnourished children, the supply of this probiotic bacterium increases the vitamin A in the intestine. Vitamins production by probiotic bacteria in gut environment is a natural way of enrichment of vitamins in human beings (Miller et al. 2013).
Immunomodulatory compounds
The immune system of the host is modulated by probiotic bacteria in the gut environment. The probiotic bacteria modulate the immune system and regulate the production of antibodies, interleukins, cytokines and lymphocytes (Nagpal et al. 2012). The probiotic bacteria react with intestinal epithelial cells and initiate a host immune response by producing immunomodulatory molecules. Due to this interaction, a controlled production of cytokines and chemokines takes place in the gut environment (Hardy et al. 2013). The probiotic bacteria modulate immunity and inflammatory gene expression results in the production of interleukins such as IL-1β and IL-8 (Plaza-Diaz et al. 2014). In a recent study reported by Kawashima et al. (2018), it is shown that the production of IgA antibody induced via IL-10 in mucosal sites of host is a host defence mechanism against the pathogens. The genetically engineered Lactococcus lactis strain-produced anti-inflammatory mediator such as IL-10 and IL-12 showed reduction of dextran sodium sulfate-induced colitis in mice models (Gupta et al. 2014). In another study reported by Ozdemir (2010), it was found that there is a reduction of IgE levels in allergic diseases. Hence, immunomodulation through probiotics is an alternative treatment option for various diseases.
Mechanism of action of bioactive molecules in the gut environment
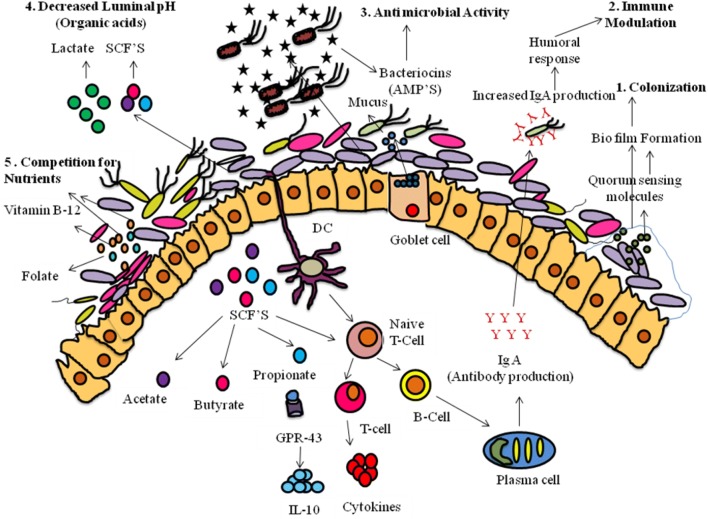
Modulation of the micro-biome in the gut atmosphere has become a promising approach or an integral part of precision medicine approach to improve host health. This strategy protects the host from a variety of infections and diseases by producing bioactive compounds and regulates the host metabolism and immune system. These bioactive molecules regulate the host health by activating various mechanisms represented in Fig. 1. To begin with, probiotic organisms after oral administration reach the gut environment where they activate the production of quorum sensing molecules. These molecules form the biofilm, a protective layer made up of lipopolysaccharide which helps as a growth substrate for colonization of the useful bacteria and prevents the pathogenic bacteria colonization (Dobson et al. 2012; Mukherjee and Ramesh 2015). Secondly, they activate dendritic cells, which in turn activate naive T cell. The produced T cells further activate the B cells resulting in production of secretory IgA. The secretory IgA helps in elimination of pathogenic bacteria resulting in humoral response. In another way, the naïve T cell differentiates into mature T cell, which produces cytokines and results in immune modulation (Derrien and van Hylckama Vlieg 2015). The mechanism by which the probiotic bacteria benefit the host by producing bacteriocins, antimicrobial peptides kills the pathogenic bacteria results in the elimination of harmful organisms, in the gut environment. The secretory IgA, bacteriocins and mucus act as primary defense mechanism in the intestinal epithelium and protect the host from invading pathogens (Corthesy et al. 2007; Derrien and van Hylckama Vlieg 2015). In fourth mechanism, they produce short-chain fatty acids (butyrate, acetate and propionate) and organic acids (lactic acid and acetic acid) help in lowering of pH in the gut environment; this condition is unfavorable for growth of the pathogenic bacteria (Schepper et al. 2017). In fifth mechanism, they produce nutrients and growth factors such as vitamins, precursors to the enzymes helping in regulation of metabolism through biochemical reactions. The vitamins produced by these bacteria are B-complex vitamins (especially vitamin B12, which is not synthesized by humans) and vitamin K which help in the growth and establishment of useful bacteria in the gut environment. Finally, the SCFAs regulate G-protein coupled receptors. In this line, the propionate binds with GPR43 receptor expressed on lymphocytes and triggers the production of IL-10, helping in resolving inflammatory responses. Butyrate also triggers the differentiation of T cell leading to the production of Interleukin such as IL-10 (Kota et al. 2018; Vitetta et al. 2015). Butyrate binds to the PPAR-γ (peroxisome proliferated activated receptor- γ) leading to β-oxidation and oxygen consumption. Hence, the reduced levels of oxygen in the gut lumen result in the anaerobic environment, which is undesirable for pathogenic bacteria (Patrice 2018). Thus, overall, the Probiotic bacteria completely influence metabolism, immune system, composition and functioning of the gut microbes.
Fig. 1.
Schematic presentation of the overall mechanisms on protection of gut environment by bioactive molecules produced by Probiotic bacteria (Kim et al. 2013; Derrien and van Hylckama Vlieg 2015). First, the ingested bacteria reach the gut environment where they then produce lipopolysaccharides for colonization. Secondly, they activate the dendritic cells which in turn activate the T cell triggering the activation and differentiation of B cells. The produced B cells secrete IgA, and helps in the elimination of pathogens. Thirdly, they produce bacteriocins which are antimicrobial peptides and kill the pathogenic bacteria. Fourthly, they prevent pathogenic bacteria by decreasing the luminal pH results from the production of organic acids. This creates undesirable conditions for growth of pathogens. Fifthly, they produce growth factors, including vitamins and exopolysaccharide (EPS) which helps for the growth and metabolic reactions in the gut environment. Finally, the metabolites acetate, butyrate and propionate trigger immune defense mechanisms
Industrial applications of probiotics and manufacturing challenges for probiotic products
In recent years, the use of probiotics has become a fascinating area due to applications in various fields such as diary, food and beverages, agriculture, health care and aqua culture (Zielinska and Kolozyn-Krajewska 2018). Once desired strain is identified for developing a probiotic product, first we have to see if the strain can be cultured at large scale, process design and control, product recovery, formulation technology, packaging of a product and finally successful incorporation into the consumer products (Fenster et al. 2019). The important criteria required for selection of an organism considered as probiotic food application include: The strain has to survive in process conditions, formulation and packaging of a product, shelf life of the product, stability of the organism, quality and safety of the organism, adherence and colonization of the intestinal epithelium, stressful conditions in the gastrointestinal tract, anti microbial activity against pathogenic strains, immune system activation, biological function in the GI tract and finally health benefits to the host (Misra et al. 2019; Fenster et al. 2019; Sanders et al. 2019). For industrial producers, the important parameters which affect the manufacturing and marketing are stability and viability of microorganisms (Sutton 2008). In case of manufacturing probiotic supplements, the viable count of the cells and shelf life of a product are important. The survival ability of the organism during process depends on the quality of raw materials, culture medium selection, cell protectants, environmental parameters and aseptic maintenance of the process (Vinderola et al. 2011; Fenster et al. 2019). The functional aspects of probiotic organism include viability in sufficient dosage levels and the production will be strain dependent (Fenster et al. 2019). In case of dietary supplements, the probiotic products are manufactured as freeze-dried powders and formulated as capsules, powders and tablets (Govender et al. 2014; Fenster et al. 2019). The parameters which influence manufacture of these dietary supplements include viable count of the cells and water activity (Vinderola et al. 2011; Grzeskowiak et al. 2011). For application of probiotic as food and beverages, the products are prepared in vegetative cell form and added to the food products (Misra et al. 2019). Hence refrigerated conditions are required for maintaining the shelf life of a product (Ranadheera et al. 2017). The fermented probiotic products maintain viable cell count, but on the other side, care should be taken to maintain the sensory profile of a product, mitigation of health risk due to pathogens and maintenance of aseptic conditions. The use of probiotics as functional food may enhance the energy source and greatly influences the performance of a product (Khaneghah and Fakhri 2019; Ranadheera et al. 2017).
Conclusions
The gut microbiota can be modulated to recover host health through some interventions, i.e., administration of probiotic organisms. This review highlighted the data related to the relationship between bioactive molecules produced by probiotic bacteria, their mechanism of action in in vivo and its impact on the health of host. This is a new and attractive phase of research for formulating probiotic bacterial composition to modulate the host immune system for protection and treatment of various ailments. In future, identification of new variants and consumption of newly formulated probiotics may be a good strategy to promote good health in future. Therefore, probiotics can be recommended as alternative bio-therapeutic agents for treatment of various infections.
Acknowledgements
The authors acknowledge the support of Vignan’s Foundation for Science Technology and Research (Deemed to be University), Vadlamudi-522213, Guntur, A.P, India.
Abbreviations
- CFU
Colony forming units
- ANVISA
National Sanitary Surveillance Agency
- FOS
Fructooligosaccharides
- GOS
Glucooligosaccharides
- XOS
Xylooligosaccharides
- LAB
Lactic acid bacteria
- PPAR-γ
Peroxisome proliferated activated receptor- γ
Compliance with ethical standards
Conflict of the interest
The authors declare no conflicts of interest.
Contributor Information
T. C. Venkateswarulu, Email: venki_biotech327@yahoo.com
S. Krupanidhi, Email: krupanidhijuly2012@gmail.com
References
- Amara AA, Shibl A. Role of Probiotics in health improvement, infection control and disease treatment and management. Saudi Pharm J. 2015;23:107–114. doi: 10.1016/j.jsps.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar MA, Kralj S, Pique AV, Leemhuis H, van der Maarel MJ, Dijkhuizen L. Inulin and levan synthesis by probiotic Lactobacillus gasseri strains: characterization of three novel fructansucrase enzymes and their fructan products. Microbiology. 2010;156(4):1264–1274. doi: 10.1099/mic.0.036616-0. [DOI] [PubMed] [Google Scholar]
- Asrar FM, O’Connor DL. Bacterially synthesized folate and supplemental folic acid are absorbed across the large intestine of piglets. J Nutr Biochem. 2005;16:587–593. doi: 10.1016/j.jnutbio.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Ates O. Systems biology of microbial exopolysaccharides production. Front Bioeng Biotechnol. 2015;3:200. doi: 10.3389/fbioe.2015.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baye K, Guyot JP, Mouquet-Rivier C. The unresolved role of dietary fibers on mineral absorption. Crit Rev Food Sci Nutr. 2017;57:949–957. doi: 10.1080/10408398.2014.953030. [DOI] [PubMed] [Google Scholar]
- Campana R, Van Hemert S, Baffone W. Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathog. 2017;9:12. doi: 10.1186/s13099-017-0162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas N, Laino JE, Delgado S, Jimenez E, Del Valle MJ, De Giori GS, Sesma F, Mayo B, Fernandez L, LeBlanc JG, Rodriguez JM. Relationships between the genome and some phenotypical properties of Lactobacillus fermentum CECT 5716, a probiotic strain isolated from human milk. Appl Microbiol Biotechnol. 2015;99:4343–4353. doi: 10.1007/s00253-015-6429-0. [DOI] [PubMed] [Google Scholar]
- Cerdo T, Garcia-Santos JA, G Bermudez M, Campoy C. The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients. 2019;11:635. doi: 10.3390/nu11030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Datta S, Sharma S, Tiwari S, Gupta DK. Health and environmental applications of gut microbiome: a review. Ecol Chem Eng S. 2017;24:467–482. [Google Scholar]
- Ciarlo E, Heinonen T, Herderschee J, Fenwick C, Mombelli M, Le Roy D, Roger T. Impact of the microbial derived short chain fatty acid propionate on host susceptibility to bacterial and fungal infections in vivo. Sci Rep. 2016;6:37944. doi: 10.1038/srep37944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon M, Bird A. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2015;7:17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthesy B, Gaskins HR, Mercenier A. Cross-talk between probiotic bacteria and the host immune system. J Nutr. 2007;137:781S–790S. doi: 10.1093/jn/137.3.781S. [DOI] [PubMed] [Google Scholar]
- Costa GN, Miglioranza LHS. Probiotics: the effects on human health and current prospects. In: Rigobelo EC, editor. Probiotics. London: In Tech Open; 2012. pp. 367–384. [Google Scholar]
- Dai ZL, Wu G, Zhu WY. Amino acid metabolism in intestinal bacteria: links between gut ecology and host health. Front Biosci. 2011;16:1768–1786. doi: 10.2741/3820. [DOI] [PubMed] [Google Scholar]
- Dai C, Zheng CQ, Jiang M, Ma XY, Jiang LJ. Probiotics and irritable bowel syndrome. World J Gastroenterol. 2013;19:5973. doi: 10.3748/wjg.v19.i36.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Wu Z, Hang S, Zhu W, Wu G. Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. MHR Basic Sci Reprod Med. 2015;21:389–409. doi: 10.1093/molehr/gav003. [DOI] [PubMed] [Google Scholar]
- Daliri EB, Lee BH. New perspectives on probiotics in health and disease. Food Sci Hum Wellness. 2015;4:56–65. [Google Scholar]
- Dalto D, Matte JJ. Pyridoxine (vitamin B6) and the glutathione peroxidase system; a link between one-carbon metabolism and anti oxidation. Nutrients. 2017;9:189. doi: 10.3390/nu9030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moura FA, Macagnan FT, da Silva LP. Oligosaccharide production by hydrolysis of polysaccharides: a review. Int J Food Sci Technol. 2015;50:275–281. [Google Scholar]
- den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien M, van Hylckama Vlieg JE. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015;23:354–366. doi: 10.1016/j.tim.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Dobson A, Cotter PD, Ross RP, Hill C. Bacteriocin production: a probiotic trait. Appl Environ Microbiol. 2012;78:1–6. doi: 10.1128/AEM.05576-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA, Sonnenburg JL. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551:648. doi: 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago L. Probiotics and Colon. Cancer Microorg. 2019;7:1–11. doi: 10.3390/microorganisms7030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Kang J, Zhang D. Microbial production of vitamin B12: a review and future perspectives. Microb Cell Fact. 2017;16:15. doi: 10.1186/s12934-017-0631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Ao H, Peng C. Gut microbiota, short-chain fatty acids, and herbal medicines. Front Pharmacol. 2018;9:1–12. doi: 10.3389/fphar.2018.01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster K, Freeburg B, Hollard C, Wong C, Rønhave Laursen R, Ouwehand AC. The production and delivery of probiotics: a review of a practical approach. Microorganisms. 2019;7:83. doi: 10.3390/microorganisms7030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick TB, Basset GJ, Borel P, Carrari F, DellaPenna D, Fraser PD, Hellmann H, Osorio S, Rothan C, Valpuesta V, Caris-Veyrat C. Vitamin deficiencies in humans: can plant science help? Plant Cell. 2012;24:395–414. doi: 10.1105/tpc.111.093120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization (FAO) Guidelines for the evaluation of Probiotics in food. FAO; London, ON, Canada: Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. 30 April–1 May 2002
- Fukuda S, Toh H, Taylor TD, Ohno H, Hattori M. Acetate-producing bifidobacteria protect the host from enteropathogenic infection via carbohydrate transporters. Gut Microbes. 2012;3:449–454. doi: 10.4161/gmic.21214. [DOI] [PubMed] [Google Scholar]
- Geirnaert A, Calatayud M, Grootaert C, Laukens D, Devriese S, Smagghe G, De Vos M, Boon N, Van de Wiele T. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci Rep. 2017;7:11450. doi: 10.1038/s41598-017-11734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghan KA, Elhafez EA, Hamouda RA. Evaluation of antioxidant and antitumor activities of Lactobacillus acidophilus bacteria isolated from Egyptian infants. Int J Pharmacol. 2014;10:282–288. [Google Scholar]
- Gill PA, van Zelm MC, Muir JG, Gibson PR. Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment Pharmacol Thera. 2018;48:15–34. doi: 10.1111/apt.14689. [DOI] [PubMed] [Google Scholar]
- Gillor O, Etzion A, Riley MA. The dual role of bacteriocins as anti- and probiotics. Appl Microbiol Biotechnol. 2008;81:591–606. doi: 10.1007/s00253-008-1726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govender M, Choonara YE, Kumar P, du Toit LC, van Vuuren S, Pillay V. A review of the advancements in probiotic delivery: conventional vs. non-conventional formulations for intestinal flora supplementation. AAPS Pharm Sci Tech. 2014;15:29–43. doi: 10.1208/s12249-013-0027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzeskowiak L, Isolauri E, Salminen S, Gueimonde M. Manufacturing process influences properties of probiotic bacteria. Br J Nutr. 2011;105:887–894. doi: 10.1017/S0007114510004496. [DOI] [PubMed] [Google Scholar]
- Gu Q, Li P. Biosynthesis of vitamins by probiotic bacteria, probiotics and prebiotics in human nutrition and health. London: In tech open; 2016. pp. 135–148. [Google Scholar]
- Gupta C, Prakash D, Gupta S. Genetically engineered probiotics. Afr J Basic Appl Sci. 2014;6:57–64. [Google Scholar]
- Hardy H, Harris J, Lyon E, Beal J, Foey A. Probiotics, prebiotics and immunomodulation of gut mucosal defences: homeostasis and immunopathology. Nutrients. 2013;5:1869–1912. doi: 10.3390/nu5061869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou N, Huo D, Dignam JJ. Prevention of colorectal cancer and dietary management. Chin Clin Oncol. 2013;2:13. doi: 10.3978/j.issn.2304-3865.2013.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indira M, Venkateswarulu TC, Chakravarthy K, Reddy AR, Prabhakar KV. Isolation and characterization of bacteriocin producing lactic acid bacteria from diary effluent. Res J Pharm Technol. 2015;8:1–6. [Google Scholar]
- Indira M, Venkateswarulu TC, Chakravarthy K, Reddy AR, Babu DJ, Kodali VP. Morphological and biochemical characterization of exopolysaccharide producing bacteria isolated from dairy effluent. J Pharm Sci Res. 2016;8:88–91. [Google Scholar]
- Indira M, Venkateswarulu TC, Prabhakar KV, Peele KA, Krupanidhi S. Isolation and characterization of bacteriocin producing Enterococcus casseliflavus and its antagonistic effect on Pseudomonas aeruginosa. Karbala Int J Mod Sci. 2018;4:361–368. [Google Scholar]
- Indira M, Venkateswarulu TC, Peele KA, Prabhakar KV, Krupanidhi S. Characterization of bacteriocin producing probiotic properties of Enterococcus casseliflavus MI001 isolated from curd sample. Curr Trends Biotechnol Pharm. 2019;13:64–71. [Google Scholar]
- Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy DN. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julendra H, Suryani AE, Istiqomah L, Damayanti E, Anwar M, Fitriani N. Isolation of lactic acid bacteria with cholesterol-lowering activity from digestive tracts of indonesian native chickens. Media Peternak. 2017;40:35–41. [Google Scholar]
- Kawashima T, Ikari N, Kouchi T, Kowatari Y, Kubota Y, Shimojo N, Tsuji NM. The molecular mechanism for activating IgA production by Pediococcus acidilactici K15 and the clinical impact in a randomized trial. Sci rep. 2018;8:5065. doi: 10.1038/s41598-018-23404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepert I, Fonseca J, Müller C, Milger K, Hochwind K, Kostric M, Fedoseeva M, Ohnmacht C, Dehmel S, Nathan P, Bartel S. D-tryptophan from probiotic bacteria influences the gut microbiome and allergic airway disease. J Allergy Clin Immunol. 2017;139:1525–1535. doi: 10.1016/j.jaci.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Kerry RG, Patra JK, Gouda S, Park Y, Shin HS, Das G. Benefaction of probiotics for human health: a review. J Food Drug Anal. 2018;26:927–939. doi: 10.1016/j.jfda.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaneghah AM, Fakhri Y. Probiotics and prebiotics as functional foods: state of the art. Curr Nutr Food Sci. 2019;15:20–30. [Google Scholar]
- Kim HJ, Kim HY, Lee SY, Seo JH, Lee E, Hong SJ. Clinical efficacy and mechanism of probiotics in allergic diseases. Korean J Pediatr. 2013;56:369. doi: 10.3345/kjp.2013.56.9.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, Takahashi T. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober MM, Bowe WP. The effect of probiotics on immune regulation, acne, and photoaging. Int J Women’s Dermatol. 2015;1:85–89. doi: 10.1016/j.ijwd.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali VP, Das S, Sen R. An exopolysaccharide from a probiotic: biosynthesis dynamics, composition and emulsifying activity. Food Res Int. 2009;42:695–699. [Google Scholar]
- Kodali VP, Lingala VK, Karlapudi AP, Indira M, Venkateswarulu TC, John Babu D. Biosynthesis and potential application of bacteriocins. J Pure Appl Microbiol. 2013;7:2933–2945. [Google Scholar]
- Kota RK, Ambati RR, Yalakurthi AK, Srirama K, Reddy PN. Recent advances in probiotics as live biotherapeutics against gastrointestinal diseases. Curr Pharma Des. 2018;24:3162–3171. doi: 10.2174/1381612824666180717105128. [DOI] [PubMed] [Google Scholar]
- Langella P, Martín R. Emerging health concepts in the probiotics field: streamlining the definitions. Front Microbiol. 2019;10:1047. doi: 10.3389/fmicb.2019.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc JG, Chain F, Martín R, Bermúdez-Humarán LG, Courau S, Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact. 2017;16:79. doi: 10.1186/s12934-017-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Xia X, Tang W, Ji J, Rui X, Chen X, Jiang M, Zhou J, Zhang Q, Dong M. Structural characterization and anticancer activity of cell-bound exopolysaccharide from Lactobacillus helveticus MB2-1. J Agric Food Chem. 2015;63:3454–3463. doi: 10.1021/acs.jafc.5b01086. [DOI] [PubMed] [Google Scholar]
- Lin Z, Xu Z, Li Y, Wang Z, Chen T, Zhao X. Metabolic engineering of Escherichia coli for the production of riboflavin. Microb Cell Fact. 2014;13:104. doi: 10.1186/s12934-014-0104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach N, Fuster-Botella D. Endurance exercise and gut microbiota: a review. J Sport Health Sci. 2017;6:179–197. doi: 10.1016/j.jshs.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowiak P, Slizewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9:1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M, Ide M, Utsumi H, Niiro T, Shimamura Y, Murata M. Production potency of folate, vitamin B12, and thiamine by lactic acid bacteria isolated from japanese pickles. Biosci Biotechnol Biochem. 2012;76:2061–2067. doi: 10.1271/bbb.120414. [DOI] [PubMed] [Google Scholar]
- Metges CC, Eberhard M, Petzke KJ. Synthesis and absorption of intestinal microbial lysine in humans and non-ruminant animals and impact on human estimated average requirement of dietary lysine. Curr Opin Clin Nutr Metab Care. 2006;9:37–41. doi: 10.1097/01.mco.0000196142.72985.d3. [DOI] [PubMed] [Google Scholar]
- Meyer TSM, Miguel ASM, Fernandez DER, Ortiz GMD. Biotechnological production of oligosaccharides—applications in the food industry. Food Prod Ind. 2015;2:25–78. [Google Scholar]
- Miller JK, Harrison MT, D’Andrea A, Endsley AN, Yin F, Kodukula K, Watson DS. β-Carotene biosynthesis in probiotic bacteria. Probiotics Antimicrob Proteins. 2013;5:69–80. doi: 10.1007/s12602-013-9133-3. [DOI] [PubMed] [Google Scholar]
- Miremadi F, Shah NP. Applications of inulin and probiotics in health and nutrition. Int Food Res J. 2012;19:1337–1350. [Google Scholar]
- Misra S, Mohanty D, Mohapatra S. Applications of probiotics as a functional ingredient in food and gut health. J Food Nutr Res. 2019;7:213–223. [Google Scholar]
- Mohammed Y, Lee B, Kang Z, Du G. Capability of Lactobacillus reuteri to produce an active form of vitamin B12 under optimized fermentation conditions. J Acad Ind Res. 2014;2:617. [Google Scholar]
- Mokoena MP. Lactic acid bacteria and their bacteriocins: classification, biosynthesis and applications against uropathogens. A mini-review. Molecules. 2017;22:1–13. doi: 10.3390/molecules22081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalto M, Curigliano V, Santoro L, Vastola M, Cammarota G, Manna R, Gasbarrini A, Gasbarrini G. Management and treatment of lactose malabsorption. World J Gastroenterol. 2006;12:187–191. doi: 10.3748/wjg.v12.i2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss JW, Williams JO, Ramji DP. Nutraceuticals as therapeutic agents for atherosclerosis. Biochimica et Biophysica Acta—Mol Basis Dis. 2018;1864:1562–1572. doi: 10.1016/j.bbadis.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Ramesh A. Bacteriocin-producing strains of Lactobacillus plantarum inhibit adhesion of Staphylococcus aureus to extracellular matrix: quantitative insight and implications in antibacterial therapy. J Med Microbiol. 2015;64:1514–1526. doi: 10.1099/jmm.0.000181. [DOI] [PubMed] [Google Scholar]
- Nagpal R, Kumar A, Kumar M, Behare PV, Jain S, Yadav H. Probiotics, their health benefits and applications for developing healthier foods: a review. FEMS Microbiol Lett. 2012;334:1–5. doi: 10.1111/j.1574-6968.2012.02593.x. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Nosaka S, Suzuki M, Nagafuchi S, Takahashi T, Yajima T, Takenouchi-ohkubo N, Iwase T, Moro I. Dietary fructo oligosaccharides up-regulate immunoglobulin A response and polymeric immunoglobulin receptor expression in intestines of infant mice. Clin Exp Immunol. 2004;137:52–58. doi: 10.1111/j.1365-2249.2004.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburg DS. Oligosaccharides and glycoconjugates in human milk: their role in host defense. J Mammary Gland Biol Neoplasia. 1996;1:271–283. doi: 10.1007/BF02018080. [DOI] [PubMed] [Google Scholar]
- Ozdemir O. Various effects of different probiotic strains in allergic disorders: an update from laboratory and clinical data. Clin Exp Immunol. 2010;160:295–304. doi: 10.1111/j.1365-2249.2010.04109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmavathi T, Bhargavi R, Priyanka PR, Niranjan NR, Pavitra PV. Screening of potential probiotic lactic acid bacteria and production of amylase and its partial purification. J Genet Eng Biotechnol. 2018;16:357–362. doi: 10.1016/j.jgeb.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan XD, Chen FQ, Wu TX, Tang HG, Zhao ZY. Prebiotic oligosaccharides change the concentrations of short-chain fatty acids and the microbial population of mouse bowel. J Zhejiang Univ Sci B. 2009;10:258. doi: 10.1631/jzus.B0820261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics: a review. J Food Sci Technol. 2015;52:7577–7587. doi: 10.1007/s13197-015-1921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Goyal A. The current trends and future perspectives of prebiotics research a review. 3 Biotech. 2012;2:115–125. [Google Scholar]
- Patel A, Shah N, Prajapati JB. Biosynthesis of vitamins and enzymes in fermented foods by lactic acid bacteria and related genera: a promising approach. Croatian J Food Sci Technol. 2013;5:85–91. [Google Scholar]
- Patrice DC. Human gut microbiome: hopes, threats and promises. Gut. 2018;67:1716–1725. doi: 10.1136/gutjnl-2018-316723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peele KA, Ch VR, Kodali VP. Emulsifying activity of a biosurfactant produced by a marine bacterium. 3 Biotech. 2016;6:177. doi: 10.1007/s13205-016-0494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pericleous M, Mandair D, Caplin ME. Diet and supplements and their impact on colorectal cancer. J gastrointest Oncol. 2013;4:409. doi: 10.3978/j.issn.2078-6891.2013.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwowarek K, Lipińska E, Hać-Szymańczuk E, Kieliszek M, Ścibisz I. Propionibacterium spp.—source of propionic acid, vitamin B12, and other metabolites important for the industry. Appl Microbiol Biotechnol. 2018;102:515–538. doi: 10.1007/s00253-017-8616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Diaz J, Gomez-Llorente C, Fontana L, Gil A. Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J Gastroenterol. 2014;20:15632. doi: 10.3748/wjg.v20.i42.15632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranadheera C, Vidanarachchi J, Rocha R, Cruz A, Ajlouni S. Probiotic delivery through fermentation: dairy vs. non-dairy beverages. Fermentation. 2017;3:67. [Google Scholar]
- Rao SS, Yu S, Fedewa A. Systematic review: dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment Pharmacol Ther. 2015;41:1256–1270. doi: 10.1111/apt.13167. [DOI] [PubMed] [Google Scholar]
- Ravisankar P, Reddy AA, Nagalakshmi B, Koushik OS, Kumar BV, Anvith PS. The comprehensive review on fat soluble vitamins. IOSR J Pharm. 2015;5:12–28. [Google Scholar]
- Rossi M, Amaretti A, Raimondi S. Folate Production by Probiotic Bacteria. Nutrients. 2011;3:118–134. doi: 10.3390/nu3010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur J nutr. 2018;57:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said HM. Intestinal absorption of water-soluble vitamins in health and disease. Biochem J. 2011;437:357–372. doi: 10.1042/BJ20110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez B, Delgado S, Blanco-Miguez A, Lourenco A, Gueimonde M, Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res. 2017;61:1600240. doi: 10.1002/mnfr.201600240. [DOI] [PubMed] [Google Scholar]
- Sanders ME, Benson A, Lebeer S, Merenstein DJ, Klaenhammer TR. Shared mechanisms among probiotic taxa: implications for general probiotic claims. Curr Opin Biotechnol. 2018;49:207–216. doi: 10.1016/j.copbio.2017.09.007. [DOI] [PubMed] [Google Scholar]
- Sanders ME, Jackson S, Schoeni JL, Vegge C, Pane M, Stahl B, Bradley M, Goldman VS, Burguiere P, Atwater JB. Improving end-user trust in the quality of commercial probiotic products. Front Microbiol. 2019;10:1–15. doi: 10.3389/fmicb.2019.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar NR, Priyanka VD, Reddy PS, Rajanikanth P, Kumar VK, Indira M. Purification and characterization of bacteriocin produced by Lactobacillus plantarum isolated from cow milk. Int J Microbiol Res. 2012;3:133–137. [Google Scholar]
- Santos F, Wegkamp A, de Vos WM, Smid EJ, Hugenholtz J. High-level folate production in fermented foods by the B12 producer Lactobacillus reuteri JCM1112. Appl Environ Microbiol. 2008;74:3291–3294. doi: 10.1128/AEM.02719-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarowska J, Choroszy-Krol I, Regulska-Ilow B, Frej-Madrzak M, Jama-Kmiecik A. The therapeutic effect of probiotic bacteria on gastrointestinal diseases. Adv Clin Exp Med. 2013;22:759–766. [PubMed] [Google Scholar]
- Savaiano DA. Lactose digestion from yogurt: mechanism and relevance. Am J Clin Nutr. 2014;99:1251S–1255S. doi: 10.3945/ajcn.113.073023. [DOI] [PubMed] [Google Scholar]
- Schepper JD, Irwin R, Kang J, Dagenais K, Lemon T, Shinouskis A, Parameswaran N, McCabe LR. Probiotics in gut-bone signaling. Understanding the gut-bone signaling axis. New york: Springer; 2017. pp. 225–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Shukla G. Metabiotics: one step ahead of probiotics; an insight into mechanisms involved in anti cancerous effect in colorectal cancer. Front Microbiol. 2016;7:1940. doi: 10.3389/fmicb.2016.01940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit G, Smit BA, Engels WJ. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol Rev. 2005;29(3):591–610. doi: 10.1016/j.femsre.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Strozzi GP, Mogna L. Quantification of folic acid in human feces after administration of Bifidobacterium probiotic strains. J Clin Gastroenterol. 2008;42:179–184. doi: 10.1097/MCG.0b013e31818087d8. [DOI] [PubMed] [Google Scholar]
- Sturme MH, Kleerebezem M, Nakayama J, Akkermans AD, Vaughan EE, De Vos WM. Cell to cell communication by autoinducing peptides in gram-positive bacteria. Antonie Van Leeuwenhoek. 2002;81:233–243. doi: 10.1023/a:1020522919555. [DOI] [PubMed] [Google Scholar]
- Sundravel S, Shanthi P, Sachdanandam P. Therapeutic potential of riboflavin, niacin and ascorbic acid on carbohydrate metabolizing enzymes in secondary endometrial carcinoma bearing rats. Mol Cel Biochem. 2006;288(1–2):73–78. doi: 10.1007/s11010-006-9120-z. [DOI] [PubMed] [Google Scholar]
- Sutton A. Product development of probiotics as biological drugs. Clin Infect Dis. 2008;46:S128–S132. doi: 10.1086/523325. [DOI] [PubMed] [Google Scholar]
- Tachedjian G, Aldunate M, Bradshaw CS, Cone RA. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res Microbiol. 2017;168:782–792. doi: 10.1016/j.resmic.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga K, Kamimura K, Takahashi K, Yokoyama J, Yamagiwa S, Terai S. Diversion colitis and pouchitis: a mini-review. World J Gastroenterol. 2018;24:1734–1747. doi: 10.3748/wjg.v24.i16.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and non starch polysaccharides. Physiol Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Lin PP, Hsieh YM, Zhang ZY, Wu HC, Huang CC. Cholesterol-lowering potentials of lactic acid bacteria based on bile-salt hydrolase activity and effect of potent strains on cholesterol metabolism in vitro and in vivo. Sci World J. 2014;2014:690752. doi: 10.1155/2014/690752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidya Prabhakar K, Ramkrishna S. Antioxidant and free radical scavenging activities of an exopolysaccharide from a probiotic bacterium. Biotechnol J. 2008;3:245–251. doi: 10.1002/biot.200700208. [DOI] [PubMed] [Google Scholar]
- Vinderola G, Binetti A, Burns P, Reinheimer J. Cell viability and functionality of probiotic bacteria in dairy products. Front Microbiol. 2011;2:1–6. doi: 10.3389/fmicb.2011.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta L, Hall S, Coulson S. Metabolic interactions in the gastrointestinal tract (git): host, commensal, probiotics, and bacteriophage influences. Microorganisms. 2015;3:913–932. doi: 10.3390/microorganisms3040913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk RJ, Reckman GA, Harmsen HJ, Priebe MG (2012) Probiotics and lactose intolerance, vol 7. Intech, pp 149–160
- Vorobjeva LI, Khodjaev EY, Vorobjeva NV. Propionic acid bacteria as probiotics. Microb Ecol Health Dis. 2008;20:109–112. [Google Scholar]
- Wang Y, Wu Y, Wang Y, Xu H, Mei X, Yu D, Wang Y, Li W. Antioxidant properties of probiotic bacteria. Nutrients. 2017;9:521. doi: 10.3390/nu9050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SC, Lin CH, Sung CT, Fang JY. Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front Microbiol. 2014;5:241. doi: 10.3389/fmicb.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JY, Kim SS. Probiotics and prebiotics: present status and future perspectives on metabolic disorders. Nutrients. 2016;8:173. doi: 10.3390/nu8030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharof MP, Lovitt RW. Bacteriocins produced by lactic acid bacteria a review article. APCBEE Procedia. 2012;2:50–56. [Google Scholar]
- Zackular JP, Baxter NT, Iverson KD, Sadler WD, Petrosino JF, Chen GY, Schloss PD. The gut microbiome modulates colon tumorigenesis. Mbio. 2013;4:e00692. doi: 10.1128/mBio.00692-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Li S, Gan RY, Zhou T, Xu DP, Li HB. Impacts of gut bacteria on human health and diseases. Int J Mol Sci. 2015;16:7493–7519. doi: 10.3390/ijms16047493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinska D, Kolożyn-Krajewska D. Food-origin Lactic acid bacteria may exhibit probiotic properties. Bio Med rRes Int. 2018;2018:1–15. doi: 10.1155/2018/5063185. [DOI] [PMC free article] [PubMed] [Google Scholar]