Abstract
Objective
Handgrip strength (HGS) exercise has been reported to reduce blood pressure in both hypertensive and normotensive patients. In this study, we evaluated the association of HGS with hypertension in a Chinese Han Population.
Methods
A total of 11,151 subjects mainly consisting of a rural population were recruited with a multi-stage sampling method in Jurong city, Jiangsu Province, China. Besides hypertension and diabetes, major chronic diseases were excluded. HGS was categorized into tertiles by age and gender. Logistic regression was used to estimate the association of HGS and hypertension with the odds ratio (OR) and 95% confidence interval (CI).
Results
From low to high tertiles of HGS, diastolic blood pressure (DBP) was significantly increased (74.52 ± 7.39, 74.70 ± 7.03, and 75.54 ± 7.01 mmHg, respectively; Ptrend = 0.001), as well as in females (Ptrend=0.003). The differences in DBP among the tertiles of HGS were still significant in females even after adjusting for covariates (Ptrend=0.048). No significant differences in systolic blood pressure (SBP) were observed among the tertiles of HGS (P>0.05). Compared to low HGS, high HGS was significantly associated with hypertension after adjustment for age and gender (adjusted OR, 1.19; 95% CI, 1.06–1.34; P =0.004). A stratified analysis showed that the significant association of high HGS and hypertension was also observed with the following factors even after adjusting for age and gender: female gender (adjusted OR, 1.25; 95% CI, 1.08–1.46; P=0.004), ages of 60–69 years (adjusted OR, 1.29; 95% CI, 1.06–1.57; P=0.011), and married (adjusted OR, 1.20; 95% CI, 1.06–1.37; P=0.005). However, no significant associations were found after adjusting for age, gender, smoking status, drinking status, body mass index, physical activity level, glucose, high- and low-density lipoprotein cholesterol, total cholesterol, and triglyceride (P>0.05).
Conclusion
The findings of the current study suggest that HGS was positively correlated with DBP in a rural population, and high HGS was associated with hypertension in females; however, the association may be modified by smoking status, drinking status, body mass index, physical activity, cholesterol level, and glucose level. Further utilization of HGS exercises to intervene in the development and prognosis of hypertension should be verified in the future.
Keywords: Handgrip strength, Hypertension, Blood pressure, Prevalence
Introduction
Hypertension, defined as the presence of a systolic blood pressure (SBP) level ≥140 mmHg and a diastolic blood pressure (DBP) level ≥90 mmHg, is a highly preventable disorder of epidemic proportions worldwide.1, 2 As one of the main causes of cardiovascular disease (CVD), hypertension accounts for ten million deaths every year,3 contributing to the heavy burden of chronic cardiovascular diseases globally.4, 5 Hypertension is more prevalent in the older population and is closely related to behavioral risk factors, such as an unbalanced diet, excess weight, tobacco consumption, alcohol use, persistent exposure to tension or stress, physical inactivity, as well as the presence of diabetes mellitus and high cholesterol levels. The prevention and management of hypertension include early detection and treatment, and minimizing behavioral risk factors.6
International and national guidelines for prevention of hypertension recommend physical activity of moderate intensity for at least 150 minutes per week, or physical activity of vigorous intensity for 75 minutes per week.7, 8 The handgrip strength (HGS) is a fast and low-cost test used for evaluation of an individual's muscle strength.9, 10 It has been reported that isometric resistance exercise is an ideal and effective exercise method to reduce the blood pressure (BP) levels in individuals with normal and high BP.11, 12 In contrast, in the elderly, a higher HGS was observed to be associated with elevated BP levels.13
Previous randomized controlled trials with small sample sizes have found that a six-to ten-week isometric handgrip training could help reduce either the SBP or DBP by 5%–10%.14, 15 However, isometric handgrip training performed for a short time or at low intensity did not induce a transient reduction in BP levels.16, 17 Fernandez et al18 suggested that the effect of the high HGS was mediated by the body mass index (BMI). However, Dong et al19 found that even after adjustment for BMI, individuals with higher HGS usually had higher, rather than lower BP levels.19
The relationship between the HGS and the prevalence of hypertension remains to be further elucidated. The primary aim of this study was to evaluate the association of the HGS and the prevalence of hypertension in a Chinese Han population.
Methods
Subjects
A community-based, cross-sectional observational study was carried out using a multi-stage sampling method in 13 townships of Jurong city, Jiangsu Province, in the south of China. A total of 11,151 subjects, aged ≥18 years, were recruited from October to November 2015. The participants were permanent residents for the duration of the study. Over 80% of the subjects were rural residents. Participants with any of the following conditions were excluded from the study: chronic heart disease, stroke, cancer, viral hepatitis, fatty liver disease, gastrointestinal disease, rheumatic arthritis, chronic kidney disease, or chronic obstructive pulmonary disease. The exclusion criteria were based on the presence of a condition, a serious physical or mental illness that would prevent the obtainment of anthropometric measurements. Finally, 7752 participants were included in this study (Fig. 1).
Fig. 1.
Flow chart of the participant inclusion in this cross-sectional study. The HGS data of ninety-six patients with chronic diseases were missing.
All participants were interviewed using a predesigned and pretested questionnaire regarding their demographic information, smoking and drinking habits, educational level, physical activity (PA), BP level, history of chronic diseases and mental health conditions, and anthropometric measurements (height and weight).
We organized a comprehensive training workshop for the staff members who collected the data, and we explained the interview techniques, practical applications, data collection tools, and the area guidelines. The study protocol was approved by the Research Ethics Committee of Nanjing Medical University (NMU03307). Written informed consent was obtained from all subjects during the interviews.
Measurement and definition
A standard face-to-face questionnaire was used to collect socio-demographic information: age, gender, marital status, educational level, smoking and drinking habits, as well as PA. Anthropometric indices, including weight (kg) and height (cm), were measured by the trained staff using standard devices. Blood samples were drawn to analyze the levels of high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), serum total cholesterol (TC), serum triglyceride (TG), and glucose (GLU) after 8 hours of overnight fasting. The blood cold chain was maintained at −20 °C. All blood samples were tested using Cobas® 6000 analyzer (Roche Diagnostics GmbH, Ibaraki-ken, Japan).
A digital sphygmomanometer (HEM-7207, OMRON Co. Ltd., Dalian, Liaoning, China) was used for BP measurement. Before the measurement, the individuals were required to sit still, silently, for 4–6 minutes. Three measurements were recorded at intervals of 30 seconds on the right arm, and the mean values of SBP and DBP were calculated. Hypertension was defined as an averaged BP level ≥140/90 mmHg, or a history of hypertension or taking antihypertensive medicine.
Weight was measured using a Libra weighing machine with closeness of 0.5 kg, and height was recorded using a steel anthropometry rod with closeness of 0.2 cm. BMI was calculated as the ratio of the weight (kg) to squared height (m2). Based on the obtained BMI, the subjects were classified into two groups: non-obese (BMI <24), and obese group (BMI ≥24). The participants who were underweight (<18.5 kg/m2 (n = 132) were very low in number, so they were merged into the non-obese group.
HGS test was used to evaluate the HGS, as an indicator of muscle strength, according to the instructions of the Institute of Medicine.20 HGS was measured using a CAMPY electronic hand dynamometer (EH101, CAMRY, Zhongshan, Guangdong, China), according to a standard procedure.11, 21, 22 Subjects were required to squeeze the dynamometer as hard as they could for more than 2 seconds; the results were recorded in kilograms. The HGS was categorized into tertiles, as low, moderate, and high HGS, in each age group of males and females separately.
Information on physical activity during the past years was collected using the Global Physical Activity Questionnaire (GPAQ).23, 24, 25 The weekly physical activity categories (domestic, gardening, sedentary, and work activities), and the corresponding time were determined using the questionnaire. The total metabolic equivalent (MET), expressed in minutes per week, was summed for the four categories of physical activity and categorized into tertiles, as low, moderate, and high physical activity.
Statistical analysis
The probability proportional to size (PPS) sampling method was used. Quantitative variables with normal distribution were expressed as mean ± standard deviation, and the differences between the groups were assessed with one-way analysis of variance (ANOVA). The frequencies of the categorical variables were compared using the Chi-squared (χ2) test. The Pearson's correlation test was applied to estimate the relationship of SBP and DBP with HGS. Logistic regression analysis was used to estimate the association between HGS and the prevalence of hypertension, with odds ratio (OR) and 95% confidence intervals (CIs), as well as adjustment for confounding factors. The SPSS software for Windows, version 24.0 (IBM Corporation, Armonk, New York, USA) was used for statistical analysis. Two-tailed P value < 0.05 was considered statistically significant.
Results
Characteristics of the participants
The socio-demographic characteristics of the study subjects are presented in Table 1. The study sample consisted of 7752 individuals aged ≥18 years; of those, 2984 were male and 4768 were female. The mean age of the subjects in the low, moderate, and high HGS tertiles was 59.6 ± 11.7, 59.2 ± 11.3, and 58.8 ± 11.1 years, respectively. The age, marital status, educational level, smoking and drinking habits, BMI, PA, and presence of hypertension, as well as the TG level, were significantly different (P < 0.05) between the HGS tertiles (Table 1).
Table 1.
Socio-demographic characteristics of study participants in different handgrip strength tertiles (n = 7752).
| Characteristics | Handgrip strength |
Statistic values | P-value | ||
|---|---|---|---|---|---|
| Low (n = 2611) | Moderate (n = 2575) | High (n = 2566) | |||
| Age, years | 59.6 ± 11.7 | 59.2 ± 11.3 | 58.8 ± 11.1 | 3.357a | 0.035 |
| Age groups, n (%) | |||||
| 18–49 years | 526 (20.1) | 552 (21.4) | 591 (23.0) | 27.499b | 0.015 |
| 50–59 years | 689 (26.4) | 699 (27.1) | 667 (26.0) | ||
| 60–69 years | 909 (34.8) | 898 (34.9) | 955 (37.2) | ||
| 70 years or above | 487 (18.7) | 426 (16.5) | 353 (13.8) | ||
| Gender, n (%) | |||||
| Men | 1003 (38.4) | 994 (38.6) | 987 (38.5) | 0.021b | 0.990 |
| Women | 1608 (61.6) | 1581 (61.4) | 1579 (61.5) | ||
| Marital status, n (%) | |||||
| Single | 76 (2.9) | 43 (1.7) | 23 (0.9) | 32.920b | <0.001 |
| Married | 2192 (84.0) | 2207 (85.7) | 2241 (87.3) | ||
| Deceased/divorced | 343 (13.1) | 325 (12.6) | 302 (11.8) | ||
| Educational level, n (%) | |||||
| Illiterate | 898 (34.4) | 716 (27.8) | 670 (26.1) | 69.367b | <0.001 |
| Primary | 824 (31.6) | 806 (31.3) | 773 (30.1) | ||
| Middle | 674 (25.8) | 787 (30.6) | 821 (32.0) | ||
| High school and above | 215 (8.2) | 266 (10.3) | 302 (11.8) | ||
| Smoking, n (%) | |||||
| Non-Smoking | 2073 (79.4) | 1961 (76.2) | 1973 (76.9) | 8.590b | 0.014 |
| Smoking | 538 (20.6) | 614 (23.8) | 593 (23.1) | ||
| Drinking, n (%) | |||||
| Non-Drinking | 1935 (74.1) | 1863 (72.3) | 1753 (68.3) | 22.407b | <0.001 |
| Drinking | 676 (25.9) | 712 (27.7) | 813 (31.7) | ||
| BMI, n (%) | |||||
| Non-obese | 1508 (57.8) | 1444 (56.1) | 1172 (45.7) | 88.701b | <0.001 |
| Obese | 1103 (42.2) | 1131 (43.9) | 1394 (54.3) | ||
| Physical activity level, n (%) | |||||
| Low | 1101 (35.7) | 721 (32.4) | 789 (32.3) | 14.330b | 0.006 |
| Moderate | 1012 (32.8) | 718 (32.3) | 845 (34.6) | ||
| High | 970 (31.5) | 787 (35.4) | 809 (33.1) | ||
| Cholesterol level, mmol/L | |||||
| HDL-C | 1.5 ± 0.4 | 1.5 ± 0.4 | 1.5 ± 0.5 | 11.500a | 0.414 |
| LDL-C | 2.9 ± 0.8 | 2.9 ± 0.77 | 2.8 ± 0.8 | 13.439a | 0.157 |
| TC | 5.1 ± 0.9 | 5.1 ± 0.9 | 5.1 ± 0.9 | 9.268a | 0.229 |
| TG | 1.6 ± 1.1 | 1.6 ± 1.2 | 1.7 ± 1.5 | 6.909a | 0.008 |
| Glucose, mmol/L | 6.2 ± 2.0 | 6.0 ± 1.7 | 6.1 ± 1.8 | 2.454a | 0.100 |
| Hypertension, n (%) | |||||
| No | 1104 (42.3) | 1112 (43.2) | 1018 (39.7) | 4.369b | 0.030 |
| Yes | 1507 (57.7) | 1463 (56.8) | 1548 (60.3) | ||
Data are presented as mean ± SD or count (%). BMI: body mass index; HDL-C: high density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol; TC: total cholesterol; TG: triglyceride.
aF value. b χ2 value.
Correlation analysis of HGS and the BP
In the normotensives, the average values of SBP in the subjects of the low, moderate, and high HGS tertiles were 123.03 ± 10.22 mmHg, 123.02 ± 9.87 mmHg, and 123.60 ± 9.71 mmHg, respectively, without statistically significant difference (P>0.05). As observed from the low to the high HGS tertile, the subjects’ DBP level significantly increased (74.52 ± 7.39 mmHg, 74.70 ± 7.03 mmHg and 75.54 ± 7.01 mmHg, respectively, with P trend = 0.001), as well as in female subjects (Ptrend =0.003) (Table S1 in the Supplementary Appendix). After adjustment for age, gender, smoking and drinking habits, BMI, PA, and levels of GLU, HDL-C, LDL-C, TC, and TG, the differences in DBP level between the subjects of the HGS tertiles remained significant in females, which was not the case for those in SBP level (Table 2).
Table 2.
Correlation between SBP, DBP and levels of handgrip strength in the normotensives (n = 3234).
| Population | Blood pressure | Handgrip strength tertiles |
Fa | P | ||
|---|---|---|---|---|---|---|
| Low | Moderate | High | ||||
| All | SBP, mmHg | 123.03 ± 10.22 | 123.02 ± 9.87 | 123.60 ± 9.71 | 0.137 | 0.872 |
| DBP, mmHg | 74.52 ± 7.39 | 74.70 ± 7.03 | 75.54 ± 7.01 | 1.605 | 0.201 | |
| Male | SBP, mmHg | 126.49 ± 8.96 | 125.13 ± 9.20 | 125.82 ± 8.36 | 2.186 | 0.113 |
| DBP, mmHg | 76.84 ± 7.38 | 75.54 ± 7.16 | 77.60 ± 6.79 | 6.183 | 0.002 | |
| Female | SBP, mmHg | 121.47 ± 10.37 | 121.99 ± 10.02 | 122.55 ± 10.13 | 0.952 | 0.386 |
| DBP, mmHg | 73.49 ± 7.16 | 74.29 ± 6.92 | 74.56 ± 6.90 | 3.033 | 0.048b | |
Data were presented as mean ± standard deviation. SBP: systolic blood pressure; DBP: diastolic blood pressure.
The univariate analysis of variance was used to compare the means of SBP and DBP among handgrip strength tertiles after adjustment for age, gender, smoking, drinking, body mass index, physical activity, fasting glucose level, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, total cholesterol, and triglyceride.
Ptrend of linear trend test.
Association analysis of HGS and the prevalence of hypertension
High HGS values were positively associated with an increased risk of hypertension in female subjects (OR: 1.16, 95% CI: 1.01–1.34, P = 0.033); however, this was not the case in male subjects (OR: 1.04, 95% CI: 0.86–1.25, P = 0.718).
After adjustment for age and gender (model 1), the adjusted OR (95% CI) of high HGS values and the prevalence of hypertension was 1.19 (1.06–1.34), with P value of 0.004. The association was confirmed in female subjects (OR: 1.25, 95% CI: 1.08–1.46, P = 0.004), but not in males. However, after an adjustment for smoking and drinking habits, BMI, and PA was added to model 1 (model 2), there was no significant association between the high HGS values and the prevalence of hypertension, both in male and female subjects. In addition, after an adjustment for levels of GLU, HDL-C, LDL-C, TC, and TG was added to model 2 (model 3), no significant association was found between the high HGS values and the prevalence of hypertension, both in male and female subjects (Table 3).
Table 3.
Association between handgrip strength and risk of hypertension.
| Population | Handgrip strength | Unadjusted |
Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||
| Entire | Low (n = 2611) | Ref | Ref | Ref | Ref | ||||
| Moderate (n = 2575) | 0.96 (0.86–1.08) | 0.512 | 0.98 (0.87–1.11) | 0.770 | 0.96 (0.85–1.08) | 0.515 | 0.97 (0.86–1.10) | 0.693 | |
| High (n = 2566) | 1.11 (1.00–1.24) | 0.056 | 1.19 (1.06–1.34) | 0.004 | 1.02 (0.90–1.15) | 0.768 | 1.03 (0.91–1.17) | 0.653 | |
| Male | Low (n = 997) | Ref | Ref | Ref | Ref | ||||
| Moderate (n = 986) | 0.89 (0.74–1.07) | 0.221 | 0.92 (0.76–1.12) | 0.398 | 0.92 (0.76–1.12) | 0.408 | 0.93 (0.76–1.14) | 0.504 | |
| High (n = 977) | 1.04 (0.86–1.25) | 0.718 | 1.10 (0.91–1.34) | 0.331 | 0.88 (0.72–1.08) | 0.213 | 0.89 (0.73–1.10) | 0.307 | |
| Female | Low (n = 1597) | Ref | Ref | Ref | Ref | ||||
| Moderate (n = 1569) | 1.01 (0.88–1.16) | 0.937 | 1.02 (0.87–1.18) | 0.817 | 0.99 (0.84–1.15) | 0.877 | 1.00 (0.86–1.18) | 0.946 | |
| High (n = 1564) | 1.16 (1.01–1.34) | 0.033 | 1.25 (1.08–1.46) | 0.004 | 1.12 (0.96–1.31) | 0.147 | 1.13 (0.96–1.33) | 0.129 |
OR: odd ratio; CI: confidence interval; Ref: reference.
Model 1: Adjusted for age and gender. Model 2: Adjusted for smoking, drinking, body mass index, physical activity and the covariates in model 1. Model 3: Adjusted for fasting glucose level, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, total cholesterol, triglyceride and the covariates in model 2.
Stratification analysis of HGS and the prevalence of hypertension
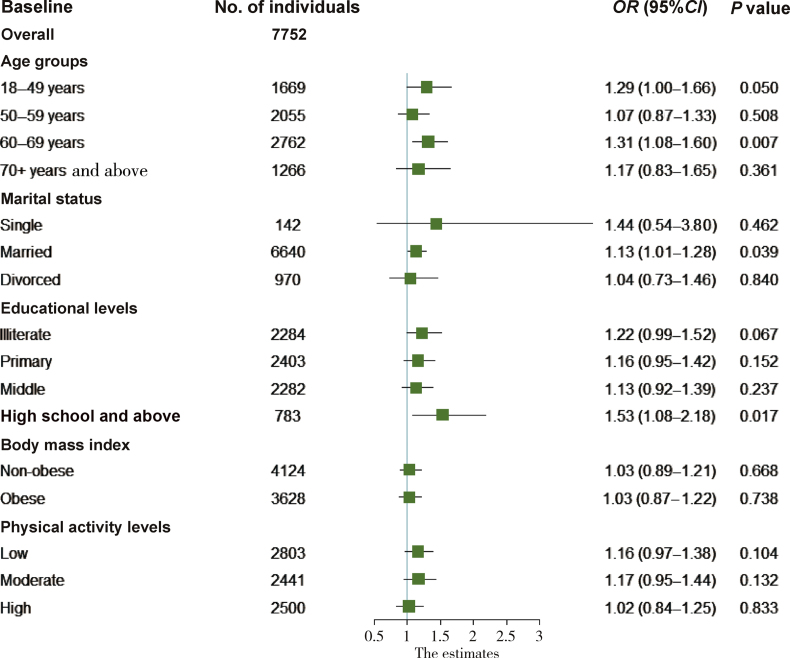
In the age groups 18–49 and 60–69 years, the high HGS values were positively associated with the prevalence of hypertension; the crude ORs (95% CIs) were 1.29 (1.00–1.66) and 1.31 (1.08–1.60), respectively, with P values less than 0.05. This association was also observed in the groups of married subjects and those with an education level higher than high school; the crude ORs (95% CIs) were 1.13 (1.01–1.28) and 1.53 (1.08–2.18), respectively, with P values of 0.039 and 0.017, respectively (Fig. 2).
Fig. 2.
Stratified analysis of the association of high HGS values with the prevalence of hypertension by age, marital status, educational level, body mass index (BMI), and physical activity level. Logistic regression analysis was used to estimate the crude OR (95% CI).
After adjustment for age and gender, the association of high HGS values with the prevalence of hypertension remained statistically significant in the age group of 60–69 years and the group of married subjects (OR:1.29, 95% CI: 1.06–1.57, P = 0.011; and OR:1.20, 95% CI: 1.06–1.37, P = 0.005, respectively) (model 1). After further adjustment for confounders in model 2 and model 3, there was no significant association observed in the abovementioned groups (Table S2 in the Supplementary Appendix).
Furthermore, no significant association of moderate HGS values with the prevalence of hypertension was found in any of the groups of age, educational level, marital status, BMI and physical activity (Fig. 3), even after adjustment for all covariates (age, gender, smoking and drinking habits, BMI, PA, and levels of GLU, HDL-C, LDL-C, TC, and TG) (Table S2 in the Supplementary Appendix).
Fig. 3.
Stratified analysis of the association of moderate HGS values with the prevalence of hypertension by age, marital status, educational level, body mass index (BMI), and physical activity level. Logistic regression analysis was used to estimate the crude OR (95% CI).
Discussion
In this study, the results indicated that the HGS was positively correlated with the DBP, but not with the SBP. A cross-sectional study of a population of 4597 subjects also showed that the HGS was positively correlated with the DBP26; however, in that study it was observed that the HGS was also significantly associated with the SBP. Another cross-sectional study in China, with a population consisting of 89,655 students, aged 13–17 years, revealed that higher HGS values were associated with an increased BP.19 Furthermore, a third cross-sectional study described that high HGS values were associated with a higher BP in the elderly, but not in young people.13
This study observed that the crude association of high HGS values with the prevalence of hypertension was significant particularly in female subjects, even after adjustment for age and gender. However, after adjustment for more confounding factors, the association was not replicated. Most of the confounding factors adjusted for in this study are risk factors of hypertension; thus, further adjustment for these covariates is necessary and should be done cautiously. Nonetheless, the results of this study might imply that the association of HGS and the prevalence of hypertension might be modified by the smoking and drinking habits, BMI, PA, and the levels of cholesterol and GLU. Furthermore, the results of this study need to be further verified in a cohort study.
Although several interventional studies have been performed, their results were not consistent. Millar et al27 observed that isometric exercise training plays a vital role in reducing the BP in individuals with normal and elevated BP. Several randomized controlled trials found that a more intense isometric HGS exercise was significantly associated with decreased BP.14, 15, 28, 29 However, another randomized controlled trial did not support the beneficial effects of isometric HGS exercise on the BP level.16
The results of this study showed that after further stratified analysis by age, marital status, educational level, BMI and PA, and after adjustment for age and gender, the high HGS values were associated with the prevalence of hypertension in the subjects of the age group of 60–69 years; however, this association was not found in elderly people, aged above 70 years. Muscle mass loss, commonly known as sarcopenia, has often been reported with aging. In the elderly, sarcopenia is a major risk factor of strength loss.30, 31, 32, 33, 34 The loss in muscle strength would affect the physical performance, functional status, and mobility, which would increase the risk of impairment occurrence, hypertension development, cardiovascular disease development, as well as death. The decrease in muscle strength occurs more rapidly than the loss of the muscle tissue or mass. The muscle strength might not be retained by maintaining or increasing the muscle mass.35 The inability to perform physical activity is one of the major risk factors for loss of muscle strength.36, 37, 38 In contrast, high HGS might be linked to heavy or aggressive labor in the rural population, and that would increase the risk of hypertension. In addition, the association between high HGS and the prevalence of hypertension was observed in the group of married subjects, which might be due to the greater level of physical activity in married people, performing many household assignments, and to the greater work-related pressure than that in single or divorced people.
Although this study was performed in a relatively large sample, there are some limitations that need to be discussed. First, the association between HGS and the prevalence of hypertension might not be clear, as this is a cross-sectional study. Further research through a prospective study would be warranted. Second, for establishing a new diagnosis of hypertension, three measures on three separate days are required. This study included the self-reported history of hypertension and three measures in one day; thus, the prevalence of hypertension might be overestimated. Third, the muscle quantity/size, which is an adequate factor for overall evaluation of HGS, was not measured in this study. Fourth, our study population had a higher proportion of older people; hence, the muscle strength in the older participants might have been lower than that in the younger participants. Despite these limitations, the initial findings of this study would warrant further research to verify the relationship between HGS and the risk of hypertension.
Conclusions
In conclusion, the current study revealed that the HGS was positively correlated with the DBP, and that high HGS values were associated with the prevalence of hypertension in female subjects; however, this association might be modified by the smoking and drinking habits, BMI, PA, and the levels of cholesterol and GLU. Further studies are needed to verify whether the HGS, an easily measurable physical fitness index, can be utilized to assess the development and prognosis of hypertension.
Conflicts of interest
The authors declare no actual or potential conflicts of interest.
Acknowledgements
This work was supported by grants from the Social Development Guidance Project of Zhenjiang (FZ2015064), the Livelihood Science and Technology Plan Project of Science and Technology Bureau in Jurong (SF2016896226), the National Natural Science Foundation of China (81573232), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We would like to thank Hailong Zhao, Lijun Zhu, Ting Zhang, Jinbo Wen and Zhengmei Fang for quality control and the staffs for working on data collecting and sorting. We would like to thank Ye Tong for helping to proofread the article.
Edited by Pei-Fang Wei
Footnotes
Peer review under responsibility of Chinese Medical Association.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cdtm.2019.05.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Pescatello L.S., Franklin B.A., Fagard R. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian A.V., Bakris G.L., Black H.R. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization A Global Brief on Hypertension. Silent Killer, Global Public Health Crisis. https://www.who.int/cardiovascular_diseases/publications/global_brief_hypertension/en/
- 4.Forouzanfar M.H., Liu P., Roth G.A. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317:165–182. doi: 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- 5.Lim S.S., Vos T., Flaxman A.D. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brook R.D., Appel L.J., Rubenfire M. Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the american heart association. Hypertension. 2013;61:1360–1383. doi: 10.1161/HYP.0b013e318293645f. [DOI] [PubMed] [Google Scholar]
- 7.Mansia G., De Backer G., Dominiczak A. 2007 ESH-ESC Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Blood Press. 2007;16:135–232. doi: 10.1080/08037050701461084. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of Health and Human Services . Department of Health and Human Services; Washington, DC: 2008. 2008 Physical Activity Guidelines for Americans; Be Active, Healthy, and Happy. [Google Scholar]
- 9.Artero E.G., Lee D.C., Lavie C.J. Effects of muscular strength on cardiovascular risk factors and prognosis. J Cardiopulm Rehabil Prev. 2012;32:351–358. doi: 10.1097/HCR.0b013e3182642688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silventoinen K., Magnusson P.K., Tynelius P., Batty G.D., Rasmussen F. Association of body size and muscle strength with incidence of coronary heart disease and cerebrovascular diseases: a population-based cohort study of one million Swedish men. Int J Epidemiol. 2009;38:110–118. doi: 10.1093/ije/dyn231. [DOI] [PubMed] [Google Scholar]
- 11.Wiley R.L., Dunn C.L., Cox R.H., Hueppchen N.A., Scott M.S. Isometric exercise training lowers resting blood pressure. Med Sci Sports Exerc. 1992;24:749–754. [PubMed] [Google Scholar]
- 12.Millar P.J., Levy A.S., McGowan C.L., McCartney N., MacDonald M.J. Isometric handgrip training lowers blood pressure and increases heart rate complexity in medicated hypertensive patients. Scand J Med Sci Sports. 2013;23:620–626. doi: 10.1111/j.1600-0838.2011.01435.x. [DOI] [PubMed] [Google Scholar]
- 13.Taekema D.G., Maier A.B., Westendorp R.G., de Craen A.J. Higher blood pressure is associated with higher handgrip strength in the oldest old. Am J Hypertens. 2011;24:83–89. doi: 10.1038/ajh.2010.185. [DOI] [PubMed] [Google Scholar]
- 14.Hess N.C., Carlson D.J., Inder J.D., Jesulola E., McFarlane J.R., Smart N.A. Clinically meaningful blood pressure reductions with low intensity isometric handgrip exercise. A randomized trial. Physiol Res. 2016;65:461–468. doi: 10.33549/physiolres.933120. [DOI] [PubMed] [Google Scholar]
- 15.Kelley G.A., Kelley K.S. Isometric handgrip exercise and resting blood pressure: a meta-analysis of randomized controlled trials. J Hypertens. 2010;28:411–418. doi: 10.1097/HJH.0b013e3283357d16. [DOI] [PubMed] [Google Scholar]
- 16.Ash G.I., Taylor B.A., Thompson P.D. The antihypertensive effects of aerobic versus isometric handgrip resistance exercise. J Hypertens. 2017;35:291–299. doi: 10.1097/HJH.0000000000001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goessler K., Buys R., Cornelissen V.A. Low-intensity isometric handgrip exercise has no transient effect on blood pressure in patients with coronary artery disease. J Am Soc Hypertens. 2016;10:633–639. doi: 10.1016/j.jash.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Díez-Fernández A., Sánchez-López M., Gulías-González R. BMI as a mediator of the relationship between muscular fitness and cardiometabolic risk in children: a mediation analysis. PLoS One. 2015;10:e0116506. doi: 10.1371/journal.pone.0116506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong B., Wang Z., Arnold L., Song Y., Wang H.J., Ma J. The association between blood pressure and grip strength in adolescents: does body mass index matter? Hypertens Res. 2016;39:919–925. doi: 10.1038/hr.2016.84. [DOI] [PubMed] [Google Scholar]
- 20.Pate R.R., Daniels S. Institute of Medicine report on fitness measures and health outcomes in youth. JAMA Pediatr. 2013;167:221–222. doi: 10.1001/jamapediatrics.2013.1464. [DOI] [PubMed] [Google Scholar]
- 21.Taylor A.C., McCartney N., Kamath M.V., Wiley R.L. Isometric training lowers resting blood pressure and modulates autonomic control. Med Sci Sports Exerc. 2003;35:251–256. doi: 10.1249/01.MSS.0000048725.15026.B5. [DOI] [PubMed] [Google Scholar]
- 22.Badrov M.B., Freeman S.R., Zokvic M.A., Millar P.J., McGowan C.L. Isometric exercise training lowers resting blood pressure and improves local brachial artery flow-mediated dilation equally in men and women. Eur J Appl Physiol. 2016;116:1289–1296. doi: 10.1007/s00421-016-3366-2. [DOI] [PubMed] [Google Scholar]
- 23.Bauman A., Bull F., Chey T. The international prevalence study on physical activity: results from 20 countries. Int J Behav Nutr Phys Act. 2009;6:21. doi: 10.1186/1479-5868-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craig C.L., Marshall A.L., Sjöström M. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 25.Haskell W.L., Lee I.M., Pate R.R. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 26.Ji C., Zheng L., Zhang R., Wu Q., Zhao Y. Handgrip strength is positively related to blood pressure and hypertension risk: results from the national health and nutrition examination survey. Lipids Health Dis. 2018;17:86. doi: 10.1186/s12944-018-0734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Millar P.J., McGowan C.L., Cornelissen V.A., Araujo C.G., Swaine I.L. Evidence for the role of isometric exercise training in reducing blood pressure: potential mechanisms and future directions. Sports Med. 2014;44:345–356. doi: 10.1007/s40279-013-0118-x. [DOI] [PubMed] [Google Scholar]
- 28.Jin Y.Z., Yan S., Yuan W.X. Effect of isometric handgrip training on resting blood pressure in adults: a meta-analysis of randomized controlled trials. J Sports Med Phys Fitness. 2017;57:154–160. doi: 10.23736/S0022-4707.16.05887-4. [DOI] [PubMed] [Google Scholar]
- 29.Carlson D.J., Inder J., Palanisamy S.K., McFarlane J.R., Dieberg G., Smart N.A. The efficacy of isometric resistance training utilizing handgrip exercise for blood pressure management: a randomized trial. Medicine (Baltim) 2016;95:e5791. doi: 10.1097/MD.0000000000005791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doherty T.J. Invited review: aging and sarcopenia. J Appl Physiol (1985) 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 31.Frontera W.R., Hughes V.A., Fielding R.A., Fiatarone M.A., Evans W.J., Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol (1985) 2000;88:1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 32.Corish C.A., Kennedy N.P. Anthropometric measurements from a cross-sectional survey of Irish free-living elderly subjects with smoothed centile curves. Br J Nutr. 2003;89:137–145. doi: 10.1079/BJN2002748. [DOI] [PubMed] [Google Scholar]
- 33.Taekema D.G., Gussekloo J., Maier A.B., Westendorp R.G., de Craen A.J. Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age Ageing. 2010;39:331–337. doi: 10.1093/ageing/afq022. [DOI] [PubMed] [Google Scholar]
- 34.Ling C.H., Taekema D., de Craen A.J., Gussekloo J., Westendorp R.G., Maier A.B. Handgrip strength and mortality in the oldest old population: the Leiden 85-plus study. CMAJ. 2010;182:429–435. doi: 10.1503/cmaj.091278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delmonico M.J., Harris T.B., Visser M. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hollmann W., Strüder H.K., Tagarakis C.V., King G. Physical activity and the elderly. Eur J Cardiovasc Prev Rehabil. 2007;14:730–739. doi: 10.1097/HJR.0b013e32828622f9. [DOI] [PubMed] [Google Scholar]
- 37.Hortobágyi T., Katch F.I., Katch V.L., LaChance P.F., Behnke A.R. Relationships of body size, segmental dimensions, and ponderal equivalents to muscular strength in high-strength and low-strength subjects. Int J Sports Med. 1990;11:349–356. doi: 10.1055/s-2007-1024817. [DOI] [PubMed] [Google Scholar]
- 38.Musselman K., Brouwer B. Gender-related differences in physical performance among seniors. J Aging Phys Act. 2005;13:239–253. doi: 10.1123/japa.13.3.239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.