Abstract
Because the study population with gliosarcoma (GSM) is limited, the understanding of this disease is insufficient. In this study, the authors aimed to determine the clinical characteristics and independent prognostic factors influencing the prognosis of GSM patients and to develop a nomogram to predict the prognosis of GSM patients after craniotomy. A total of 498 patients diagnosed with primary GSM between 2004 and 2015 were extracted from the 18 Registries Research Data of the Surveillance, Epidemiology, and End Results (SEER) database. The median disease-specific survival (DSS) was 12.0 months, and the postoperative 0.5-, 1-, and 3-year DSS rates were 71.4%, 46.4% and 9.8%, respectively. We applied both the Cox proportional hazards model and the decision tree model to determine the prognostic factors of primary GSM. The Cox proportional hazards model demonstrated that age at presentation, tumour size, metastasis state and adjuvant chemotherapy (CT) were independent prognostic factors for DSS. The decision tree model suggested that age <71 years and adjuvant CT were associated with a better prognosis for GSM patients. The nomogram generated via the Cox proportional hazards model was developed by applying the rms package in R version 3.5.0. The C-index of internal validation for DSS prediction was 0.67 (95% confidence interval (CI), 0.63 to 0.70). The calibration curve at one year suggested that there was good consistency between the predicted DSS and the actual DSS probability. This study was the first to develop a disease-specific nomogram for predicting the prognosis of primary GSM patients after craniotomy, which can help clinicians immediately and accurately predict patient prognosis and conduct further treatment.
Subject terms: Surgical oncology, Risk factors
Introduction
Gliosarcoma (GSM) is a rare malignant brain tumour composed of both glial and sarcomatous elements, the incidence of which is between 1% and 8% of all malignant gliomas1–3. GSM was first described by Stroebe in 1895 as a variant of glioblastoma (GBM) and gained wide acceptance after Feigin et al. and Rubinstein et al. published their papers presenting several patients with this malignant tumour in detail4–6.
Due to the low incidence of GSM, there are few studies describing the patient characteristics, treatment regimen and prognosis of GSM. As a variant of GBM, primary GSMs are often managed in accordance with GBM guidelines (the Stupp protocol)7. However, even after receiving standardized treatment, the prognosis of GSM patients remains dismal, with a median overall survival ranging from 6.6 to 18.5 months8–11.
In this study, retrospective data including a total of 498 patients who underwent craniotomy between 2004 and 2015 were reviewed from the Surveillance, Epidemiology, and End Results (SEER) database. The clinical characteristics and independent prognostic factors were analysed by applying large patient numbers. A prognostic disease-specific nomogram was constructed and validated based on retrospective patient data from the SEER database.
The nomogram is a multivariate visualization prediction model that can incorporate different variables affecting prognosis12. Recently, the nomogram has been widely used to predict the prognosis of patients with malignant tumours13–16. However, to our knowledge, no published literature has proposed a nomogram to predict the prognosis of primary GSM patients after craniotomy. Therefore, our study intended to develop a nomogram that can be applied to individually assess the survival time of patients with primary GSM after craniotomy and to discuss different factors influencing the prognosis of GSM patients.
Results
Patients’ clinicopathologic characteristics
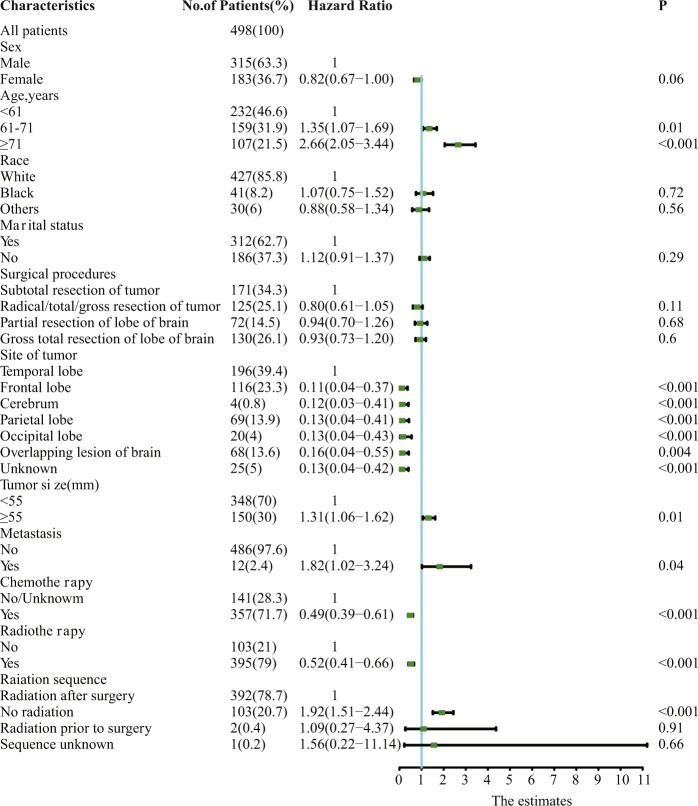
The study population consisted of 498 patients diagnosed with primary GSM receiving craniotomy. A flowchart of the case selection criteria of patients is shown in Fig. 1. Patient, tumour and surgical characteristics, including sex, age, race, marital status, surgical procedures, site of the tumour, tumour size, metastasis, chemotherapy and radiotherapy information, are displayed in Fig. 2. Most of the patients were male (315, 63.3%). Tumour metastasis was rare (12, 2.4%). The temporal lobe was more susceptible to tumours than other lobes (196, 39.4%).
Figure 1.
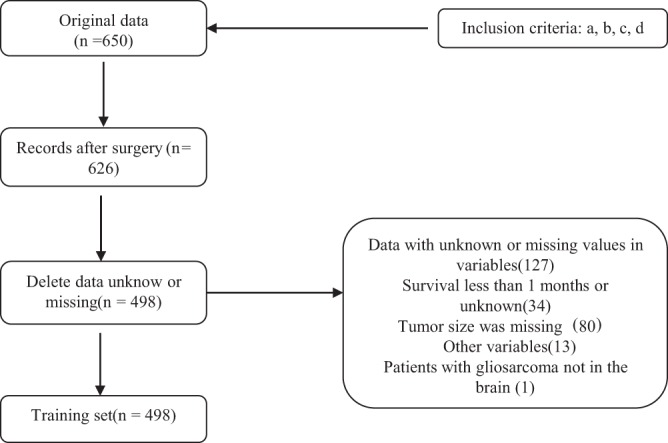
Flowchart of the selection criteria of patients with primary GSM.
Figure 2.
Patient and tumour characteristics and the univariate analysis of these factors on DSS (hazard ratio ± 95% confidence interval).
Disease-specific survival (DSS) and independent prognostic factors in the dataset
The median DSS was 12.0 months (range, 1.0 to 137 months), and the postoperative 0.5-, 1-, and 3-year DSS rates were 71.4%, 46.4% and 9.8%, respectively.
The univariate analysis is shown in Fig. 2. The results demonstrated that age at presentation, site of the tumour, tumour size, metastasis state, adjuvant chemotherapy (CT) and adjuvant radiotherapy (RT) were significantly associated with GSM patient survival. There was no significant difference regarding sex, race, marital status or surgical procedure.
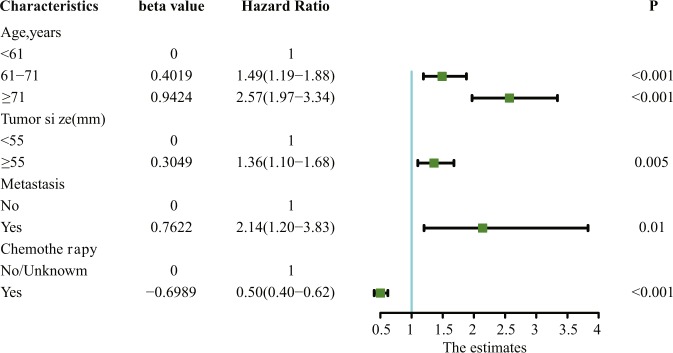
The results of the multivariate analysis are displayed in Fig. 3. Age at presentation (61–71 years: hazard ratio (HR) 1.49, 95% confidence interval (CI) 1.19–1.88, p < 0.001; ≥71 years: HR 2.57, 95% CI 1.97–3.34, P < 0.001), tumour size (≥55 mm: HR 1.36, 95% CI 1.10–1.68, P = 0.005), metastasis (HR 2.14, 95% CI 1.20–3.83, P = 0.01), and adjuvant CT (HR 0.50, 95% CI 0.40–0.62, P < 0.001) remained significant independent prognostic factors predicting GSM survival. Figure 4 displays the Kaplan-Meier DSS curves reflecting the independent prognostic factors for the patients with primary GSM.
Figure 3.
Multivariate analysis of different factors on DSS (hazard ratio ± 95% confidence interval).
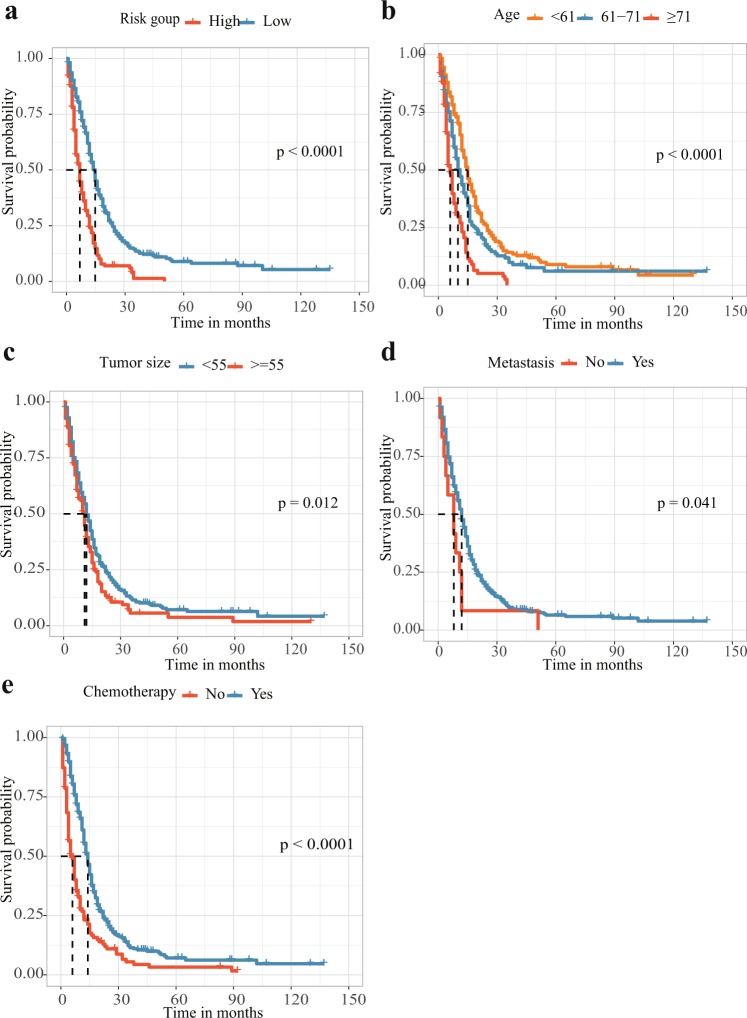
Figure 4.
Kaplan-Meier DSS curves for patients with primary GSM according to different prognostic factors. (a) The disease-specific survival curve according to risk scores stratified into a high score group and a low score group by the average risk score. (b–e) Kaplan-Meier DSS curves for patients with primary GSM according to (b) age at presentation, (c) tumour size, (d) metastasis state and (e) adjuvant chemotherapy.
Prognostic nomogram for DSS and the decision tree model
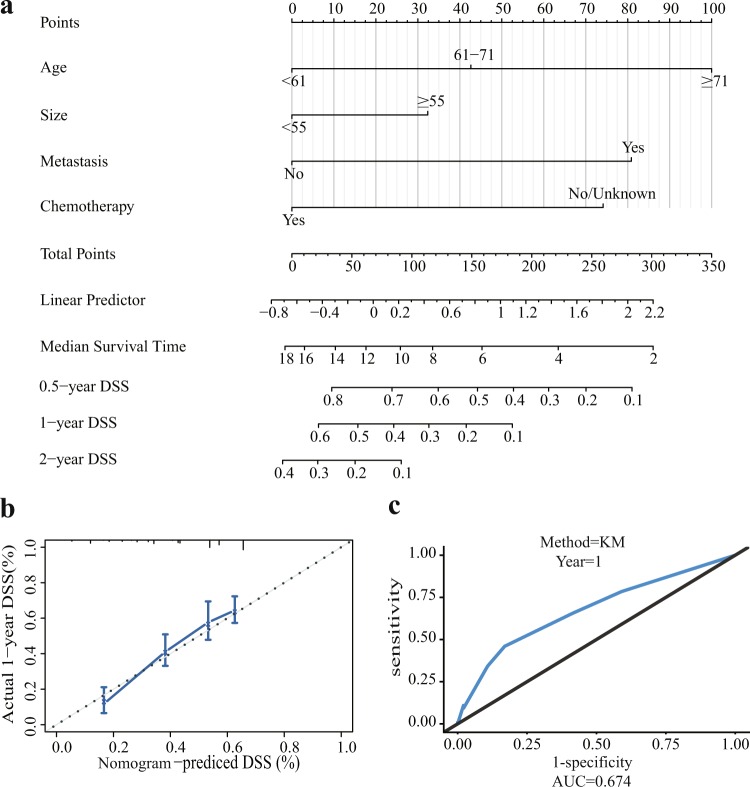
The nomogram generated via the Cox proportional hazards model included four independent prognostic factors influencing DSS after optimization by the Akaike information criterion (AIC) protocol, which is shown in Fig. 5. The C-index of internal validation for DSS prediction was 0.67 (95% CI, 0.63 to 0.70). The calibration curve for the probability of postoperative DSS at 1 year suggested that there was good consistency between the predicted DSS probability and the actual DSS probability in the dataset (Fig. 5). The receiver operating characteristic (ROC) curve and area under the curve (AUC) are displayed in Fig. 5. The AUC (0.67) indicated good accuracy of the one-year prognosis prediction of this model.
Figure 5.
Nomogram, the internal calibration curve, and the ROC curve. (a) Nomogram for predicting the 0.5-, 1-, and 2-year disease-specific survival probabilities in patients with primary GSM following craniotomy. (b) The internal calibration curve for predicting 1-year disease-specific survival probability is displayed. The nomogram-predicted probability of DSS is plotted on the x-axis, and the actual probability of DSS is plotted on the y-axis. (c) The ROC curve shows the sensitivity and specificity of disease-specific survival prediction by the nomogram.
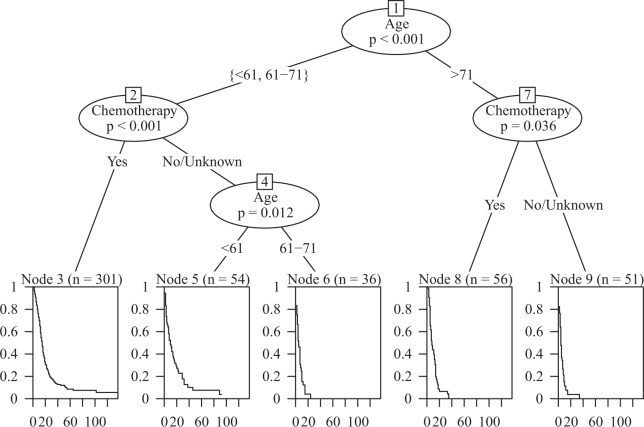
Figure 6 displays the decision tree model and two significant parameters influencing GSM survival (age and adjuvant CT).
Figure 6.
The decision tree model and two important parameters influencing GSM survival.
Discussion
Due to the rarity of this disease, data concerning the patient characteristics of primary GSM are lacking. Most previous studies have been based on single institutional experience, and the results do not represent the actual situation. Our results showed that the median age at diagnosis was over 60 years (61 years), and most of the patients were male (63.1%). The results were consistent with those from three other studies whose sample sizes included more than 50 patients2,17,18. The temporal lobe was more susceptible to tumours than other lobes (196, 39.3%). Most other previous studies also reported a tendency of temporal lobe involvement by GSM2,17,19,20. Ma R et al.8 reported that the tumours were most likely to involve the frontal and parietal lobes. However, there were only 33 patients in this study.
The multivariate analysis demonstrated that age at presentation, tumour size, metastasis state, and adjuvant CT were independent prognostic factors for DSS. Several other studies have also concluded that age at diagnosis was a significant prognostic factor and that a younger age was associated with a better prognosis2,8,18. To our knowledge, there have been no previous studies suggesting that tumour size is a prognostic factor. Our results showed that smaller tumours implied a better prognosis. Regarding the metastatic state, our study suggested that patients with tumour metastasis had a worse prognosis, which was verified in another study21.
There are no standardized management protocols for GSM. Generally, maximal surgical resection and adjuvant therapy are recommended22. Kozak et al. suggested that biopsy alone resulted in worse survival than either subtotal resection or gross total resection (GTR)2. Another study found that GTR resulted in better survival than subtotal resection or biopsy in GSM patients18. Our series did not find a significant difference in prognosis based on the surgical procedure. Regarding adjuvant therapy, trimodality therapy is considered the most effective method for GBM7. For low-grade gliomas (LGGs), the effect of adjuvant chemotherapy or radiotherapy alone was compared, and one study suggested that CT alone was associated with better survival than RT alone in patients with LGGs who received craniotomy23. Concerning GSM, previous studies have reached different conclusions. Some studies have concluded that chemotherapy is a prognostic factor10,11,24, while some have demonstrated that radiotherapy affects prognosis2,17, and others have indicated that trimodality therapy is the most beneficial for prognosis8,9,18. Our series found a significant correlation between chemotherapy and patient prognosis. We summarize several studies discussing the prognostic factors of GSM patients in Table 1.
Table 1.
Studies reporting survival data and prognostic factors for GSM patients.
| Study | Year of publication | No. of patients | Survival data | Prognostic factors |
|---|---|---|---|---|
| Kozak et al.2 | 2009 | 353 |
9 months (median OS) |
Younger age, RT, Extent of resection |
| Singh et al.11 | 2012 | 22 | 18.5 months (median OS) | TMZ |
| Walker et al.10 | 2013 | 46 | 12.5 months(median OS) | TMZ |
| Rath et al.24 | 2015 | 27 | 16.7 months (median OS) | TMZ |
| Castelli et al.17 | 2016 | 75 | 13 months (median OS) | RT, Treatment at recurrence |
| Adeberg et al.9 | 2016 | 37 | 13.4 months (median OS) | Trimodality therapy |
| Ma et al.8 | 2017 | 33 | 6.6 months (median OS) |
Age <50 years, Trimodality therapy |
| Frandsen et al.18 | 2018 | 1102 | 10.7 months (median OS) |
Age <65 years, Female sex, Fewer comorbidities, Trimodality therapy, GTR |
| Our series | 498 | 12.0 months (median DSS) |
Age at presentation, Tumour size, Metastasis state, Chemotherapy |
Although it is generally believed that the prognosis of GSM patients is poor, there are still reports of GSM patients with a relatively good prognosis. Huo Z et al.25 reported two cases of primary GSM with a prolonged prognosis (130 months and 48 months). Both patients received complete tumour resection and postoperative adjuvant therapy without any evidence of tumour recurrence or metastasis. Another case report presented a female GSM patient who was in stable condition at 31 months after the initial diagnosis26. Tumour resection and concomitant adjuvant therapy were performed after the initial diagnosis. Another surgery and second-line chemotherapy (ifosfamide, carboplatin, and etoposide) were conducted after tumour recurrence at 8 months. The authors discussed the feasibility of unconventional chemotherapy in the treatment of GSM.
Many prognostic models have been reported for different types of tumours. Breast cancer is the most common tumour in women, and the prognosis varies greatly. Phung MT et al.27 conducted a systematic review of studies discussing the prognostic models of breast cancer and identified 58 relevant models between 1982 and 2016. Within these 58 models, many methods of model development were applied. The most commonly used method was the Cox proportional hazards regression (n = 32). Other kinds of methods included an artificial neural network (n = 6), a decision tree (n = 4), logistic regression (n = 3), the Bayesian method (n = 3), a multistate model (n = 2), a support vector machine (n = 2) and others (n = 6). Four models applied a nomogram as the presentation form. When assessing discrimination ability, the C-index/AUC was the most commonly used method. Another systematic review of predictive models for resectable pancreatic cancer reported that within the 16 developed models, 11 used the Cox regression method28. There are also reports of the application of machine learning in the development of a clinical prognostic model29,30. However, the Cox proportional hazards regression method is still the most widely used method when establishing prognostic models.
In this study, we applied both the Cox proportional hazards model and the decision tree model to determine the prognostic factors of primary GSM and developed a prognostic DSS nomogram. By applying this nomogram, clinicians can immediately and accurately predict patient prognosis, which can help conduct further treatment after craniotomy. Regarding the decision tree model, we found that age and chemotherapy were important nodes for prognostic judgement. A younger age and adjuvant chemotherapy were associated with better survival for GSM patients.
We know that the ability to accurately predict patient outcome is important, yet the statistical methodology to assess the accuracy of these predictive models seems to be insufficient. Schumacher M et al.31 illustrated that the Brier score and the prediction error curves based on it are valuable for assessing the predictive performance of prognostic classification schemes through the analysis of two studies on node-positive breast cancer patients. The same study provided a more comprehensive perspective for clinical researchers to conduct these prognosis prediction studies and could help researchers select more appropriate statistical models based on the prediction error curves. The authors compared the predictive ability of different statistical methods (fuzzy inference, logistic regression, classification and regression tree) in another study32.
There are several limitations to our study. As a retrospective study, a selection bias was unavoidable. The use of the open access data from the SEER database provided a large amount of patients and surgical information, but several important factors affecting patient prognosis, including molecular/pathological information, were not available through this database. It is generally recognized that molecular pathological data, such as MGMT, are also associated with patient prognosis18,33. Thus, the prognostic factors we analysed based on the SEER database were not complete. The prognostic disease-specific nomogram developed in this study should undergo further improvements after adding these relevant data. Additionally, due to the rarity of this disease, we could not find sufficient clinical data to externally validate this nomogram.
Material and Methods
Patients and study design
A total of 498 patients receiving craniotomy between 2004 and 2015 were extracted from the 18 Registries Research Data of the SEER database. All patients were diagnosed with GSM by a histopathological examination. The variables included sex, age at diagnosis, race, marital status at diagnosis, surgical procedures, tumour size, primary site, metastasis state, and adjuvant therapy. The end of the follow-up was Dec. of 2015, and the primary endpoint was cause-specific death.
The inclusion criteria were as follows: (a) Primary site of the tumour: brain (CS Schema v0204+: brain); (b) Histologic type: GSM (ICD-O-3: 9442); (c) The tumour was the first and there was no other malignant tumour history (sequence number: one primary only; first malignant primary indicator: yes); and (d) The patient received surgery.
The exclusion criteria were as follows: (a) Survival was less than 1 month or unknown (according to clinical practice, patients who die within one month after craniotomy usually die of surgical complications; therefore, it may not be appropriate to incorporate these patients into a prognostic analysis.); (b) Tumour size was missing; (c) One patient had GSM that was not in the brain; and (d) Another variable was unknown or missing. The exclusion process is shown in Fig. 1.
Statistical analysis
The continuous variables were transformed into categorical variables to match with the nomogram. The best cut-off points of continuous variables were identified with X-tile34. Categorical variables were grouped according to clinical reality. The DSS rate and the median DSS were calculated with the life table method.
Both univariate and multivariate Cox proportional hazard models were applied to calculate the HRs and their 95% CIs to analyse different prognostic variables associated with DSS35. Variables were included in the multivariate analysis if they reached a p value of ≤0.20 on the univariate analysis. These prognostic factors were screened with a Cox proportional hazard model adopting the bidirectional elimination method and were optimized with the AIC protocol36. The risk scores were then calculated according to the following formula: risk score = β1 × 1 + β2 × 2+ … +βnXn (β, regression coefficient; X, prognostic factors). Kaplan-Meier curves were plotted to compare DSS on account of different prognostic factors.
A nomogram was developed based on the independent prognostic factors and by using the rms package in R version 3.5.0 (http://www.r-project.org/). The discrimination of the nomogram was assessed by Harrell’s C-index, which could estimate the probability between the observed and predicted DSS37. A random resampling procedure (bootstrapping) with 1,000 resamples was used for internal validation. The ROC curve and the AUC were evaluated using the survivalROC package in R version 3.5.0 to assess the accuracy of one-year prognosis prediction. We also performed the decision tree model by using the party package in R version 3.5.0 to analyse the prognostic factors from other perspectives. P < 0.05 was considered statistically significant.
Ethical declaration
This article does not contain any experiments on humans as well as animals and/or the use of human tissue samples performed by any of the authors.
Conclusion
Our study was the first to develop a disease-specific nomogram to predict the prognosis of primary GSM patients after craniotomy based on retrospective patient data from the SEER database. This predictive model included four independent prognostic factors influencing DSS: age at presentation, tumour size, metastasis state, and adjuvant chemotherapy. Further research is needed to improve this nomogram by analysing more comprehensive prognostic data, and the effectiveness of this model should be evaluated in future clinical applications. Apart from the Cox proportional hazard model, we also performed the decision tree model to analyse the prognostic factors and determined that age and adjuvant CT were important prognostic factors.
Supplementary information
Acknowledgements
The authors thank the Surveillance, Epidemiology, and End Results (SEER) database for providing open data. We would like to thank Prof. Bing Jiang and Prof. Zhixiong Liu for their assistance in this research. This work was supported by the National Natural Science Foundation of China (No. 81703622), the China Postdoctoral Science Foundation (No. 2018M633002), and the Hunan Provincial Natural Science Foundation of China (No. 2018JJ3838).
Author Contributions
S.S.F. and Q.C. conceived and designed the study. S.S.F., H.B.L. and Q.C. performed data mining and statistical analyses. F.F., J.L. and H.C. interpreted the results of the statistical analyses. Z.W.X., K.Y., X.S.Z. and T.T.C. prepared the figures and tables. S.S.F. drafted the initial manuscript. Q.C. made critical comments and revised the initial manuscript. S.S.F. and Q.C. have primary responsibility for the final content. All authors reviewed and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-47211-7.
References
- 1.Galanis E, et al. Clinical outcome of gliosarcoma compared with glioblastoma multiforme: North Central Cancer Treatment Group results. Journal of neurosurgery. 1998;89:425–430. doi: 10.3171/jns.1998.89.3.0425. [DOI] [PubMed] [Google Scholar]
- 2.Kozak KR, Mahadevan A, Moody JS. Adult gliosarcoma: epidemiology, natural history, and factors associated with outcome. Neuro-oncology. 2009;11:183–191. doi: 10.1215/15228517-2008-076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, et al. Brachium Pontis Gliosarcoma With Well-Differentiated Cartilaginous Tissue: A Case Report. Medicine. 2015;94:e1735. doi: 10.1097/MD.0000000000001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stroebe H. Uber Entstehung und Bau der Gehirngliome. Beitr Pathol Anat Allg Pathol. 1895;18:405–486. [Google Scholar]
- 5.Feigin IH, Gross SW. Sarcoma arising in glioblastoma of the brain. The American journal of pathology. 1955;31:633–653. [PMC free article] [PubMed] [Google Scholar]
- 6.Rubinstein LJ. The development of contiguous sarcomatous and gliomatous tissue in intracranial tumours. The Journal of pathology and bacteriology. 1956;71:441–459. doi: 10.1002/path.1700710219. [DOI] [PubMed] [Google Scholar]
- 7.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 8.Ma, R., Alexe, D. M., Boeris, D. & Pereira, E. Primary gliosarcoma: epidemiology, clinical presentation, management and survival. Journal of neurosurgical sciences, 10.23736/S0390-5616.17.04077-2 (2017). [DOI] [PubMed]
- 9.Adeberg S, et al. Radiotherapy plus concomitant temozolomide in primary gliosarcoma. Journal of neuro-oncology. 2016;128:341–348. doi: 10.1007/s11060-016-2117-x. [DOI] [PubMed] [Google Scholar]
- 10.Walker GV, Gilbert MR, Prabhu SS, Brown PD, McAleer MF. Temozolomide use in adult patients with gliosarcoma: an evolving clinical practice. Journal of neuro-oncology. 2013;112:83–89. doi: 10.1007/s11060-012-1029-7. [DOI] [PubMed] [Google Scholar]
- 11.Singh G, et al. A study of clinico-pathological parameters and O(6)-methylguanine DNA methyltransferase (MGMT) promoter methylation status in the prognostication of gliosarcoma. Neuropathology: official journal of the Japanese Society of Neuropathology. 2012;32:534–542. doi: 10.1111/j.1440-1789.2012.01297.x. [DOI] [PubMed] [Google Scholar]
- 12.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:1364–1370. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 13.Choi Seo Hee, Park Seung Woo, Seong Jinsil. A nomogram for predicting survival of patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Radiotherapy and Oncology. 2018;129(2):340–346. doi: 10.1016/j.radonc.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Song Wei, Lv Chang-guang, Miao Dong-liu, Zhu Zhi-gang, Wu Qiong, Wang Yong-gang, Chen Lei. Development and validation of a nomogram for predicting survival in patients with gastrointestinal stromal tumours. European Journal of Surgical Oncology. 2018;44(10):1657–1665. doi: 10.1016/j.ejso.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Jiang S, et al. Prognosis and nomogram for predicting postoperative survival of duodenal adenocarcinoma: A retrospective study in China and the SEER database. Scientific reports. 2018;8:7940. doi: 10.1038/s41598-018-26145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu YP, et al. Prognostic Nomograms Stratify Survival of Patients with Hepatocellular Carcinoma Without Portal Vein Tumor Thrombosis After Curative Resection. The oncologist. 2017;22:561–569. doi: 10.1634/theoncologist.2016-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castelli J, et al. Prognostic and therapeutic factors of gliosarcoma from a multi-institutional series. Journal of neuro-oncology. 2016;129:85–92. doi: 10.1007/s11060-016-2142-9. [DOI] [PubMed] [Google Scholar]
- 18.Frandsen J, et al. Patterns of care and outcomes in gliosarcoma: an analysis of the National Cancer Database. Journal of neurosurgery. 2018;128:1133–1138. doi: 10.3171/2016.12.JNS162291. [DOI] [PubMed] [Google Scholar]
- 19.Salvati M, et al. Gliosarcomas: analysis of 11 cases do two subtypes exist? Journal of neuro-oncology. 2005;74:59–63. doi: 10.1007/s11060-004-5949-8. [DOI] [PubMed] [Google Scholar]
- 20.Jain A, et al. Analysis of Outcomes of Multidisciplinary Management of Gliosarcoma: A Single-Center Study, 2000-2013. World neurosurgery. 2017;106:30–36. doi: 10.1016/j.wneu.2017.06.073. [DOI] [PubMed] [Google Scholar]
- 21.Pietschmann S, et al. An individual patient data meta-analysis on characteristics, treatments and outcomes of glioblastoma/gliosarcoma patients with metastases outside of the central nervous system. PloS one. 2015;10:e0121592. doi: 10.1371/journal.pone.0121592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang T, et al. CGCG clinical practice guidelines for the management of adult diffuse gliomas. Cancer letters. 2016;375:263–273. doi: 10.1016/j.canlet.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, et al. Comparison of Adjuvant Radiation Therapy Alone and Chemotherapy Alone in Surgically Resected Low-Grade Gliomas: Survival Analyses of 2253 Cases from the National Cancer Data Base. World neurosurgery. 2018;112:e812–e822. doi: 10.1016/j.wneu.2018.01.163. [DOI] [PubMed] [Google Scholar]
- 24.Rath GK, et al. Clinical outcome of patients with primary gliosarcoma treated with concomitant and adjuvant temozolomide: A single institutional analysis of 27 cases. Indian journal of cancer. 2015;52:599–603. doi: 10.4103/0019-509X.178407. [DOI] [PubMed] [Google Scholar]
- 25.Huo Z, et al. Primary gliosarcoma with long-survival: report of two cases and review of literature. International journal of clinical and experimental pathology. 2014;7:6323–6332. [PMC free article] [PubMed] [Google Scholar]
- 26.Kalita Ondřej, Zlevorová Miloslava, Megová Magdalena, Vaverka Miroslav, Trojanec Radek, Tučková Lucie. A Patient with Primary Intraventricular Gliosarcoma and Long-term Survival – a Case Report. Klinicka onkologie. 2016;29(6):454–459. doi: 10.14735/amko2016454. [DOI] [PubMed] [Google Scholar]
- 27.Phung MT, Tin Tin S, Elwood JM. Prognostic models for breast cancer: a systematic review. BMC cancer. 2019;19:230. doi: 10.1186/s12885-019-5442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strijker M, et al. Systematic review of clinical prediction models for survival after surgery for resectable pancreatic cancer. The British journal of surgery. 2019;106:342–354. doi: 10.1002/bjs.11111. [DOI] [PubMed] [Google Scholar]
- 29.Paik ES, et al. Prediction of survival outcomes in patients with epithelial ovarian cancer using machine learning methods. Journal of gynecologic oncology. 2019;30:e65. doi: 10.3802/jgo.2019.30.e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang CQ, Gardiner L, Wang H, Hueman MT, Chen D. Creating Prognostic Systems for Well-Differentiated Thyroid Cancer Using Machine Learning. Frontiers in endocrinology. 2019;10:288. doi: 10.3389/fendo.2019.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schumacher M, Graf E, Gerds T. How to assess prognostic models for survival data: a case study in oncology. Methods of information in medicine. 2003;42:564–571. doi: 10.1055/s-0038-1634384. [DOI] [PubMed] [Google Scholar]
- 32.Schwarzer G, Nagata T, Mattern D, Schmelzeisen R, Schumacher M. Comparison of fuzzy inference, logistic regression, and classification trees (CART). Prediction of cervical lymph node metastasis in carcinoma of the tongue. Methods of information in medicine. 2003;42:572–577. doi: 10.1055/s-0038-1634385. [DOI] [PubMed] [Google Scholar]
- 33.Kang SH, et al. O6-methylguanine DNA methyltransferase status determined by promoter methylation and immunohistochemistry in gliosarcoma and their clinical implications. Journal of neuro-oncology. 2011;101:477–486. doi: 10.1007/s11060-010-0267-9. [DOI] [PubMed] [Google Scholar]
- 34.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 35.Pan Y, Lu L, Chen J, Zhong Y, Dai Z. Analysis of prognostic factors for survival in patients with primary spinal chordoma using the SEER Registry from 1973 to 2014. Journal of orthopaedic surgery and research. 2018;13:76. doi: 10.1186/s13018-018-0784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) Psychological methods. 2012;17:228–243. doi: 10.1037/a0027127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrell FE, Jr., Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. doi: 10.1001/jama.1982.03320430047030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.