Abstract
Adequate analgesia is essential whenever pain might occur in animal experiments. Unfortunately, the selection of suitable analgesics for mice in bone-linked models is limited. Here, we evaluated two analgesics – Tramadol [0.1 mg/ml (Tlow) vs. 1 mg/ml (Thigh)] and Buprenorphine (Bup; 0.009 mg/ml) – after a pre-surgical injection of Buprenorphine, in a mouse-osteotomy model. The aim of this study was to verify the efficacy of these opioids in alleviating pain-related behaviors, to provide evidence for adequate dosages and to examine potential side effects. High concentrations of Tramadol affected water intake, drinking frequency, food intake and body weight negatively in the first 2–3 days post-osteotomy, while home cage activity was comparable between all groups. General wellbeing parameters were strongly influenced by anesthesia and analgesics. Model-specific pain parameters did not indicate more effective pain relief at high concentrations of Tramadol. In addition, ex vivo high-resolution micro computed tomography (µCT) analysis and histology analyzing bone healing outcomes showed no differences between analgesic groups with respect to newly formed mineralized bone, cartilage and vessels. Our results show that high concentrations of Tramadol do not improve pain relief compared to low dosage Tramadol and Buprenorphine, but rather negatively affect animal wellbeing.
Subject terms: Bone, Experimental models of disease, Behavioural methods, Animal behaviour, Animal disease models
Introduction
Untreated or insufficiently treated pain hampers animal welfare and may have diverse and uncontrollable effects on an organism, such as impaired wound healing, blood flow disorders, or immunosuppression1–3. Therefore, adequate pain management is essential for ethical and scientific reasons whenever pain might occur in animal experiments. Unfortunately, data on the efficacy of specific analgesic treatments in commonly used surgical mouse models is still scarce2,4.
Human patients with fractures are exposed to experiences such as injury and pain5. Acute pain ranging from moderate to severe is observed in emergency departments when patients arrive with e.g. long bone fractures6. In rodent models of closed femur fractures with a stable pin fixation, the pain peak is expected at day 2 post-operatively and might be comparable to the pain experienced by human patients7. Even though the acute pain in a fracture is reduced by manual fixation in the animal model, the impact catalogue of the EU-Directive 2010/63/EU classifies stable osteotomies as moderately painful procedures; therefore, reliable analgesic treatment is essential in these models.
Unfortunately, the selection of suitable analgesics for mice in bone healing research is limited, since concerns have been raised about potential adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs) on the initial, inflammatory phase of bone healing8,9.
The opioid Buprenorphine and the opioid analogue Tramadol are applied widely in many fields of research, although empirical data on effective doses for rats and mice are rare, and thus dosage recommendations vary substantially in the literature. Buprenorphine is a commonly used fast acting and potent opioid that is mainly administered s.c. or i.p. in dosages of 0.05–0.75 mg/kg every 12 h although recent studies indicate the short half-life and recommend the administration at every 4–6 h10. Tramadol, for example, is used in high dosages for bone cancer models (>50–100 mg/kg s.c.)11, but appears also effective in lower concentrations to treat acute pain12. The application of Tramadol via injections is questionable due to its short half-life of 1–2 h13,14, which should be taken into account when designing studies on the efficacy of Tramadol in order to avoid drawing misleading conclusions15. Concentrations recommended for oral administration of Tramadol range from 0.025 mg/ml up to 1 mg/ml16–20. Nevertheless, to our knowledge, no evidence-based recommendations exist for the treatment of osteotomy pain with Tramadol or Buprenorphine. Therefore, the need for evidence-based and empirical data on the dosage and effectiveness of analgesics in bone research is apparent and further studies must be enrolled.
Here, we performed a refinement study embedded in a basic research study of a mouse-osteotomy model21. We evaluated two commonly used pain management protocols, Tramadol (0.1 mg/ml = Tlow vs. 1 mg/ml = Thigh) and Buprenorphine (0.009 mg/ml = Bup) administered via drinking water, after an initial pre-operative injection of Buprenorphine (0.03 mg/kg, s.c.), for their efficacy and side effects on experimental readouts in a mouse-osteotomy model. We asked the research question whether the application of Tramadol or Buprenorphine in commonly used dosages via drinking water represents a continuous, stress-free method of administering effective analgesia in the mouse-osteotomy model with no adverse impact on fracture healing in the model used.
Results
Drinking frequency and water intake are affected by type and concentration of opioid
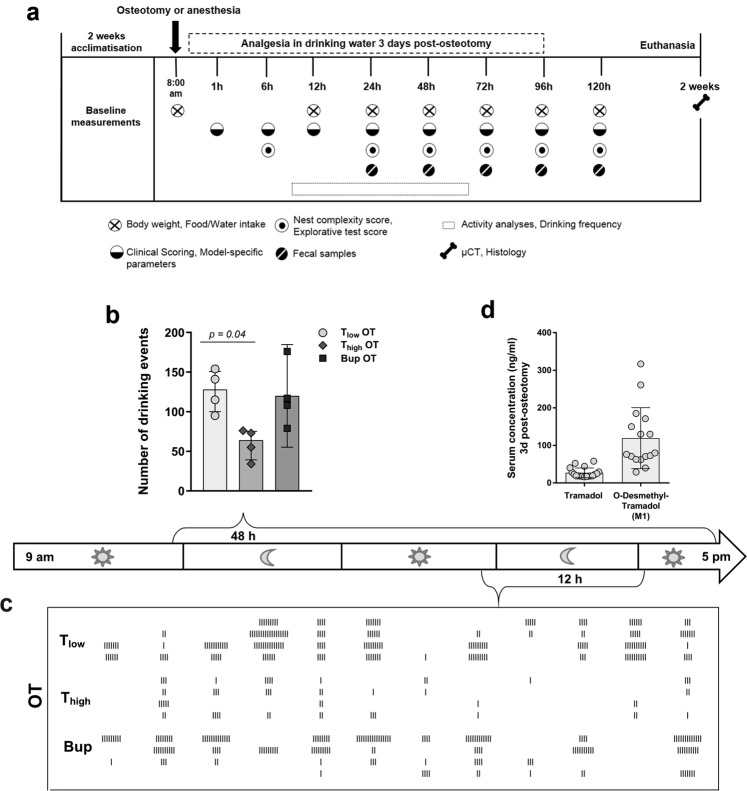
Overall water intake was reduced compared to baseline in the Thigh osteotomy (OT) group at 48 h and 72 h after surgery (Table 1, for other groups see Supplementary Table S1). Prior to surgery or start of treatment, the baseline of the drinking frequency over 48 h was assessed for n = 8 mice, which resulted in a median of 182 events in 48 h (interquartile range: 170.5, 207). Video analysis for 48 h starting 9 h after osteotomy enabled the examination of the drinking frequency and therefore the monitoring of continuous analgesia up-take (Fig. 1b,c). After start of the experiment, the number of drinking events decreased significantly in the Thigh OT group compared to the Tlow OT groups (Kruskal-Wallis test and Dunn’s multiple comparison test; H = 7.42; exact p = 0.01; adjusted p = 0.04 − Tlow vs. Thigh) and showed a non-significant tendency for reduction compared to the Bup OT (adjusted p > 0.99 Tlow vs. Bup, p = 0.07 Bup vs. Thigh) (Fig. 1b). To evaluate the impact of the anesthesia and the treated drinking water alone on the outcome parameters, two control groups were used in the study. In the anesthesia (AN) group, animals underwent isoflurane anesthesia and were treated with either high or low dose Tramadol or Buprenorphine via drinking water for 3 days. In the drinking water (DW) group, animals received either high or low dose Tramadol or Buprenorphine via drinking water over 3 days. In the AN and DW groups, the drinking frequency was slightly higher in the Bup groups compared to the T groups (Supplementary Fig. S1). The depiction of individual drinking events indicates a decline in the drinking frequency in the Thigh OT group between 36 h and 48 h post-osteotomy (Fig. 1c).
Table 1.
Food and water intake per cage for the osteotomy groups.
| Food intake (g) Median (Min – Max) | Water intake (ml) Median (Min – Max) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Groups | 0 h | 24 h | 48 h | 72 h | 96 h | 0 h | 24 h | 48 h | 72 h | 96 h |
| Tlow OT |
7.69 (7.5–8.8) |
5.09 (4.4–5.8) |
7.92 (6.4–9.7) |
7.63 (5.4–10.1) |
10.25 (3.0–14.5) |
9.26 (7.4–9.9) |
7.26 (3.8–11.7) |
9.88 (6.2–11.3) |
10.67 (10.5–13.2) |
7.90 (7.4–10.0) |
| Thigh OT |
9.05 (8.5–9.2) |
4.56 (2.8–14.2) |
3.75 (2.9–4.0) |
8.05 (5.0–9.0) |
11.76 (10.2–16.8) |
9.38 (8.5–11.0) |
10.19 (8.7–12.5) |
5.35 (3.1–11.1) |
5.69 (5.0–6.3) |
9.52 (9.1–9.9) |
| Bup OT |
8.54 (8.3–9.7) |
6.84 (5.2–9.8) |
4.77 (4.0–6.1) |
11.92 (10.1–17.1) |
8.97 (5.8–12.5) |
9.15 (8.2–10.2) |
8.65 (4.5–11.7) |
7.38 (4.5–11.6) |
11.34 (10.0–12.7) |
9.80 (9.4–10.9) |
N = 4 cages (with 2 mice per cage). See Supplementary Table S1 for values of control groups (AN, DW).
Figure 1.
Drinking frequency is reduced concentration-dependent by Tramadol. (a) Study design. Overview on time points and measurements of the different parameters. (b) Total number of drinking events for 48 h was assessed via video recording. Data are shown for OT groups as scatter dot plot and bar with median ± interquartile ranges (n = 4). For group differences Kruskal-Wallis test and Dunn’s multiple comparison test were applied; adjusted p-values are indicated. Results of AN and DW groups are provided as Supplementary Fig. S1. (c) Depiction of the drinking events over 12 h between 36 h and 48 h post-osteotomy. Each line indicates one drinking event (n = 4). (d) Concentrations of Tramadol and M1 3 days post-osteotomy were analyzed in sera from mice treated with the same regime as the Tlow OT group. Data are shown as scatter dot plot and bar with mean ± SD (n = 18).
Tlow Tramadol and M1 serum concentrations appear sufficient
To verify sufficient intake of Tramadol, sera from Tlow, OT mice euthanized 3 days after osteotomy were analyzed for Tramadol and O-Desmethyl-Tramadol – the analgesic active/effective metabolite (M1) – concentrations. The mean serum concentration of Tramadol was 27.1 ng/ml (±12.9 ng/ml) and the mean M1 concentration was 119.6 ng/ml (±81.1 ng/ml; Fig. 1d).
High concentrations of Tramadol in the drinking water impact body weight and food intake
As a parameter of general wellbeing, body weight was measured every 24 h and normalized to the initial body weight directly before osteotomy (baseline). Animals of all treatment groups lost weight compared to baseline values, regardless of whether they underwent surgery or not. The body weights of all groups undergoing OT were significantly reduced at 24 h after surgery compared to the initial body weight before surgery and recovered on the following days depending on the analgesia protocol (one sample t test; exact p-values are listed in Supplementary Table S2) (Fig. 2a). Bup OT and Tlow OT mice reached initial body weight levels (≥100%) at 72 h post-osteotomy. In contrast, Thigh OT mice showed significant decreased body weight until 120 h after osteotomy. Significant group differences were found at 24, 48, 72 and 96 h post-osteotomy, F (14, 100) = 26.21 (p < 0.001) (One-way ANOVA with Bonferroni’s multiple comparisons test; selected pairs comparison; adjusted p-values are indicated in Fig. 2a). In detail, mice of the Bup OT group revealed significant lower fold changes compared to the Thigh OT group at 24 h, 48 h, 72 h and 96 h, respectively and the Tlow OT at 24 h. Additionally, at 72 h and 96 h after surgery, differences were observed between the Tlow OT and Thigh OT groups. At 24 h after anesthesia and/or start of the analgesic treatments, the AN and DW groups showed significant lower body weights compared to the initial body weight, which paralleled the observations from the OT groups (Fig. 2b,c). Overall positive body weight development was more pronounced in the DW groups (reaching 100% − Tlow 72 h, Thigh 96 h, Bup 48 h) (Fig. 2b) than in the AN groups (reaching 100% − Tlow 96 h, Thigh 120 h, Bup 72 h) (Fig. 2c) or OT groups (reaching 100% – Tlow 96 h, Thigh 120 h, Bup 96 h) (Fig. 2a). There was a significant difference between the AN groups (F (14, 45) = 12.16; p < 0.001) and DW groups (F (14, 45) = 23.39; p < 0.001) at different time points (One-way ANOVA; adjusted p-values are indicated in Fig. 2b). In the AN groups, Bup-treated animals showed significant higher body weights than Tlow (24 h and 48 h) and Thigh (48 h and 72 h) treated animals. This was also observed in the DW groups (Bup vs. Tlow or T at 24 h and 48 h; Bup vs. Thigh at 24 h, 48 h and 72 h). Additionally, Tlow DW groups showed significant differences towards the Thigh DW groups at 72 h and 96 h. Intra-group differences over time were mostly significant for all OT groups and the Tlow/Thigh AN and DW groups when applying a repeated measures two-way ANOVA with a Tukey’s multiple comparisons test (Interaction: F (40, 192) = 5.59; p < 0.001; Time: F (4, 192) = 130.8; p < 0.001; Fold change body weight – column factor: F (10, 48) = 25.84; p < 0.001; adjusted p-values for each comparison per group are listed in Supplementary Table S3).
Figure 2.
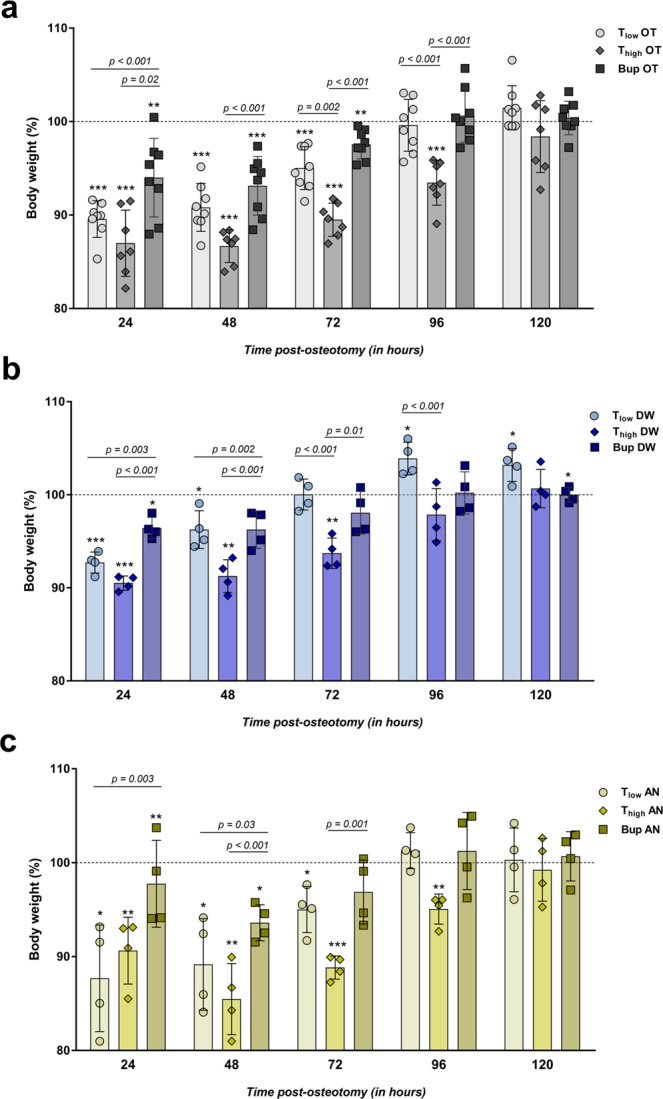
Body weight development is affected by type and concentration of opioid. (a–c) Body weight was measured every 24 h and normalized to the initial weight before surgery (=100%). Graphs depict the osteotomy groups (a, OT) and the control groups including anesthesia and analgesics via the drinking water (b, AN) and only analgesics via the drinking water (c, DW). Data are shown as scatter dot plot and bar with mean ± SD for n = 8 (OT) and n = 4 (AN, DW). One sample t test was used to determine statistical significance towards the initial body weight (hypothetical mean = 100; exact p-values are listed in Supplementary Table S2); p-values are indicated with *p < 0.05, **p < 0.01 and ***p < 0.001. For group differences One-way ANOVA with Bonferroni’s multiple comparisons test was performed; adjusted p-values are indicated.
Food intake was measured daily before and after osteotomy. All OT groups showed a reduced food intake 24 h post-osteotomy that remained low for the Bup OT and Thigh OT groups for 48 h or 72 h, respectively (Table 1). Similar trends were observed in the AN and DW groups (Supplementary Table S1).
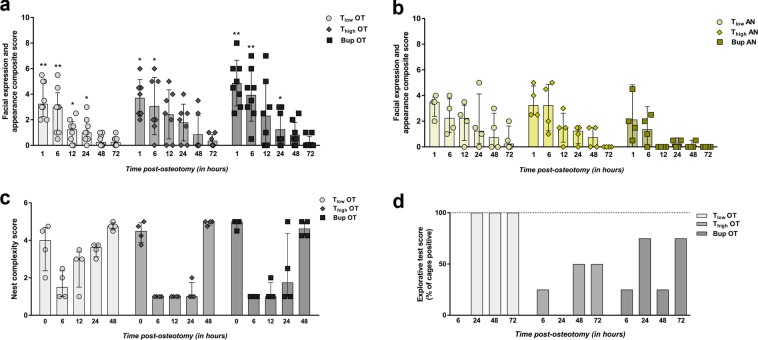
Composite pain score (facial expression + appearance) decreases constantly over time in all OT groups and is strongly influenced by anesthesia and analgesia protocol
A composite score consisting of parameters of facial expression and overall appearance was determined by transferring the mice individually to an observation box at 1, 6, 12, 24, 48 and 72 h post-osteotomy. In order to familiarize the mice with the procedure, scoring was performed at three time points (9 a.m., 3 p.m. and 8 p.m.) on the day before surgery or treatment (baseline). The baseline score was 0 for all mice. After surgery/treatment, OT groups showed a significant increase of the score at 1 and 6 h independent of the treatment and compared to the baseline score of 0 (Wilcoxon signed rank test; hypothetical median = 0; exact p-values are listed in Supplementary Table S4) (Fig. 3a). AN groups showed higher scores than baseline at several time points in the immediate post-anesthesia phase that were not statistical significant (Fig. 3b). DW groups scored continuously low as baseline (=0). Treatment group comparison (Tlow, Thigh vs. Bup) at every time point (selected pairs comparison) using Kruskal-Wallis test (non-parametric) revealed significant differences in medians within the OT groups (H = 68.66; app. p < 0.001) and AN groups (H = 41.92; app. p < 0.001). The following Dunn’s multiple comparison test showed no significance. As shown in the graphs, the median scores reached nearly baseline values at 48 h for the Tlow OT group and 72 h for the Thigh OT and Bup group. This was comparable to the Thigh AN group (72 h) while median scores of Tlow AN reached baseline values at 72 h and Bup AN at 12 h.
Figure 3.
General wellbeing parameters are reduced in osteotomy groups and influenced by anesthesia and analgesia. (a,b) Mice were transferred individually to observation boxes, allowed to acclimate for 1 min and were observed for 3 min. Facial expression and appearance composite score was assessed in the OT (a) and AN (b) groups at 1, 6, 12, 24, 48 and 72 h post-osteotomy/post-anesthesia. DW groups were continuously scored with 0. Measured scores are shown as scatter dot plot and bar with median ± interquartile range for n = 8 (OT) and n = 4 (AN). Wilcoxon signed rank test was used to determine statistical significance towards the initial score (hypothetical median = 0; exact p-values are listed in Supplementary Table S4); p-values are indicated with *p < 0.05, **p < 0.01 and ***p < 0.001. (c) Nest complexity score of the OT groups are depicted for 0, 6, 12, 24 and 48 h post-osteotomy. Data are shown as scatter dot plot and bar with median ± interquartile range for n = 4 (cages). (d) The explorative test score is depicted for the OT groups as mean – percentage of cages that were determined positive for n = 4 (cages). Nest complexity and explorative test scores are provided in Supplementary Fig. S2.
Nest building and explorative behavior are influenced by type and concentration of opioid
The nest complexity score was used to detect changes in general behavior that might indicate changes in wellbeing as described previously22. At 7 a.m., 1 h before surgery or treatment, the baseline nest complexity score, determined for all groups, reached almost the maximum score of 5. Normalization of the scores towards the baseline score was not performed to show the variance of the scores between the groups in absolute numbers per cage. Therefore, the baseline scores (time point = 0) are depicted within the graph (Fig. 3c). Post-osteotomy all OT groups showed a decline in the nest complexity score towards 1 (Fig. 3c). Tlow OT groups recovered faster between 12 h and 48 h than the Thigh OT and Bup groups comparing the medians. A statistically significant difference in group medians was found (Kruskal-Wallis test; non-parametric; H = 48.51; app. p < 0.001) while the Dunn’s multiple comparison test revealed no significance between the selected pairs (p > 0.99). However, strong differences can be seen between the Tlow OT and Thigh OT group at 12 h and 24 h. Animals from AN and DW groups showed a comparable decline at 6 h, but achieved the initial state more continuously and faster over the following 42 h (Supplementary Fig. S2).
Explorative behavior scores are presented in the percentage of cages that were scored positive (100% = four cages scored positive). In general, the scores were more variable in the OT groups than in the other treatment groups (Fig. 3d). While all animals showed explorative behavior in the baseline measures prior to surgery, explorative behavior declined towards zero at 6 h after surgery. Tlow OT recovered to stable values at 24 h (=100%). Animals from Thigh OT showed reduced explorative behavior over time compared to Tlow OT mice. Bup OT groups demonstrated varying behavior between a mean of 25% (one cage out of four positive) and 75% (three cages out of four positive). AN groups showed comparable results to the OT groups while DW groups were highly explorative with a mean score of 100% (Supplementary Fig. S2).
Additionally, the time spent in the nest (resting time) was assessed for each individual and normalized to the total recorded time. As shown in Supplementary Fig. S2, there was no significant difference between the OT groups (Kruskal-Wallis test; H = 2.67; exact p = 0.28). There was a slight decrease of the relative time spent in nest with the AN and DW groups compared to the OT groups. In order to track the normal time in the nest over 48 h, baseline measurements were performed prior to surgery or treatments for n = 8 mice. The median of the relative time in nest was 47.05% (interquartile range: 43.13%, 48.18%), indicating an increase in resting time for all groups after surgery or treatments compared to normal conditions.
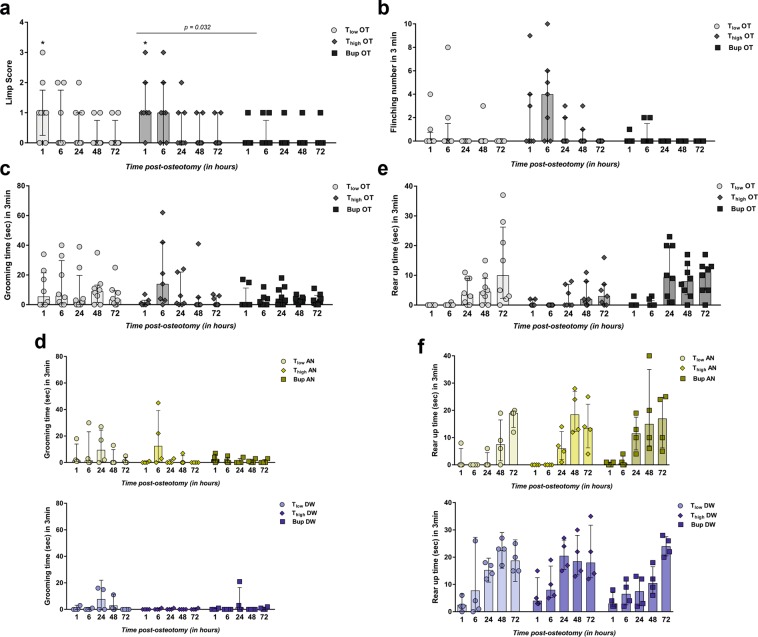
Model-specific pain behavior are reduced by Buprenorphine and low concentrations of Tramadol
To assess model-specific pain behaviors, a limp score (limping and dragging), the frequency of flinching, the duration of grooming the operated leg as well as the rear up duration, i.e., time standing on both hind limbs in 3 min, were determined. Limp score and flinching are described only for OT animals as AN and DW groups showed no positive limp scores or flinching. Baseline limp score and flinching were assessed prior to osteotomy, revealing no positive limping or dragging (score = 0) and no flinching. Mice of the Tlow OT and Thigh OT group showed significant higher limp scores at 1 h post-osteotomy compared to the baseline scores (=0) (Wilcoxon signed rank test; hypothetical median = 0; exact p-values are listed in Supplementary Table S5) (Fig. 4a). Group medians were statistically significant (Kruskal-Wallis test; non-parametric; H = 29.45; app. p < 0.009) between the Bup OT and Thigh OT groups (Dunn’s multiple comparison test; selected pairs comparison; p = 0.032). In general, the Bup OT and Tlow OT groups showed a median score of 0 at 6 h post-osteotomy compared to the Thigh OT group with a median of 1 that was reduced towards the baseline at 12 h post-osteotomy. Flinching was not statistically significantly increased in the OT groups compared to the baseline (=0) (Wilcoxon signed rank test; hypothetical median = 0; p > 0.25 except Thigh OT at 6 h p = 0.063) (Fig. 4b). The Kruskal-Wallis test showed a significant difference in the group medians (H = 32.28; app. p < 0.004) while the Dunn’s multiple comparison test revealed no significance between the selected pairs (p > 0.99; except Bup OT vs. Thigh OT p = 0.1 and Tlow OT vs. Bup OT p = 0.15). Nevertheless, flinching was observed more often in the Thigh OT group (median = 4) compared to the Tlow OT and Bup OT groups (median both = 0) at 6 h (Fig. 4b).
Figure 4.
Higher concentrations of Tramadol do not further ameliorate model-specific pain-related parameters. (a) The limp score included limping and dragging. Only the scores for the OT groups are shown in the graph as AN and DW groups showed no positive score. Measured scores are shown as scatter dot plot and bar with median ± interquartile range for n = 8. Wilcoxon signed rank test was used to determine statistical significance towards the initial score (hypothetical median = 0; exact p-values are listed in Supplementary Table S5); p-values are indicated with *p < 0.05, **p < 0.01 and ***p < 0.001. For group differences, Kruskal-Wallis test with Dunn’s multiple comparison test for selected pairs (time point) was performed; adjusted p-values are indicated. (b) Number of flinching per 3 min observation are depicted for OT groups (no flinching was observed in AN and DW groups). (c,d) Graphs show grooming time during 3 min for OT (c) and AN and DW (d) groups. (e,f) Rear up time during 3 min is shown for OT (e) and AN and DW (f) groups. (b–f) Data are shown as scatter dot plot and bar with median ± interquartile range for n = 8 (OT) and n = 4 (AN, DW).
Baseline measurements of leg grooming duration showed durations between 0 and 4 sec (n = 8); no statistical test was performed for group comparisons. However, grooming time was prolonged in OT groups for up to 3 days after osteotomy, with no difference between the three groups (Kruskal-Wallis test; non-parametric; H = 15.28; app. p = 0.36) (Fig. 4c). Interestingly, increased grooming was also seen in Thigh AN, Tlow AN and Tlow DW mice (Fig. 4d).
Normal rear up time was determined during baseline measurements (n = 8) with a median = 14.2 sec and a range from 8 to 27 sec. No statistical test was performed for comparison with baseline. Rear up was shown only rarely by OT groups at 1 h and 6 h post-osteotomy (Fig. 4e), which is in line with the results of the AN groups (Fig. 4f). Between 24 h and 96 h, rear up time increased in the OT as well as in the AN and DW groups (Fig. 4f). Statistical differences in the OT group medians were found (Kruskal-Wallis test; non-parametric; H = 60.03; app. p < 0.001) while Dunn’s multiple comparison test revealed no significance between the selected pairs (p > 0.99; except Bup OT vs. Thigh OT p = 0.36). In general, rear up was more apparent in the Bup OT and Tlow OT compared to the Thigh OT group.
Physiological stress response varies in regard to osteotomy surgery and analgesic treatment
To evaluate stress non-invasively, FCMs were determined. Samples were collected during the animals’ stay in the observation boxes after spontaneous and voluntary defecation (no samples at 1 h, 6 h and 12 h post-osteotomy/post-anesthesia). Consequently, different numbers of fecal samples were collected per time point and group. FCM concentration peaked at 24 h post-osteotomy in the OT groups, with no significant differences between groups (Kruskal-Wallis test; H = 3.82; p = 0.15; Fig. 5a) and declined immediately at 48 h to values lower than DW mice (Fig. 5c). Nevertheless, FCM concentrations in AN and DW groups were also slightly elevated at 24 h and declined over time (Fig. 5b,c).
Figure 5.
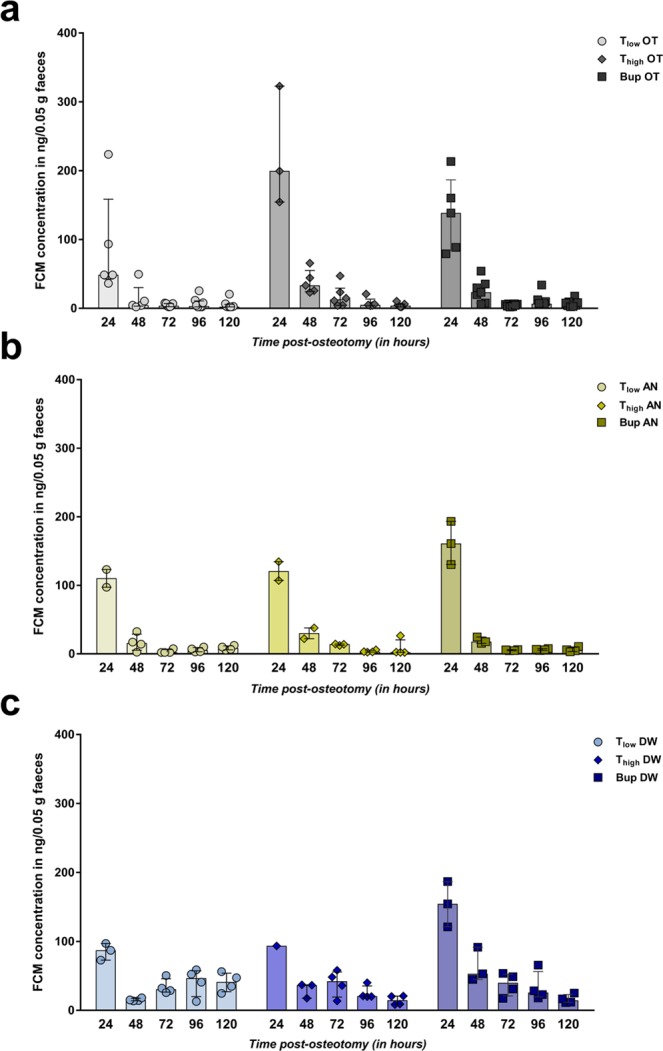
Assessment of adrenocortical activity indicate varying physiological stress responses. (a–c) Fecal corticosterone metabolite (FCM) concentrations were analyzed in all groups: OT (a), AN (b) and DW (c) group. Data are shown as scatter dot plot and bar with median ± interquartile range for n = 8 (OT) and n = 4 (AN, DW).
Opioid analgesia does not significantly affect fracture healing outcome
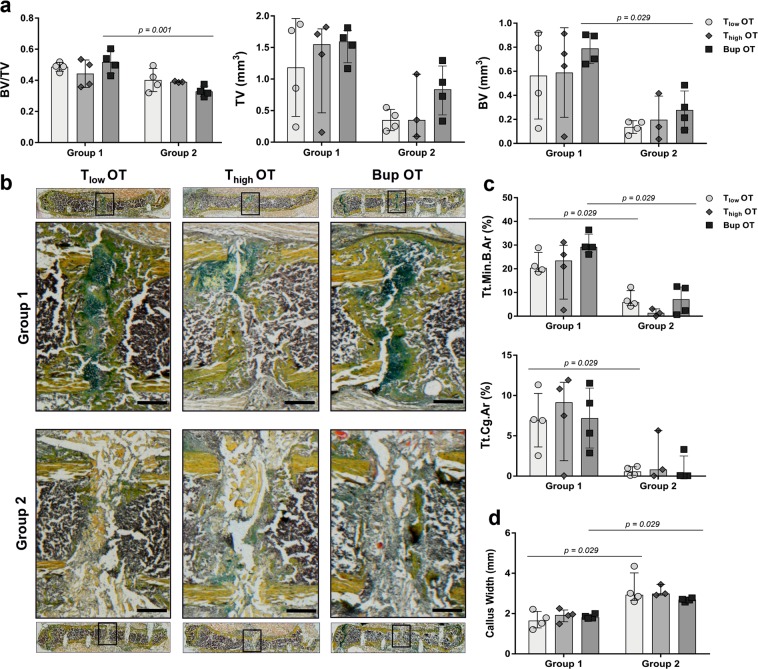
In order to evaluate the potential side effects of opioid pain management on fracture healing outcomes, we performed ex vivo µCT, histomorphometric analysis and vessel staining via immunofluorescence. Within the protocol, animals from each OT group were assigned randomly to one of two groups receiving different treatment of the osteotomy gap. While in group 1, the osteotomy gap was empty, a scaffold was applied in group 2 that inhibits fracture healing, as we have shown previously21. In the present study, we focused on potential differences within group 1 or 2 to evaluate the potential influence of the pain management regime. The total volume (TV), bone volume (BV) and bone volume fraction (BV/TV) were determined during ex vivo µCT analyses. The BV revealed no significant differences between the analgesics groups within the treatment groups (Kruskal-Wallis test; selected pairs; H = 10.01; p = 0.75) while the medians of TV and BV/TV were significant (Kruskal-Wallis test; selected pairs; TV: H = 11.18; p = 0.05; BV/TV: H = 13.05; p = 0.02), but not in the Dunn’s multiple comparison test (p > 0.99) (Fig. 6a). Moreover, we detected a significant difference in the Bup OT group between group 1 and 2 (Mann-Whitney U test; p-values indicated in graphs) (Fig. 6a). Histomorphometric results analyzing the total mineralized bone (Tt.Md.B.Ar) and cartilage area (Tt.Cg.Ar) as well as the callus width were significantly different in group medians within one fracture gap treatment group and between the analgesic protocols (Kruskal-Wallis test; selected pairs; Tt.Md.B.Ar: H = 16; p = 0.007; Tt.Cg.Ar: H = 11.05; p = 0.05; Callus width: H = 17.89; p = 0.003). Dunn’s multiple comparison test showed no significance (p > 0.99) (Fig. 6b–d). Within group 1, Bup OT revealed a higher Tt.Md.B.Ar compared to the T OT groups, but conversely, Thigh OT exhibits slightly more cartilage within the fracture gap. Between the treatment groups, significant differences were found for Tlow OT and Bup OT, showing significant higher amounts of mineralized bone and cartilage in group 1 compared to group 2 (Mann-Whitney U test; p-values indicated in graphs) (Fig. 6c). This was also observed for the callus width, which was also significant higher in the Thigh OT group 2 compared to group 1 (Fig. 6d).
Figure 6.
Fracture healing outcomes are not affected by type and concentration of opioid. (a) Using ex vivo µCT, the bone volume (BV), the total volume (TV) and the bone volume fraction (BV/TV) were determined. (b) Exemplary images for Movat’s pentachrome staining allowing for the differentiation between tissues: yellow/orange = mineralized bone; light yellow = scaffold; green = cartilage; purple/brown = bone marrow and cells. Scale bars = 500 µm. (c) Histomorphometry was performed for quantitative assessment of different tissue components – mineralized bone (Tt.Min.B.Ar), cartilage (Tt.Cg.Ar) and (d) callus width. (a–d) Data are shown as scatter dot plot and bar with median ± interquartile range for n = 3–4. Differences between group 1 and 2 within one analgesic group were determined using the Mann-Whitney U test. Exact p-values are indicated in the graphs.
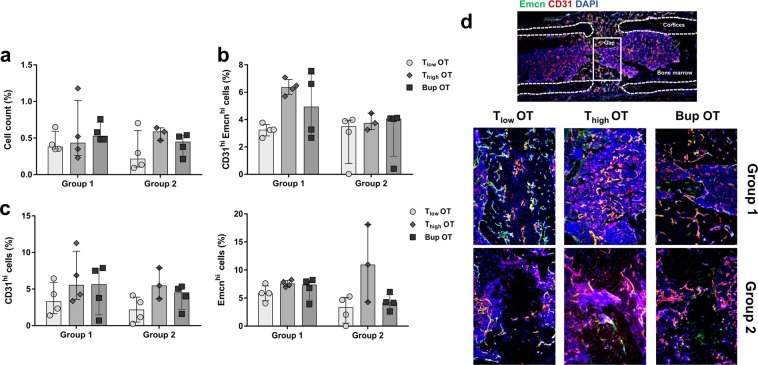
To evaluate vessel formation within the fracture gap, staining was performed for CD31 and endomucin (Emcn), two common endothelial markers. In a first step, the relative cell count was determined within the fracture gap (Fig. 7a). No significant differences were identified between analgesic protocols or treatment groups (Kruskal-Wallis test; H = 4.65; p = 0.46). This was also observed for CD31 and Emcn alone (Kruskal-Wallis test; CD31: H = 6; p = 0.31; Emcn: H = 10.85; p = 0.054) (Fig. 7c). Therefore, the obvious higher amount of Emcnhi cells in the Thigh OT group 2 seems to be an effect of random sampling. Additionally, there were no differences between treatment groups for CD31hi Emcnhi (Kruskal-Wallis test; H = 9.54; p = 0.089) although the Dunn’s multiple comparison test showed a significance in group 1 between the T groups (p = 0.04) (Fig. 7b).
Figure 7.
Vessel formation is comparable between treatment groups. Immunofluorescence staining was performed to measure (a) the cell count, (b) the number of double-positive cells (CD31hi Emcnhi) and (c) the number of CD31hi and Emcnhi cells alone in the osteotomy gap. Data was normalized to the total analyzed area and are shown as scatter dot plot and bar with median ± interquartile range n = 3–4. (d) Exemplary immunofluorescence vessel staining (CD31 = red, Emcn = green, DAPI = blue). Scale bars = 200 µm.
Unexpected observations of the study
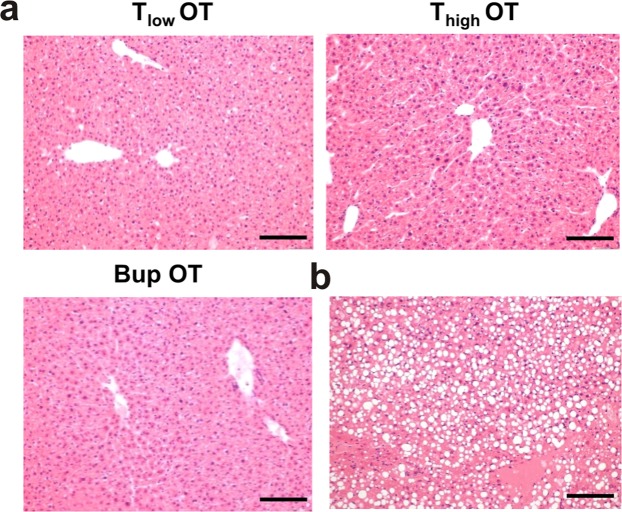
One animal from the Thigh OT group was euthanized 2 days after osteotomy due to reaching a humane endpoint. Autopsy revealed a hepatomegaly. In order to exclude the influence of the pain medication, this liver as well as the livers of all other animals from the OT group were assessed via histology; the results revealed a high-grade fatty liver in the respective animal (Fig. 8b) and no changes in the others (beside mild hepatitis due to Helicobacter infection) (Fig. 8a).
Figure 8.
Liver histology reveal no differences between groups. (a) Histology of the livers from all OT animals (n = 4) was performed as one animal needed to be euthanized 48 h after osteotomy; this animal showed a high-grade fatty liver (b). Scale bars = 200 µm.
Discussion
This study evaluated three used analgesia protocols, Tramadol (0.1 mg/ml = Tlow vs. 1 mg/ml = Thigh) and Buprenorphine (0.009 mg/ml) administered via drinking water, in a mouse osteotomy model after a pre-operative injection of Buprenorphine. Analgesia, anesthesia, surgery and testing had clear effects on the measured parameters assessed. All protocols tested reduced pain without side effects on fracture healing outcome. Nevertheless, mice that were treated with Thigh showed more signs of reduced wellbeing and pain compared to mice treated with Tlow or Buprenorphine.
Daily water intake in female B6 mice is about 3–6 ml23, which resembles our baseline values. After osteotomy water intake was reduced but remained sufficient for adequate analgesia in groups receiving Buprenorphine or Tlow. In contrast, water intake and drinking frequency were reduced significantly in animals that received Thigh after surgery. While overall, water intake was not distinctly reduced in controls without surgery receiving Thigh compared to other analgesia protocols, drinking frequency during the first 2 days was low compared to animals that received a lower dose or Buprenorphine. Comparable effects were observed in a previous study: in contrast to sufficient overall 24 h intake of Tramadol-treated water after laparotomy in female B6 mice, drinking frequency was distinctly reduced in the acute post-surgical phase (<4 h)24. Reduced water intake might result in insufficient concentrations of Tramadol or M1, the analgesic metabolite of Tramadol, and, therefore, in insufficient pain relief. However, based the amount of water intake, the total amount of Tramadol intake can be calculated and compared between the Tlow and Thigh groups. Considering a total water up-take at 48 h for two mice with a median of 9.88 ml (Tlow) and 5.35 ml (Thigh), the calculated up-take of Tramadol per mouse would be 0.49 mg (Tlow) compared to 2.68 mg (Thigh) which is a 5-fold difference and indicates a sufficient up-take of Tramadol in Thigh although the water intake amount was reduced. Nevertheless, this calculation does not include the timing of the water intake over 24 h and the short half-life of Tramadol14. It can be assumed that the bitter taste or aversive side effects of Thigh result in reduced willingness to ingest. As effective drinking water administration relies on frequent and sufficient water intake, sweetened drinking water as well as application of pain medications via the drinking water prior to surgery might be used to avoid the observed effect and to ensure sufficient intake16,25. In addition, the voluntary ingestion via nut paste, jelly26 or by syringe feeding can be an alternative in order to ensure the adequate intake, although animals should be used to the food or technique beforehand27.
We determined the serum levels of Tramadol and M1 following administration of Tlow. The M1 value was found to be nearly three times as high than the human minimal effective serum level of M128. Nevertheless, analgesic serum concentrations of Tramadol or M1 in mice are not known; we can therefore only assume that mice received sufficient doses.
Weight reduction, and reduced food intake, might be indicators of pain10,29 but are also known side effects of analgesics10. All animals lost weight after the start of experiments, suggesting an effect of treatment but also of the testing procedure. This tendency was stronger after osteotomy and anesthesia – a result that can possibly be explained by a loss of fluid during anesthesia and the observed drop in food and water intake after anesthesia. The impact of surgery on these parameters was minor compared to the effect of analgesia and anesthesia only. Interestingly, animals treated with Thigh lost more body weight and recovered weight more slowly compared to the other groups. This effect was seen in surgery and controls, and can therefore be more likely attributed to the side effects, of Thigh and less likely to pain due to osteotomy.
Composite scores comparable to the scoring system used, have been applied successfully for pain evaluation in other mouse models (e.g.10,30). In our study, scores increased during the acute post-anesthesia and post-osteotomy phase. Nevertheless, the increase in scores towards mean scores of 3–6 was significant compared to baseline in the surgery groups only and scores of osteotomized animals recovered slower towards baseline levels compared with anesthesia groups. In a previous study, we showed that, after ovariectomy, B6 mice that did not receive pain treatment reached mean composite scores of 4.5, while animals that received effective pain treatment after surgery reached mean scores of 1.5–2.510. Compared to the increase in scores after anesthesia and analgesia without surgery, osteotomized animals showed only slightly higher scores, hinting at very mild residual post-surgical pain.
The influence of anesthesia is in line with findings that subunits of the score are not pain-specific. An increase in the MGS, part of the pain scoring used in our study, after anesthesia is also described by Miller et al.31.
High nest scores – an indicator of good general wellbeing30,32,33 – were observed during baseline. Nest scores were reduced in all groups after the start of experiments. This reduction in nest building is a known side effect of anesthesia and opioid analgesia32,34. Nevertheless, the impact on nest building was less severe in animals that received Tlow compared to Thigh or Buprenorphine, hinting at fewer side effects on wellbeing. Changes in activity might have been the underlying cause of reduced nest building. Groups that scored higher in nest scores in general also spent less time inactive in their nest.
The explorative test used in our study is a modification of the time-to-integrate-to-nest test (TINT)35. Mice observed in pilot studies rarely started to integrate provided nest building material into their existing nest within 10 min as described by Rock et al., therefore we assessed exploration of new nest material rather than nest building behavior. Exploration might be a useful measure of activity and anxiety36 and therefore an interesting parameter to assess overall wellbeing in mice. In the explorative test, all mice scored positive in baseline, animals that received analgesia only showed a comparable high level of explorative behavior during the observation time. After anesthesia, exploration was reduced in all OT groups. This reduction seemed to be stronger in animals that received Thigh, suggesting a negative effect of the higher dose on wellbeing.
Assessment of limp scores and rearing behavior to quantify altered gait and weight bearing are commonly used in bone fracture, neuropathy or cancer pain studies37–40. Additionally, grooming in terms of an increased tending to the painful limb and flinching as a pain-specific symptom is applied41–43. No limping, flinching or guarding was observed during baseline or in the AN and DW groups. Limping and flinching occurred rarely after osteotomy. Rear up time was shorter in surgery groups compared to AN and DW groups. These changes are therefore clearly connected to the surgery and do not represent a side effect of analgesia or anesthesia.
We cannot discriminate if these changes are due to functional impairment, due to the fixator, or residual osteotomy pain. Minville et al. reported that mice with a fractured tibia have a significantly higher “subjective pain” score, defined as reduced use of fractured limb with regards to limping and weight bearing, than sham-operated animals40. This score was reduced with an opioid + NSAID combination, which proves the pain specificity of the parameters. In other studies using fractured femurs, flinching occurs more frequently than in our model when animals are not treated with analgesia42,43. In contrast to our model, these studies used an internal pin to fixate the closed fracture.
Regardless of dose, animals that received Tramadol showed higher limp scores and more flinching than animals that received Buprenorphine. Thigh animals showed a tendency towards more limping and flinching and less rearing and a slower recovery of behaviors. Buprenorphine treatment seemed to be slightly superior to Tramadol.
Guarding behavior, even though observed typically in rodent bone fracture pain models41–44, was not observed in our mice. Again, the lack of could therefore be a sign of sufficient or acceptable pain relief. Grooming of the wounded leg, which might be a sign of pain45, occurred in all animals, but was increased mainly in the operated and anesthetized animals. As both groups received a dressing spray to close the wound, it is assumed that grooming is not due to pain but rather induced by the sticking spray on the animal’s skin.
Measuring FCM is a non-invasive technique to monitor adrenocortical activity46–49. At 24 h after start of the experiments, elevated FCM levels were observed with no significant differences between groups. The increase in FCM concentrations in AN and DW groups on day one could be explained by frequent handling, which has been shown to potentially induce an acute stress response in mice50,51.
Finally, we evaluated the influence of Tramadol and Buprenorphine on fracture healing outcomes. No differences were found between analgesic groups in formation of mineralized bone, cartilage and vessels. In addition, we reproduce our previous finding on applying a scaffold to inhibit fracture healing21. Negative effects of opioids on bone homeostasis in humans have been described only in case of long-term administration, and include a high incidence of spontaneous fractures related to, e.g., loss of bone mineral density due to direct effects on osteoclasts and osteoblasts or indirect effects via the endocrine system52–54. However, results from animal experiments do not clearly indicate any influence of either Tramadol or Buprenorphine on bone homeostasis and/or regeneration55,56.
Within the study only female B6 mice were used without determination of the estrous cycle status. Therefore, we are not able to exclude potential hormonal influences, which have been also shown to be strain-dependent57. In addition, conclusion towards male mice should be carefully taken. Moreover, the presented analgesia protocols could be further refined by sweetening the medicated drinking water for better water intake as well as the use of local anesthesia during surgery.
Although we cannot rule out minor, short-lasting pain in the post-surgery phase, this study provides evidence for pain relief after osteotomy in female B6 mice when administering analgesics stress-free and continuously via the drinking water. Additionally, potential overdosing of Tramadol can lead to changes in wellbeing without further pain relief. Nevertheless, as we have not compared oral with injection protocols, we cannot evaluate if the injection of analgesics or the combination of injection and and oral administration for several post-surgical days might have been superior, i.e. reduces remaining signs of pain further, compared to the oral protocols. Therefore, further model-specific studies on pain management should be performed to provide evidence-based refinement of analgesia protocols, to enhance animal wellbeing, reproducibility and translation.
Methods
Animals, housing and study design
The study was performed in accordance with the German Animal Welfare Act and was approved by the local Berlin state authority – “Landesamt für Gesundheit und Soziales” (LaGeSo; permit numbers: G0039/16 and G0111/13). A total of 48 female C57BL/6 N mice aged 10 weeks was purchased from Charles River Laboratories (Sulzfeld, Germany). Only female mice are used routinely in our osteotomy model as bone healing is slower in female mice than in males, allowing for a stepwise, complete bone healing within a time period of 21 days27,58,59. The mean body weight was 21.3 ± 1.3 g. Mice were housed in a conventional (non-SPF) facility, habituated to the housing room in groups of 8–10 animals for 1 week in Eurostandard Type lll clear-transparent plastic cages and afterwards randomly split into groups of two for another week before experiments started. Mice were housed in Eurostandard Type II clear-transparent plastic cages (two animals per cage) covered with a wire lid and built-in u-shaped feed hopper and closed with a filter top. As bedding material, fine wood chips (Lignocel FS 14, J. Rettenmaier & Söhne GmbH + Co. KG, Germany) and nesting material (Envirodri®, Shepherd Specialty Papers, USA) was provided. Due to the external fixator, houses and pipes are contraindicated in order to avoid injuries. Food (Standard mouse diet, Ssniff Spezialdiäten, Germany) and tap water was available ad libitum, and room temperature was constant, at between 20 and 22 °C with a humidity of 45–50%. The light/dark cycle was a 12/12-h cycle with lights on at 0600 hours and off at 1800 hours. Cage changing was performed weekly during the acclimatisation phase (two weeks prior to start) by the experimenter; animals were tail handled. Nesting material was transferred to new cages when dry and clean. All experimenters were female (see60).
The study design (Fig. 1a) began with baseline measurements during the week prior to the osteotomy. Cages were allocated randomly to three post-operative treatment groups: Tramadol (Drops, Grünenthal GmbH) was applied in two different concentrations: Tlow = 0.1 mg/ml and Thigh = 1 mg/ml, Buprenorphine (Temgesic, RB Pharmaceuticals Limited) was applied with Bup = 0.009 mg/ml. Analgesics were administered via drinking water (tap water) for three consecutive days directly after osteotomy or anesthesia. The medicated water was not changed during treatment time13. Eight animals of each analgesic regime were osteotomized (OT), four animals of each regime underwent anesthesia only for 15 min and received analgesics post-anesthesia (AN) and four animals of each regime received only analgesics (DW; Supplementary Table S6). Two weeks after surgery, osteotomized animals were anesthetized with ketamine and xylazine (i.p. ketamine 120 mg/kg, xylazine 16 mg/kg). Once depth of anesthesia was achieved, blood was withdrawn from the heart and euthanasia was conducted by cervical dislocation.
Osteotomy
All animals received Buprenorphine (0.03 mg/kg) s.c. as analgesic 1 h prior to surgery. Anesthesia was induced at 2.5% isoflurane (CP-Pharma, Germany) in a transparent Plexiglas box where anesthetic gas was provided using a rodent inhalation anesthesia system (Ohmeda Isotec 4, DRE, USA). Once the animal was asleep and breathing independently deeply and continuously, it was moved to a heating mat (37 °C) and anesthesia was maintained via a nose mask at 1.5% isoflurane. Eye ointment and clindamycin (0.02 ml) were applied s.c. After shaving and disinfecting the left femur area with an alcoholic iodine solution (Braunoderm, B. Braun, Germany), osteotomy was performed as described earlier20. In short, a lateral longitudinal incision of the skin (2 mm) along an imaginary line from knee to hip followed by a blunt preparation of the femur between the Musculus vastus lateralis and Musculus biceps femoris while protecting the subjacent nerve. Pin placement (0.45 mm diameter) was performed through the connector bar of the external fixator (MouseExFix, RISystem, Davos, Switzerland) by serially drilling to place the fixator laterally parallel to the femur. A 0.70-mm osteotomy gap was created between the middle pins using a Gigli wire saw (RISystem, Davos, Switzerland). According to the protocol of the basic research study, the osteotomy gap was either flushed with NaCl and left empty for group 1 or filled with a PBS-soaked bovine collagen-I scaffold, Lyostypt® for group 2 (B. Braun Vet Care GmbH, Tuttlingen, Germany). After skin closure, the wound was covered with a permeable spray dressing, the mice were given pre-warmed NaCl (0.2 ml) s.c. and then returned to their cages. Recovery from anesthesia was conducted under an infrared radiator and was monitored closely. Surgery was performed by two trained veterinarians. Approximately 45 min after surgery, cages were transferred back to the housing room for further analgesic treatment and behavioral assessment. General scoring and humane endpoints were applied as recommended and summarized in Lang et al.27.
Body weight, behavioral home cage analyses and food/water intake
Animals were weighed daily starting 1 day prior to surgery and continuing until the day of euthanasia. Food and water intake were determined for each cage every 12 h. For each analgesic treatment group, 8 animals (4 OT, 2 AN, 2 DW) were filmed in their home cages starting for 48 h at 6 p.m. (9 h after osteotomy). This time point was chosen as we expected the pre-emptive Buprenorphine treatment to deliver a pain relief for up to 8 h. Cages were filmed from above (at a distance of ~ 1.5 m) with an infrared sensitive USB 3.0 Monochrome-Camera (The Imaging Source Europe GmbH, Germany). Cage grids were removed for better visibility and plastic walls elevated to prevent escape. Mice were kept with their familiar partner, provided with nesting material, food and water. One mouse in every cage was marked with black stripes (Edding permanent marker 3000) on the tail. An infrared light was turned on during dark cycles. Videos were analyzed manually by one blinded observer with event-logging software (BORIS - Behavioral Observation Research Interactive Software). The drinking frequency of each mouse was assessed manually, where the contact of a mouse’s snout with the bottle tip was counted as one drinking event and continuous drinking for a longer time was also counted as one drinking event. Time spent active was measured (defined as animal moving outside of the nest) and used to calculate resting time in the nest.
Tramadol/M1 analysis in serum
There is no available data on the minimum effective serum concentration of Tramadol in mice; Evangelista et al. only recently published data on the pharmacokinetics of Tramadol with different application routes13. Due to this lack of knowledge, sera were additionally collected and analyzed during an unrelated parallel study in our lab. Serum from mice (n = 18) euthanized 3 days after osteotomy and treated with Tramadol via drinking water at a concentration of 0.1 mg/ml (comparable to analgesic regime of the Tlow group) were analyzed by TOXILAB Ludwigsburg for Tramadol and O-Desmethyl-Tramadol (M1) concentrations. The analysis was conducted based on an established method to determine the concentration of Tramadol and M1 in urine. Inter- and intra-assay controls and QM were applied.
Clinical, behavioral and model-specific pain scoring
Animal scoring was performed by two researchers blinded to the groups’ analgesic treatment. To assess the facial expression and appearance composite score, mice were transferred individually to a transparent plastic observation box, allowed to acclimate for 1 min and were observed for 3 min. Scoring was performed according to Jirkof et al.61 but slightly adapted (Supplementary Table S7). A maximum score of 11 could be reached. The nest complexity score was assessed in the home cage using the naturalistic system developed by Hess et al.22. To assess motivation to interact with an object in the home cage, we performed an explorative test. A ball of four Envirodri® stripes was put in the home cage and the animals were observed for 1 min by a blinded observer. The outcome was either negative (score = 0) with no interaction or positive (score = 1) if there was an interaction with the provided material. Interaction was defined as gnawing, fraying, carrying, holding with forelimbs, rolling and intensive sniffing of the material. As model-specific pain or severity parameters, several established behaviors related to gait and weight bearing were observed40,43,62. Mice were transferred individually in standard cages covered with a filter top and given an acclimation time of 15 min before taking a video for 3 min. Offline video analysis was performed by one blinded observer. The frequency of limping and dragging with the operated leg (=limp score) as well as total time an animal spent with grooming the operated leg was measured in seconds (see Supplementary Table S8). Additionally, the frequency of flinching with the left hind limb was recorded. Flinching is characterized as a rapid, repetitive lifting of the affected limb40. The total time of remaining in a rear up position with weight bearing apparently on both legs was determined in seconds.
Measurement of fecal corticosterone metabolites (FCMs)
Following observation, fecal samples were collected from the observation box, and urine was carefully absorbed with a facial tissue. Fecal samples were stored at −20 °C immediately after collecting. FCM analysis was carried out as described and validated previously46,63. In short, the material was dried at 70 °C, homogenized with a mortar and weighed. A portion of 0.05 mg of each sample was vortexed for 2 min in 1 ml 80% methanol followed by a centrifugation step (2,500 × g for 15 min). Supernatants were stored at −80 °C and analyzed using a 5α-pregnane-3β,11β,21-triol-20-one enzyme immunoassay63.
Ex vivo µCT of osteotomized femora
Three-dimensional (3D) bone formation was analyzed after euthanasia using high-resolution µCT. Osteotomized femora were fixed in 4% PFA and ascending glucose solutions. After removal of the pins and external fixator, femora were scanned with an isotropic voxel size of 10.5 μm (Viva40 micro-CT, Scanco Medical AG®, Switzerland, 70 KVp, 114 μA). The scan axis coincided with the diaphyseal axis of the femora while 191 slides were scanned between the middle pins to include the complete osteotomy gap. The provided software package (Scanco®, Switzerland) was used for 3D reconstruction, post-processing and analyses. The osteotomy gap was defined per sample from half broken up cortical bone to the other before the volume of interest (VOI) was defined manually excluding the cortical bone. A fixed global threshold of 240 mg HA/cm3 was applied for the automatic 3D callus tissue analysis. The total volume (TV, mm3), the total bone volume (BV, mm3) and the bone volume fraction (BV/TV) were included for evaluation. Analysis was performed blinded to treatment.
Histology and immunofluorescence of osteotomized femora
In order to investigate undecalcified bones, embedding and slice preparation was conducted as described previously21. In detail, femora were fixed for 6 h in 4% PFA followed by an ascending sugar solution treatment (10%, 20%, 30%) for 24 h, respectively, and cryo-embedding with SCEM medium (Sectionlab, Japan) and subsequent storing at −80 °C. A specific cryofilm (Sectionlab, Japan) was used to provide slices (7 mm) with a cryotom and storage at −80 °C. Slices were air-dried for 20 min and fixed with 4% PFA prior to every histological staining. Movat’s Pentachrome staining was conducted using an already published protocol20. Pictures were taken with a LSM 710 confocal microscope (Carl Zeiss, Jena, Germany) and analysed using ImageJ. For immunofluorescence staining, the slides were air dried for 20 min, rehydrated with PBS and blocked with PBS/5% FCS for 30 min. Primary PECAM-1 antibody (goat anti-mouse; R&D Systems; AF3628) was diluted 1:50 in PBS/5% FCS/0.1% Tween 20 and incubated for 2 h. After washing with PBS/0.1% Tween 20, secondary antibody (donkey anti-goat A568; Life Technologies/ThermoScientific; A-11057) was diluted 1:500 in PBS/5% FCS/0.1% Tween 20 and applied for 1 h. A subsequent washing step was followed by a blocking step with PBS/10% normal goat serum. Primary Endomucin antibody (rat anti-mouse Endomucin; Santa Cruz; V.7C7) diluted 1:500 in PBS/5% FCS/0.1% Tween 20 was applied for 2 h. After washing secondary antibody (goat anti-rat A647; Life Technologies/ThermoScientific; A-21247) was used for 1 h. A washing step and DAPI (1:500; 1 mg/ml in PBS) completed the staining. Pictures were taken with a Keyence fluorescence microscope BZ 9000 using the DAPI, TexasRed and Cy5 channels. Image analysis was performed using ImageJ by a treatment-blinded researcher.
Liver histology
The liver of each osteotomized mouse was collected after euthanasia, frozen, and stored at −80 °C. Before embedding in paraffin, the livers were fixed in 4% PFA for 24 h and then transferred to the Institute of Veterinary Pathology at the Department of Veterinary Medicine FU Berlin. The livers were paraffin embedded and HE stained. The samples were assessed histologically blinded to the analgesic treatment to assess any possible influence of the analgesia on the liver.
Statistical analysis
Samples size calculation for primary endpoints (composite score, model-specific pain score) was based on the resource equation approach with focus in the 3 groups with osteotomy and different analgesia (min. number per group = 4; max. number per group = 8). For secondary endpoints such as water intake (ml) and BV/TV (bone volume fraction), power analysis was possible, calculated via G*Power 3.1 program and compared to the group sizes calculated via the resource equation approach. For more detailed information please see Animal Study Registry (Bf3R, Germany, 10.17590/asr.0000113).
The number of animals is either stated in the text, figure legend or indicated as a scatter dot plot in the graphs. Statistical analysis was carried out with GraphPad Prism V.7 software. As a first step, data sets were tested for Gaussian distribution using Shapiro-Wilk normality test (n < 8) or Kolmogorov-Smirnov normality test (n > 4). Data with n ≤ 4 were assumed as non-parametric. Only the body weight showed a Gaussian distribution compared to all other data sets. Therefore, the body weight is presented as mean ± SD and normalized to the initial body weight. To statistically compare the measured body weight to the initial body weight, one sample t test was performed (hypothetical mean = 100). One-way ANOVA with Bonferroni’s multiple comparisons test (selected pairs) was used to determine group differences. Changes over time were assessed with a repeated-measure two-way ANOVA with a Tukey’s multiple comparisons test. Normalization to baseline measurements was not useful in the other datasets. Where reasonable, baseline measurements are stated in the text or depicted in the graphs (time point = 0). Data with no Gaussian distribution are shown as median ± interquartile range. To determine statistical differences towards the baseline measurements, Wilcoxon signed rank test was used. As non-parametric statistical test for group differences, the Kruskal-Wallis test with Dunn’s multiple comparison test was applied for selected pairs. All important numbers and adjusted p-values are either stated in the text, the graphs or are listed in the Supplement.
Supplementary information
Acknowledgements
The authors would like to thank Manuela Jakstadt and Theresia Reding Graf for excellent technical assistance and Juliane Unger and Margarete Arras for their support. This study was funded by the German Federal Institute for Risk Assessment (BfR) and the German Centre for the Protection of Laboratory Animals (Bf3R), Berlin, Germany (project no.: 1328-542; RefineMOMo) and the Deutsche Forschungsgemeinschaft (DFG) as part of the research group “Severity Assessment for animal based research FOR 2591”. We acknowledge support with regard to the publication fee from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité – Universitätsmedizin Berlin. These funding bodies did not have any role in designing the study, in collecting, analysing and interpreting the data, in writing this manuscript, and in deciding to submit it for publication. A.L., F.B. and C.T.R. are members of Berlin-Brandenburg research platform BB3R. A.L., F.B. and K.S.B. are active members of Charité3R.
Author Contributions
Study design: A.L., P.J., K.S.B. and F.B. Data collection, analysis and interpretation: all authors; Drafting manuscript: A.L., P.J. and M.D. Revising manuscript: K.S.B., F.B., C.T.R., R.P. and R.K. Approved final version of manuscript: all authors. A.L. and P.J. are responsible for the integrity of the manuscript.
Data Availability
The authors declare that all data supporting the findings of this study are available within the paper and its supplementary information file. Further information on the study design and the protocols are available in the Animal Study Registry (Bf3R, Germany; 10.17590/asr.0000113). Further information are made available by the authors upon request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Paulin Jirkof and Mattea Durst contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-47186-5.
References
- 1.Carbone L, Austin J. Pain and Laboratory Animals: Publication Practices for Better Data Reproducibility and Better Animal Welfare. PloS one. 2016;11:e0155001. doi: 10.1371/journal.pone.0155001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jirkof P. Side effects of pain and analgesia in animal experimentation. Lab animal. 2017;46:123–128. doi: 10.1038/laban.1216. [DOI] [PubMed] [Google Scholar]
- 3.Peterson NC, Nunamaker EA, Turner PV. To Treat or Not to Treat: The Effects of Pain on Experimental Parameters. Comparative medicine. 2017;67:469–482. [PMC free article] [PubMed] [Google Scholar]
- 4.Flecknell P. Rodent analgesia: Assessment and therapeutics. Veterinary journal (London, England: 1997) 2018;232:70–77. doi: 10.1016/j.tvjl.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Santy J, Mackintosh C. A phenomenological study of pain following fractured shaft of femur. Journal of Clinical Nursing. 2001;10:521–527. doi: 10.1046/j.1365-2702.2001.00506.x. [DOI] [PubMed] [Google Scholar]
- 6.Minick P, et al. Long-bone fracture pain management in the emergency department. Journal of emergency nursing: JEN: official publication of the Emergency Department Nurses Association. 2012;38:211–217. doi: 10.1016/j.jen.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Bove SE, Flatters SJ, Inglis JJ, Mantyh PW. New advances in musculoskeletal pain. Brain research reviews. 2009;60:187–201. doi: 10.1016/j.brainresrev.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharyya T, Levin R, Vrahas MS, Solomon DH. Nonsteroidal antiinflammatory drugs and nonunion of humeral shaft fractures. Arthritis and rheumatism. 2005;53:364–367. doi: 10.1002/art.21170. [DOI] [PubMed] [Google Scholar]
- 9.Cottrell J, O’Connor JP. Effect of Non-Steroidal Anti-Inflammatory Drugs on Bone Healing. Pharmaceuticals. 2010;3:1668. doi: 10.3390/ph3051668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jirkof P, Tourvieille A, Cinelli P, Arras M. Buprenorphine for pain relief in mice: repeated injections vs sustained-release depot formulation. Laboratory animals. 2015;49:177–187. doi: 10.1177/0023677214562849. [DOI] [PubMed] [Google Scholar]
- 11.Mouedden ME, Meert TF. Pharmacological evaluation of opioid and non-opioid analgesics in a murine bone cancer model of pain. Pharmacology, biochemistry, and behavior. 2007;86:458–467. doi: 10.1016/j.pbb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Aydin ON, et al. The antinociceptive effects of systemic administration of tramadol, gabapentin and their combination on mice model of acute pain. Agri: Agri. 2012;24:49–55. doi: 10.5505/agri.2012.31032. [DOI] [PubMed] [Google Scholar]
- 13.Evangelista Vaz R, et al. Preliminary pharmacokinetics of tramadol hydrochloride after administration via different routes in male and female B6 mice. Veterinary anaesthesia and analgesia. 2018;45:111–122. doi: 10.1016/j.vaa.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Matthiesen T, Wohrmann T, Coogan TP, Uragg H. The experimental toxicology of tramadol: an overview. Toxicology letters. 1998;95:63–71. doi: 10.1016/S0378-4274(98)00023-X. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe AM, Kennedy LH, Na JJ, Nemzek-Hamlin JA. Efficacy of Tramadol as a Sole Analgesic for Postoperative Pain in Male and Female Mice. Journal of the American Association for Laboratory Animal Science: JAALAS. 2015;54:411–419. [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrnthaller C, et al. Complement C3 and C5 deficiency affects fracture healing. PloS one. 2013;8:e81341. doi: 10.1371/journal.pone.0081341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.GV-SOLAS. Fachinformation Schmerztherapie bei Versuchstieren aus dem Ausschuss für Anästhesie der GV-SOLAS. (2015).
- 18.Haffner-Luntzer M, et al. Midkine-deficiency delays chondrogenesis during the early phase of fracture healing in mice. PloS one. 2014;9:e116282. doi: 10.1371/journal.pone.0116282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heilmann A, et al. Systemic treatment with the sphingosine-1-phosphate analog FTY720 does not improve fracture healing in mice. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2013;31:1845–1850. doi: 10.1002/jor.22426. [DOI] [PubMed] [Google Scholar]
- 20.Schlundt C, et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone. 2018;106:78–89. doi: 10.1016/j.bone.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Lang A, et al. Collagen I-based scaffolds negatively impact fracture healing in a mouse-osteotomy-model although used routinely in research and clinical application. Acta Biomaterialia. 2019 doi: 10.1016/j.actbio.2018.12.043. [DOI] [PubMed] [Google Scholar]
- 22.Hess SE, et al. Home Improvement: C57BL/6J Mice Given More Naturalistic Nesting Materials Build Better Nests. Journal of the American Association for Laboratory Animal Science: JAALAS. 2008;47:25–31. [PMC free article] [PubMed] [Google Scholar]
- 23.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32:435–443. doi: 10.1023/A:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evangelista Vaz, R. E. A. Evaluation of the analgesic efficacy of a 25 mg/kg Tramadol s.c. injection-oral combination in a surgical model in C57BL/6J mice. Journal of the American Association for Laboratory Animal Science: JAALAS, Unpublished (2017).
- 25.Rapp AE, et al. Analgesia via blockade of NGF/TrkA signaling does not influence fracture healing in mice. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2015;33:1235–1241. doi: 10.1002/jor.22892. [DOI] [PubMed] [Google Scholar]
- 26.Khan SN, et al. The temporal role of leptin within fracture healing and the effect of local application of recombinant leptin on fracture healing. Journal of orthopaedic trauma. 2013;27:656–662. doi: 10.1097/BOT.0b013e3182847968. [DOI] [PubMed] [Google Scholar]
- 27.Lang A, Schulz A, Ellinghaus A, Schmidt-Bleek K. Osteotomy models - the current status on pain scoring and management in small rodents. Laboratory animals. 2016;50:433–441. doi: 10.1177/0023677216675007. [DOI] [PubMed] [Google Scholar]
- 28.Lehmann KA, Kratzenberg U, Schroeder-Bark B, Horrichs-Haermeyer G. Postoperative patient-controlled analgesia with tramadol: analgesic efficacy and minimum effective concentrations. Clin J Pain. 1990;6:212–220. doi: 10.1097/00002508-199009000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Morton DB, Griffiths PH. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet Rec. 1985;116:431–436. doi: 10.1136/vr.116.16.431. [DOI] [PubMed] [Google Scholar]
- 30.Arras M, Rettich A, Cinelli P, Kasermann HP, Burki K. Assessment of post-laparotomy pain in laboratory mice by telemetric recording of heart rate and heart rate variability. BMC Vet Res. 2007;3:16. doi: 10.1186/1746-6148-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller A, Kitson G, Skalkoyannis B, Leach M. The effect of isoflurane anaesthesia and buprenorphine on the mouse grimace scale and behaviour in CBA and DBA/2 mice. Applied animal behaviour science. 2015;172:58–62. doi: 10.1016/j.applanim.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jirkof P, et al. Assessment of postsurgical distress and pain in laboratory mice by nest complexity scoring. Laboratory animals. 2013;47:153–161. doi: 10.1177/0023677213475603. [DOI] [PubMed] [Google Scholar]
- 33.Gaskill, B. N., Karas, A. Z., Garner, J. P. & Pritchett-Corning, K. R. Nest building as an indicator of health and welfare in laboratory mice. J Vis Exp, 51012, 10.3791/51012 (2013). [DOI] [PMC free article] [PubMed]
- 34.Darbyshire A. An Assessment of the Safety of Recuvyra following Topical Administration in Mice - Abstracts of Scientific Presentations: 2015 AALAS National Meeting Phoenix, Arizona. Journal of the American Association for Laboratory Animal Science: JAALAS. 2015;54:568–668. [Google Scholar]
- 35.Rock ML, et al. The time-to-integrate-to-nest test as an indicator of wellbeing in laboratory mice. J Am Assoc Lab Anim Sci. 2014;53:24–28. [PMC free article] [PubMed] [Google Scholar]
- 36.Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–818. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Attal N, Jazat F, Kayser V, Guilbaud G. Further evidence for ‘pain-related’ behaviours in a model of unilateral peripheral mononeuropathy. Pain. 1990;41:235–251. doi: 10.1016/0304-3959(90)90022-6. [DOI] [PubMed] [Google Scholar]
- 38.Luger NM, et al. Osteoprotegerin diminishes advanced bone cancer pain. Cancer Res. 2001;61:4038–4047. [PubMed] [Google Scholar]
- 39.Minville V, Fourcade O, Mazoit JX, Girolami JP, Tack I. Ondansetron does not block paracetamol-induced analgesia in a mouse model of fracture pain. British journal of anaesthesia. 2011;106:112–118. doi: 10.1093/bja/aeq277. [DOI] [PubMed] [Google Scholar]
- 40.Minville V, Laffosse JM, Fourcade O, Girolami JP, Tack I. Mouse model of fracture pain. Anesthesiology. 2008;108:467–472. doi: 10.1097/ALN.0b013e3181649333. [DOI] [PubMed] [Google Scholar]
- 41.Majuta LA, Longo G, Fealk MN, McCaffrey G, Mantyh PW. Orthopedic surgery and bone fracture pain are both significantly attenuated by sustained blockade of nerve growth factor. Pain. 2015;156:157–165. doi: 10.1016/j.pain.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jimenez-Andrade JM, et al. Nerve growth factor sequestering therapy attenuates non-malignant skeletal pain following fracture. Pain. 2007;133:183–196. doi: 10.1016/j.pain.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 43.Koewler NJ, et al. Effects of a monoclonal antibody raised against nerve growth factor on skeletal pain and bone healing after fracture of the C57BL/6J mouse femur. J Bone Miner Res. 2007;22:1732–1742. doi: 10.1359/jbmr.070711. [DOI] [PubMed] [Google Scholar]
- 44.Freeman KT, et al. A fracture pain model in the rat: adaptation of a closed femur fracture model to study skeletal pain. Anesthesiology. 2008;108:473–483. doi: 10.1097/ALN.0b013e3181649351. [DOI] [PubMed] [Google Scholar]
- 45.Leach MC, et al. The assessment of post-vasectomy pain in mice using behaviour and the Mouse Grimace Scale. PloS one. 2012;7:e35656. doi: 10.1371/journal.pone.0035656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Touma C, Palme R, Sachser N. Analyzing corticosterone metabolites in fecal samples of mice: a noninvasive technique to monitor stress hormones. Hormones and behavior. 2004;45:10–22. doi: 10.1016/j.yhbeh.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Goldschlager GB, Gillespie VL, Palme R, Baxter MG. Effects of multimodal analgesia with LowDose buprenorphine and meloxicam on fecal glucocorticoid metabolites after surgery in New Zealand white rabbits (Oryctolagus cuniculus) J Am Assoc Lab Anim Sci. 2013;52:571–576. [PMC free article] [PubMed] [Google Scholar]
- 48.Hohlbaum K, et al. Impact of repeated anesthesia with ketamine and xylazine on the well-being of C57BL/6JRj mice. PloS one. 2018;13:e0203559. doi: 10.1371/journal.pone.0203559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Resch Marlene, Neels Tania, Tichy Alexander, Palme Rupert, Rülicke Thomas. Impact assessment of tail-vein injection in mice using a modified anaesthesia induction chamber versus a common restrainer without anaesthesia. Laboratory Animals. 2018;53(2):190–201. doi: 10.1177/0023677218786982. [DOI] [PubMed] [Google Scholar]
- 50.Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemporary topics in laboratory animal science/American Association for Laboratory Animal Science. 2004;43:42–51. [PubMed] [Google Scholar]
- 51.Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ. Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav Immun. 2008;22:105–113. doi: 10.1016/j.bbi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coluzzi F, Pergolizzi J, Raffa RB, Mattia C. The unsolved case of “bone-impairing analgesics”: the endocrine effects of opioids on bone metabolism. Therapeutics and Clinical Risk Management. 2015;11:515–523. doi: 10.2147/tcrm.S79409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gotthardt F, et al. Bone mineral density and its determinants in men with opioid dependence. Journal of Bone and Mineral Metabolism. 2017;35:99–107. doi: 10.1007/s00774-015-0732-9. [DOI] [PubMed] [Google Scholar]
- 54.Hirst A, Knight C, Hirst M, Dunlop W, Akehurst R. Tramadol and the risk of fracture in an elderly female population: a cost utility assessment with comparison to transdermal buprenorphine. The European Journal of Health Economics. 2016;17:217–227. doi: 10.1007/s10198-015-0673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hedenqvist P, et al. Carprofen neither reduces postoperative facial expression scores in rabbits treated with buprenorphine nor alters long term bone formation after maxillary sinus grafting. Research in Veterinary Science. 2016;107:123–131. doi: 10.1016/j.rvsc.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 56.Vivian B. Evaluation of Osteoporosis Risk Associated with Chronic Use of Morphine, Fentanyl and Tramadol in Adult Female Rats. Current Drug Safety. 2011;6:159–163. doi: 10.2174/157488611797579267. [DOI] [PubMed] [Google Scholar]
- 57.Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes, brain, and behavior. 2007;6:192–200. doi: 10.1111/j.1601-183X.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- 58.Haffner-Luntzer M, Kovtun A, Rapp AE, Ignatius A. Mouse Models in Bone Fracture Healing Research. Current Molecular Biology Reports. 2016;2:101–111. doi: 10.1007/s40610-016-0037-3. [DOI] [Google Scholar]
- 59.Mehta M, et al. A 5-mm femoral defect in female but not in male rats leads to a reproducible atrophic non-union. Archives of orthopaedic and trauma surgery. 2011;131:121–129. doi: 10.1007/s00402-010-1155-7. [DOI] [PubMed] [Google Scholar]
- 60.Sorge Robert E, Martin Loren J, Isbester Kelsey A, Sotocinal Susana G, Rosen Sarah, Tuttle Alexander H, Wieskopf Jeffrey S, Acland Erinn L, Dokova Anastassia, Kadoura Basil, Leger Philip, Mapplebeck Josiane C S, McPhail Martina, Delaney Ada, Wigerblad Gustaf, Schumann Alan P, Quinn Tammie, Frasnelli Johannes, Svensson Camilla I, Sternberg Wendy F, Mogil Jeffrey S. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nature Methods. 2014;11(6):629–632. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- 61.Jirkof P, Tourvieille A, Cinelli P, Arras M. Buprenorphine for pain relief in mice: repeated injections vs sustained-release depot formulation. Laboratory Animals. 2014;49(3):177–187. doi: 10.1177/0023677214562849. [DOI] [PubMed] [Google Scholar]
- 62.Luger NM, et al. Efficacy of systemic morphine suggests a fundamental difference in the mechanisms that generate bone cancer vs inflammatory pain. Pain. 2002;99:397–406. doi: 10.1016/S0304-3959(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 63.Touma C, Sachser N, Mostl E, Palme R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. General and comparative endocrinology. 2003;130:267–278. doi: 10.1016/S0016-6480(02)00620-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the paper and its supplementary information file. Further information on the study design and the protocols are available in the Animal Study Registry (Bf3R, Germany; 10.17590/asr.0000113). Further information are made available by the authors upon request.