Abstract
Objective
The aim of this study was to explore the relationship between fasting glucose levels and all-cause and cause-specific mortality in Chinese population.
Methods
The role of fasting blood glucose levels as a predictor of all-cause and cause-specific mortality was estimated in 9930 participants from four Chinese general populations with a 20-year follow-up. Multivariate Cox proportional hazard models were used to identify the relationship between fasting glucose and mortality.
Results
There were 1471 deaths after a median follow-up of 20.2 years (a total of 187,374 person-years), including 310 cardiovascular deaths, 581 cancer deaths, and 580 other-cause deaths. After adjustment for age, sex, urban or rural, northern or southern of China, types of work, education level, physical exercise, smoking status, drinking status, body mass index, systolic blood pressure, and serum total cholesterol at baseline, the hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality in the fasting blood glucose categories of <60, 60–69, 70–79, 90–99, 100–109, 110–125, and ≥126 mg/dl were 1.38 (1.04–1.84), 1.20 (1.01–1.43), 1.18 (1.03–1.36), 1.18 (0.99–1.41), 1.48 (1.16–1.88), 1.17 (0.84–1.62), and 2.23 (1.72–2.90), respectively, in contrast to the reference group (80–89 mg/dl). The HRs and 95% CIs for cardiovascular disease mortality in these groups were 2.58 (1.44–4.61), 1.41 (0.95–2.10), 1.56 (1.15–2.11), 1.29 (0.88–1.89), 1.36 (0.78–2.37), 1.05 (0.52–2.11), and 2.73 (1.64–4.56), respectively.
Conclusions
Both low and high fasting glucose were significantly associated with increased risk of all-cause and cardiovascular mortality in Chinese general population.
Keywords: Fasting blood glucose, Mortality, Cardiovascular disease, Cohort study
Introduction
Diabetes mellitus and higher fasting blood glucose (FBG) level have been associated with a higher risk of cardiovascular disease (CVD) morbidity and mortality,1, 2, 3, 4 as well as all-cause mortality.5, 6, 7 However, most previous studies used the lowest FBG category as reference and did not analyze the association between low FBG and mortality specifically.8 Several studies have shown that low FBG significantly correlates with higher rates of cardiovascular disease and all-cause mortality, and the J-shape or U-shape relationships between fasting glucose levels and mortality have been found in these studies.9, 10, 11 The Women's Health Initiative Study however indicated that low FBG levels (<80 mg/dl) were not significantly associated with survival in specific cardiovascular disease events or all-cause mortality in postmenopausal women.12 Therefore, the aim of this study was to explore the relationship between fasting glucose levels and all-cause and cause-specific mortality in Chinese population. In addition, we have also explored the possible dose–response relationship between blood glucose level and mortality across the full range of FBG values.
Materials and methods
Study population
The participants were from The People's Republic of China–United States of America (PRC-USA) collaborative study of cardiovascular and cardiopulmonary epidemiology, which was initiated in 1981 under the PRC-USA governmental cooperation in science and technology. Detailed information about the study populations have been reported elsewhere.13, 14, 15, 16 Briefly, the initial cross-sectional study involved adult men and women from 4 urban and rural populations in Beijing and Guangzhou, of which Beijing is located in northern China and Guangzhou is located in southern China. The Beijing urban population included workers from 5 factories from the capital iron and steel complex. The Beijing rural population included farmers from 11 farm brigades in Shijingshan Agricultural District. The Guangzhou urban population included workers from 8 company workshops from the Guangzhou Shipyard Company. Guangzhou rural population included farmers from 14 agricultural villages in Panyu County. Thus, our study had a sample size of at least 2000 adults (1000 men and 1000 women) aged 35–54 years from each population. In order to compare the data with other studies in China, the Chinese task force decided to expand the study population to include subjects up to 59 years of age, thus, in the present analysis, participants between 35 and 59 years were included.17 A baseline survey, including data collection on risk factors for major cardiovascular and cardiopulmonary diseases, was completed during the autumn of 1983–1984. This study with multiple follow-ups was approved by the Cardiovascular Institute and Fuwai Hospital Ethics Committee. Written informed consent was obtained from all participants.
Data collection
All surveys were done in facilities at or adjacent to work sites of the participants. The demographic information, lifestyle risk factors, and personal medical history were collected through a standardized questionnaire. In addition to the survey items, physical examination data, including height, weight, and blood pressure, were measured at baseline. Height without shoes was measured to the nearest 1 centimeter by using a standard right angle device. Weight was measured to the nearest 0.1 kilogram by using a spring balance. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Blood pressure was measured three times at one session using a standard mercury sphygmomanometer and mean of the three measurements was used. All staff involved in administering the survey were trained and had passed a qualification test according to a uniform and standard manual of operation. As plans for field work developed, a joint Beijing-Guangzhou quality control committee was established to monitor and enhance performance.13
Fasting blood specimens after an overnight fast were obtained in 1983–1984 for measurement of serum glucose and lipids. Blood samples were centrifuged and serum was separated within 3 hours. Samples were then transported to the laboratories in Beijing and Guangzhou for analysis. Both laboratories were standardized by the Lipid Standardization Program of the U.S. National Heart, Lung, and Blood Institute and Center for Disease Control and Prevention (CDC). The fasting glucose was measured by the enzymatic method (SmithKline Instruments, Inc, Sunnyvale, CA, USA), and total cholesterol was measured with a high-performance enzymic reagent (Boehringer-Mannheim Diagnostics, Indianapolis, IN, USA) with the Abbott Laboratories (Abbott Park, Ill) ABA200 bichromatic analyzer (Abbott Park, Illinois, USA).18
Follow-up and outcomes
The first follow-up for coronary heart disease (CHD) and stroke events and all-cause mortality was done 4 years later, in 1987–1988, following which, the cohort was followed up every 2 years until the date of death or December 31, 2005. Death certificates or hospital records were obtained from next-of-kin of the study participants or from the local death registration department. This vital information was reviewed and verified by the joint Beijing-Guangzhou endpoint assessment committee and was then coded by the staff according to the International Classification of Disease, 9th Edition (ICD-9). The CVD mortality in this study was defined as fatal myocardial infarction, CHD death, sudden cardiac death, or fatal stroke. Cancer mortality was defined as per codes 140 to 208, and other-cause mortality included all non-CVD and non-cancer deaths in this analysis. More details of follow-up information have been explained in the previous publication.17
Statistical analysis
Data of baseline investigations were entered into computer readable files, edited in China, and then sent to the United States for standardization. In the present analysis, FBG levels were categorized as <60, 60–69, 70–79, 80–89, 90–99, 100–109, 110–125, and ≥126 mg/dl. We calculated mean values and proportions of characteristics of the participants at baseline for categories of fasting glucose levels, and tested for differences in baseline characteristics between FBG categories with one-way analysis of variance for continuous variables with normal distribution, and with χ2 tests for categorical variables. We then used Cox proportional hazards models to determine hazard ratios (HR) and 95% confidence intervals (CI) for all-cause and cause-specific mortality by fasting glucose categories using the group with fasting glucose level of 80–89 mg/dl as the reference group. All the analyses were from univariate models by stepwise adjustment of the following confounding variables: age at baseline, sex, urban or rural, north or south, types of work (mental, light manual or heavy manual), education levels (illiteracy, primary school, junior middle school, high school or at least some college), physical exercise (never, occasionally or regular), smoking (defined as having smoked at least one cigarette per day for 1 year or more), drinking (defined as drinking alcohol at least once a week in the past 12 months), BMI, systolic blood pressure (SBP), and serum total cholesterol. Restricted cubic spline models were used to detect the possible dose–response relationships between FBG and the risk of death. We additionally performed sensitivity analysis by excluding deaths in the first 3 years, and participants whose BMI was less than 18.5 kg/m2 at baseline. A two tailed P-value less than 0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
A total of 11,325 participants were enrolled in the baseline investigation. Participants who had CHD (n = 14) or stroke (n = 67) history at baseline or participants who did not provide blood specimens (n = 1261) were excluded. We further excluded participants with classic hypoglycemia with FBG < 50 mg/dl (n = 53) in the present analysis and then 9930 participants remained, including 4902 men and 5028 women. Participants had a mean age of 45.8 ± 6.1 years at baseline, a mean FBG value of 81.9 ± 19.2 mg/dl, and a mean BMI of 22.0 ± 3.4 kg/m2. The baseline characteristics of the population according to fasting glucose categories are shown in Table 1. Overall, participants in lower fasting glucose categories were more likely to be younger, to be from the southern parts of China, and to live in rural areas and engaged in heavy manual work. In addition, participants with lower fasting glucose were more likely to have lower SBP, lower diastolic blood pressure (DBP), and lower total cholesterol levels.
Table 1.
Characteristics of the study population at baseline according to the baseline levels of fasting glucose (n = 9930).
| Fasting glucose categories (mg/dl) |
P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| <60 | 60–69 | 70–79 | 80–89 | 90–99 | 100–109 | 110–125 | ≥126 | ||
| n | 351 | 1486 | 3229 | 2903 | 1151 | 397 | 212 | 201 | |
| Age, years, mean ± SD | 44.3 ± 5.9 | 44.2 ± 6.0 | 45.3 ± 6.0 | 46.3 ± 6.0 | 46.9 ± 5.8 | 47.4 ± 6.0 | 47.8 ± 5.8 | 48.6 ± 5.9 | <0.0001 |
| Female, n (%) | 186 (53.0) | 746 (50.2) | 1597 (49.5) | 1423 (49.0) | 608 (52.8) | 226 (56.9) | 130 (61.3) | 112 (55.7) | 0.0005 |
| Urban, n (%) | 136 (38.7) | 697 (46.9) | 1777 (55.0) | 1777 (61.2) | 701 (60.9) | 219 (55.2) | 112 (52.8) | 111 (55.2) | <0.0001 |
| Northern, n (%) | 144 (41.0) | 651 (43.8) | 1656 (51.3) | 1643 (56.6) | 728 (63.2) | 287 (72.3) | 164 (77.4) | 174 (86.6) | <0.0001 |
| Current smoker, n (%) | 173 (49.3) | 751 (50.5) | 1556 (48.2) | 1313 (45.2) | 507 (44.0) | 193 (48.6) | 97 (45.8) | 97 (48.3) | 0.0110 |
| Alcohol drinker, n (%) | 96 (27.4) | 384 (25.8) | 790 (24.5) | 702 (24.2) | 301 (26.2) | 94 (23.7) | 43 (20.3) | 44 (21.9) | 0.3807 |
| Type of work, n (%) | <0.0001 | ||||||||
| Mental | 22 (6.3) | 76 (5.1) | 273 (8.5) | 260 (9.0) | 104 (9.0) | 32 (8.1) | 18 (8.5) | 9 (4.5) | |
| Light manual | 107 (30.5) | 504 (33.9) | 1269 (39.3) | 1365 (47.0) | 563 (48.9) | 204 (51.4) | 119 (56.1) | 119 (59.2) | |
| Heavy manual | 222 (63.2) | 906 (61.0) | 1687 (52.2) | 1278 (44.0) | 484 (42.1) | 161 (40.6) | 75 (35.4) | 73 (36.3) | |
| Education, n (%) | <0.0001 | ||||||||
| Illiteracy | 87 (24.8) | 335 (22.5) | 713 (22.1) | 680 (23.4) | 276 (24.0) | 113 (28.5) | 63 (29.7) | 67 (33.3) | |
| Primary school | 164 (46.7) | 699 (47.0) | 1468 (45.5) | 1311 (45.2) | 559 (48.6) | 190 (47.9) | 113 (53.3) | 96 (47.8) | |
| Junior middle school | 75 (21.4) | 315 (21.2) | 658 (20.4) | 570 (19.6) | 197 (17.1) | 68 (17.1) | 28 (13.2) | 27 (13.4) | |
| High school | 23 (6.6) | 119 (8.0) | 310 (9.6) | 265 (9.1) | 85 (7.4) | 23 (5.8) | 5 (2.4) | 8 (4.0) | |
| At least some college | 2 (0.6) | 18 (1.2) | 80 (2.5) | 77 (2.7) | 34 (3.0) | 3 (0.8) | 3 (1.4) | 3 (1.5) | |
| Exercise, n (%) | <0.0001 | ||||||||
| Never | 315 (89.7) | 1278 (86.0) | 2712 (84.0) | 2364 (81.4) | 943 (81.9) | 325 (81.9) | 180 (84.9) | 167 (83.1) | |
| Occasionally | 19 (5.4) | 102 (6.9) | 216 (6.7) | 243 (8.4) | 78 (6.8) | 36 (9.1) | 8 (3.8) | 18 (9.0) | |
| Regular | 17 (4.8) | 106 (7.1) | 301 (9.3) | 296 (10.2) | 130 (11.3) | 36 (9.1) | 24 (11.3) | 16 (8.0) | |
| BMI, kg/m2, mean ± SD | 20.8 ± 2.9 | 21.2 ± 3.0 | 21.6 ± 3.2 | 22.2 ± 3.3 | 22.9 ± 3.6 | 23.4 ± 3.8 | 23.9 ± 4.0 | 23.9 ± 3.6 | <0.0001 |
| SBP, mmHg, mean ± SD | 114.7 ± 17.0 | 115.2 ± 17.6 | 117.7 ± 18.2 | 120.4 ± 18.7 | 123.5 ± 20.0 | 123.9 ± 20.4 | 128.4 ± 22.4 | 127.6 ± 22.0 | <0.0001 |
| DBP, mmHg, mean ± SD | 74.8 ± 11.5 | 75.0 ± 11.3 | 76.1 ± 11.2 | 77.6 ± 11.4 | 79.4 ± 11.7 | 79.0 ± 12.1 | 80.0 ± 13.3 | 80.5 ± 12.4 | <0.0001 |
| TC, mmol/L, mean ± SD | 4.1 ± 1.0 | 4.3 ± 0.9 | 4.5 ± 0.9 | 4.7 ± 1.0 | 4.8 ± 1.0 | 4.8 ± 1.0 | 4.9 ± 1.0 | 5.0 ± 1.3 | <0.0001 |
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; TC: total cholesterol; SD: standard deviation.
There were 1471 deaths after a median follow-up of 20.2 years (a total of 187,374 person-years) with a follow-up rate of 93.7%, including 310 CVD deaths, 581 cancer deaths, and 580 other-cause deaths. The all-cause and cause-specific mortality rate per 1000 person-years according to the baseline fasting glucose categories and HR assessed by Cox proportional hazards models are shown in Table 2. The all-cause and CVD mortality rates per 1000 person-years both in lower (<80 mg/dl) and higher fasting glucose categories were higher than FBG in the range of 80–89 mg/dl. All-cause mortality rates per 1000 person-years in the FBG categories of <60, 60–69, 70–79, 80–89, 90–99, 100–109, 110–125, and ≥126 mg/dl were 8.40, 7.08, 7.41, 7.02, 8.66, 11.00,10.12, and 19.76, respectively. The CVD mortality rates in the same groups were 2.10, 1.31, 1.64, 1.35, 1.95, 2.06, 2.28, and 5.44, respectively. Cox proportional hazards models indicated that both low and high fasting glucose levels were significantly associated with the risk of all-cause and CVD mortality compared with those having FBG in the range of 80–89 mg/dl after adjustments for age, sex, urban or rural, northern or southern China, type of work, education level, physical exercise, smoking status, drinking status, BMI, SBP, and serum total cholesterol at baseline. The HRs and 95% CIs for all-cause mortality in the FBG categories of <60, 60–69, 70–79, 90–99, 100–109, 110–125, and ≥126 mg/dl were 1.38 (1.04–1.84), 1.20 (1.01–1.43), 1.18 (1.03–1.36), 1.18 (0.99–1.41), 1.48 (1.16–1.88), 1.17 (0.84–1.62), and 2.23 (1.72–2.90) in contrast to that for the reference group (80–89 mg/dl). The HRs and 95% CIs for CVD mortality in these groups were 2.58 (1.44–4.61), 1.41 (0.95–2.10), 1.56 (1.15–2.11), 1.29 (0.88–1.89), 1.36 (0.78–2.37), 1.05 (0.52–2.11), and 2.73 (1.64–4.56), respectively. Multivariable restricted cubic spline regression model showed that continuous variation in FBG was related to all-cause (P = 0.0016) and CVD mortality (P = 0.0003) in a nonlinear manner, suggesting that there is an adjusted dose–response association between continuous FBG and all-cause mortality as well as CVD mortality. However, we did not find a significant relationship between low fasting glucose and cancer or other-cause mortality. Sensitivity analysis showed that the results remained unchanged when we excluded deaths in the first 3 years and participants whose BMI was less than 18.5 kg/m2 at baseline, respectively.
Table 2.
Hazard ratios (95% CI) of all-cause and cause-specific mortality according to fasting glucose levels at baseline.
| Fasting glucose categories (mg/dl) |
||||||||
|---|---|---|---|---|---|---|---|---|
| <60 | 60–69 | 70–79 | 80–89 | 90–99 | 100–109 | 110–125 | ≥126 | |
| n | 351 | 1486 | 3229 | 2903 | 1151 | 397 | 212 | 201 |
| Person-years | 6668.10 | 28252.40 | 61133.27 | 55013.28 | 21591.08 | 7272.06 | 3951.47 | 3491.82 |
| All causes | ||||||||
| No. of deaths | 56 | 200 | 453 | 386 | 187 | 80 | 40 | 69 |
| Mortality ratea | 8.40 | 7.08 | 7.41 | 7.02 | 8.66 | 11.00 | 10.12 | 19.76 |
| Model 1 | 1.17 (0.88–1.55) | 1.00 (0.85–1.19) | 1.06 (0.92–1.21) | 1 (ref) | 1.24 (1.04–1.47) | 1.57 (1.24–2.00) | 1.43 (1.03–1.98) | 2.87 (2.22–3.72) |
| Model 2 | 1.41 (1.06–1.86) | 1.21 (1.02–1.44) | 1.17 (1.02–1.34) | 1 (ref) | 1.19 (1.00–1.42) | 1.54 (1.21–1.96) | 1.32 (0.96–1.83) | 2.43 (1.88–3.15) |
| Model 3 | 1.30 (0.98–1.73) | 1.16 (0.97–1.37) | 1.15 (1.01–1.32) | 1 (ref) | 1.19 (1.00–1.42) | 1.47 (1.15–1.87) | 1.22 (0.88–1.69) | 2.21 (1.70–2.87) |
| Model 4 | 1.38 (1.04–1.84) | 1.20 (1.01–1.43) | 1.18 (1.03–1.36) | 1 (ref) | 1.18 (0.99–1.41) | 1.48 (1.16–1.88) | 1.17 (0.84–1.62) | 2.23 (1.72–2.90) |
| CVD | ||||||||
| No. of deaths | 14 | 37 | 100 | 74 | 42 | 15 | 9 | 19 |
| Mortality ratea | 2.10 | 1.31 | 1.64 | 1.35 | 1.95 | 2.06 | 2.28 | 5.44 |
| Model 1 | 1.55 (0.88–2.74) | 0.97 (0.65–1.44) | 1.22 (0.90–1.64) | 1 (ref) | 1.45 (0.99–2.11) | 1.54 (0.88–2.68) | 1.68 (0.84–3.35) | 4.19 (2.53–6.93) |
| Model 2 | 1.94 (1.09–3.44) | 1.21 (0.82–1.80) | 1.38 (1.02–1.86) | 1 (ref) | 1.38 (0.95–2.02) | 1.48 (0.85–2.59) | 1.50 (0.75–3.00) | 3.39 (2.04–5.62) |
| Model 3 | 2.15 (1.21–3.83) | 1.28 (0.86–1.91) | 1.43 (1.06–1.94) | 1 (ref) | 1.28 (0.87–1.87) | 1.28 (0.73–2.23) | 1.19 (0.59–2.39) | 2.57 (1.54–4.28) |
| Model 4 | 2.58 (1.44–4.61) | 1.41 (0.95–2.10) | 1.56 (1.15–2.11) | 1 (ref) | 1.29 (0.88–1.89) | 1.36 (0.78–2.37) | 1.05 (0.52–2.11) | 2.73 (1.64–4.56) |
| Cancer | ||||||||
| No. of deaths | 23 | 87 | 185 | 166 | 69 | 27 | 13 | 11 |
| Mortality ratea | 3.45 | 3.08 | 3.03 | 3.02 | 3.20 | 3.71 | 3.29 | 3.15 |
| Model 1 | 1.14 (0.74–1.76) | 1.02 (0.78–1.32) | 1.00 (0.81–1.24) | 1 (ref) | 1.06 (0.80–1.41) | 1.24 (0.82–1.86) | 1.09 (0.62–1.91) | 0.98 (0.52–1.86) |
| Model 2 | 1.32 (0.85–2.04) | 1.18 (0.91–1.53) | 1.09 (0.88–1.34) | 1 (ref) | 1.04 (0.78–1.37) | 1.22 (0.81–1.84) | 1.04 (0.59–1.82) | 0.87 (0.46–1.64) |
| Model 3 | 1.16 (0.75–1.80) | 1.09 (0.84–1.42) | 1.05 (0.85–1.29) | 1 (ref) | 1.06 (0.80–1.40) | 1.22 (0.81–1.84) | 1.02 (0.58–1.79) | 0.88 (0.46–1.67) |
| Model 4 | 1.14 (0.74–1.78) | 1.09 (0.84–1.42) | 1.04 (0.85–1.29) | 1 (ref) | 1.06 (0.80–1.40) | 1.22 (0.81–1.84) | 1.02 (0.58–1.81) | 0.89 (0.47–1.70) |
| Other causesb | ||||||||
| No. of deaths | 19 | 76 | 168 | 146 | 76 | 38 | 18 | 39 |
| Mortality ratec | 2.85 | 2.69 | 2.75 | 2.65 | 3.52 | 5.23 | 4.56 | 11.17 |
| Model 1 | 1.01 (0.62–1.65) | 1.01 (0.76–1.33) | 1.04 (0.83–1.29) | 1 (ref) | 1.33 (1.00–1.75) | 1.97 (1.38–2.82) | 1.70 (1.04–2.77) | 4.37 (3.07–6.23) |
| Model 2 | 1.24 (0.76–2.02) | 1.24 (0.94–1.64) | 1.16 (0.93–1.45) | 1 (ref) | 1.27 (0.97–1.68) | 1.93 (1.35–2.76) | 1.55 (0.95–2.53) | 3.61 (2.53–5.15) |
| Model 3 | 1.10 (0.67–1.80) | 1.15 (0.87–1.52) | 1.13 (0.91–1.42) | 1 (ref) | 1.31 (0.99–1.73) | 1.86 (1.30–2.66) | 1.44 (0.88–2.36) | 3.26 (2.28–4.67) |
| Model 4 | 1.19 (0.72–1.94) | 1.21 (0.91–1.60) | 1.17 (0.93–1.46) | 1 (ref) | 1.30 (0.98–1.72) | 1.86 (1.30–2.67) | 1.40 (0.85–2.29) | 3.29 (2.29–4.71) |
Model 1 did not adjust any variables; Model 2 adjusted for age and sex; Model 3 additionally adjusted for urbanization (urban or rural), region (north or south), types of work (mental, light manual or heavy manual), education level (illiteracy, primary school, junior middle school, high school or at least some college), exercise habit (never, occasionally or regular), smoking status (current smoker or not), drinking status (current drinker or not) and body mass index; Model 4 additionally adjusted for systolic blood pressure and serum total cholesterol at baseline. CI: confidence interval; CVD: cardiovascular disease; ref: reference.
a,c The unit of mortality rate is per 1000 person-years. b Includes all non-CVD and non-cancer deaths.
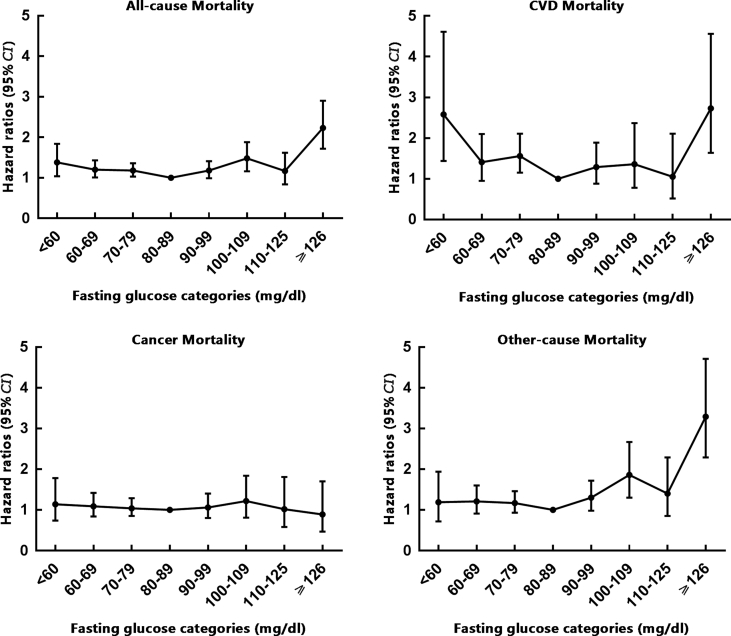
We also found J-shape relationships between fasting glucose and all-cause and CVD mortality across the full range of FBG values even when we adjusted multiple potential confounding variables (Fig. 1). Both lower fasting glucose level (<80 mg/dl) and higher fasting glucose level (≥126 mg/dl) increased the risk of all-cause and CVD mortality, and FBG in the range of ≥126 mg/dl posed the highest risk for all-cause and CVD mortality.
Fig. 1.
Multivariate-adjusted hazard ratios (95% CI) for all-cause and cause-specific mortality according to fasting glucose categories at baseline. aAdjusted for age, sex, urbanization (urban or rural), region (north or south), types of work (mental, light manual or heavy manual), education level (illiteracy, primary school, junior middle school, high school or college), exercise habits (never, occasionally or regular), smoking status (current smoker or not), drinking status (current drinker or not), body mass index, systolic blood pressure and serum total cholesterol at baseline.
Discussion
In this prospective cohort study conducted in Chinese population for a median of 20.2 years corresponding 187,374 person-years follow-up, we found that both low and high fasting glucose levels were significantly associated with higher risk of all-cause and CVD mortality. J-shaped relationships between fasting glucose and all-cause and CVD mortality across the full range of FBG values were also found in our study.
Our findings are consistent with the results of several previous studies.9, 10, 11 Wei et al9 reported that the association between low fasting plasma glucose (FPG) and mortality was strong, independent, and graded across strata of low FPG during an average follow-up of 8 years in US adults. For example, the multivariate-adjusted relative risks (with 95% CIs) of low FPG (<80 mg/dl) for CVD and all-cause mortality were 2.7 (1.7–4.3) and 2.0 (1.4–2.9) in all study subjects. Wei et al9 also found that the relation between low FPG and cancer mortality was weak and nonsignificant, which is consistent with our study. Wen et al10 assessed mortality risks at different levels of FBG in Taiwan of China and found that the lowest FBG group, 50–75 mg/dl, had a significant 2-fold risk of mortality from all causes and they further found an overall J-shaped relationship between all-cause mortality and levels of FBG, with significant increase in mortality, starting at ≥110 mg/dl as well as ≤75 mg/dl.10 Wandell et al11 found a J-curved risk of long-term mortality in relation to FBG values during a 26-year follow-up among 2300 participants in Sweden. This J-shaped relationship between glucose levels and mortality was also found in the Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe (DECODE) study, which showed a 1.2-fold increase in the risk of all-cause mortality among those with FBG ≤81 mg/dl.19 Recently, Mongraw-Chaffin et al12 indicated that low fasting glucose at baseline was not significantly associated with survival from specific cardiovascular disease events or all-cause mortality, but the participants in this study were all postmenopausal women, aged between 50 and 79 years at baseline, as the authors reported that participants with low fasting glucose at baseline were more likely to have used hormone therapy in their study.12 Hormone replacement therapy may be an important confounding factor because it could affect glucose metabolism,20 but unfortunately, the study did not adjust this factor in multivariable models. Variations in the source population or in analysis may lead to different results in other studies.
In recent years, several studies have shown that low hemoglobin A1c (HbA1c) is associated with a higher risk of death, providing further evidence for the association between low fasting glucose and mortality. National Health and Nutrition Examination Survey Ⅲ (NHANES Ⅲ) found HbA1c <4.0% versus 5.0%–5.4% was associated with an increased risk of all-cause mortality (HR: 2.90; 95% CI: 1.25 to 6.76) after multivariable adjustment.21 A prospective cohort study of 13,288 participants in the Atherosclerosis Risk in Communities (ARIC) Study showed that HbA1c <5.0% was associated with a significantly increased risk of all-cause mortality (HR: 1.32, 95% CI: 1.13–1.55) compared with HbA1c of 5.0–5.7%.22 Since fasting glucose and HbA1c share a similar clinical purpose, these results add to evidence that low fasting glucose values may be a generalized marker of mortality risk in the general population.
The exact mechanism underlying the association between low fasting glucose and the high risk of mortality remains unclear. Wei et al9 supposed that severely low fasting glucose may induce brain damage and dysfunction. However, brain damage occurs only when classic hypoglycemia occurs, with FBG <50 mg/dl,23 and we have excluded participants with FBG <50 mg/dl in the present analysis. Another possibility is that low fasting glucose could be a marker of malignant disease,8 but there was no significant association between low FBG and cancer mortality in our study, nor in the study by Wei et al9 or other population-based cohort studies.24, 25 For sensitivity analysis, we additionally excluded deaths in the first 3 years, and the results did not change at lower glucose range, which indicated that positive association of low FBG with high mortality risk is unlikely to be due to the presence of serious illness. In our study, participants with lower fasting glucose were more likely to have lower BMI, lower SBP, lower DBP, and lower total cholesterol levels, which suggest that low FBG may be a marker of poor nutritional status. Nevertheless, the association of low FBG with all-cause or CVD mortality remained significant after adjustment for BMI, SBP, and total cholesterol levels. Moreover, we found that results remained unchanged when we excluded participants with BMI <18.5 kg/m2 at baseline.
This study has several strengths. First, our study was conducted in a younger population with longer follow-up than those of many other studies, which adds estimates in another population of special interest for the development of cardiometabolic risk. Second, the inclusion of rural population adds a generalization, as most studies in the US and Europe focused only on urban centers. Third, half of the participants in our study came from factories with complete and detailed medical records, and the other half from rural areas without large migration, which ensured a high follow-up rate in our study. Our study also has several limitations that need to be mentioned. First, the study did not record diabetes history and the history of antidiabetic drugs at baseline, although the prevalence of diabetes in China from the 80's to the early 90's of the last century was very low. The prevalence of diabetes in the Chinese population was less than 1% in 1980 and 2.5% in a subsequent national survey conducted in 1994.26, 27 It can be speculated that the rate of awareness and medication rate of diabetes mellitus at that time was minimal and low FBG in this study is unlikely to be associated with diabetes treatment. Second, we assumed that low fasting glucose was linked to poor nutritional status, but our study lacks data on diet and nutrition in all populations.
Conclusion
We found that both low (<80 mg/dl) and high (≥126 mg/dl) fasting glucose levels were significantly associated with increased risk of all-cause and cardiovascular diseases mortality in Chinese general population from the cohort of PRC-USA Collaborative Study. More attention should be paid to the J-shape relationships between fasting glucose and all-cause and CVD mortality. Further studies are needed to clarify the mechanism underlying the association between low fasting glucose and high risk of mortality.
Conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgments
This study was supported by the United States National Heart, Lung, and Blood Institute (grants NO-1HV12243 and NO-1HV8112); the People's Republic of China Ministry of Science and Technology from the 8th to 10th National Five-Year Plans Projects (grants 85-915-01-01, 96-906-02-01, and 2001BA703B01).
Edited by Jing-Ling Bao
Footnotes
Peer review under responsibility of Chinese Medical Association.
Contributor Information
Lian-Cheng Zhao, Email: zhaolch@163.com.
Xiao-Qing Liu, Email: drxqliu@21cn.com.
References
- 1.Lawes C.M., Parag V., Bennett D.A. Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes Care. 2004;27:2836–2842. doi: 10.2337/diacare.27.12.2836. [DOI] [PubMed] [Google Scholar]
- 2.Brunner E.J., Shipley M.J., Witte D.R., Fuller J.H., Marmot M.G. Relation between blood glucose and coronary mortality over 33 years in the Whitehall Study. Diabetes Care. 2006;29:26–31. doi: 10.2337/diacare.29.01.06.dc05-1405. [DOI] [PubMed] [Google Scholar]
- 3.Sarwar N., Gao P., Seshasai S.R. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park C., Guallar E., Linton J.A. Fasting glucose level and the risk of incident atherosclerotic cardiovascular diseases. Diabetes Care. 2013;36:1988–1993. doi: 10.2337/dc12-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barr E.L., Boyko E.J., Zimmet P.Z., Wolfe R., Tonkin A.M., Shaw J.E. Continuous relationships between non-diabetic hyperglycaemia and both cardiovascular disease and all-cause mortality: the Australian diabetes, obesity, and lifestyle (AusDiab) study. Diabetologia. 2009;52:415–424. doi: 10.1007/s00125-008-1246-y. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y., Cai X., Mai W., Li M., Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ. 2016;355:i5953. doi: 10.1136/bmj.i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bragg F., Holmes M.V., Iona A. Association between diabetes and cause-specific mortality in rural and urban areas of China. J Am Med Assoc. 2017;317:280–289. doi: 10.1001/jama.2016.19720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wändell P.E., Theobald H. The association between low fasting blood glucose value and mortality. Curr Diabetes Rev. 2007;3:274–279. doi: 10.2174/1573399810703040274. [DOI] [PubMed] [Google Scholar]
- 9.Wei M., Gibbons L.W., Mitchell T.L., Kampert J.B., Stern M.P., Blair S.N. Low fasting plasma glucose level as a predictor of cardiovascular disease and all-cause mortality. Circulation. 2000;101:2047–2052. doi: 10.1161/01.cir.101.17.2047. [DOI] [PubMed] [Google Scholar]
- 10.Wen C.P., Cheng T.Y., Tsai S.P., Hsu H.L., Wang S.L. Increased mortality risks of pre-diabetes (impaired fasting glucose) in Taiwan. Diabetes Care. 2005;28:2756–2761. doi: 10.2337/diacare.28.11.2756. [DOI] [PubMed] [Google Scholar]
- 11.Wandell P.E., Theobald H. The association between blood glucose value and long-term mortality. Diabetes Metab. 2005;31:588–594. doi: 10.1016/s1262-3636(07)70235-5. [DOI] [PubMed] [Google Scholar]
- 12.Mongraw-Chaffin M., LaCroix A.Z., Sears D.D. A prospective study of low fasting glucose with cardiovascular disease events and all-cause mortality: the Women's Health Initiative. Metabolism. 2017;70:116–124. doi: 10.1016/j.metabol.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.People's Republic of China--United States Cardiovascular and Cardiopulmonary Epidemiology Research Group An epidemiological study of cardiovascular and cardiopulmonary disease risk factors in four populations in the People's Republic of China. Baseline report from the P.R.C.-U.S.A. Collaborative Study. Circulation. 1992;85:1083–1096. doi: 10.1161/01.cir.85.3.1083. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z., Wu X., Stamler J. A north-south comparison of blood pressure and factors related to blood pressure in the People's Republic of China: a report from the PRC-USA Collaborative Study of Cardiovascular Epidemiology. J Hypertens. 1994;12:1103–1112. [PubMed] [Google Scholar]
- 15.Li Y., Stamler J., Xiao Z., Folsom A., Tao S., Zhang H. Serum uric acid and its correlates in Chinese adult populations, urban and rural, of Beijing. The PRC-USA Collaborative Study in Cardiovascular and Cardiopulmonary Epidemiology. Int J Epidemiol. 1997;26:288–296. doi: 10.1093/ije/26.2.288. [DOI] [PubMed] [Google Scholar]
- 16.Zhou B., Rao X., Dennis B.H. The relationship between dietary factors and serum lipids in Chinese urban and rural populations of Beijing and Guangzhou. PRC-USA Cardiovascular and Cardiopulmonary Research Group. Int J Epidemiol. 1995;24:528–534. doi: 10.1093/ije/24.3.528. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y., Liu X., Li X. Estimation of 10-year risk of fatal and nonfatal ischemic cardiovascular diseases in Chinese adults. Circulation. 2006;114:2217–2225. doi: 10.1161/CIRCULATIONAHA.105.607499. [DOI] [PubMed] [Google Scholar]
- 18.Siedel J., Hagele E.O., Ziegenhorn J., Wahlefeld A.W. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem. 1983;29:1075–1080. [PubMed] [Google Scholar]
- 19.DECODE Study Group, European Diabetes Epidemiology Group Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care. 2003;26:688–696. doi: 10.2337/diacare.26.3.688. [DOI] [PubMed] [Google Scholar]
- 20.Coelingh B.H., Verhoeven C., Zimmerman Y., Visser M., Foidart J.M., Gemzell-Danielsson K. Pharmacodynamic effects of the fetal estrogen estetrol in postmenopausal women: results from a multiple-rising-dose study. Menopause. 2017;24:677–685. doi: 10.1097/GME.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 21.Carson A.P., Fox C.S., McGuire D.K. Low hemoglobin A1c and risk of all-cause mortality among US adults without diabetes. Circ Cardiovasc Qual Outcomes. 2010;3:661–667. doi: 10.1161/CIRCOUTCOMES.110.957936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aggarwal V., Schneider A.L., Selvin E. Low hemoglobin A(1c) in nondiabetic adults: an elevated risk state? Diabetes Care. 2012;35:2055–2060. doi: 10.2337/dc11-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohseni S. Neurologic damage in hypoglycemia. Handb Clin Neurol. 2014;126:513–532. doi: 10.1016/B978-0-444-53480-4.00036-9. [DOI] [PubMed] [Google Scholar]
- 24.Yamagata H., Kiyohara Y., Nakamura S. Impact of fasting plasma glucose levels on gastric cancer incidence in a general Japanese population: the Hisayama study. Diabetes Care. 2005;28:789–794. doi: 10.2337/diacare.28.4.789. [DOI] [PubMed] [Google Scholar]
- 25.Rapp K., Schroeder J., Klenk J. Fasting blood glucose and cancer risk in a cohort of more than 140,000 adults in Austria. Diabetologia. 2006;49:945–952. doi: 10.1007/s00125-006-0207-6. [DOI] [PubMed] [Google Scholar]
- 26.National Diabetes Research Group A mass survey of diabetes mellitus in a population of 300,000 in 14 provinces and municipalities in China. Zhonghua Nei Ke Za Zhi. 1981;20:678–683. [in Chinese] [PubMed] [Google Scholar]
- 27.Pan X.R., Yang W.Y., Li G.W., Liu J. Prevalence of diabetes and its risk factors in China, 1994. National Diabetes Prevention and Control Cooperative Group. Diabetes Care. 1997;20:1664–1669. doi: 10.2337/diacare.20.11.1664. [DOI] [PubMed] [Google Scholar]