Abstract
Several studies have previously reported propolis, or its constituents, to inhibit tumour angiogenesis. The anti-angiogenic activity of two Indonesian stingless bee propolis extracts from Sulawesi Island on vascular cells were assessed. Sample D01 was obtained from the outer side of bee hives, while D02 was from the inner side of the same hives. The extracts were profiled by using liquid chromatography coupled to high resolution mass spectrometry. The anti-angiogenic capacity was assessed on HUVECs and placenta-derived pericytes by cell viability, multi-channel wound healing, and CoCl2 based-hypoxia assays. The exact chemical composition has not been confirmed. The most abundant compounds in Indonesian sample D01 seem to be unusual since they do not immediately fall into a clear class. Two of the most abundant compounds have elemental compositions matching actinopyrones. Identification on the basis of elemental composition is not definitive but compounds in D01 are possibly due to unusually modified terpenoids. Sample D02 has abundant compounds which include four related diterpenes with differing degrees of oxygenation and some sesquiterpenes. However, again the profile is unusual. The anti-angiogenic assays demonstrated that D01 elicited a strong cytotoxic effect and a considerable anti-migratory activity on the vascular cells. Although D02 demonstrated a much weaker cytotoxic effect on the cell lines compared to D01, it elicited a substantial protective effect on the pericytes against CoCl2-induced dropout in an experiment to mimic a micro-environment commonly associated with angiogenesis and tumour growth. These results demonstrate modulatory effects of these propolis samples in vascular cells, which requires further investigation.
Keywords: Analytical chemistry, Natural product chemistry, Organic chemistry, Pharmaceutical chemistry, Cancer research, Cell biology, Pharmaceutical science, Physiology, Indonesian propolis, Anti-angiogenesis, HUVECs, Pericyte dropout, LC-MS
1. Introduction
Among many existing natural products propolis is the most ambiguous in terms of the source, as it is derived from both plants and animals. Propolis is a natural resinous material that honey bees produce by collecting exudates and materials of various parts of the plant, such as flower buds, leaf buds, and tree barks, then mixing them with beeswax and several bee enzymes (Sforcin and Bankova, 2011). Propolis is characterized as a lipophilic material that is hard, brittle and breakable when cold but, soft, malleable and considerably adhesive when warm (Marcucci, 1995). It possesses unique herbaceous aromatic scents with various colorations, including yellow, green, brown, and red (Marcucci, 1995; Fokt et al., 2010). In nature, propolis is effectively used by honey bees as a protective material as it is attached in many parts of their hive to defend the colony from predators and pathogens (Umthong et al., 2011). Ina addition, propolis is beneficial to fix damages in the hive, refine internal walls, preserve internal heat of the hive, and envelop the corpses of intruders that die inside the hive, which forms aseptic coffins and halts the decay (Bankova et al., 2000).
Generally, propolis contains biochemicals from the class of flavonoids, phenolic acid derivatives, cinnamic acids derivatives, terpenes/terpenoids, tannins, alkaloids and bee enzymes (Xu et al., 2009; Kumazawa et al., 2004). However, due to the nature of propolis, which relates to its plant origin, the bioactive compounds within propolis of one area may be different from propolis of another area, depending on the climate, seasons and environmental conditions (Kujumgiev et al., 1999; Bankova et al., 1998). In consequence, it results to various bioactivities it possesses. Currently, there are many scientific reports on the bioactivities of propolis from both in vitro and in vivo studies, such as anti-viral (in vitro and animal model in vivo) (Shimizu et al., 2008; Shen et al., 2013; Bankova et al., 2014), anti-bacterial (in vitro, human model in vivo and ex vivo) (Velikova et al., 2000; Boisard et al., 2015; Duailibe et al., 2007; Dziedzic et al., 2013), anti-protozoal (in vitro and animal model in vivo) (Pontin et al., 2008; Almutairi et al., 2014; Siheri et al., 2014), anti-inflammatory (in vitro, animal model in vivo and ex vivo) (Mirzoeva and Calder, 1996; Naito et al., 2007), immunomodulatory (in vitro and animal model in vivo) (Li et al., 2015; Tao et al., 2014), anti-oxidant (in vitro and animal model in vivo) (Sun et al., 2000; Moreira et al., 2008; Miguel et al., 2010), cardioprotective (animal model in vivo) (Alyane et al., 2008; Daleprane and Abdalla, 2013), etc.
Aside from those bioactivities, studies have also shown potential anti-angiogenic properties of propolis and its pure compounds through multiple cellular signaling pathways due to the synergistic contributions of different phytochemicals (Daleprane and Abdalla, 2013). Kunimasa et al. (2011) reported that the ethanolic extract of Brazilian brown propolis promoted an inactivation of extracellular signal–regulated kinases ½ (ERK1/2), an activation of caspase-3 and cleavage of poly ADP-ribose polymerase (PARP) and lamin A/C in an in vitro study, which are all responsible for cell apoptosis. Some propolis crude samples and pure compounds have been shown as evident to suppress angiogenesis by inhibiting vascular endothelial cell growth factor (VEGF) expression (e.g. the ethanolic extract of Chinese red propolis in an in vitro study using HUVEC (Izuta et al., 2009) and CAPE in an in vitro study using human breast adenocarcinoma (MDA-MB-231) cells (Wu et al., 2011)). An additional report by Daleprane and Abdalla (2013) showed that the anti-angiogenic effect of the Brazilian red propolis corresponded with a decrease in hypoxia inducible factor 1-alpha (HIF-1α) protein expression in endothelial cells under hypoxic conditions. Moreover, an increase in the von Hippel–Lindau tumour suppressor protein level (pVHL), and an attenuation of VEGF gene expression was observed, which diminished the migration and sprouting of endothelial cells (Daleprane and Abdalla, 2013). Interestingly, Xuan et al. (2011) discovered that the ethanolic extract of Brazilian brown propolis could modulate both proliferation and apoptosis of endothelial cells in a dose-dependent manner. They reported that a lower concentration of the ethanolic extract of propolis (12.5 μg/ml) could induce proliferation by decreasing the expression of p53 and ROS levels. Alternatively, high concentrations of the ethanolic extract of propolis (25 and 50 μg/ml) led to apoptosis by increasing the level of p53 and ROS at 24 hours while reducing the mitochondrial membrane potential level at all times.
As a potent anti-angiogenic compound, propolis may potentially have remarkable effects towards cancer cells, as both cancer and angiogenesis are intertwined with each other. However, the mechanism of action of propolis in eliminating cancer cells may not always work through the angiogenesis pathway. Aside from affecting angiogenesis, some studies have found that various propolis samples and pure compounds can block particular oncogene signalling pathways, increase apoptosis, decrease the cancer stem cell population and modulate the tumour microenvironment (Sawicka et al., 2012; Chan et al., 2013). Furthermore, Alizadeh et al. (2015) investigated the chemoprotective effects of the ethanolic extract of Iranian poplar-type propolis on N-methyl-N-nitro-N-nitrosoguanidine-(MNNG-)-induced-gastric cancer in rats, with a significant reduction in nuclear/cytoplasmic ratio, epithelial stratification, structural abnormality and Beta-catenin and Bcl-2 protein expressions.
Despite all of these therapeutic potentials, propolis from around the world are still poorly characterized. This phenomenon is also one of the main reasons why propolis has not been included in mainstream drugs yet, in spite of its increasing popularity and scientific findings (Bankova, 2005). It is uncertain whether the bioactivities that have been found in some propolis samples would be found in other samples as well. Therefore, preliminary research on the sample of interest to determine its unique biological activities is warranted before advancing to its utilization. In this study, two Indonesian propolis samples from Sulawesi Island, D01 and D02, were tested on several cell lines for their potential as an anti-angiogenic drug in tumour microenvironment. Two cell lines, human umbilical vein endothelial cells (HUVECs) and pericytes, were used for this objective, while MDA-MB-231 cells was used as a comparison to see how these samples affect cancer cells as a hint of their further applicability in treating tumour angiogenesis. HUVECs and pericytes are being used as a model of the microvascular environment, whereas the MDA-MB-231 cell line is a common model in cancer research.
2. Material and method
2.1. Materials
Indonesian propolis samples (D01 & D02) were a gift from Dr. Muhamad Sahlan from the Department of Chemical Engineering, Universitas Indonesia. D01 and D02 are classified as pacific-type brown propolis from Trigona sp. and were harvested in North Luwu Regency, South Sulawesi Province, Indonesia. D01 was harvested from the inner side of the hive, while D02 was from the outer side of the same hive. Human umbilical vein endothelial cells (HUVECs) and placenta-derived pericytes were from Promocell, Heidelberg, Germany. The MDA-MB-231 human breast cancer cell line was a gift from Fahy Gurteen UK Ltd, Cambridge, United Kingdom. Caffeic acid phenethyl ester (CAPE) was from Abcam, Cambridge, UK, and pinocembrin was from Sigma-Aldrich, HaverHill, UK. Endothelial basal medium 2 and supplement kit were from Promocell, Heidelberg, Germany. Pericyte growth medium and supplement kit were from Promocell, Heidelberg, Germany. Dulbecco's Modified Eagle Medium (DMEM), foetal bovine serum (FBS), antibiotic-antimycotic, trypsin/EDTA solution, trypan blue solution (0.4 %), human recombinant VEGF and 13 mm Thermanox cell culture coverslips were from Gibco Life Science, Paisley, UK. Phosphate Buffered Saline (PBS) was from Oxoid Ltd, Basingstoke, UK. Dimethyl Sulfoxide (DMSO), cobalt chloride (CoCl2), and Taxol were from Sigma-Aldrich, Gillingham, UK. The CCK-8 solution was from Dojindo Molecular Technologies, Inc., Japan. Fibroblast growth factor was from Prospec, Rehovot, Israel.
2.2. Preparation of propolis
Both samples were extracted at Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow. The extraction was performed in an ethanolic-based extraction system. Briefly, 15 grams of raw propolis was macerated in 1 litre of absolute ethanol with sonication in 37 °C for an hour. The solution was then filtered and the process was repeated two more times to achieve an increased yield in the filtrate. Following the extraction process, the filtrate was then dried and ca 10 grams of dried filtrate was obtained. To prepare the extracts of the samples from Ghana, Nigeria, Saudi Arabia, and New Zealand 50 mg of each sample was extracted with 5 ml of ethanol by sonication for 1 hour at 37 °C. The extraction solution was blow to dryness, weighed and then made up in methanol to give a 1 mg/ml solution.
2.3. LC-MS profiling of the propolis samples
Propolis extracts were dissolved in methanol (1 mg/ml) and 10μl of the solutions were injected into the LC-MS. High resolution mass spectra were obtained using an linear trap quadropole orbitrap mass spectrometry (LTQ Orbitrap MS) (Thermo Fisher, Hemel Hempstead UK) in negative ion mode with a needle voltage of -4.0 kV, and a sheath gas flow as well as an auxiliary gas flow of 50 and 10 arbitrary units, respectively. Separation was carried out on an ACE-C18 column (150 × 3 mm, 3 μm) (HiChrom, Reading, UK) with 0.1%v/v formic acid in water as mobile phase A and 0.1%v/v formic acid in acetonitrile (ACN) as mobile phase B at a flow rate of 300 μl/min. The gradient programme was as follows: 0 min (25% B), 30 min (100% B), 35 min (100% B), 36 min 25% B, 41 min 25% B. The data obtained were extracted by using m/zMine 2.20 (Pluskal et al., 2010) and then searched against a database compiled from the Dictionary of Natural Products (Buckingham, 1993). Putative identities were assigned according to mass matching to elemental composition to within 3 ppm. The extracted data was analysed using Orthogonal Partial Least Squares Discriminant Analysis (OPLSDA) using Simca P 14.1 (Umetrics, Sweden).
2.4. Cell culture
HUVECs were cultured in endothelial cell basal medium-2, supplemented with 1% antibiotic-antimycotic solution (ANTI-ANTI) and a supplement kit, according to manufacturer instructions. This supplemented medium is henceforth referred to as endothelial cell growth medium (EGM-2). For general usage, HUVECs were incubated at 37 °C with humidified air containing 5% CO2. In this study, assays to HUVECs were performed using the basal medium to optimally monitor the comparative effect of additional growth factors. Cells were used in passages 2 to 8 for this study.
Pericytes were cultured in a similar way as HUVECs but using pericyte growth medium (PGM). This medium was supplemented with 1% ANTI-ANTI solution and a supplement mix. To perform downstream assays, the complete medium is used instead of the titrated medium due to the absence of basal medium in Promocell pericyte growth medium product. Passage 2 to 8 were used in this study.
MDA-MB-231 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% FBS and 1% ANTI-ANTI. Generally, MDA-MB-231 cells were cultured in a similar way as HUVECs and pericytes. Complete medium was used for downstream assays. This was to ensure the growth environment resembles realistic conditions where cancer cells can fully proliferate. Passage 3 to 9 were used in this study.
2.5. Cell viability assay
To measure cell viability following the propolis treatment, a cell counting kit-8 (CCK-8) solution was used. CCK-8 is a sensitive colorimetric assay for determining the number of viable cells in cell proliferation and cytotoxicity assays. The detection sensitivity of CCK-8 is increased than other tetrazolium salts, such as MTT, XTT, MTS or WST-1. Although the calibration curve for certain cell lines did not exist at the time of the experiment, using CCK-8 is still useful to provide us an accurate proxy of cell viability following the drug treatment compared to the vehicle controls in the experiments.
To perform the cell viability assay, HUVECs, pericytes, and MDA-MB-231 cells were trypsinised, counted and seeded onto 96 well plates at a density of 2,500 cells per well for HUVECs and pericytes, and 10,000 cells per well for MDA-MB-231 cells. The plates were then incubated for 24 hours to allow the cells to begin proliferating. The drug testing step was performed by changing the medium using an appropriate medium with the addition of different concentrations of DMSO-diluted drug samples. The dilution of drug samples into the medium was in the ratio of 1:1000 to minimise possible DMSO toxicity to the cells, and the total volume of medium-drug solution in one well is 100 μl. For the experimental controls, 0.1% DMSO was used as a vehicle control, while Taxol was used as a positive control. We use Taxol as a positive control because from preliminary investigations (i.e., literature study, preliminary experiments), we observed that the drug samples (propolis) may possess anti-angiogenic properties. Besides, we also used both CAPE and pinocembrin as drug samples—two of the most common propolis pure compounds—to show a comparability between the propolis samples and the pure compounds. The cells were incubated for 48 hours and following incubation 10 μl of CCK-8 solution was added to each well, and plates were further incubated for 2 hours in darkness at 37 °C. Lastly, the absorbance at 450 nm was then measured with a 96-well plates reader. To avoid interference from background colorimetric effects, plates of the blank sample containing just medium were also tested. The blank control absorbance values were then subtracted from the treatment values to calculate the corrected absorbance values of wells.
In addition to performing the standard cell viability assay, we also modified the experiment in order to provide evidence regarding how the drug samples/propolis interact on selected cells. The modification included the addition of growth factors in HUVEC experiments and the addition of CoCl2 to generate hypoxia conditions in pericyte experiments. For experiments that involved growth factor effects in HUVECs, each well was supplemented with 20 ng/ml of VEGF and 50 ng/ml of fibroblast growth factor (FGF) when testing the drug samples. For the hypoxia experiment of pericyte, CoCl2 was added to each well 2 hours after changing the medium prior to 24 hours incubation.
2.6. Wound healing/migration assay
HUVECs, pericytes, and MDA-MB-231 cells were trypsinised, counted and seeded onto 13mm diameter Thermanox coverslips placed in 24-well plates at a density of 20,000 cells per well for HUVEC and pericytes, and 40,000 cells per well for MDA-MB-231 cells. The plates were incubated until they became confluent (approximately 5 days). Once confluent, each well was treated with 10 μg/ml of mitomycin-C two hours prior to the wounding process in order to stop cell proliferation. The coverslips were then wounded using a specifically designed mechanical wounder, which produces 11 lesions with 400 μm distance between the lesions. Next, they were rinsed with PBS three times before being placed into a 24-well plate containing fresh corresponding medium and appropriate concentration of the drug samples. The total volume of medium and drug in one well is 400 μl. To establish the time zero control, some wells were fixed immediately after wounding using 500 μl methanol for 5 minutes. Then, the plates were further incubated for 8 hours before finally being fixed. Following fixation, a photomicrograph of each coverslip was taken and the denuded area was analysed using ImageJ software to quantify the ability of the cells to migrate over 8 hours.
2.7. Statistical analysis
For statistical analysis, Microsoft Excel and Graphpad Prism 5 software were used. Ordinary one-way analysis of variance (ANOVA) with a Tukey's or Dunnett's multiple comparisons test was performed to compare the mean of each treatment group with the mean of the control (vehicle and positive control). An unpaired two-tailed t-test was performed to compare any significant difference between the treatment. Data are always reported as mean ± SD. Significance is reported as a * which represents stimulatory effect (* for P < 0.05, ** for P < 0.01, and *** for P < 0.001) and # which represents inhibitory or cytotoxic effect (# for P < 0.05, ## for P < 0.01, and ### for P < 0.001). A * sign is also used to indicate a level of significance in the t-test experiments (* for P < 0.05, ** for P < 0.01, and *** for P < 0.001).
3. Results
3.1. LC-MS profiles of D01 and D02
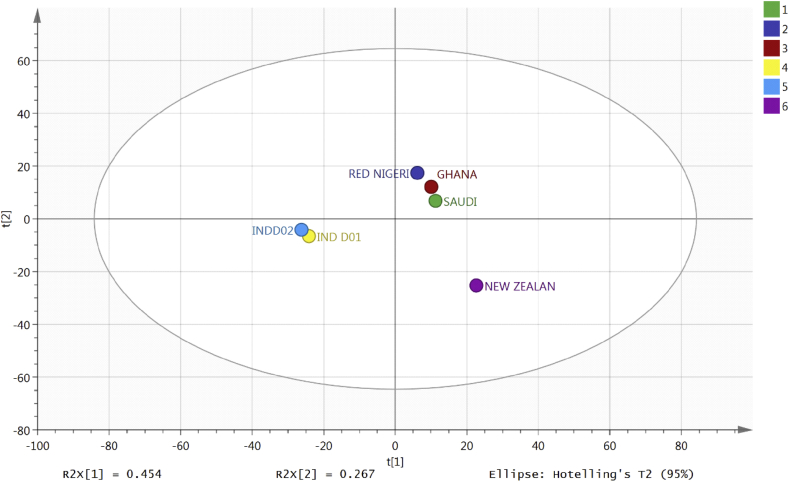
Fig. 1 shows a comparison of the two Indonesian samples with samples of propolis from Nigeria, Ghana, New Zealand and Saudi Arabia using the 950 most abundant components in the samples to construct an OPLSDA model. It was putatively found that the Indonesian samples are similar to each other but very different from the other samples which contain abundant flavonoids. Although the Indonesian samples were similar to each other in comparison to the Nigerian, Saudi, Ghanaian and New Zealand samples, they differ from each other and Tables 1 and 2 show the ten tentatively identified components with putative identifications and elemental compositions in each sample. It is observed that these identified chemicals are the most abundant components of each sample, but further confirmation is needed to claim such findings.
Fig. 1.
Separation for six propolis samples using OPLSDA based on the 950 most abundant compounds in the samples.
Table 1.
The 10 tentatively identified components in sample D01 in negative ion mode. Putatively identified according to accurate mass <3 ppm from assigned elemental composition.
| m/z | Rt min | Elemental Comp. | Putative identification |
|---|---|---|---|
| 399.2542 | 23.6 | C25H36O4 | Actinopyrone A |
| 447.2755 | 22.3 | C26H40O6 | Amphidinolide X |
| 445.2599 | 16.7 | C26H38O6 | Acetoxy-acetoxymethyl-acetoxymethylene-dimethyhexadecatetraene |
| 445.26 | 16.1 | C26H38O6 | Acetoxy-acetoxymethyl-acetoxymethylene-dimethylhexadecatetraene |
| 385.2389 | 21.7 | C24H34O4 | Abietatrienediol Di-Acetate |
| 445.2597 | 17 | C26H38O6 | Acetoxyacetoxymethylacetoxymethylenedimethylhexadecatetraene |
| 451.213 | 17.5 | C27H32O6 | Arisugacin E; 3-Ketone |
| 447.2754 | 20.8 | C26H40O6 | Amphidinolide X |
| 449.2549 | 14.3 | C25H38O7 | Daucanetriol Trimethoxybenzoyl |
| 433.2598 | 24.5 | C25H38O6 | Abeo-dihydroxy-eremophilenolide 1-O-(2-Methylbutanoyl), 9-O-(3-methylbutanoyl) |
Table 2.
The 10 tentatively identified components in sample D02 in negative ion mode.
| m/z | Rt min | Elemental Comp. | Putative identification |
|---|---|---|---|
| 351.2178 | 16.3 | C20H32O5 | 11(15->1)-Abeo-4(20),11-taxadiene-5,9,10,13,15-pentol |
| 315.0512 | 10.6 | C16H12O7 | 2-Acetyl-1,3,6,8-tetrahydroxyanthraquinone; 1′R-Alcohol |
| 363.1817 | 8.9 | C20H28O6 | 19(4->3)-Abeo-4,5-epoxy-1,6,7,14-vouacapanetetrol |
| 381.1922 | 7.4 | C20H30O7 | 11(15->1)-Abeo-5,20:13,15-diepoxy-11-taxene-2,4,7,9,10-pentol |
| 349.202 | 9.9 | C20H30O5 | 7(8->9)-Abeo-1,9-dihydroxy-11(13)-eremophilen-8,12-olide; (1?,7?,9?)-form, 1-O-(3-Methylbutanoyl) |
| 303.1966 | 19.4 | C19H28O3 | Acalycixeniolide K |
| 317.2121 | 22.1 | C20H30O3 | 6,8(14),15-Abietatriene-3,11,17-triol |
| 319.1916 | 14.4 | C19H28O4 | 7(8->9)-Abeo-9-hydroxy-11(13)-eremophilen-8,12-olide; (7?,9?,10?)-form, 2-Methylpropanoyl |
| 333.2072 | 17.5 | C20H30O4 | 20(10->9)-Abeo-6,16-dihydroxy-19,10-kauranolide |
| 289.1809 | 16.4 | C18H26O3 | 2-Alkyl-5-hydroxy-4H-1-benzopyran-4-ones; 5-Hydroxy-2-nonyl-4H-1-benzopyran-4-one, 2?,3-Dihydro |
3.2. Effect of the samples on cell viability
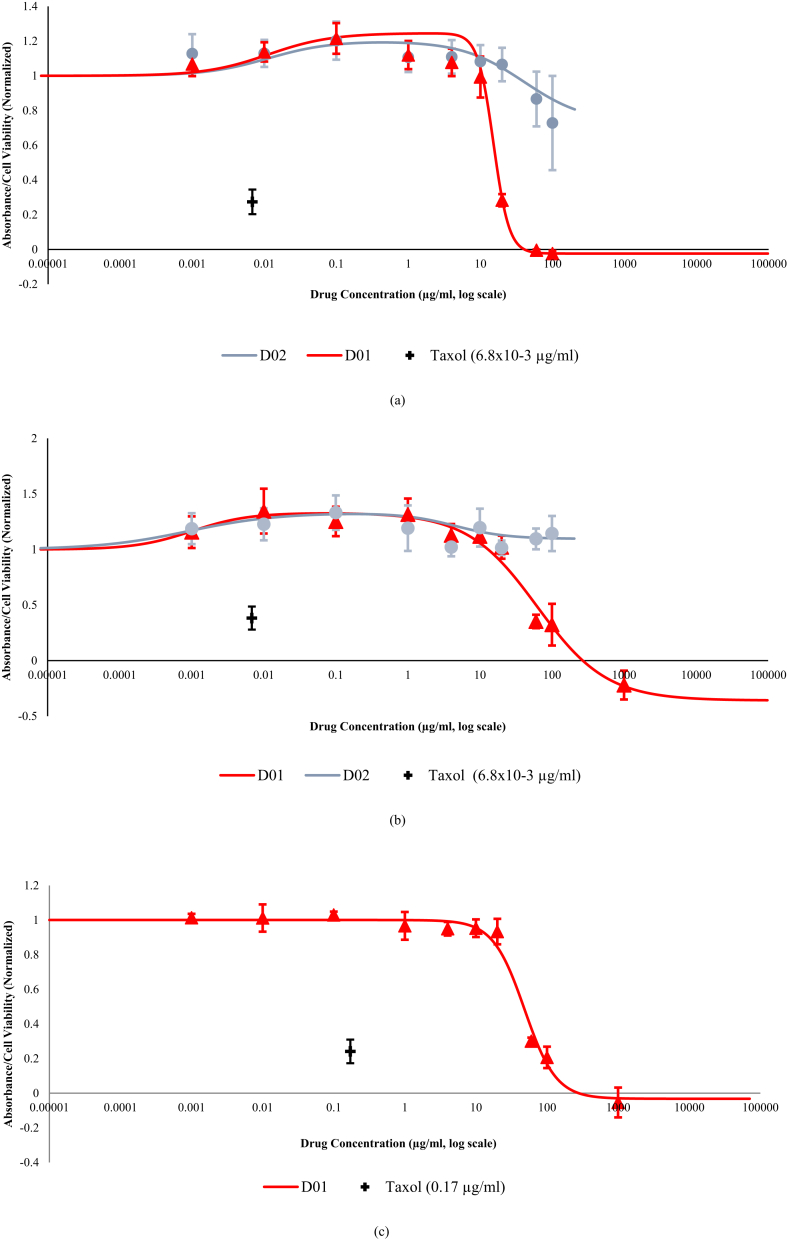
In Fig. 2, dose-response curves of the effects of different concentrations of the samples towards the viability of the three cell lines are shown. Between the two crude extracts, D01 exhibited a much stronger inhibitory or cytotoxic effect on HUVECs, pericytes and MDA-MB-231 when compared to D02. Furthermore, it can be seen from Fig. 2 that D02 could not reach a maximum inhibition at the highest soluble concentration of this study (D02 is not soluble in 1000 μg/ml concentration), resulting in non-converged D02 curves on the graph plots of the three cells.
Fig. 2.
Cell Viability of (a). HUVEC (b). pericytes and (c). MDA-MB 231 cells when Treated with Propolis Extracts. The cells were treated with various concentrations of D01 and D02. After 48 hours of incubation, 10μl/well of the CCK-8 solution was added and the absorbance at 450nM was measured. Data are expressed as absorbance values with normalization to the basal values of cells with DMSO as the control, which show means ±SD and represent biological replicates. Taxol was used as a positive control of anti-angiogenesis to be compared with the samples. The best fit curve was produced by analyzing the predicted residual error sum of squares (PRESS). Bullets signify the actual average experimental data. The experiment was repeated three times with five technical replicates in each experiment. Data of D02 did not produce a converged curve on HUVECs' and pericytes' curves. Furthermore, D02 barely exhibited an inhibitory or cytotoxic effect on MDA-MB-231 cells (the plot is not depicted for efficiency).
For the experiments on HUVECs, although D01 did not demonstrate an inhibitory or cytotoxic effect until 10 μg/ml, the graph shows a steep decline after the respective value with a hill coefficient of about 4 (Fig. 2a). However, the inhibitory or cytotoxic potential of D01 to pericytes and its Hill coefficient are drastically reduced compared to the value in the HUVEC experiment (Fig. 2b). In terms of the IC50 value, the biological activity of CAPE towards both cells is actually more potent than D01 (Table 3a-b. The graphs of CAPE and pinocembrin are not shown). However, the inhibitory or cytotoxic effect of D01 may or may not be derived from the interaction of its pure compounds. If it actually came from just one or two single compound(s), the biological activity of that unknown compound(s) may be higher than CAPE, provided the fact that one single propolis pure compound may constitute a tiny percentage of the total volume. The comparison between D01's and the pure compounds' results towards the three cell lines are shown in Table 3.
Table 3.
IC50 of D01 and pure compounds on (a). HUVECs (b). pericytes and (c). MDA-MB-231 cells (n = 3). The graphs of CAPE and pinocembrin are not shown.
| Sample |
IC50. 1 |
IC50. 2 |
IC50. 3 |
Average ± Standard Deviation |
| (a) | ||||
| D01 (μg/ml) | 15.7 | 13.6 | 14.7 | 14.7 ± 1.01 |
| CAPE (μg/ml) | 0.68 | 0.92 | 0.73 | 0.78 ± 0.13 |
| Pinocembrin (μg/ml) |
18.1 |
13.7 |
20.7 |
17.5 ± 3.55 |
| (b) | ||||
| D01 (μg/ml) | 67.2 | 58.8 | 55.2 | 60.4 ± 6.14 |
| CAPE (μg/ml) | 4.42 | 4.14 | 3.19 | 3.91 ± 0.64 |
| Pinocembrin (μg/ml) |
41.8 |
38.5 |
29.6 |
36.6 ± 6.33 |
| (c) | ||||
| D01 (μg/ml) | 53.4 | 53.1 | 41.7 | 49.4 ± 6.69 |
| CAPE (μg/ml) | 35.9 | 42.1 | 43.8 | 40.6 ± 4.17 |
| Pinocembrin (μg/ml) | 35.9 | 39.8 | 45 | 40.2 ± 4.52 |
A unique feature of the dose-response curves of D01 on HUVECs and pericytes is their tendency to be biphasic. While it has been proven that D01 exhibited a strong inhibitory or cytotoxic activity towards both cells, the graphs do not decline until they increase first, indicating a slight proliferative effect of D01 in the lower concentrations. In contrast, the results of D01 on MDA-MB-231 do not produce a biphasic curve even though they show a relatively similar pattern to what happened to HUVECs and pericytes (Fig. 2c). These results provide a further proxy about the modulatory effect of propolis on different cells, which needs to be further investigated.
3.3. The propolis samples and pure compounds have no effect on VEGF and FGF activities
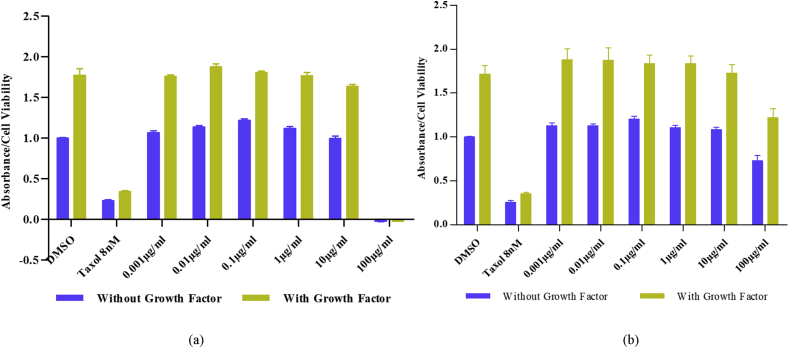
To further investigate whether the samples may have interactions with the growth factors of endothelial cells, additional cell viability assays were performed by adding 20 ng/ml of VEGF and 50 ng/ml of bFGF to the HUVEC experiment. The vehicle control in this experiment was the growth factors-enhanced DMSO-treated cells. The results of this experiment were compared to another group which the cells were not enhanced with additional growth factors. By comparing these results, a hypothesis about a possible interaction between the propolis samples and the endothelial cell growth factors may be generated. If the inhibition percentage of propolis on the growth factors-enhanced HUVECs was increased when compared to the non-growth factors-enhanced HUVECs, there may be a possible nexus between the propolis samples suppressive activities and the growth factors activities on HUVECs. Fig. 3 illustrates the cell viability results of both conditions and points to them having a similar trend. However, the inhibition percentages in both conditions do not produce a significantly statistical difference, thus negating the hypothesis (calculation not shown).
Fig. 3.
Effect of The Propolis Samples on The Growth Factors-Enhanced HUVECs. HUVECs were treated with VEGF (20 ng/ml) and bFGF (50 ng/ml), and also the various concentration of (a) D01 and (b) D02. After 48 hours of incubation, 10μl/well of CCK-8 solution was added and the absorbances at 450nM were measured. These data are compared with the absorbance of HUVECs treated without growth factors but with the same concentration of propolis samples, which have similar graphic trends with HUVEC data in Fig. 2. Data are expressed as absorbance values with normalization to the basal values of cells with DMSO as the control and represent biological replicates. The experiment was repeated two times with five technical replicates in each experiment.
3.4. Effect of the samples on migratory activity of the cells
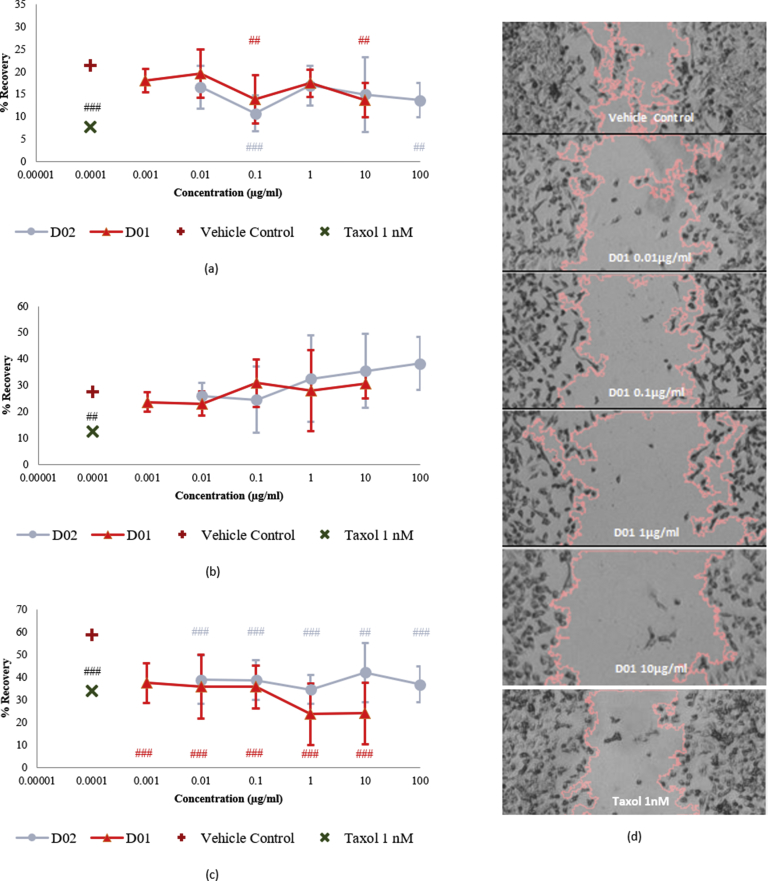
Although D01 and D02 performed remarkably different in the cell viability assays, their results in the wound healing assays are barely distinctive and show a similar downward trend at almost the same potency. Between 0.01 – 10 μg/ml, both D01 and D02 were able to inhibit the migratory activity of HUVECs in the half capacity of Taxol 1 nm (Fig. 4a). On the other hand, both extracts suppressed the cancer cells in a comparable level to Taxol 1 nm for all tested concentrations (Fig. 4c). Of important notes, D01 at 1 and 10 μg/ml were even stronger than taxol 1 nm in inhibiting MDA-MB-231 cell migration (35% inhibition by D01 at 1 and 10 μg/ml versus 25% inhibition by taxol 1 nm as shown in Fig. 4c). However, none of these results to both cells produces a dose-response curve, as most of them fluctuate between certain values. Aside from those results, they did not induce a significant inhibition to pericyte migration (Fig. 4b).
Fig. 4.
Wound Healing/Cell Migration Rate of (a). HUVECs (b). pericytes and (c). MDA-MB-231 cells when Exposed to Propolis Samples for 8 Hours. In 24 well-plates, the cells were grown until confluent before wounding by a mechanical wounder to produce 11 lesions on the cell monolayers. 10 μg/ml final concentration of mitomycin-C was added 2 hours prior to wounding. Next, the cells were treated with D01 and D02 at the concentrations where they do not induce cytotoxicity. DMSO 0.1% was used as the vehicle control. After 8 hours, all wells were fixed using methanol and the resulted images were analyzed by ImageJ. Data are expressed as average % recovery values compared to the time-zero control with no normalization and represent biological replicates. An ordinary one-way ANOVA was performed with a Dunnett's multiple comparisons test where the mean of each group was compared with the mean of the vehicle control group for each drug separately. Significance is reported as a * which represents a recovery effect (* for P < 0.05, ** for P < 0.01, and *** for P < 0.001) and a # which represents an inhibitory effect (# for P < 0.05, ## for P < 0.01, and ### for P < 0.001). The experiment was repeated two times with four technical replicates in each experiment. Figure (d) is the example of photomicrograph of wound healing experiment using D01 on MDA-MB-231 cells.
3.5. D02 retained the cell viability of CoCl2-treated pericytes
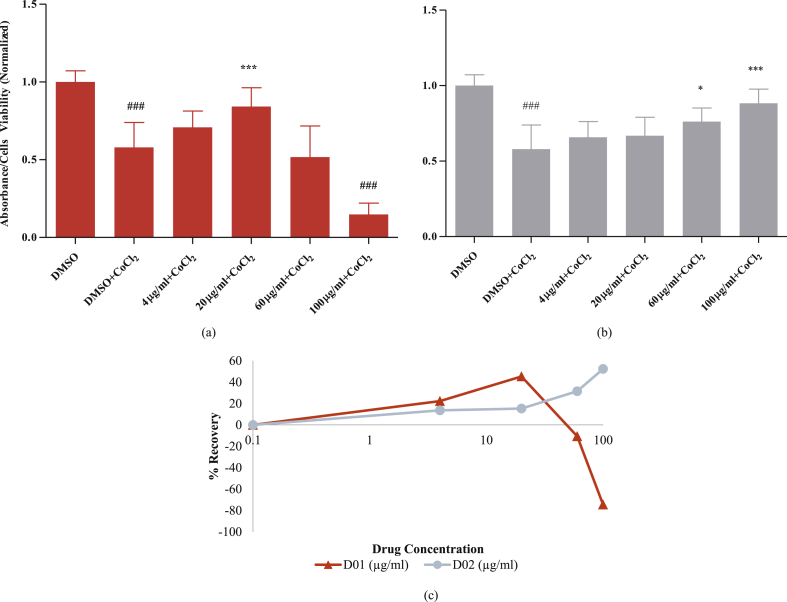
Aside from investigating cytotoxicity and migratory profiles resulted on the three cell lines after treated with the propolis samples, the effect of the samples in protecting pericytes against hypoxia is also of interest since hypoxia induced-pericyte dropout is a common phenomenon in tumour angiogenesis (Xian et al., 2006; Raza et al., 2010). CoCl2 addition was performed to mimic hypoxic condition in the cell culture Wu and Yotnda (2011). The results of this experiment demonstrate that D02 potentially counteracted the CoCl2-induced pericyte loss by delivering a consistent protection in a dose-dependent manner as depicted by Fig. 5. If the assumption of CoCl2-induced hypoxia in this experiment was correct, this result may lead to a hypothesis that D02 can ameliorate angiogenesis-related hypoxic microenvironment in tumours, even though its direct inhibitory or cytotoxic activity on HUVECs and pericytes was not strong.
Fig. 5.
Effect of Propolis Samples on CoCl2-induced Pericyte Loss. Pericytes were treated with various concentrations of (a) D01 and (b) D02. After 2 hours, each well, except the control wells, were further added with CoCl2, giving the final concentration of 250μM. After 24 hours of incubation, 10μl/well of CCK-8 solution was added and the absorbances of 450nM was measured. Data are expressed as absorbance values with normalization to the basal values of cells with DMSO as the control, which show means ±SD and represent biological replicates. An ordinary one-way ANOVA was performed with a Tukey's multiple comparisons test. The DMSO + CoCl2 group was compared to the vehicle control group (DMSO), and the other treatments were compared to the DMSO + CoCl2 group in order to assess the effect of propolis treatment against pericyte dropout, either stimulatory/recovery (*) or inhibitory/cytotoxic (#). Significance is reported as a * which represents a stimulatory effect (* for P < 0.05, ** for P < 0.01, and *** for P < 0.001) and # which represents an inhibitory or cytotoxic effect (# for P < 0.05, ## for P < 0.01, and ### for P < 0.001). While in (c), the protection percentage of propolis-samples-treated pericytes after experiencing a CoCl2-induced decline (pericyte loss) is depicted. Data were obtained by calculating the percentage of change between the data of each sample to the control data (samples which were treated by CoCl2 only). The experiment was repeated two times with five technical replicates in each experiment.
4. Discussion
4.1. Analysis of propolis extracts
The most abundant tentatively identified compounds in Indonesian sample D01 from LC-MS analysis seem to be unusual in that they do not obviously fall into a class such as flavonoids or terpenoids. Two of the most abundant compounds have elemental compositions matching actinopyrones which are terpenoid derivatives and antibiotic compounds isolated from Streptomyces pactum (Yano et al., 1986). The putative presence of these antibiotic compounds justifies the role of D01 as an antiseptic inside the hive of its former colony. Although, it is not possible to be definitive of identity on the basis of elemental composition, but it would seem the compounds in D01 are possibly modified terpenoids. On the other hand, D02 has abundant compounds which fit better with various classes of compound and putatively include four related diterpenes with differing degrees of oxygenation and some sesquiterpenes. These results conform to the common characteristic of Indonesian propolis which usually contains terpenes and terpenoids (Trusheva et al., 2011). The plant source of the terpenes of these samples is possibly Mangifera indica which is common in the area of harvesting. However, the exact chemical composition has not been confirmed, and a fuller phytochemical exploration will be conducted in the future.
4.2. Indonesian propolis is potentially anti-angiogenic in vitro
Recent literature has demonstrated that propolis has anti-angiogenic activity both in vivo and in vitro. Many papers already reported its possible mechanisms in suppressing angiogenesis and tumour-related angiogenesis. One of the possible pathways is by inducing apoptosis in vascular cells and upregulating the level of some apoptosis-related markers such as p53, ROS, and caspase-3 (Xuan et al., 2011; Kunimasa et al., 2011). An Additional possible mechanism is suppression of VEGF gene expression, which is one of the principal regulators of angiogenesis, as reported by Daleprane and Abdalla (2013), and Izuta et al. (2009).
This study provides a hypothesis that Indonesian propolis from Sulawesi Island may have an anti-angiogenic activity. D01 was quite effective in suppressing HUVEC viability at 10 μg/ml and above with a relatively low IC50 value and a high Hill coefficient (approximately 4), in a dose-dependent manner. Furthermore, the Hill coefficient of D01 is much larger than of CAPE and pinocembrin (0.75 and 0.8, respectively—the graphics are not shown) although the IC50 of D01 is much higher than CAPE's. From a statistical point of view, the steeper the curve means the more potent the drug at small increases in concentration. In general, this number may indicate a positive cooperativity among ligand-binding sites between the cells and D01, leading to a higher expected therapeutic effectiveness compared to drugs with hill coefficient 1 or less i.e. no cooperativity between ligand-binding site. This hypothesis may have a relevancy to a natural product like propolis whose diverse compounds allow interactions and modulations of its activities towards cells. From the result of compound analysis, modified terpenes are presumably the major contributors of this bioactivity. In fact, there are some reports about antiangiogenic activities of terpenes and terpenoids through several pathways (Kim et al., 2000; Sogno et al., 2009).
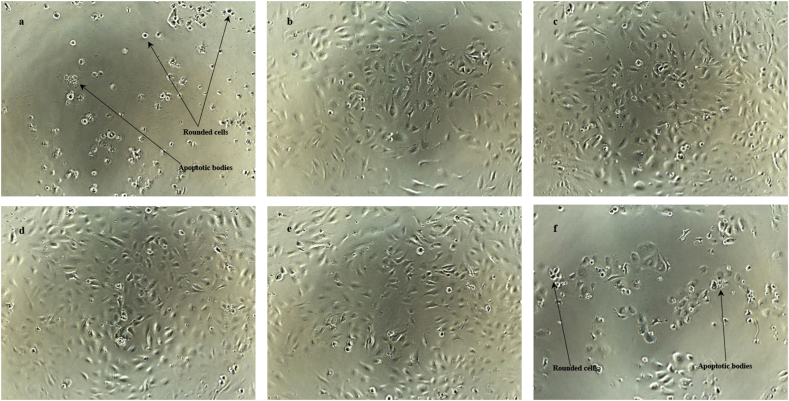
In Fig. 6, the morphological observations of HUVECs upon exposure to different concentrations of D01 corroborate the cell viability assay results. The lower results of the cell viability assay, which relate to the higher concentrations of samples, were reflected by more rounded cells with compromised cell density. In contrast, control cells demonstrated a dense normal cell population with very few rounded cells.
Fig. 6.
Photomicrographs of HUVECs after the incubation with D01 for 48 hours. HUVECs were treated with various concentrations of D01: (a) 100 μg/ml, (b) 10 μg/ml, (c) 1 μg/ml, (d) 0.1 μg/ml, (e) DMSO, and (f) 8nM Taxol. The images were taken after 48 hours of incubation and before addition of CCK-8 solution using Nikon Phase Contrast ELWD 0.3 microscope at 80X magnification.
An increased rounded cell population in a cell culture may suggest two common phenomena in the cells: apoptosis and mitotic arrest. Both phenomena induce a formation of rounded cells in the late stage of their mechanism. The presence of rounded cells in apoptosis is due to the breakdown of proteinaceous cytoskeleton by caspases (Bohm, 2003). Alternatively, the rounded cell phenomenon in a mitotic arrest represents cells in metaphase, which cannot advance to the next step of mitosis. Rounded cells normally occur in mitosis when cells down-regulate focal adhesions and increase their cortical tension and intracellular pressure (Stewart et al., 2011). Furthermore, a mitotic cell rounding is also involved in tissue organization, development, and homeostasis of dividing cells (Luxenburg et al., 2011; Kondo and hayashi, 2013).
Between those two phenomena, apoptosis is a more common explanation behind propolis’ suppressing action to vascular cells. In this study, it is also confirmed from the results of the growth factors-enhanced experiment on HUVECs. The relatively similar results between the two groups (with and without additional growth factors) compared to their respective vehicle control indicates an absent of cell cycle arrest, which signifies the insignificant effect of D01 towards microtubule dynamics.
On the contrary, Taxol presented a significant difference between the two groups, where the suppression of viability to the group with additional growth factors was stronger than the group without additional growth factors, confirming their function as an anti-mitotic drug. This hypothesis is substantiated by the results of the wound healing assay. Even though the results of the wound healing experiment of D01 are statistically significant in suppressing the cell migration of HUVECs—which strongly relates to microtubule dynamics—, they did not show a dose-response relationship. The absence of dose-response relationship in a migration assay demonstrates an indication of the indirect effect of the samples to microtubule dynamics, which needs to be further investigated. As a result of this hypothesis, the possibility of direct apoptosis as the main mechanism of D01 to suppress the HUVECs viability is now more plausible to prove.
In addition to the rounded cells, the indication of apoptosis in the present study can also be observed from the apoptotic bodies on the HUVECs’ photomicrographs (Fig. 6) especially at the higher concentrations of D01. These results may further strengthen the occurrence of apoptosis in the HUVECs following the treatment by D01.
Furthermore, provided the fact that D01 potentially suppress HUVEC viability, the analysis of its activity to pericytes was interesting. Pericytes are another major player of angiogenesis, which promote vessel stability by covering endothelial cells and producing chemical signals to maintain the attachment of both cells (Hall, 2006). The role of pericytes in normal vessels is also reflected in tumour vessels. Erber et al. (2004) showed that targeting only VEGFR-2 for inhibiting angiogenesis is not effective to degrade tumour cells because the endothelial cells are protected and stabilized by pericyte coverage. Interestingly, VEGF inhibition may also increase pericyte recruitment in tumour vessels (Benjamin et al., 1999), which may lead to pericyte-mediated resistance to VEGF-related anti-angiogenic therapies (Raza et al., 2010). Thus, achieving both endothelial cell and pericyte degradation in tumour vasculatures is important to simultaneously prevent their reformation and survival signals (Bergers et al., 2003).
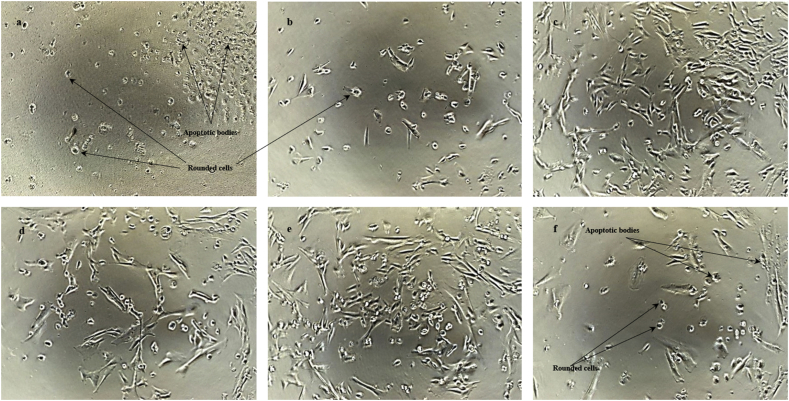
The findings in this work suggest that D01 has a potential inhibitory or cytotoxic effect to pericytes, although not as strong as to HUVECs, which may support the evidence of its therapeutic effects on tumour angiogenesis. From the respective figures (Fig. 7), it can be presumed that the mechanism of D01 in suppressing pericyte viability is tantamount to the mechanism on HUVECs. The presence of rounded cells with suspended cell extension and low cell density was common, especially at the higher concentrations of the samples. Based on the previous analysis, direct apoptosis may also be the main mechanism of D01 to reduce pericyte viability.
Fig. 7.
Photomicrographs of pericytes after the incubation with D01 for 48 hours. Pericytes were treated with various concentrations of D01: (a) 1000 μg/ml, (b) 100 μg/ml, (c) 10 μg/ml, (d) 1 μg/ml, (e) DMSO, and (f) 8nM Taxol. The images were taken after 48 hours of incubation and before addition of CCK-8 solution using Nikon Phase Contrast ELWD 0.3 microscope at 80X magnification.
4.3. Protective activity of propolis on CoCl2-induced pericyte loss
As an inherent aspect of inflammation, hypoxia plays an important role in the pathogenesis of many inflammation-related diseases including cancer. In the tumour microenvironment, hypoxia initiates tumour angiogenesis, which provides cancer cells with sufficient oxygen and nutrients. In addition, hypoxia affects destabilization of the vessels by inducing pericyte loss, which makes the endothelial cell sprouting possible. Unlike endothelial cells, the role of pericytes in tumour angiogenesis remains controversial. Although pericyte recruitment is perceived to promote cancer cell survival by stabilizing tumour vasculature as explained in the previous section, there are actually some contradictions whether pericyte recruitment in tumour vessels ameliorate or worsen the clinical conditions. Alternatively, instead of stabilizing vessels to support cancer cells, pericyte recruitment is important to restrict further vessel sprouting by—again—stabilizing the vessels. In addition, tumours with lower pericyte density are usually distinguished by a compact vasculature with active endothelial cell proliferation (Eberhard et al., 2000). Stabilization of the vessels may also limit tumour cell metastasis by reducing the leakiness of intratumoural blood vessels (Xian et al., 2006; Raza et al., 2010).
From this perspective, maintaining pericyte attachment to the vessels and preventing any pericyte loss is paramount to avoid unwanted angiogenesis in the tumour microenvironment. Therefore, protection of pericytes from hypoxia-induced loss can be one of the proposed mechanisms to inhibit tumour angiogenesis. In these experiments, CoCl2 was used to mimic hypoxic condition by inducing and stabilizing HIF-1/3α (Wu and Yotnda, 2011). The administration of CoCl2 to the pericytes in these experiments led to a major decline of their viability, which may relate to the “pericyte dropout” phenomenon in several angiogenesis diseases e.g. cancer, diabetic retinopathy, atherosclerosis and even in some cases of Alzheimer's disease (Data is represented in Fig. 5; The CoCl2-treated cells show a decline compared to the vehicle control).
D01 and D02 showed positive results by increasing the cell viability of CoCl2-treated pericytes when compared to the control. Interestingly, while D01 just restored the cell viability in certain concentrations, Indonesian propolis sample D02 was able to protect pericytes against CoCl2-induced loss in a dose-dependent manner. Thus, while D02 seemed to be ineffective to block HUVEC and pericyte proliferation, it may possess another bioactivity, which can synergise with anti-angiogenic drugs by protecting pericytes from hypoxia-induced loss.
While the exact mechanism of D02 in protecting pericytes against hypoxia-induced damage remain unknown, it may include a destabilization of HIF-1α (Daleprane and Abdalla, 2013) and a reduction of reactive oxygen species (ROS) by the abundant anti-oxidant compounds in propolis (Xuan et al., 2011). However, the chelation of CoCl2 by propolis samples may also become a robust alternative argument in this regard. Hence, a confirmation of their mechanism of action is still a top priority before confirming such a claim.
4.4. Indonesian propolis samples induce potential modulatory activities on different cell lines
It is interesting to notice that D01 induced a different pattern of activities on HUVECs, pericytes, and MDA-MB-231 compared to the pure compounds: CAPE and pinocembrin. One aspect worth highlighting is D01's weaker cytotoxic activity on pericytes compared to MDA-MB-231 cells as shown in Table 3b-c (IC50 62.35 μg/ml vs 48.23 μg/ml, respectively). In the case of CAPE and pinocembrin, their order of cytotoxic strength to these three cell lines is HUVECs > pericytes > MDA-MB-231. However, the order for D01 is HUVECs > MDA-MB-231 > pericytes. In addition, D01 has a distinct characteristic compared to of CAPE and pinocembrin, where it elicits a moderate elimination to HUVECs, a weaker activity to pericytes, but a considerably similar potency to destroy MDA-MB-231 cells (Table 3). This finding may correspond to the question of how to deal with pericytes in tumour vasculature as discussed in the previous section. In the case where pericyte population needs to be maintained in order to prevent endothelial cell sprouting, administration of D01 may aid by suppressing HUVECs population and moderately eliminating cancer cells, at a level where it is not severely cytotoxic to pericytes. For example, administration of 32 μg/ml D01 extract (the median of IC50 to HUVECs and MDA-MB-231) may suppress 97% HUVECs, 33% MDA-MB-231 and 28% pericytes. The potency of D01 in inhibiting the cell migration on both HUVECs and MDA-MB-231 cells, but not on pericytes, also supports this hypothesis.
Unlike D01, D02 appeared to be unsuitable as a cytotoxic agent. However, its excellent result in protecting pericytes from hypoxia-induced-damage provides a sign of its distinct bioactivity, which possibly derives from different constituents. In observing this fact, another idea that can be generated is to mix D01 and D02 in the correct ratio to produce a synergistic therapy on the tumour microenvironment. By that strategy, it may strengthen each other's potency and provide a better approach to eliminate cancer. Nevertheless, this hypothesis needs to be proven by identifying the respective pure compounds and how they interact with each other in the tumour microenvironment.
5. Conclusion
From a scientific perspective, many useful natural lead compounds await discovery as less than 10% of the world's biodiversity has been evaluated for potential biological activity (Cragg and Newman, 2005). However, the challenge to access, standardize and test this natural chemical diversity based on an analytical chemistry and pharmacology approach needs to be addressed before any claim is confirmed. As a complementary drug whose perspective is to benefit from a synergistic effect of multiple compounds, a combinatorial approach to co-administer both natural drug and conventional drug may also contribute to a novel strategy to deliver a better treatment.
From the results of the cell viability and wound healing assays of HUVECs and pericytes, the potency to develop D01 as an anti-proliferative and anti-migratory compound for preventing cancer angiogenesis is a rational option to explore. Albeit the unclarity of analysis of D01 pure compounds, most of them could be classified as modified terpenoids which are possibly the reason of their putative anti-angiogenic activities. Though it may not be as strong as Taxol at the same concentrations, D01 would be a good candidate as a complementary drug alongside certain existing anti-cancer drugs e.g. Taxol, Vincristine, Vinblastine. The administration of propolis as an adjuvant to reduce the complications of chemotherapy and increase the effectiveness of the treatment has been assessed by some studies (Padmavathi et al., 2006; Suzuki et al., 2002; Takara et al., 2007). The application of propolis as a chemotherapy adjuvant is also supported by the evidence that propolis administration to humans and rats is generally safe and does not induce detectable adverse effects (Jasprica et al., 2006; Watanabe et al., 2011). The idea is also strengthened by D02's protective activity towards pericytes which can be administered together with D01 to produce a further synergistic effect for tackling tumour angiogenesis.
It is interesting to note that both D01 and D02 have distinct activities toward vascular and cancer cells, even though they are produced from the same colony. Furthermore, both samples also have a slight difference of component although most of them are putatively under the class of terpenes and terpenoids. The unique characteristic of D01 and D02, in both chemical and pharmacological perspectives, has emerged new questions for understanding their exact mechanism and signalling on vascular and cancer cells. The outstanding questions that remain are: how can this difference happen despite being made by the same colony? Does the colony make the component of each sample intentionally different? Does the difference of the component relate to their distinct bioactivities? The answers to these questions can lead to a better understanding on how to harness, not only these two specific samples, but propolis in general as a source of novel therapy. In the later stage, the elucidation of their mechanisms of action can be enhanced using molecular docking and chemogenomic techniques in order to predict the interaction between the pure compounds and their specific targets. Nevertheless, an exact identification of their pure compounds is mandatory before conducting such studies. Although the components of the two samples responsible for their biological activities were not fully investigated in this research, present study opens a new perspective for further investigation. In conclusion, a continuation of this research is necessary to further develop both propolis samples to the stage of drug discovery, more specifically for the medical world, the economy and the society of Indonesia.
Declarations
Author contribution statement
Muhammad Iqbal: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Tai-Ping Fan: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
David Watson: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Samya Alenezi, Khaled Saleh: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Muhamad Sahlan: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by The Indonesian Endowment Fund for Education, Indonesia.
Competing interest statement
The authors declare no conflict of interest.
Additional information
Data associated with this study is available on request from the authors.
Acknowledgements
The authors acknowledge Dr. Gavin Jarvis of the Department of Physiology, Development and Neuroscience of the University of Cambridge for his input in generating dose-response curves of this work.
Contributor Information
Muhammad Iqbal, Email: muhammad.iqbal.marzuki@gmail.com.
Muhamad Sahlan, Email: sahlan@che.ui.ac.id.
References
- Alizadeh A.M., Afrouzan H., Dinparast-Djadid N., Sawaya A.C., Azizian S., Hemmati H.R., Mohagheghi M.A., Erfani S. Chemoprotection of MNNG-initiated gastric cancer in rats using Iranian propolis. Arch. Iran. Med. 2015;18(1):18–23. [PubMed] [Google Scholar]
- Almutairi S., Eapen B., Chundi S.M., Akhalil A., Siheri W., Clements C., Fearnley J., Watson D.G., Edrada-Ebel R. New anti-trypanosomal active prenylated compounds from african propolis. Phytochem Lett. 2014;10:35–39. [Google Scholar]
- Alyane M., Kebsa L.B.W., Boussenane H.N., Rouibah H., Lahouel M. Cardioprotective effects and mechanism of action of polyphenols extracted from propolis against doxorubicin toxicity. Pak. J. Pharm. Sci. 2008;21(3):201–209. [PubMed] [Google Scholar]
- Bankova V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005;100(1-2):114–117. doi: 10.1016/j.jep.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Bankova V., Galabov A.S., Antonova D., Vilhelmova N., Di Perri B. Chemical composition of Propolis Extract ACF and activity against herpes simplex virus. Phytomedicine. 2014;21(11):1432–1438. doi: 10.1016/j.phymed.2014.04.026. [DOI] [PubMed] [Google Scholar]
- Bankova V.S., de Castro S.L., Marcucci M.C. Propolis: recent advances in chemistry and plant origin. Apidologie. 2000;31(1):3–15. [Google Scholar]
- Bankova V., Boudourova-Krasteva G., Popov S., Sforcin J.M., Funari S.R.C. Seasonal variations of the chemical composition of Brazilian propolis. Apidologie. 1998;29(4):361–367. [Google Scholar]
- Benjamin L.E., Golijanin D., Itin A., Pode D., Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J. Clin. Investig. 1999;103(2):159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G., Song S., Meyer-Morse N., Bergsland E., Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Investig. 2003;111(9):1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm I. Disruption of the cytoskeleton after apoptosis induction with autoantibodies. J. Autoimmun. 2003;36:183–189. doi: 10.1080/0891693031000105617. [DOI] [PubMed] [Google Scholar]
- Boisard S., Le Ray A.M., Landreau A., Kempf M., Cassisa V., Flurin C., Richomme P. Antifungal and antibacterial metabolites from a French poplar type propolis. Evid. Based Complement Alternat. Med. 2015;2015:319240. doi: 10.1155/2015/319240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham J. CRC Press; Boca Raton: 1993. Dictionary of Natural Products. [Google Scholar]
- Chan G.C., Cheung K.W., Sze D.M. The immunomodulatory and anticancer properties of propolis. Clin. Rev. Allergy Immunol. 2013;44(3):262–273. doi: 10.1007/s12016-012-8322-2. [DOI] [PubMed] [Google Scholar]
- Cragg G.M., Newman D.J. Biodiversity: a continuing source of novel drug leads. Pure Appl. Chem. 2005;77:7–24. [Google Scholar]
- Daleprane J.B., Abdalla D.S. Emerging roles of propolis: antioxidant, cardioprotective, and antiangiogenic actions. Evid. Based Complement Alternat. Med. 2013;2013:175135. doi: 10.1155/2013/175135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duailibe S.A., Gonçalves A.G., Ahid F.J. Effect of a propolis extract on Streptococcus mutans counts in vivo. J. Appl. Oral Sci. 2007;15(5):420–423. doi: 10.1590/S1678-77572007000500009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziedzic A., Kubina R., Wojtyczka R.D., Kabała-Dzik A., Tanasiewicz M., Morawiec T. The antibacterial effect of ethanol extract of polish propolis on mutans streptococci and lactobacilli isolated from saliva. Evid. Based Complement Alternat. Med. 2013;2013:681891. doi: 10.1155/2013/681891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard A., Kahlert S., Goede V., Hemmerlein B., Plate K.H., Augustin H.G. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res. 2000;60(5):1388–1393. [PubMed] [Google Scholar]
- Erber R., Thurnher A., Katsen A.D., Groth G., Kerger H., Hammes H.P., Menger M.D., Ullrich A., Vajkoczy P. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18:338–340. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- Fokt H., Pereira A., Ferreira A.M., Cunha A., Aguiar C. How do bees prevent hive infections? The antimicrobial properties of propolis. In: Mendez-Vilas A., editor. Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. Formatex Research Center; Badajoz: 2010. pp. 481–493. [Google Scholar]
- Hall P. Review of the pericyte during angiogenesis and its role in cancer and diabetic retinopathy. Toxicol. Pathol. 2006;34(6):763–775. doi: 10.1080/01926230600936290. [DOI] [PubMed] [Google Scholar]
- Izuta H., Shimazawa M., Tsuruma K., Araki Y., Mishima S., Hara H. Bee products prevent VEGF-induced angiogenesis in human umbilical vein endothelial cells. BMC Complement Altern. Med. 2009;9:45. doi: 10.1186/1472-6882-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasprica I., Mornar A., Debeljak Z., Smolcic-Bubalo A., Medic-Saric M., Mayer L., Romic Z., Bucan K., Balog T., Sobocanec S., Sverko V. In vivo study of propolis supplementation effects on antioxidative status and red blood cells. J. Ethnopharmacol. 2006;110(3):548–554. doi: 10.1016/j.jep.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Kim M.S., Lee Y.M., Moon E.J., Kim S.E., Lee J.J., Kim K.W. Anti-angiogenic activity of torilin, a sesquiterpene compound isolated from Torilis japonica. Int. J. Cancer. 2000;87:269–275. [PubMed] [Google Scholar]
- Kondo T., Hayashi S. Mitotic cell rounding accelerates epithelial invagination. Nature. 2013;494(7435):125–129. doi: 10.1038/nature11792. [DOI] [PubMed] [Google Scholar]
- Kumazawa S., Hamasaka T., Nakayama T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004;84(3):329–339. [Google Scholar]
- Kujumgiev A., Tsvetkova I., Serkedjieva Y., Bankova V., Christov R., Popov S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol. 1999;64(3):235–240. doi: 10.1016/s0378-8741(98)00131-7. [DOI] [PubMed] [Google Scholar]
- Kunimasa K., Ahn M.R., Kobayashi T., Eguchi R., Kumazawa S., Fujimori Y., Nakano T., Nakayama T., Kaji K., Ohta T. Brazilian propolis suppresses angiogenesis by inducing apoptosis in tube-forming endothelial cells through inactivation of survival signal ERK1/2. Evid. Based Complement Alternat. Med. 2011;2011:870753. doi: 10.1093/ecam/nep024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Wei K., Yang S., Yang Y., Zhang Y., Zhu F., Wang D., Zhu R. Immunomodulatory effects of taishan Pinus massoniana pollen polysaccharide and propolis on immunosuppressed chickens. Microb. Pathog. 2015;78:7–13. doi: 10.1016/j.micpath.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Luxenburg C., Pasolli H.A., Williams S.E., Fuchs E. Developmental roles for Srf, cortical cytoskeleton and cell shape in epidermal spindle orientation. Nat. Cell Biol. 2011;13(3):203–214. doi: 10.1038/ncb2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcucci M.C. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26(2):83–99. [Google Scholar]
- Miguel M.G., Nunes S., Dandlen S.A., Cavaco A.M., Antunes M.D. Phenols and antioxidant activity of hydro-alcoholic extracts of propolis from Algarve, South of Portugal. Food Chem. Toxicol. 2010;48(12):3418–3423. doi: 10.1016/j.fct.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Mirzoeva O.K., Calder P.C. The effect of propolis and its components on eicosanoid production during the inflammatory response. Prostagl. Leukot. Essent. Fat. Acids. 1996;55(6):441–449. doi: 10.1016/s0952-3278(96)90129-5. [DOI] [PubMed] [Google Scholar]
- Moreira L., Dias L.G., Pereira J.A., Estevinho L. Antioxidant properties, total phenols and pollen analysis of propolis samples from Portugal. Food Chem. Toxicol. 2008;46(11):3482–3485. doi: 10.1016/j.fct.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Naito Y., Yasumuro M., Kondou K., Ohara N. Antiinflammatory effect of topically applied propolis extract in carrageenan-induced rat hind paw edema. Phytother Res. 2007;21:452–456. doi: 10.1002/ptr.2093. [DOI] [PubMed] [Google Scholar]
- Padmavathi R., Senthilnathan P., Chodon D., Sakthisekaran D. Therapeutic effect of paclitaxel and propolis on lipid peroxidation and antioxidant system in 7,12 dimethyl benz(a)anthracene-induced breast cancer in female Sprague Dawley rats. Life Sci. 2006;78:2820–2825. doi: 10.1016/j.lfs.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Pluskal T., Castillo S., Villar-Briones A., Orešič M. MZmine 2: modular framework for processing, visualizing, and analyzing massspectrometry-based molecular profile data. BMC Bioinf. 2010;11:395. doi: 10.1186/1471-2105-11-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontin K., Da Silva Filho A.A., Santos F.F., Silva M.L., Cunha W.R., Nanayakkara N.P., Bastos J.K., de Albuqueque S. In vitro and in vivo antileishmanial activities of a Brazilian green propolis extract. Parasitol. Res. 2008;103(3):487–492. doi: 10.1007/s00436-008-0970-z. [DOI] [PubMed] [Google Scholar]
- Raza A., Franklin M.J., Dudek A.Z. Pericytes and vessel maturation during tumor angiogenesis and metastasis. Am. J. Hematol. 2010;85(8):593–598. doi: 10.1002/ajh.21745. [DOI] [PubMed] [Google Scholar]
- Sawicka D., Car H., Borawska M.H., Niklinski J. The anticancer activity of propolis. Folia Histochem. Cytobiol. 2012;50(1):25–37. doi: 10.2478/18693. [DOI] [PubMed] [Google Scholar]
- Sforcin J.M., Bankova V. Propolis: is there a potential for the development of new drugs? J. Ethnopharmacol. 2011;133(2):253–260. doi: 10.1016/j.jep.2010.10.032. [DOI] [PubMed] [Google Scholar]
- Shen H., Yamashita A., Nakakoshi M., Yokoe H., Sudo M., Kasai H., Tanaka T., Fujimoto Y., Ikeda M., Kato N., Sakamoto N., Shindo H., Maekawa S., Enomoto N., Tsubuki M., Moriishi K. Inhibitory effects of caffeic acid phenethyl ester derivatives on replication of hepatitis C virus. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0082299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Hino A., Tsutsumi A., Park Y.K., Watanabe W., Kurokawa M. Anti-influenza virus activity of propolis in vitro and its efficacy against influenza infection in mice. Antivir. Chem. Chemother. 2008;19(1):7–13. doi: 10.1177/095632020801900102. [DOI] [PubMed] [Google Scholar]
- Siheri W., Igoli J.O., Gray A.I., Nasciemento T.G., Zhang T., Fearnley J., Clements C.J., Carter K.C., Carruthers J., Edrada-Ebel R., Watson D.G. The isolation of antiprotozoal compounds from Libyan propolis. Phytother Res. 2014;28(12):1756–1760. doi: 10.1002/ptr.5194. [DOI] [PubMed] [Google Scholar]
- Sogno I., Vannini N., Lorusso G., Cammarota R., Noonan D.M., Generoso L., Sporn M.B., Albini A. Anti-angiogenic activity of a novel class of chemopreventive compounds: oleanic acid terpenoids. Recent Results Canc. Res. 2009;181:209–212. doi: 10.1007/978-3-540-69297-3_19. [DOI] [PubMed] [Google Scholar]
- Stewart M.P., Helenius J., Toyoda Y., Ramanathan S.P., Muller D.J., Hyman A.A. Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding. Nature. 2011;469:226–230. doi: 10.1038/nature09642. [DOI] [PubMed] [Google Scholar]
- Sun F., Hayami S., Haruna S., Ogiri Y., Tanaka K., Yamada Y., Ikeda K., Yamada H., Sugimoto H., Kawai N., Kojo S. In vivo antioxidative activity of propolis evaluated by the interaction with vitamins C and E and the level of lipid hydroperoxides in rats. J. Agric. Food Chem. 2000;48(5):1462–1465. doi: 10.1021/jf990594t. 2000. [DOI] [PubMed] [Google Scholar]
- Suzuki I., Hayashi I., Takaki T., Groveman D.S., Fujimiya Y. Antitumor and anticytopenic effects of aqueous extracts of propolis in combination with chemotherapeutic agents. Cancer Biother. Radiopharm. 2002;17(5):553–562. doi: 10.1089/108497802760804781. [DOI] [PubMed] [Google Scholar]
- Takara K., Fujita M., Matsubara M., Minegaki T., Kitada N., Ohnishi N., Yokoyama T. Effects of propolis extract on sensitivity to chemotherapeutic agents in HeLa and resistant sublines. Phytother Res. 2007;21:841–846. doi: 10.1002/ptr.2165. [DOI] [PubMed] [Google Scholar]
- Tao Y., Wang D., Hu Y., Huang Y., Yu Y., Wang D. The immunological enhancement activity of propolis flavonoids liposome in vitro and in vivo. Evid. Based Complement Alternat. Med. 2014;2014:483513. doi: 10.1155/2014/483513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusheva B., Popova M., Koendhori E.B., Tsvetkova I., Naydenski C., Bankova V. Indonesian propolis: chemical composition, biological activity and botanical origin. Nat. Prod. Res. 2011;25(6):606–613. doi: 10.1080/14786419.2010.488235. [DOI] [PubMed] [Google Scholar]
- Umthong S., Phuwapraisirisan P., Puthong S., Chanchao C. In vitro antiproliferative activity of partially purified Trigona laeviceps propolis from Thailand on human cancer cell lines. BMC Complement Altern. Med. 2011;11:37. doi: 10.1186/1472-6882-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velikova M., Bankova V., Sorkun K., Houcine S., Tsvetkova I., Kujumgiev A. Propolis from the Mediterranean region: chemical composition and antimicrobial activity. Z. Naturforsch. C Biosci. 2000;55(9-10):790–793. doi: 10.1515/znc-2000-9-1019. [DOI] [PubMed] [Google Scholar]
- Watanabe M.A., Amarante M.K., Conti B.J., Sforcin J.M. Cytotoxic constituents of propolis inducing anticancer effects: a review. J. Pharm. Pharmacol. 2011;63(11):1378–1386. doi: 10.1111/j.2042-7158.2011.01331.x. [DOI] [PubMed] [Google Scholar]
- Wu D., Yotnda P. Induction and testing of hypoxia in cell culture. J. Vis. Exp. 2011;54:2899. doi: 10.3791/2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Omene C., Karkoszka J., Bosland M., Eckard J., Klein C.B., Frenkel K. Caffeic acid phenethyl ester (cape), derived from a honeybee product propolis, exhibits a diversity of anti-tumour effects in pre-clinical models of human breast cancer. Cancer Lett. 2011;308(1):43–53. doi: 10.1016/j.canlet.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian X., Håkansson J., Ståhlberg A., Lindblom P., Betsholtz C., Gerhardt H., Semb H. Pericytes limit tumor cell metastasis. J. Clin. Investig. 2006;116:642–651. doi: 10.1172/JCI25705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Luo L., Chen B., Fu Y. Recent development of chemical components in propolis. Front. Biol. 2009;4(4):385. [Google Scholar]
- Xuan H., Zhao J., Miao J., Li Y., Chu Y., Hu F. Effect of Brazilian propolis on human umbilical vein endothelial cell apoptosis. Food Chem. Toxicol. 2011;49(1):78–85. doi: 10.1016/j.fct.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Yano K., Yokoi K., Sato J., Oono J., Kouda T., Ogawa Y., Nakashima T. Actinopyrones A, B and C, new physiologically active substances. J. Antibiot (Tokyo) 1986;39(1):32–37. doi: 10.7164/antibiotics.39.32. [DOI] [PubMed] [Google Scholar]