Abstract
Alleviation of cadmium-induced root genotoxicity and cytotoxicity by calcium chloride (CaCl2) in faba bean (Vicia faba L. var. minor) seedlings were studied. Faba bean seeds were treated with H2O or 2% CaCl2 for 6 h before germination. Seeds were then exposed to 0 and 50 µM CdCl2 concentrations for 7 days. Genotoxic damaging effects of Cd was examined through the determination of the mitotic index (MI), chromosomal aberrations (CA) and micronucleus (MN) in the meristem cells of faba bean roots. Similarly, effects of Cd stress on metal accumulation, total membrane lipid contents, total fatty acid composition (TFA), lipid peroxidation as indicated by malondialdehyde production, soluble protein and non-protein thiols (NP-SH) contents, hydrogen peroxide production and the activities of superoxide dismutase (SOD), catalase (CAT) and guaiacol peroxidase (GPX) were evaluated after 7 days of Cd stress in the seedling roots. Cd stress resulted in the reduction of MI, in addition to MN formation and CA induction in the roots of non-primed seeds (treated with H2O). Moreover, Cd induced lipid peroxidation, H2O2 overproduction and loss of membrane lipid amount and soluble protein content, and changes in the TFA composition in roots of faba bean seedlings. SOD activity declined, but CAT and GPX activities increased. However, seed pre-treatment with CaCl2 attenuated the genotoxic and cytotoxic effects of Cd on Vicia faba roots. The results showed that CaCl2 induced reduction of Cd accumulation, improved cell membrane stability and increased the antioxidant defence systems, thus reducing and alleviating Cd genotoxicity and oxidative damage.
Keywords: Cadmium, Genotoxicity, Membrane lipid, Oxidative stress, Roots, Seed priming
Introduction
Cadmium (Cd) is a highly toxic and non-essential metal which enters the environment mainly from anthropogenic activities, such as mining, industrial and municipal wastes and widespread use of phosphate fertilizers (Dong et al. 2007). Because of its high mobility and solubility in water (Vig et al. 2003), Cd represents a threat to plant, animal and human health (Filipic 2012; Singh and Prasad 2014). In plants, Cd is a strong phytotoxic metal, which can cause various changes at physiological, biochemical and genomical processes (Steinkellner et al. 1998; Moussa and El-Gamal 2010). It is well documented that Cd causes growth inhibition through root growth limitation, reduction in leaf photosynthesis, disordering mineral nutrition, water imbalance (Andosch et al. 2012; Li et al. 2012), decreased mitotic index (MI), and by inducing chromosomal abnormalities in the meristematic cells (Fusconi et al. 2006). As other heavy metals, high Cd doses enhance the production of reactive oxygen species (ROS) leading to cell apoptosis and death by inducing damage to major classes of macromolecules in plant, mainly proteins, membrane lipid (Nouairi et al. 2006) and DNA (Seth et al. 2008; Shi et al. 2016).
Plants possess a highly efficient antioxidative defence system with an array of enzymatic (including superoxide dismutase, catalase and guaiacol peroxidase) and non-enzymatic constituents present in all plant cells (Benavides et al. 2005; Foyer and Noctor 2005) to scavenge ROS and alleviate their deleterious effects. However, previous studies showed that Cd inhibit the antioxidant capacity of plants, thus declining plant growth (Guo et al. 2009; Farooq et al. 2013).
Many approaches have been used to alleviate abiotic stress symptoms in crop plants. Seed priming, is one the oldest used methods (Heydecker and Coolbear 1977). Osmoprimed alfaalfa seeds exhibit enhanced and improved tolerance to salt stress (Yacoubi et al. 2013), while seed-soaking in spermidine provide protection against Cd stress in wheat (Tajti et al. 2018). In previous studies, it has been shown that calcium can also be an important candidate to promote plant development and confer protection against heavy metals stress (Wan et al. 2011; Jan et al. 2015; Abd_Allah et al. 2017). In fact, calcium was shown to be involved in the regulation of plant cell metabolism, gene expression and signal transduction of the environmental stimuli in plants (Rentel and Knight 2004), increasing the tolerance of plants against several abiotic stresses, such as Cd (Farzadfar et al. 2013; Ranty et al. 2016).
The present study was performed to determine if Ca pre-treatment of Vicia faba L. var. minor seeds, provide protection against Cd-induced genotoxicity and cytotoxicity effects in the roots of faba bean.
Materials and methods
Plant material and germination conditions
The uniform seeds of faba bean (Vicia faba L. var. minor) were surface sterilized with 0.5% NaOCl solution, washed repeatedly with several changes of sterile distilled water and then divided into two parts. For priming, one half of seeds was soaked in 2% CaCl2 solution for 6 h, the other half was soaked in H2O (control). In Petri dishes with four layers of sterile Whatman filter papers, the seeds with or without priming were treated for 7 days (d) with 20 mL of CdCl2 solution at the following concentrations: 0 and 50 µM. Both treatments had 12 replicates (dishes) with 10 seeds per dish. The CdCl2 solution was refreshed every day for maintaining its concentration. The seeds were allowed to germinate in the dark for 3 d at 25 °C, and then transferred into a growth chamber at a day/night cycle (10/14 h); at 25/18 °C, respectively, relative humidity between 60 and 70%. At the end of the 7-day treatments, germination percentage, root weight and length were measured, roots of seedlings were collected, weighed and frozen in liquid nitrogen, and stored at − 80 °C until further analysis.
Determination of Cd content
The root dry weight (DW) was digested with a mixture HNO3–HClO4 (3:1, v/v). After total evaporation, 0.5% of HNO3 was added, and Cd level in the digest was analyzed using an atomic absorption spectrophotometry (Varian SpectrAA 220 FS).
Cytological analysis
To study the genotoxic effects of Cd on faba bean root tip cells, the primary root tips were collected and transferred overnight in the Carnoy fixation solution mixture, containing ethanol and glacial acetic acid (3:1, v/v) at 4 °C and then stored in 70% ethanol in the dark. After successive washings in distilled water, fixed root tips were incubated in HCl 1 N (5–6 min at 60 °C). The root cap was removed and root meristematic tissues were stained with 1% aceto-orcein solution, squashed on slides and finally examined with use of a light microscope (model BX41; Olympus, Japan) under 1000 × magnification. The micronucleus frequency (MN), cell mitotic index (MI) and chromosomal aberration (CA) frequency were examined and counted microscopically on Vicia faba root tip squashes. Ten root tips were used in each treatment. At least 1000 cells in squash preparations from three separate root tips were scored.
Lipid peroxidation
Malondialdehyde (MDA) content is a widely used method to measure the level of lipid peroxidation in cell membranes. According to Karabal et al. (2003), about 200 mg of root fresh tissues were crushed and homogenized with of 1 mL of 5% trichloroacetic acid (TCA) solution. The homogenates were transferred to tubes and centrifuged at 10,000g for 15 min. Equal volumes of supernatant aliquot and reagent [0.5% thiobarbituric acid (TBA) in 20% TCA solution] were added into a new tubes and incubated at 96 °C for 30 min and then cooled into ice bath. After cooling and centrifuging (10,000g for 5 min), the absorbance at 532 nm was read and the value for the non-specific absorption at 600 nm was substracted. MDA level was quantified using the extinction coefficient of 155 mM−1 cm−1.
Membrane lipid extraction and fatty acid analysis
Total membrane lipid from root seedlings was extracted according to the method of Bligh and Dyer (1959). Fresh roots were fixed in boiling H2O (for 5 min) to denature phospholipases (Douce 1964) and then crushed in chloroform:methanol mixture (1:1, v/v). The homogenate was centrifuged at 3000g for 15 min. The lower chloroformic phase containing lipids was removed and evaporated at 40 °C under vacuum using a rotary evaporator. Fatty acids from total membrane lipid contents were methylated by the method of Metcalfe et al. (1966). Fatty acids methyl esters were separated and quantified with a Hewlett Packard chromatography model 4890D fitted with a 30 m × 0.25 mm × 0.25 µm film thickness fused silica capillary column (HP-Innowax; agilent, USA) coupled to a flame ionization detector (FID), maintained at 230 and 250 °C, respectively. Nitrogen was used as the carrier gas at 1 mL/min with split injector system (split ratio 1:100/200 °C). The amount of fatty acids was determining by using the heptadecanoic acid (C17:0) as internal standard. Calculation of fatty acids amounts was done using an integrator (HP model 3390A; Agilent).
Enzyme assays
Root samples (200 mg FW) were homogenized in 1.5 mL pre-cooled 50 mM potassium phosphate buffer (pH 7.0) containing 0.2 mM Na2EDTA and 2% polyvinypyrrolidine (w/v) at 4 °C. The homogenate was squeezed through two layers of muslin, centrifuged for 20 min at 12,000g at 4 °C and the obtained supernatant was used for the assay of SOD, CAT and GPX.
The assay of SOD was performed according to Xu et al. (2010). The absorbance was determined at 560 nm. One unit of SOD activity was defined as the content of enzyme required to inhibit by 50% the photochemical reduction rate of nitrobluetetrazolium (NBT).
CAT activity was determined in a reaction mixture composed of 25 mM potassium phosphate buffer (pH 7.0), 12.5 mM H2O2 and the enzyme extract. The decrease in absorbance of H2O2 within 2 min at 240 nm was recorded. The CAT activity was expressed as µmol H2O2 min−1 mg−1 protein and estimated with an extinction coefficient of 39.4 mM−1 cm−1 (Aebi 1984).
GPX activity was determined by measuring the oxidation of guaiacol (extinction coefficient 26.6 mM−1 cm−1) at 470 nm as described by Urbanek et al. (1991). The reaction mixture contained 25 mM of potassium phosphate buffer (pH 7.0, containing 0.1 mM EDTA), 10 mM H2O2 and 0.05% guaiacol.
Soluble protein amount was estimated by the method of Bradford (1976), using bovine serum albumin (Sigma chemicals) as a standard.
Determination of non-protein thiols
Non-protein thiols (NP-SH) was determined with Ellman’s Reagent (Devos et al. 1992). NP-SH were extracted by homogenizing roots in 2 mL ice-cold 5% sulfosalicylic acid solution (w/v). After centrifugation at 10,000g at 4 °C for 30 min, 300 µl of the supernatant was added to 1.2 mL of 0.1 M potassium phosphate buffer (pH 7.6). After obtaining a stable absorbance at 412 nm, 25 µl of 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) solution (6 mM DTNB in 5 mM EDTA and 0.1 M phosphate buffer solution, pH 7.6) was added and the increase in absorbance at 412 nm was recorded.
Evaluation and histochemical detection of H2O2
Root tissues were homogenized with 5% TCA solution. The homogenate was centrifuged at 12,000g for 15 min. After reaction with potassium iodide (KI), H2O2 production was detected and determined according to Sergiev et al. (1997). The reaction mixture consisted of the root extract, 2.5 mM potassium-phosphate buffer (pH 7.0) and 0.5 M KI. After incubation in the dark for 1 h, the content of H2O2 was recorded spectrophotometrically at 390 nm by reference to a standard curve prepared with H2O2 solution.
For in vivo detection of H2O2, roots attached to the plants were stained for 45 min in KI/starch reagent [0.1 M KI and 4% (v/v) starch, pH 5.0] (Schützendübel et al. 2001). Stained roots were photographed under a binocular (Leica 56E).
Statistical analysis
The statistical analysis was performed by one–way variance analysis (ANOVA); Duncan test was used for separation of the means at a 0.05 probability level, and data were analysed using SPSS software (Version 20.0).
Results
Cd effects on root growth and soluble protein content of faba bean seedlings
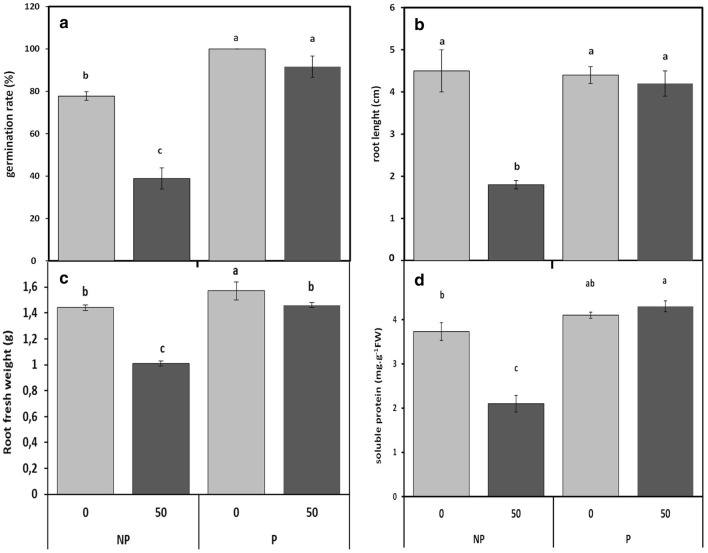
Several parameters, including germination rate, root length, biomass (FW), Cd and soluble protein contents of faba bean roots were used to determine CdCl2 effects, during germination of non-primed and CaCl2-primed seeds. Exposure of Vicia faba seedlings to 50 µM Cd resulted in a marked reduction (51%) in germination rate as compared to controls. However, a substantial improvement by 28% and 17% in germination rate was noticed due to CaCl2 pre-treatment under 0 and 50 µM Cd treatments, respectively, as compared to the non-primed seeds (Fig. 1a).
Fig. 1.
Cadmium effect on germination rate (a), root length (b), root fresh weight (c) and soluble protein content (d) in roots of faba bean seedlings, pretreated with 2% CaCl2 (P) or H2O (NP) for 6 h, and germinated for 7 days under 0 or 50 µM Cd. Values are the means of at least 12 replications ± SE. The data followed by different letters are significantly different at P ≤ 0.05
The roots of Vicia faba showed a marked decrease in root length (60%) under Cd stress compared with controls. While, in CaCl2 pre-treated seeds, Cd doses (0 and 50 µM) have no effect on the root length (Fig. 1b).
In response to 50 µM Cd, the biomass (FW) of Vicia faba roots was significantly lower than that of the control ones (Fig. 1c). However, results showed that CaCl2 pre-treatment can be effective in improving root growth of Vicia faba both under the presence (50 µM) or absence (0 µM) of Cd (Fig. 1c).
The accumulation of Cd in roots of faba bean seedlings is shown in Table 1. Under Cd stress (50 µM), CaCl2 seed pre-treatment reduced Cd accumulation in roots by 24% as compared to the non-primed seeds.
Table 1.
Cadmium content (µg g−1 DW) in roots of faba bean seedlings, pretreated with 2% CaCl2 (P) or with H2O (NP) for 6 h, and germinated for 7 days under 0 or 50 µM Cd
| Cd µM | Cd content (µg g−1 DW) | |
|---|---|---|
| NP | 0 | ND |
| 50 | 97.6 ± 7.1*,a | |
| P | 0 | ND |
| 50 | 74.2 ± 4.9b |
ND not detected
*Values are the means of five replications ± SE. The data followed by different letters are significantly different at P ≤ 0.05
The changes in soluble protein contents also indicated significant effects of Cd treatment on roots of non-primed and primed faba bean seedlings (Fig. 1d). Root protein concentration significantly decreased by 46% under Cd stress (50 µM) in non-primed seedlings compared to the control. However, at the same Cd dose, protein content were significantly increased by 15% in the roots of CaCl2-primed Vicia faba seedlings in comparison with that of the control (Fig. 1d).
Genotoxic effect of Cd in root tips of faba bean seedlings
Mitotic index (MI), micronucleus frequency (MN) and chromosomal aberration percentage (CA) were performed to estimate genotoxic effects of CdCl2 (50 µM) during mitotic activity (Table 2).
Table 2.
The cell mitotic index (MI), micronucleus (MN), and chromosomal aberration (CA) frequency in the root tip meristem cells of faba bean seedlings, pre-treated with 2% CaCl2 (P) or with H2O (NP) for 6 h, and germinated for 7 days under 0 or 50 µM Cd
| Cd (µM) | MI (%) | MN (‰) | CA (‰) | |
|---|---|---|---|---|
| NP | 0 | 12.7 ± 2.2*,a | 0 ± 00 | 0 ± 0.0 |
| 50 | 8.7 ± 0.3b | 24.6 ± 0.3a | 16.2 ± 0.2a | |
| P | 0 | 13.2 ± 0.1a | 0 ± 0.0 | 0 ± 0.0 |
| 50 | 13.9 ± 0.2a | 2.4 ± 0.1b | 1.04 ± 0.5b |
*3000 cells analysed per treatment. Mean ± SE. The data followed by different letters are significantly different at P ≤ 0.05
The root meristem lost 32% of their mitotic activity under 50 µM Cd. However, in CaCl2-primed Vicia faba seedlings, Cd treatment led to an increase in the MI compared to the control (Table 2).
Abnormalities noticed during mitotic activity were also assessed with micronucleus (MN) induction. Table 2 shows that the MN frequency increase highly in Cd-treated root tips of non-primed seedlings, while in CaCl2-primed plants, a lower MN formation was detected under Cd stress (2.4 ± 0.1‰).
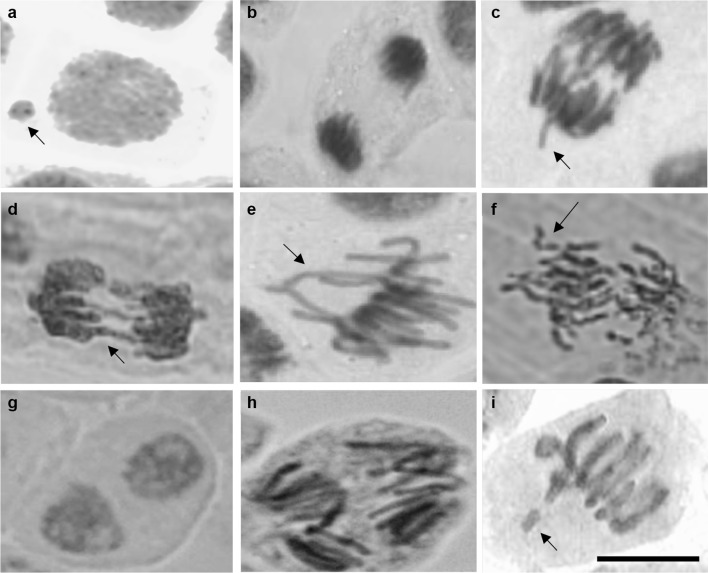
Concomitant to MN induction, the diverse chromosomal aberrations (CA) were also observed in the meristematic cells of Cd-treated Vicia faba roots at different mitotic stages (Table 2). e.g., sticky chromosomes, chromosome laggards, anaphase–telophase bridges and breaks (Fig. 2).
Fig. 2.
Representative mitotic abnormalities in apical meristem cells of faba bean Cd-treated roots. a micronuclei in interphase; b sticky telophase; c, d chromosome bridges in anaphase; e disoriented chromosomes at metaphase; f fragmented chromosomes at anaphase; g binucleate cell; h multipolar anaphase; i metaphase with chromosome fragments. Bar = 50 µm
Impact of Cd on H2O2 content and lipid peroxidation in roots
In the present study, it was found that treatment with 50 µM Cd enhanced H2O2 content by 60% in roots of non-primed Vicia faba seedlings as compared to the control (Fig. 3b), while only a slight increase in it (17%) was detected when 50 µM of CdCl2 was preceded by seed-soaking with CaCl2 (Fig. 3b). To further validate our results, the H2O2 histochemical detection in roots of Vicia faba roots was determined (Fig. 3a).
Fig. 3.
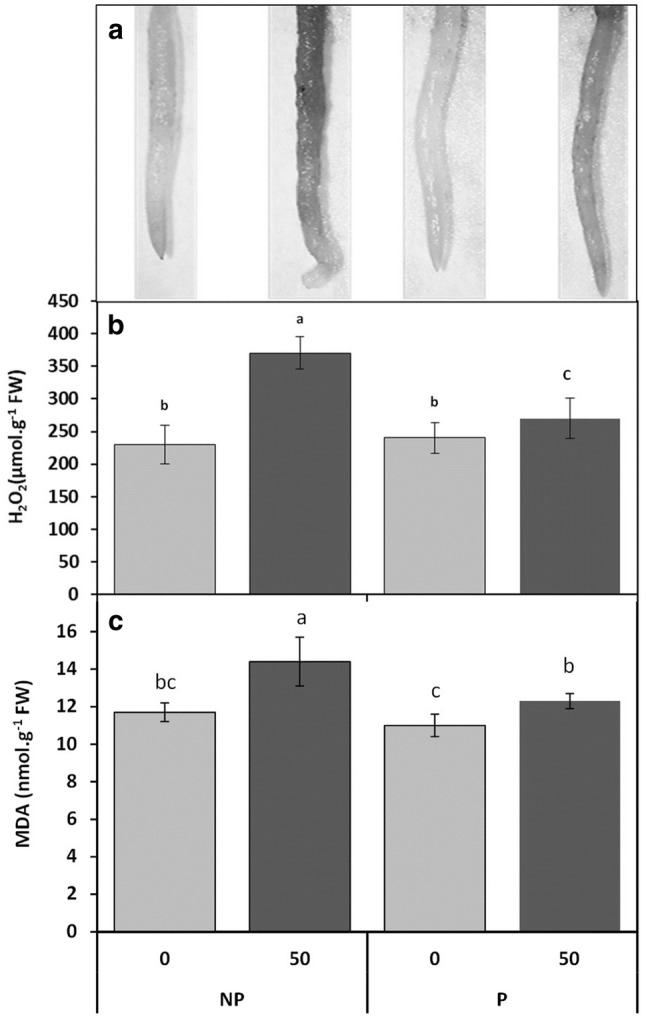
Histochemical detection (a) and accumulation of H2O2 (b) and MDA content (c) in roots of faba bean seedlings, pretreated with 2% CaCl2 (P) or with H2O (NP) for 6 h, and germinated for 7 days under 0 or 50 µM Cd. Values are the means of at least 10 replications ± SE. The data followed by different letters are significantly different at P ≤ 0.05
MDA is one of the final products of cell membrane lipid injury and accumulates when plants are exposed to oxidative damage. Similar trend was observed for MDA levels as it was described for H2O2 amount. Cd treatment (50 µM) enhanced MDA content by 23% and 5% in roots of non-primed and primed Vicia faba seedlings respectively, compared to the control (Fig. 3c).
Changes in root membrane lipid content and fatty acid composition
In the presence of 50 µM Cd, the level of total membrane lipid content (TL) content was reduced by approximately 25% in the roots of non-primed Vicia faba seedlings, while a slight decrease in it (11%) was observed when faba bean seedlings were exposed to 50 µM Cd after CaCl2 seed-soaking, compared to the control (Fig. 4).
Fig. 4.
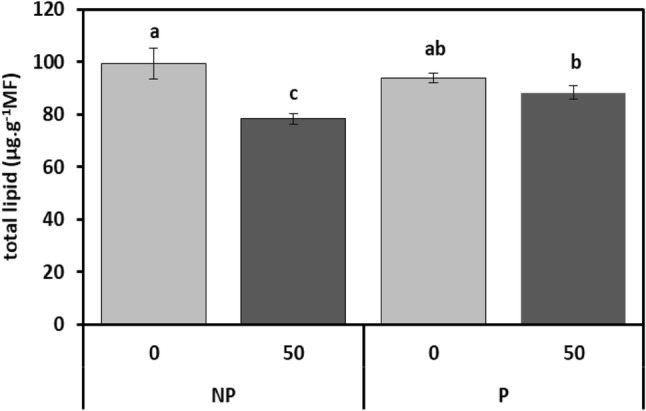
Total membrane lipid (TL) content in roots of faba bean seedlings, pretreated with 2% CaCl2 (P) or with H2O (NP) for 6 h, and germinated for 7 days under 0 or 50 µM Cd. Values are the means of six replications ± SE. The data followed by different letters are significantly different at P ≤ 0.05
Table 3 showed the fatty acid composition of TL. The detected root membrane lipids in Vicia faba seedlings are palmitic (C16:0), hexadecadienoic (C16:2), stearic (C18:0), oleic (C18:1), linoleic (C18:2) and linolenic (C18:3) acids. This composition was also dominated by the presence of a high level of C18:2, approximately 42% of the total fatty acids amount in roots of faba bean control plants. Under Cd stress, the level of C18:2, C16:0 and C16:2 decreased by 26, 30 and 81% respectively, while that of C18:1 increased. However, the fatty acid composition remained similar to the control, in roots of CaCl2-primed Vicia faba seedlings (Table 3) under 50 µM cadmium dose.
Table 3.
Total fatty acid composition (%) in roots of faba bean seedlings, pre-treated with 2% CaCl2 (P) or with H2O (NP) for 6 h, and germinated for 7 days under 0 or 50 µM Cd
| Cd(µM) | C16:0 | C16:1 | C16:2 | C18:0 | C18:1 | C18:2 | C18:3 | |
|---|---|---|---|---|---|---|---|---|
| NP | 0 | 16.17 ± 1.01*,a | 0.76 ± 0.29a | 10.30 ± 2.09a | 7.73 ± 2.66a | 2.25 ± 0.18b | 42.26 ± 2.09a | 3.96 ± 0.19a |
| 50 | 11.38 ± 0.76b | 0.29 ± 0.04b | 1.91 ± 2.57b | 7.21 ± 1.92a | 17.62 ± 0.80a | 31.36 ± 2.05b | 4.56 ± 0.87a | |
| P | 0 | 20.69 ± 1.62a | 1.03 ± 0.04a | 10.46 ± 3.69a | 10.71 ± 0.83a | 2.69 ± 0.22b | 45.77 ± 3.30a | 3.19 ± 0.63a |
| 50 | 18.46 ± 2.12a | 0.83 ± 0.25a | 12.77 ± 3.12a | 9.34 ± 1.05a | 1.94 ± 0.44b | 41.31 ± 4.62a | 3.33 ± 0.55a |
*Values are the means of six replications ± SE. The data followed by different letters are significantly different at P ≤ 0.05
Effect of Cd on antioxidative enzymes (SOD, CAT and GPX) and antioxidants (non- protein thiols)
As shown in Fig. 5a, SOD activity was notably decreased in the roots of non-primed faba bean seedlings treated with 50 µM Cd. However, Cd has no significant effect on the SOD activity in roots of CaCl2-primed seedlings treated with 0 or 50 µM Cd, and remained unchanged as compared to controls (Fig. 5a).
Fig. 5.
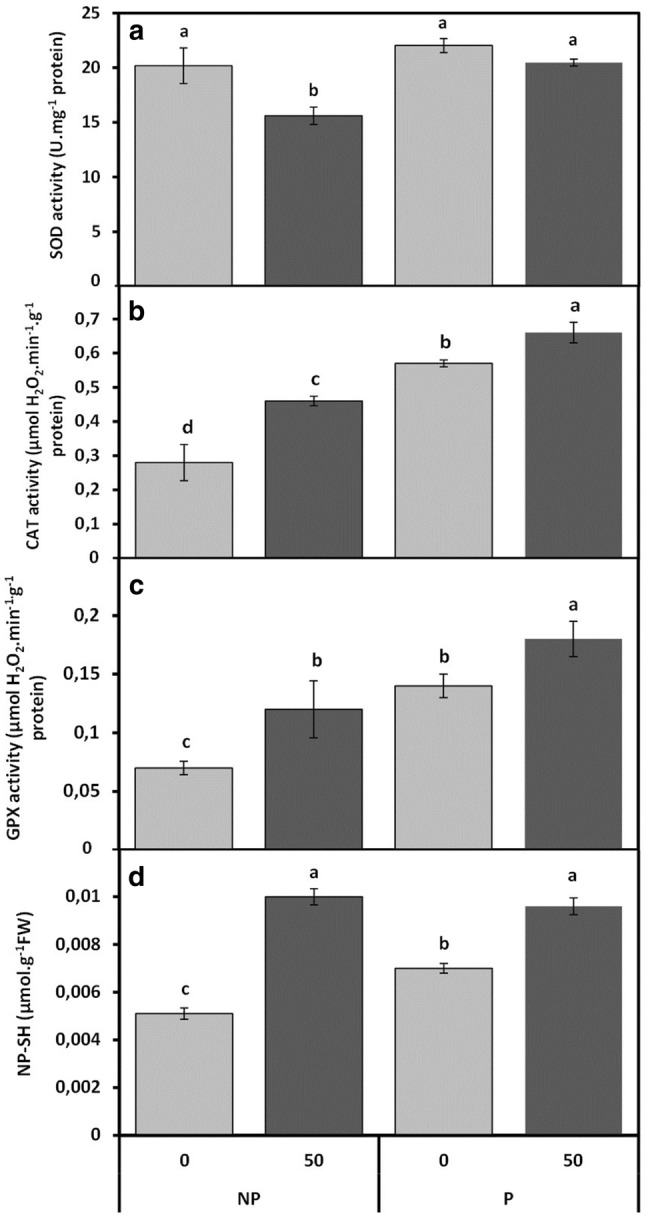
Cadmium effects on SOD (a), CAT (b), GPX (c) activities and non-protein thiols (NP-SH) content (d) in roots of faba bean seedlings, pre-treated with 2% CaCl2 (P) or with H2O (NP) for 6 h, and germinated for 7 days under 0 or 50 µM Cd. Values are the means of at least six replications ± SE. The data followed by different letters are significantly different at P ≤ 0.05
As shown in Fig. 5b and c, total activities of both CAT and GPX enhanced significantly upon the exposure to 50 µM Cd in roots of non-primed Vicia faba seedlings, and the CAT and GPX activities further increased about 124 and 143% respectively under 50 µM Cd treatment in roots of primed faba bean seedlings as compared with the controls (Fig. 5b, c). This additive effect is due to the stimulatory effect of CaCl2 priming, as in only primed seedlings, the CAT and GPX activities showed an enhancement of about 89% and 85% respectively.
The accumulation of non-protein thiols (NP-SH), the most common non-enzymatic antioxidant, significantly enhanced after 50 µM Cd treatment, by 100% and 86% respectively in roots of non-primed and primed Vicia faba seedlings (Fig. 5d) as compared with control. Interestingly, CaCl2 pre-treatment alone also increased the accumulation of NP-SH by 37% compared to the control (Fig. 5d).
Discussion
Cd is a persistent and hazardous heavy metal which is harmful to food safety and human health (Patrick 2003). Its accumulation affects and hampers the germination process (Rahoui et al. 2010; Ahmad et al. 2012), growth and development by affecting various physiological and biochemical metabolism (Wang et al. 2011; Asgher et al. 2015).
The inhibition of root growth under Cd stress, may be due to the fact that the root is the first organ to contact the heavy metal and be involved in the absorption process. In fact, the root has important role in the immobilization of heavy metals such as Cd by means of the extracellular wall and carbohydrates (Amirjani 2012). Mondal (2013) reported that the decrease of root growth may be due to direct interaction of cadmium with some hydrolytic enzymes, which plays a crucial role in transporting the nutritional compounds to the primary roots. It was also mentioned that the decrease in root biomass and length may be due to the direct inhibition of cell division or elongation (Mondal 2013).
The mitotic index (MI) reflects cell division frequency and is considered to be an important root growth factor. The result of our study revealed that 50 µM Cd diminished MI and induced various mitotic disturbances in the root meristem of Vicia faba (Table 2). Such a significant decrease in MI suggest that Cd exposure obstruct cells to enter into cell division, and could be due to inhibition of DNA synthesis (Patlolla and Tchounwou 2005) and/or due to blocking the G2-phase in which tubulin is intended for the formation and establishment of mitotic spindle (Mahoney et al. 2006). In the present work, it was showed that in roots of Vicia faba seedlings, 50 µM Cd induced a significant presence of MN (Table 2). The MN induction can result from chromosome breaks, which induce chromosome fragments or by the inability of the entire chromosomes to migrate during anaphase (Albertini et al. 2000). Thus, the induction of MN in root meristems proposes that Cd was either a spindle inhibitor and therefore aneugenic or a clastogen agent (Klein et al. 2007), which indicate indirect evidence of Cd genotoxicity (Fusconi et al. 2007). Moreover, induction of chromosome fragments observed in this work reporting chromosome-type breaks and DNA deterioration in root meristem cells, substantiate the potential clastogenic effect of Cd (Zhang and Yang 1994; Gichner et al. 2004).
It is well established that the decrease in root elongation, can be related to the decrease in the apical root meristem activity and cellular elongation (Ma et al. 2003). In our experiment, 50 µM Cd treatment decelerated the MI proportionally to the root growth inhibition and induced peroxidation and loss of membrane lipid content. Cd concentration (50 µM) led to 75% of TL values on average compared to the control and a significant increase of MDA level (23% as compared to the control). Moreover, fatty acid composition in membrane cells of Vicia faba L. var. minor roots was also affected by Cd treatment and could be a signal that there are some deleterious effects on fatty acids biosynthesis pathway. In 50 µM Cd-treated faba bean roots seedlings, the amount of C18:1 increased, but C18:2 decreased. This could be an indication that the desaturase (Δ12 fatty acid desaturase) activity, involved in the conversion and transformation of C18:1 → C18:2, was affected (Nouairi et al. 2006; Nguyen et al. 2016).
Previous works have shown that Cd stress obviously increases the H2O2 content in plant tissues (Rodríguez-Serrano et al. 2006; Singh et al. 2008). Vitória et al. (2001) found that the increased level of H2O2, may be associated with a lower SOD activity because H2O2 can oxidize the -SH groups of the enzymes and become inactive. In our study, Cd treatment (50 µM) also significantly increased intracellular H2O2 production and, negative linear relationships were observed between the H2O2 content and the activity SOD in faba bean roots (r2 = − 0.671, p < 0.01). The comparison of the three enzyme activities (SOD, CAT and GPX) showed that the lower SOD activity to Cd treatment might be compensated by the enhanced activities of CAT and GPX (Fig. 5b, c), showing that these two enzymes were functioning concurrently to remove H2O2 in seedlings roots after Cd exposure. Moreover, it has been demonstrated that an increase of some antioxidant enzymes activities can be assigned to the induced expression of their genes, possibly mediated by ROS (Fatima and Ahmad 2005; Singh and Prasad 2014). However, summarizing the results, it can be concluded that under Cd stress, the antioxidant capacity may not be sufficient to decrease the deleterious effect of oxidative damage. Excessive level of H2O2 in roots of faba bean seedlings may induce destructive effects on cellular and molecular components by peroxidising cell membrane lipids, oxidizing proteins and nucleic acids (Gill and Tuteja 2010; Sharma et al. 2012). Moreover, in this investigation, it was found that Cd inhibited soluble protein contents in root of Vicia faba seedlings. It might be the consequence of more oxidative effect under heavy metal stress (Ali et al. 2015). Decreased protein level due to Cd may be attributed to enhancement of protein alteration by protease or other catabolic enzymes related to protein metabolism (Seneviratne et al. 2017).
In another way, the pre-treatment of Vicia faba seeds with 2% CaCl2 solution for 6 h, improved the germination rate and growth of 7-day-old faba bean seedlings under the presence (50 µM) or absence (0 µM) of Cd. Moreover, in our study, we showed that CaCl2 pre-treatment has a protective role on several investigated parameters, such as root membrane lipid contents, the stability of fatty acid profiles, soluble protein content and mitotic index. This improvement could be due to the positive role of calcium by attenuating the harmful and toxic effects of Cd on plant growth. For instance, it has been shown that Ca2+ signal the cells to enter into early mitotic cycle (Rajendra et al. 2005). Moreover, Ca2+ may favour cell expansion and elongation and finally enhancing plant growth (Hernández and Almansa 2002). Thus, it can be suggested that the protective effect of CaCl2 pre-treatment on roots of CdCl2 treated faba bean seedlings may be attributed to increased cell division (as indicated by MI) within apical root meristems.
Many investigations have indicated that seed priming with CaCl2 could act as a physiological seed pre-treatment to enhance plant tolerance (Upadhyaya et al. 2011; Chengbin et al. 2013; Ahlam and Basalah 2015; Farooq et al. 2017). These findings suggest that Ca2+ plays an important role in alleviating and preventing the damage to faba bean seedlings sustained under heavy metal stress. Comparable alleviation activity by applied CaCl2 seed pre-treatment has been reported for different stress conditions (Tamimi 2016; Madany and Khalil 2017; Malgorzata et al. 2017).
Indeed, the enhancement of MDA and H2O2 content in roots of non-primed faba bean seedlings and their observed lower values in CaCl2 pre-treated seedlings under 50 µM Cd, is confirmation of the protective role of CaCl2 against Cd toxicity and ROS damage.
In this study, it was determined that CaCl2 seed pre-treatment significantly increased soluble protein content under Cd stress. The accumulation of protein during heavy metal treatment may provide a reserve form of nitrogen (N) that helps in osmotic adjustment (Parvaiz et al. 2016). The increased total soluble protein in roots of CaCl2 pre-treated seedlings may be due to increased amino acids especially proline as one of the most characteristic metabolic consequences of heavy metal stress (Al-Beltagi and Mohamed 2013), this may represent a major biochemical adaptation, along with membrane stabilization and ROS scavenging (Bandurska 2001). The role of Ca in enhancing soluble protein content may also be attributed to de novo synthesis of stress-related proteins such as metal-chelating polypeptides (Ahsan et al. 2007), lowering of enzyme denaturation and decreased proteolysis that were disturbed during stress (Parvaiz et al. 2016).
Moreover, in roots of CaCl2 pre-treated faba bean seedlings, Cd treatment resulted in the high activation of CAT and GPX activities. CAT and GPX are important enzymes that scavenge H2O2 detoxification in cells (Choudhury and Panda 2004). Furthermore, GPX enzymes, which is involved in lignin biosynthesis pathway, might build up a physical barrier attenuated metal toxicity (Gupta et al. 2015). This phenomenon is in good agreement with our results. Indeed, our study showed that CaCl2 seed pre-treatment reduced Cd accumulation (by 24%) in faba bean roots (Table 1). This protection provided by calcium (under 50 µM Cd), can be partly explained by the highest GPX activity in comparison with the non-primed seeds. Otherwise, experimental findings in some plant species demonstrate that roots with elevated content of lignin and suberin may be more impermeable to Cd and therefore more resistant to its absorption, penetration into cells and translocation (Lux 2010; Lux et al. 2011).
Moreover, in our study, a considerable increase of NP-SH contents (non-enzymic antioxidants) was observed in roots of CaCl2 primed seeds under 50 µM Cd dose. Therefore, it is possible that CaCl2 seed priming enhances other alternative antioxidant systems, which could be involved in lowering Cd toxicity and might be responsible for lower lipid peroxidation and consequently more stability of cell membrane lipids and fatty acid composition (Table 3). This can be involved in a better vacuolar compartmentation of cadmium-phytochelatin (Cd-PC) complexes (Sharma et al. 2016). Moreover, faba bean primed seedlings exhibited high amounts of NP-SH (which are precursors in phytochelatins synthesis) during 50 µM Cd exposure, showing its capability to tolerate cellular metal ions load, and probably reduced the free availability of metal ions in cytosol. This mechanism can be a strategy aimed to avert heavy metal cytotoxicity. The enhanced content of NP-SH may also be due to stimulation of sulphate reduction pathway such as adenosine-5′-phosphosulfate (APS) reductase and serine acetyl transferase (Nocito et al. 2007; Singh and Tuteja 2011) in roots of CaCl2 pre-treated faba bean seedlings.
Concluding remarks
In the present study, CaCl2 seed pre-treatment alleviated Cd induced genotoxicity and cytotoxicity in Vicia faba roots. Cd induced oxidative stress was lower in roots of primed seedlings. CaCl2 seed pre-treatment provide Cd tolerance to plants. Our results suggest that involvement of CaCl2 in the alleviation of Cd toxicity is manifested by (1) reducing cadmium uptake, (2) adjusting the membrane stability, (3) better performance of the enzymatic and non-enzymatic antioxidant defense systems.
Thus, CaCl2 priming can serve as an effective seed pre-treatment for the improvement of seedling vigor, and enhancement of physiological and metabolic processes that may increase the tolerance of plants to abiotic stress.
Acknowledgements
The authors are grateful for the financial support provided by the Tunisian Ministry of Higher Education and Scientific Research.
Authors contributions
IN: Assistant Professor, KJ and SE: PhD students, carried out the experimental work and performed all laboratory analyses. IN: conceived the study and wrote the first draft of manuscript. KZ: Associate Professor and HM: Professor, supervised the laboratory experiments and analyses. All authors contributed to the study and gave final approval to publish the manuscript in its current form.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abd_Allah EF, Hashem A, Alqarawia AA, Wirth S, Egamberdieva D. Calcium application enhances growth and alleviates the damaging effects induced by Cd stress in sesame (Sesamum indicum L.) J Plant Interact. 2017;12:237–243. doi: 10.1080/17429145.2017.1319500. [DOI] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Ahlam KM, Basalah MO. The active role of calcium chloride on growth and photosynthetic pigments of cowpea “Vigna unguiculata L. (Walp)” under salinity stress conditions. Am Eurasian J Agric Environ Sci. 2015;15:2011–2020. [Google Scholar]
- Ahmad I, Akhtar MJ, Zahir ZA, Jamil A. Effect of cadmium on seed germination and seedling growth of four wheat (Triticum aestivum L.) cultivars. Pak J Bot. 2012;5:1569–1574. [Google Scholar]
- Ahsan N, Lee SH, Lee DG, Lee H, Lee SW, Bahk JD, Lee BH. Physiological and protein profiles alternation of germinating rice seedlings exposed to nacute cadmium toxicity. C R Biol. 2007;330:735–746. doi: 10.1016/j.crvi.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Al-beltagi H, Mohamed H. Alleviation of cadmium toxicity in Pisum sativum L. seedlings by calcium chloride. Not Bot Horti Agrobo. 2013;41:157–168. doi: 10.15835/nbha4118910. [DOI] [Google Scholar]
- Albertini RJ, Anderson D, Douglas GR, Hagmar L, Hemminki K, Merlo F, Natarajan AT, Norppa H, Shuker DE, Tice R, Waters MD, Aitio A. IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. International Programme on Chemical Safety. Mutat Res. 2000;463:111–172. doi: 10.1016/S1383-5742(00)00049-1. [DOI] [PubMed] [Google Scholar]
- Ali S, Chaudhary A, Rizwan M, Anwar HT, Adrees M, Farid M, Irshad MK, Hayat T, Anjum SA. Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.) Environ Sci Pollut R. 2015;22:10669–10678. doi: 10.1007/s11356-015-4193-4. [DOI] [PubMed] [Google Scholar]
- Amirjani M. Effects of cadmium on wheat growth and some physiological factors. Int Forest Soil Eros. 2012;2:50–58. [Google Scholar]
- Andosch A, Affenzeller MJ, Lütz C, Lütz-Meindl U. A freshwater green alga under cadmium stress: ameliorating calcium effects on ultrastructure and photosynthesis in the unicellular model Micrasterias. J Plant Physiol. 2012;169:1489–1500. doi: 10.1016/j.jplph.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Asgher M, Khan MIR, Anjum NA, Khan NA. Minimising toxicity of cadmium in plants-role of plant growth regulators. Protoplasma. 2015;252:399–413. doi: 10.1007/s00709-014-0710-4. [DOI] [PubMed] [Google Scholar]
- Bandurska H. Proline accumulation during hardening and its involvement in reducing membrane injuries in leaves subjected to severe osmotic stress. Acta Physiol Plant. 2001;23:483–490. doi: 10.1007/s11738-001-0059-0. [DOI] [Google Scholar]
- Benavides MP, Gallego SM, Tomar ML. Cadmium toxicity in plants. Braz J Plant Physiol. 2005;17:21–34. doi: 10.1590/S1677-04202005000100003. [DOI] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chengbin X, Li X, Zhang L. The effect of calcium chloride on growth, photosynthesis and antioxidant responses of Zoysia japonica under drought conditions. PLoS ONE. 2013;8(7):e68214. doi: 10.1371/journal.pone.0068214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury S, Panda SK. Role of salicylic acid in regulating cadmium induced oxidative stress in Oryza sativa L. roots. Bulg J Plant Physiol. 2004;30:95–110. [Google Scholar]
- Devos CHR, Vonk MJ, Vooijs R, Schat H. Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol. 1992;98:853–858. doi: 10.1104/pp.98.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Mao WH, Zhang GP, Cai Y. Root excretion and plant tolerance to cadmium toxicity. Plant Environ J. 2007;53:193–200. [Google Scholar]
- Douce R. Identification et dosage de quelques glycerophosphatides dans des souches normales et tumorales descosoneres cultivés in vitro. C R Acad Sci. 1964;259:3066–3068. [Google Scholar]
- Farooq MA, Ali S, Hameed A, Ishaque W, Mahmood K, Iqbal Z. Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol Environ Saf. 2013;96:242–249. doi: 10.1016/j.ecoenv.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Farooq S, Hussain M, Jabran K, Hassan W, Rizwan MS, Yasir TA. Osmopriming with CaCl2 improves wheat (Triticum aestivum L.) production under water-limited environments. Environ Sci Pollut Res. 2017;24:13638–13649. doi: 10.1007/s11356-017-8957-x. [DOI] [PubMed] [Google Scholar]
- Farzadfar S, Zarinkamar F, Modarres-Sanavy SAM, Hojati M. Exogenously applied calcium alleviates cadmium toxicity in Matricaria chamomilla L. Plants. Environ Sci Pollut Res. 2013;20:1413–1422. doi: 10.1007/s11356-012-1181-9. [DOI] [PubMed] [Google Scholar]
- Fatima RA, Ahmad M. Certain antioxidant enzymes of Allium cepa as biomarkers for the detection of toxic heavy metals in wastewater. Sci Total Environ. 2005;346:256–273. doi: 10.1016/j.scitotenv.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Filipic M. Mechanisms of cadmium induced genomic instability. Mutat Res. 2012;733:69–77. doi: 10.1016/j.mrfmmm.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusconi A, Repetto O, Bona E, Massa N, Gallo C, Dumas-Gaudot E, Berta G. Effects of cadmium on meristem activity and nucleus ploidy in roots of Pisum sativum L. cv Frisson seedlings. Environ Exp Bot. 2006;58:253–260. doi: 10.1016/j.envexpbot.2005.09.008. [DOI] [Google Scholar]
- Fusconi A, Gallo C, Camusso W. Effects of cadmium on root apical meristem of Pisum sativum L.: cell viability, cell proliferation and microtubule pattern as suitable markers for assessment of stress pollution. Mutat Res Genet Toxicol Environ Mutagen. 2007;632:9–19. doi: 10.1016/j.mrgentox.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Gichner T, Patkova Z, Szakova J, Demnerova K. Cadmium induces DNA damage in tobacco roots, but no DNA damage, somatic mutations or homologous recombination in tobacco leaves. Mutat Res. 2004;559:49–57. doi: 10.1016/j.mrgentox.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Guo B, Liang Y, Zhu Y. Does salicylic acid regulate antioxidant defense system, cell death, cadmium uptake and partitioning to acquire cadmium tolerance in rice? J Plant Physiol. 2009;166:20–31. doi: 10.1016/j.jplph.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Gupta DK, Palma JM, Corpas FJ. Reactive oxygen species and oxidative damage in plants under stress. Berlin: Springer; 2015. pp. 1–22. [Google Scholar]
- Hernández JA, Almansa MS. Short-term effects of salt stress on antioxidant systems and leaf water relations of pea plants. Physiol Plant. 2002;115:251–257. doi: 10.1034/j.1399-3054.2002.1150211.x. [DOI] [PubMed] [Google Scholar]
- Heydecker W, Coolbear P. Seed treatments for improved performance survey and attempted prognosis. Seed Sci Technol. 1977;5:353–425. [Google Scholar]
- Jan I, Rab A, Sajid M. Influence of calcium chloride on storability and quality of apple fruits. Pak J Agric Sci. 2015;52:115–122. [Google Scholar]
- Karabal E, Yucel M, Oktem HA. Antioxidant responses of tolerant and sensitive barley cultivars to boron toxicity. Plant Sci. 2003;164:925–933. doi: 10.1016/S0168-9452(03)00067-0. [DOI] [Google Scholar]
- Klein CB, Leszczynska J, Hickey TC, Rossman T. Further evidence against a direct genotoxic mode of action for arsenic induced cancer. Toxicol Appl Pharmacol. 2007;222:289–297. doi: 10.1016/j.taap.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Di Z, Han X, Yang X. Elevated CO2 improves root growth and cadmium accumulation in the hyperaccumulator Sedum alfredii. Plant Soil. 2012;354:325–334. doi: 10.1007/s11104-011-1068-4. [DOI] [Google Scholar]
- Lux A. Does diversity in root structure affect the diversity in cadmium uptake by plants? Opinion paper. Agrochimica. 2010;54:342–352. [Google Scholar]
- Lux A, Vaculík M, Martinka M, Lišková D, Kulkarni MG, Stirk WA, Van Staden J. Cadmium induces hypodermal periderm formation in the roots of the monocotyledonous medicinal plant Merwilla plumbea. Ann Bot. 2011;107:285–292. doi: 10.1093/aob/mcq240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Baskin TI, Brown KM, Lynch JP. Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness. Plant Physiol. 2003;131:1381–1390. doi: 10.1104/pp.012161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madany M, Khalil R. Seed priming with ascorbic acid or calcium chloride mitigates the adverse effects of drought stress in sunflower (Helianthus annuus L.) seedlings. Egypt J Exp Biol (Bot) 2017;13:119–133. [Google Scholar]
- Mahoney NM, Goshima G, Douglass AD, Vale RD. Making microtubules and mitotic spindles in cells without functional centrosomes. Curr Biol. 2006;16:564–569. doi: 10.1016/j.cub.2006.01.053. [DOI] [PubMed] [Google Scholar]
- Malgorzata K, Olga FS, Katarzyna G, Agnieszka W, Jan S. CaCl2 treatment improves drought stress tolerance in barley (Hordeum vulgare L.) Acta Physiol Plant. 2017;39:41. doi: 10.1007/s11738-016-2336-y. [DOI] [Google Scholar]
- Metcalfe D, Schmitz A, Pelka RJ. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem. 1966;38:524–535. doi: 10.1021/ac60235a044. [DOI] [Google Scholar]
- Mondal NK. Effect of varying cadmium stress on chickpea (Cicer arietinum L.) seedlings: an ultrastructural study. Ann Environ Sci. 2013;7:59–70. [Google Scholar]
- Moussa HR, El-Gamal SM. Effect of salicylic acid pretreatment on cadmium toxicity in wheat. Biol Plant. 2010;54:315–320. doi: 10.1007/s10535-010-0054-7. [DOI] [Google Scholar]
- Nguyen QT, Kisiala A, Andreas P, Neil Emery RJ, Narine S. Soybean seed development: fatty acid and phytohormone metabolism and their interactions. Curr Genom. 2016;17:241–260. doi: 10.2174/1389202917666160202220238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocito FF, Lancilli C, Giacomini B, Sacchi GA. Sulfur metabolism and cadmium stress in higher plants. Plant Stress. 2007;1:142–156. [Google Scholar]
- Nouairi I, Ammar WB, Youssef NB, Daoud DBM, Ghorbal MH, Zarrouk M. Comparative study of cadmium effects on membrane lipid composition of Brassica juncea and Brassica napus leaves. Plant Sci. 2006;170:511–519. doi: 10.1016/j.plantsci.2005.10.003. [DOI] [Google Scholar]
- Parvaiz A, Arafet AAL, Elsayed FAA, Abeer H, Maryam S, Naser AA, Salih G. Calcium and potassium supplementation enhanced growth, osmolyte secondary metabolite production, and enzymatic antioxidant machinery in cadmium-exposed chickpea (Cicer arietinum L.) Front Plant Sci. 2016;7:513. doi: 10.3389/fpls.2016.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlolla A, Tchounwou P. Cytogenetic evaluation of arsenic trioxide in Sprague-Dawley rats. Mutat Res. 2005;587:126–133. doi: 10.1016/j.mrgentox.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Patrick L. Toxic metals and antioxidants: part II. The role of antioxidants in arsenic and cadmium toxicity. Altern Med Rev. 2003;8:106–128. [PubMed] [Google Scholar]
- Rahoui S, Chaoui A, El Ferjani E. Membrane damage and solute leakage from germinating pea seed under cadmium stress. J Hazard Mater. 2010;178:1128–1131. doi: 10.1016/j.jhazmat.2010.01.115. [DOI] [PubMed] [Google Scholar]
- Rajendra P, Sujatha NH, Sashidkar RB, Subramanyam C, Davendranath D, Gunasekaran B, Aradhya RSS, Bhaskaran A. Effects of power frequency electromagnetic fields on growth of germinating Vicia faba L., the broad bean. Electromagn Biol Med. 2005;24:39–54. doi: 10.1081/JBC-200055058. [DOI] [Google Scholar]
- Ranty B, Aldon D, Cotelle V, Galaud JP, Thuleau P, Mazars C. Calcium sensors as key hubs in plant responses to biotic and abiotic stresses. Front Plant Sci. 2016;7:327. doi: 10.3389/fpls.2016.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentel MC, Knight MR. Oxidative stress-induced calcium signaling in Arabidopsis. Plant Physiol. 2004;135:1471–1479. doi: 10.1104/pp.104.042663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gómez M, Del Río LA, Sandalio LM. Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots: imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ. 2006;29:1532–1544. doi: 10.1111/j.1365-3040.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Schützendübel A, Schwanz P, Teichmann T, Langenfeld-Heyser GK, Godbold DL, Polle A. Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in scots pine roots. Plant Physiol. 2001;127:887–898. doi: 10.1104/pp.010318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne M, Rajakaruna N, Rizwan M, Madawala HMSP, Ok YS, Vithanage M. Heavy metal-induced oxidative stress on seed germination and seedling development: a critical review. Environ Geochem Health. 2017 doi: 10.1007/s10653-017-0005-8. [DOI] [PubMed] [Google Scholar]
- Sergiev L, Alexieva E, Karanov E. Effect of spermine, atrazine and combination between them on some endogenous protective systems and markers in plants. C R Acad Bulg Sci. 1997;51:121–124. [Google Scholar]
- Seth CS, Misraa V, Chauhan LKS, Singh RR. Genotoxicity of cadmium on root meristem cells of Allium cepa: cytogenetic and comet assay approach. Ecotoxicol Environ Saf. 2008;71:711–716. doi: 10.1016/j.ecoenv.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012;2012:e217037. [Google Scholar]
- Sharma SS, Dietz KJ, Mimura T. Vacuolar compartmentalization as indispensable component of heavy metal detoxiification in plants. Plant Cell Environ. 2016;39:1112–1126. doi: 10.1111/pce.12706. [DOI] [PubMed] [Google Scholar]
- Shi Q, Wang J, Zou J, Jiang Z, Wu H, Wang J, Jiang W, Liu D. Cadmium localization and its toxic effects on root tips of barley. Zemdirbyste Agric. 2016;103:151–158. doi: 10.13080/z-a.2016.103.020. [DOI] [Google Scholar]
- Singh A, Prasad SM. Effect of agro-industrial waste amendment on Cd uptake in Amaranthus caudatus grown under contaminated soil: an oxidative biomarker response. Ecotoxicol Environ Saf. 2014;100:105–113. doi: 10.1016/j.ecoenv.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Singh GS, Tuteja N. Cadmium stress tolerance in crop plants: probing the role of sulfur. Plant Signal Behav. 2011;6:215–222. doi: 10.4161/psb.6.2.14880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh HP, Batish DR, Kaur G, Arora K, Kohli RK. Nitric oxide (as sodium nitroprusside) supplementation ameliorates Cd toxicity in hydroponically grown wheat roots. Environ Exp Bot. 2008;63:158–167. doi: 10.1016/j.envexpbot.2007.12.005. [DOI] [Google Scholar]
- Steinkellner H, Mun-Sik K, Helma C, Ecker S, Ma TH, Horak O, Kundi M, Knasmuller S. Genotoxic effects of heavy metals: comparative investigation with plant bioassays. Environ Mol Mutag. 1998;31:183–191. doi: 10.1002/(SICI)1098-2280(1998)31:2<183::AID-EM11>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Tajti J, Janda T, Majláth I, Szalai G, Pál M. Comparative study on the effects of putrescine and spermidine pretreatment on cadmium stress in wheat. Ecotoxicol Environ Saf. 2018;148:546–554. doi: 10.1016/j.ecoenv.2017.10.068. [DOI] [PubMed] [Google Scholar]
- Tamimi MS. Effect of seed priming on growth and physiological traits of five Jordanian wheat (Triticum aestivum L.) landraces under salt stress. J Biosci Agric Res. 2016;11:906–922. doi: 10.18801/jbar.110116.111. [DOI] [Google Scholar]
- Upadhyaya H, Panda SK, Dutta BK. CaCl2 improves post-drought recovery potential in Camellia sinensis (L) O. Kuntze. Plant Cell Rep. 2011;30:495–503. doi: 10.1007/s00299-010-0958-x. [DOI] [PubMed] [Google Scholar]
- Urbanek H, Kuzniak-Gebarowska E, Herka K. Elicitation of defense responses in bean leaves by Botrytis cinerea polygalacturonase. Acta Phys Plant. 1991;13:43–50. [Google Scholar]
- Vig K, Megharaj M, Sethunathan N, Naidu R. Bioavailability and toxicity of cadmium to microorganisms and their activities in soil: a review. Adv Environ Res. 2003;8:121–135. doi: 10.1016/S1093-0191(02)00135-1. [DOI] [Google Scholar]
- Vitória AP, Lea PJ, Azevedo RA. Antioxidant enzymes responses to cadmium in radish tissues. Phytochemistry. 2001;57:710–715. doi: 10.1016/S0031-9422(01)00130-3. [DOI] [PubMed] [Google Scholar]
- Wan G, Najeeb U, Jilani G, Naeemand MS, Zhou W. Calcium invigorates the cadmium-stressed Brassica napus L. plants by strengthening their photosynthetic system. Environ Sci Pollut Res Int. 2011;18:1478–1486. doi: 10.1007/s11356-011-0509-1. [DOI] [PubMed] [Google Scholar]
- Wang Y, Qian Y, Hu H, Xu Y, Zhang H. Comparative proteomic analysis of Cd-responsive proteins in wheat roots. Acta Physiol Plant. 2011;33:349–357. doi: 10.1007/s11738-010-0554-2. [DOI] [Google Scholar]
- Xu J, Yin HX, Li YL, Liu XJ. Nitric oxide is associated with long-term zinc tolerance in Solanum nigrum. Plant Physiol. 2010;154:1319–1334. doi: 10.1104/pp.110.162982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoubi R, Job C, Belghazi M, Chaibi W, Job D. Proteomic analysis of the enhancement of seed vigour in osmoprimed alfalfa seeds germinated under salinity stress. Seed Sci Res. 2013;23:99–110. doi: 10.1017/S0960258513000093. [DOI] [Google Scholar]
- Zhang Y, Yang X. The toxic effects of cadmium on cell division and chromosomal morphology of Hordeum vulgare. Mutat Res. 1994;312:121–126. doi: 10.1016/0165-1161(94)90016-7. [DOI] [PubMed] [Google Scholar]