Abstract
Fluconazole is used to treat hematogenous Candida meningoencephalitis in preterm and term infants. To characterize plasma and central nervous system exposure, an adult fluconazole physiologically‐based pharmacokinetic (PBPK) model was scaled to infants, accounting for age dependencies in glomerular filtration and metabolism. The model was optimized using 760 plasma samples from 166 infants (median postmenstrual age (range) 28 weeks (24–50)) and 27 cerebrospinal fluid (CSF) samples from 22 infants (postmenstrual age 28 weeks (24–33)). Simulations evaluated achievement of the surrogate efficacy target of area under the unbound concentration‐time curve ≥ 400 mg • hour/L over the dosing interval in plasma and CSF using dosing guidelines. Average fold error of predicted concentrations was 0.73 and 1.14 for plasma and CSF, respectively. Target attainment in plasma and CSF was reached faster after incorporating a loading dose of 25 mg/kg. PBPK modeling can be useful in exploring CNS kinetics of drugs in children.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Fluconazole is an antifungal agent widely used for treatment and prevention of Candida meningitis in preterm and term infants. Although the ratio of cerebrospinal fluid to plasma is known to exceed 0.8 in adults, fluconazole's central nervous system (CNS) distribution has not been characterized in infants.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ We leveraged physiologically‐based pharmacokinetic (PBPK) modeling to characterize fluconazole's CNS distribution in preterm and term infants and used this to evaluate the suggested dosing regimens for CNS infections in this population.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ PBPK model simulations suggest a fluconazole CNS to plasma concentration ratio of ~1 for preterm and term infants and support the addition of a loading dose for treatment of Candida meningitis.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
☑ This study exemplifies the use of PBPK modeling to characterize a drug's CNS distribution in pediatric populations using opportunistic sampling and can possibly be applied to predict the distribution of drugs with similar hypothesized CNS penetration.
Hematogenous Candida meningoencephalitis (HCME), a manifestation of invasive candidiasis in the central nervous system (CNS), is a leading cause of neurodevelopmental impairment and death in infants.1 Infant prematurity is a principal risk factor, and most infants have other predisposing factors increasing their risk for HCME, such as the use of prolonged broad‐spectrum antibiotics and/or steroids.2, 3, 4 There are no antifungal dosing recommendations for infants < 1 month of age approved by the US Food and Drug Administration, prompting a crucial need for dosing guidance to reach rapid exposure in both plasma and cerebrospinal fluid (CSF) for this vulnerable infant population.
Fluconazole is a commonly prescribed antifungal for infants in neonatal intensive care units and is used for the prevention and treatment of invasive candidiasis, including HCME.5 In adults, fluconazole is well absorbed (oral bioavailability > 90%), exhibits excellent penetration of the CNS with CSF concentrations ≥ 80% of the corresponding concentration in plasma, and is largely eliminated by the kidneys with minimal metabolism via uridine 5′‐diphospho‐glucuronosyl‐transferase 2B7 (UGT2B7).6, 7, 8, 9, 10 In infants, fluconazole displays high oral bioavailability (model‐derived estimate of 100%), and population pharmacokinetic (PopPK) models have identified age and serum creatinine as covariates in explaining interindividual variability of clearance.11, 12 Although widely used in infants, there have been no studies to characterize fluconazole's CNS penetration in this population, and thus optimal dosing for HCME is unknown.
The CNS penetration of fluconazole has not been characterized in infants, likely because of the challenges with CSF sampling in this population. CSF sampling requires invasive methods such as lumbar puncture, which is associated with an increased risk of head bleeds during the first days of life.13 In addition, low sample volume availability in infants precludes the more robust sampling necessary for characterizing CNS disposition.14 One approach to overcoming CSF sampling challenges is physiologically‐based pharmacokinetic (PBPK) modeling, which allows for the characterization of age‐dependent changes in renal function and metabolism as well as parameterization of physiological spaces relevant to drug disposition, such as the CNS.15 PBPK models can be evaluated with clinical data and then used to optimize dosing based on target plasma and CSF exposure. However, these challenges in sampling have likely hindered PBPK modeling of CSF exposure, as only one study to date has used CSF samples from a pediatric population to evaluate a PBPK model to predict CSF pharmacokinetics (PKs).16
The objective of this study was to develop a PBPK model of fluconazole in preterm and term infants that accurately predicts CSF concentrations to inform dosing for the treatment of HCME. This PBPK model was scaled to preterm and term infants from a previously developed PBPK model using plasma data from a study in preterm infants, henceforth referred to as the prophylaxis study, and then externally evaluated using plasma data from a second clinical study of preterm and term infants, henceforth referred to as the Pediatric Pharmacology Research Unit (PPRU) study.12, 17, 18 CSF concentration predictions were evaluated using CSF samples obtained from both studies, and the model was used to evaluate dosing guidelines.
Methods
Clinical data
Data for model development came from two clinical studies referred to as the prophylaxis and PPRU studies. The prophylaxis study (ClinicalTrials.gov no. NCT00734539) was a multicenter, randomized, placebo‐controlled study that included extremely low birth weight preterm infants (< 750 g).18 Infants were given 6 mg/kg intravenous (i.v.) or oral doses twice weekly, with treatment beginning <5 days after birth and continuing for 6 weeks.18 Samples taken with a serum creatinine recorded value of > 2 mg/dL were excluded from this analysis, and only i.v. data were considered for this study. The PPRU study (ClinicalTrials.gov no. NCT00514358) included data from an open‐label, multicenter study that enrolled both preterm and term infants and stratified enrollment by gestational age (GA) and postnatal age (PNA), as well as an open‐label PK study enrolling both preterm and term infants at a single center.12 All studies were approved by institutional review boards at participating sites, and informed consent was obtained from parents or legal guardians.12, 18 Dosing occurred per routine clinical care.12 Dosing and demographic data for both the prophylaxis and PPRU study populations are presented in Table 1. For both studies, 54% of plasma samples were scavenged or leftover from blood drawn as part of routine clinical care.12, 18 A total of 27 CSF samples from 22 infants were scavenged from CSF obtained from lumbar punctures performed as part of routine clinical care for infants in both studies. The prophylaxis study samples were analyzed via a high‐performance liquid chromatography/tandem mass spectrometry method developed by a central laboratory (OpAns, LLC, Durham, NC; see Supplementary Material S1), and the PPRU study's analytical method has been previously published.19
Table 1.
Population demographics of infants used in model development
| Population characteristic | Prophylaxis study18 | PPRU study12 | Infants with CSF dataa |
|---|---|---|---|
| Number of infants | 120 | 46 | 22 |
| Number of samples | 489b | 298c | 27 |
| Number of doses | 11 (1–13) | 7 (1–61) | 12 (1–13) |
| Dose, mg/kgd | 6.0 (2.8–8.1) | 2.9 (0.8–12.5) | 5.9 (1.9–7.3) |
| Actual dose, mgd | 4.3 (1.9–18.0) | 4.2 (1.4–85.0) | 4.6 (2.8–9.0) |
| Dosing frequency, hr | 89 (0–433) | 24 (0–272) | 73 (0–167) |
| Gestational age, weeks | 24.9 (22.6–28.7) | 26.0 (24.0–40.0) | 25.5 (22.6–37.0) |
| Postnatal age,e days | 19.0 (3.0–46.0) | 21.0 (2.0–93.0) | 18.0 (7.0–50.0) |
| Postmenstrual age,e weeks | 27.6 (23.7–35.1) | 30.0 (24.7–49.6) | 27.9 (23.7–33.1) |
| Weight,e g | 720 (345–2680) | 1155 (451–7138) | 780 (490–2350) |
| Serum creatinine, mg/dL | 0.8 (0.1–2.0) | 0.4 (0.1–1.9) | 0.7 (0.2–2.0) |
| Sex, % | |||
| Male | 36.7 | 54.3 | 45.5 |
| Unknown/not reported | 0 | 6.5 | 0 |
| Ethnicity,f % | |||
| Not Hispanic or Latino | 86.7 | 82.6 | 81.8 |
| Hispanic or Latino | 13.3 | 8.7 | 18.2 |
| Unknown/not reported | 0 | 8.7 | 0 |
| Race,f % | |||
| White or Caucasian | 40.0 | 47.8 | 45.4 |
| Black or African American | 52.5 | 37.0 | 45.5 |
| American Indian or Alaska native | 5.8 | 0 | 9.1 |
| Asian | 1.7 | 4.3 | 0 |
| Native Hawaiian/other Pacific Islander | 0 | 2.2 | 0 |
| More than one race | 0 | 0 | 0 |
| Unknown/not reported | 0 | 8.7 | 0 |
Data reported as median (range) for continuous variables.
CSF, cerebrospinal fluid; PPRU, Pediatric Pharmacology Research Unit.
aPreviously unpublished data. bOf the 489 samples used in model development, 464 were plasma samples, and 25 were CSF samples. Oral samples from this study were excluded from analysis. cOf the 298 samples used in model development, 296 were plasma samples, and 2 were CSF samples. dMedian (range) was calculated across all administered doses. eMedian (range) was calculated across all sample records. fFor the prophylaxis study, the maternal ethnicity and race were recorded.
PBPK model development
The PBPK modeling software used was PK‐Sim (version 7.1, Open Systems Pharmacology Suite, open‐systems‐pharmacology.com; see Supplementary Material S2 for more information). Perfusion‐rate limitation was assumed with each compartment represented as well stirred. A previously developed fluconazole PBPK model was scaled from adults to preterm infants.17 Briefly, the adult base model, developed in PK‐Sim, assumed clearance occurred 85% renally and 15% hepatically via UGT2B7 mechanisms.6, 17, 20 Adult renal clearance via the glomerular filtration rate (GFR) was predicted using a GFR fraction to account for tubular reabsorption or secretion as defined by Eq. (1):
| (1) |
where the empiric CLR is the literature renal clearance value in adults, and the expected CLR is the clearance as a result of GFR if there was no tubular reabsorption (i.e., fraction unbound × normal GFR).17 A GFR fraction of less than one indicates tubular reabsorption, whereas a GFR fraction of greater than one indicates tubular secretion. UGT2B7 clearance values were obtained from literature.6 Additional methods and equations for model scaling can be found in Supplementary Material S1.
The preterm infant population from PK‐Sim was used to develop virtual infant populations. To examine if the infant cohort from these two studies was similar with respect to distribution and clearance to the cohort from the previously developed pediatric PBPK model, the dose‐normalized and time‐normalized prophylaxis study plasma data were used for the optimization of lipophilicity, UGT2B7 clearance, and GFR fraction using the Nelder–Mead algorithm.17, 21 In addition, the PK‐Sim Standard, Rodgers and Rowland, Schmitt, Poulin and Thiel, and the Berezhkovskiy methods were evaluated for the calculation of partition coefficients and cellular permeability values. Similarly, CSF data were used to optimize the brain interstitial to plasma partition coefficient to determine if the partition coefficient algorithm accurately captured this ratio. Data were dose‐normalized by the actual weight‐based dose and time‐normalized by accounting for the difference between the actual and simulated time of dose administration. All relevant parameter distributions were used as default values in PK‐Sim. The exception was the addition of population variability for fraction unbound, which was included assuming a normal distribution and coefficient of variation of 25%.
The brain was modeled using the following four default subcompartments: blood, plasma, interstitial fluid (representing the CSF), and intracellular compartments.22 These subcompartments were linked to the entire PBPK model through arterial and venous blood compartments.22 The brain blood flow, volume, and weight were all scaled by age using a step function for preterm and term infants as implemented in PK‐Sim.22 Drug permeability into the brain was determined by Eq. (2):
| (2) |
where P is the specific organ permeability, MWeff is the effective molecular weight, and logP is the lipophilicity.22 Because the modeling of oral dosing in preterm infant populations is not yet available in PK‐Sim, oral doses for the PPRU study and those preceding a CSF sample were modeled as i.v. boluses of equal amounts, corresponding to a reported 100% model‐derived oral bioavailability in preterm infants.11, 22 Of all the administered doses, only 15% were oral.
A local sensitivity analysis was performed following a simulation of an infant with the median demographics from the prophylaxis study to determine which of the model input parameters, when altered by 10%, caused a ≥10% change in simulated plasma area under the plasma concentration‐time curve from the time of the first dose to the time of the second dose (AUCDose1‐Dose2) or half‐life.22
Model evaluation
The adult model was evaluated using digitized CSF data from adults with cryptococcal meningitis receiving mostly 800 mg daily oral doses of fluconazole (see Supplementary Material S1 and Figure S2). After scaling the model to children, the pediatric model was evaluated using plasma and CSF data from both the prophylaxis and PPRU studies. A virtual population of 1,000 infants was simulated in PK‐Sim based on the infant demographics for each study. Changes in growth and maturation were accounted for in these virtual infants during the course of the entire simulation time period. Because the PPRU study had wide variations in dosing, a simulation was performed for each infant based on that infant's specific dosing regimen. The number of observed samples falling outside of the 90% prediction interval was calculated. The accuracy of model predictions was explored by calculating the average fold error (AFE) of the observed to median simulated concentration for all samples according to Eq. (3):
| (3) |
Model acceptance criteria were defined as simulated concentrations with AFE within twofold of observed values.
Because there are no estimates of fluconazole clearance for infants determined through intensive sampling and noncompartmental methods, the PBPK clearance estimates were compared with estimates obtained from an external evaluation of two PopPK models previously developed for the prophylaxis and PPRU trials as well as to clearance listed in the Food and Drug Administration product label (mean [% coefficient of variation] 0.180 [35%] and 0.218 [31%] mL/minute/kg at 1.5 and 7.5 days from birth, respectively, for infants 26–29 weeks GA).7, 11, 12, 23 Infants in the virtual PBPK population were stratified by postmenstrual age (PMA), the sum of both GA and PNA. Each PBPK group's mean clearance was compared with the mean clearance from the PopPK model and product label value. To compare clearance values, the ratio of the means was calculated along with a standard deviation ratio (ratioSD) according to Eq. (4):
| (4) |
where sd(observed) and sd(PBPK) are the standard deviations of the PopPK or product label and PBPK clearance values, and mean(observed) and mean(PBPK) are the averages of the PopPK or product label and PBPK values.24
Dose–exposure assessment
Model simulations were performed to evaluate current Infectious Disease Society of America (IDSA) recommendations of 12 mg/kg/day i.v. over 14 days for the treatment of Candida and CNS infection in preterm infants.25 The addition of an i.v. loading dose for treatment was evaluated because PopPK models developed in infants have shown the need for a loading dose to achieve efficacy within the first 24 hours.12, 26
Target attainment was defined using the surrogate efficacy target of area under the concentration‐time curve over 24 hours (fAUC) ≥ 400 mg • hour/L. This was chosen assuming an fAUC/minimum inhibitory concentration (MIC) ≥ 50, a ratio associated with clinical efficacy in plasma, and an MIC of 8 mg/L, which is the Clinical and Laboratory Standards Institute sensitivity breakpoint for all Candida species.27, 28, 29 There are no known targets established for CSF exposure, so these were assumed equal to plasma given the ~1:1 CSF‐to‐plasma ratio observed in adults.8, 9
Throughout the prophylaxis study, Candida isolates were collected at study days 0–7, study days 8–28, and study days 29–49.30 MIC values were tested using the Clinical and Laboratory Standards Institute method for all positive Candida cultures.30 Simulations of target attainment for the prophylaxis study were performed using the actual median MIC values at each study period and a target fAUC:MIC ≥ 50.
Results
CSF data
A total of 27 CSF samples were obtained as part of lumbar punctures performed per clinical care from 22 infants from the prophylaxis and PPRU studies (Table 1). All samples were above the limit of quantitation (0.01 and 0.1 mg/L for the prophylaxis and PPRU study, respectively).19 CSF concentrations ranged from 0.1–9.6 mg/L and were obtained 3.3–219.3 hours from the last dose (Figures 1 and S1 ). One infant who had a CSF sample taken within 5 minutes of a plasma sample (1,470 and 1,475 minutes after the last dose for the plasma and CSF sample, respectively) had a CSF‐to‐plasma ratio of 0.98.
Figure 1.
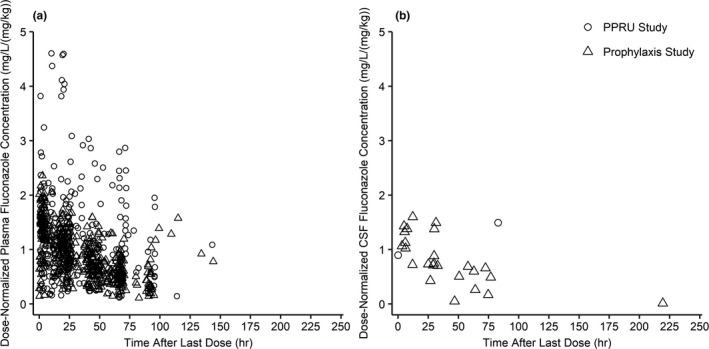
Observed plasma and CSF dose‐normalized concentration after last fluconazole dose for preterm and term infants. Dose‐normalized concentration vs. time from last dose observed from preterm and term infants enrolled in the prophylaxis and PPRU studies following various treatment and prophylactic fluconazole dosing regimens for plasma (a) and CSF (b) samples. CSF, cerebrospinal fluid; hr, hour; PPRU, Pediatric Pharmacology Research Unit.
PBPK model development
Lipophilicity and UGT2B7 clearance optimization (to 1.10 and 0.005 1/minute, respectively) using the prophylaxis study data resulted in very similar values as those optimized in a previous pediatric fluconazole PBPK model, and so the original values were retained.17 The optimized GFR fraction of 0.30 for this model differed from the previous model's finding of 0.17, suggesting less tubular reabsorption.17 Brain interstitial fluid to plasma partition coefficient optimization yielded values within 10% of those calculated using the Rodgers and Rowland method, so the Rodgers and Rowland values were retained.31 The resulting median fraction of drug excreted unchanged in urine after five half‐lives (150 hours) was 0.9. See Supplementary Material S2 and Table S1 for a list of model parameters.
A sensitivity analysis revealed that of all physicochemical parameters evaluated, the plasma AUCDose1‐Dose2 and half‐life were most sensitive to lipophilicity. No other parameters caused a ≥ 10% change in key PK parameters.
Model evaluation
For plasma, 78% and 84% of the samples from the prophylaxis and PPRU studies, respectively, fell within the 90% prediction interval (Figures 2 and S3 ). The CSF samples were generally well captured, with some clear outlying points. For CSF, 16/25 (64%) samples for the prophylaxis study and 1/2 (50%) samples for the PPRU study fell within the 90% model prediction interval. To understand individual model misspecification, trends in AFE were evaluated. The overall AFE was 0.73 and 1.14 for plasma and CSF concentrations, respectively, demonstrating that the median predicted concentrations generally fell within twofold of the observed values for both studies. When calculating AFE by infant, the AFE for infants with a greater number of samples was more likely to fall within the twofold range (Figure 3). There may be an increasing trend in misspecification with PMA as determined by AFE (Figure 3), as the majority of data used in model development came from preterm infants in the prophylaxis study with a PMA < 35 weeks. There were no differences in misspecification between samples taken after an i.v. vs. oral dose. AFE trended downward with increasing serum creatinine but still generally fell within the twofold range (Figure 3).
Figure 2.
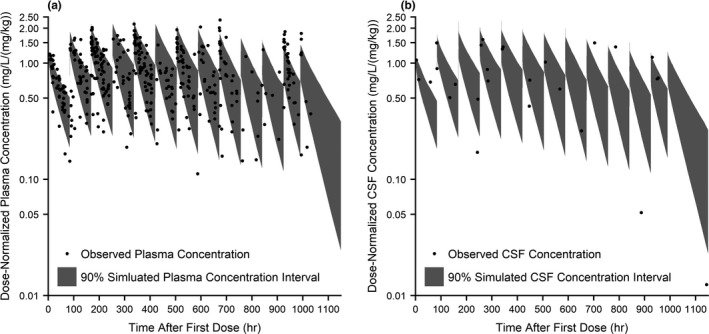
Population simulations (n = 1,000) of plasma and CSF fluconazole concentration following prophylactic dosing (6 mg/kg twice weekly) in preterm infants. Population simulations of plasma (a) and CSF (b) are shown overlaid with observed time‐normalized and dose‐normalized data. The shaded regions are the 5–95% range in concentration from 1,000 simulated infants reflective of the prophylaxis study demographics. CSF, cerebrospinal fluid; hr, hour.
Figure 3.
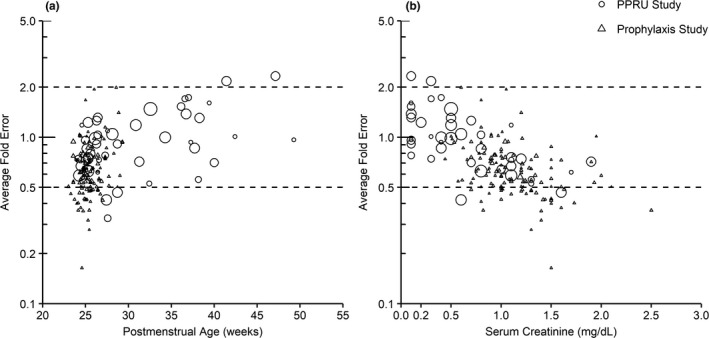
Average fold error of predicted to simulated median fluconazole plasma concentration in preterm and term infants. Average fold error of the predicted to observed plasma concentration is plotted against postmenstrual age (a) and serum creatinine (b) for each infant in the prophylaxis and PPRU studies following various treatment and prophylactic fluconazole dosing regimens. Symbol size is scaled according to the number of observed samples obtained from each infant. Dashed lines represent the twofold error range. Predicted concentration was obtained from a simulated population of 1,000 virtual infants for the prophylaxis study and populations of 100 virtual infants for each individual infant in the PPRU study. PPRU, Pediatric Pharmacology Research Unit.
Clearance from a simulated population of 1,000 infants increased from 0.003 to 0.074 L/hour/kg with increasing PMA, with greater variability observed in older infants (Figure 4). The mean ratio was calculated of PBPK‐derived clearance to clearance derived from a previously developed PopPK model as well as to clearance reported in product labeling for infants 26–29 weeks GA.7, 23 With the exception of the 26‐week PMA group, the mean ratios fell within the twofold range (Figure 5). PBPK‐derived clearance was higher than PopPK estimates but lower than product label values. Volume of distribution estimates from the PopPK model were in accordance with the PBPK model, with 100% of PopPK estimates falling within twofold of the PBPK estimates (Figure S4).
Figure 4.
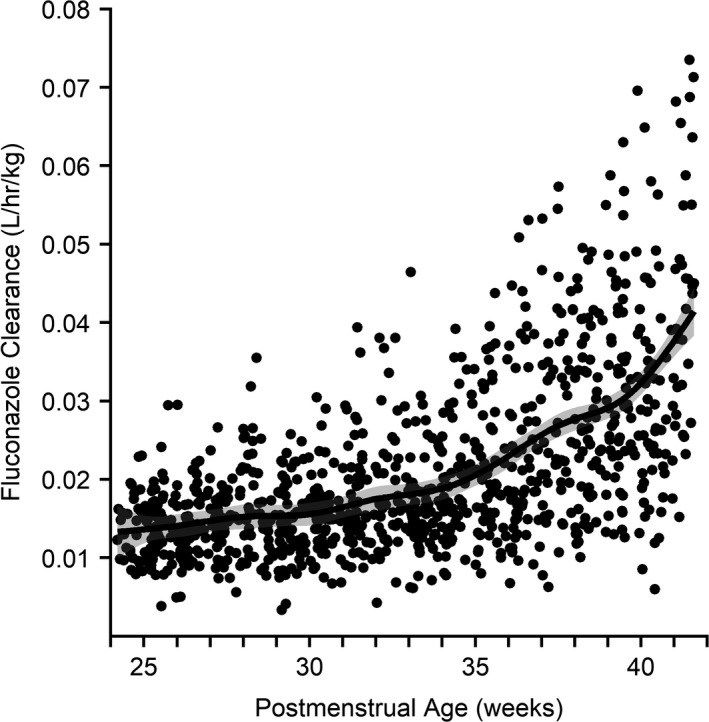
Model‐derived fluconazole clearance vs. postmenstrual age. Individual fluconazole clearance values at steady state from a simulated population (n = 1,000) of virtual preterm and term infants are plotted against postmenstrual age. The line represents the Loess line as calculated by the generalized additive model with the corresponding 95% confidence interval represented by the shaded region.
Figure 5.
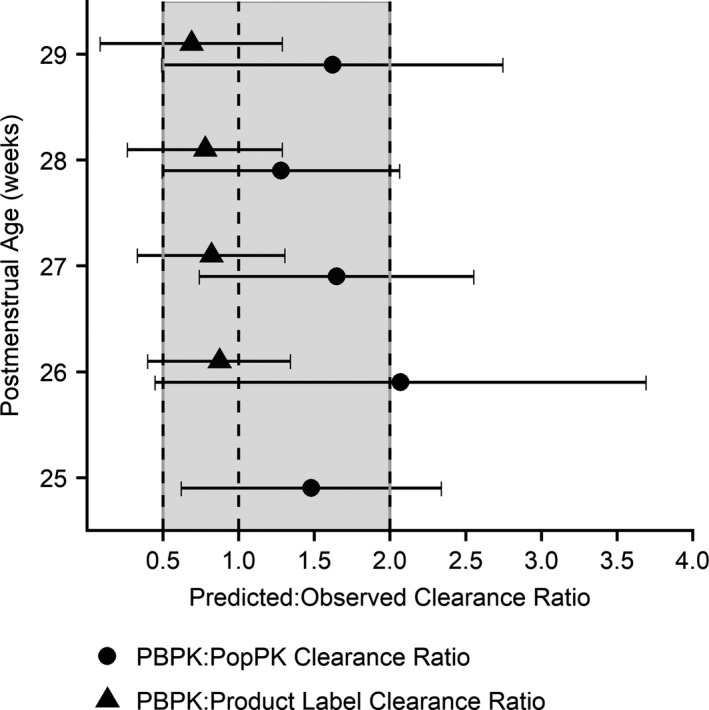
Comparison of PBPK model‐derived fluconazole clearance to clearance derived from a PopPK model and clearance reported by the product label. Circles represent the mean ratio of PBPK‐derived clearance to PopPK‐derived clearance estimates from a previous study.11, 18, 23 The model parameters from the PPRU study PopPK model were used to generate the PopPK‐derived individual empirical Bayesian clearance estimates. Triangles represent the mean ratio of PBPK‐derived clearance to clearance values reported by the fluconazole product label for infants 26–29 weeks gestational age.7 Tails represent ± 1 standard deviation ratio24 and the shaded region represents the twofold error range. Mean ratios were calculated as the mean predicted (PBPK) value divided by the mean observed (PopPK or product label) value, and standard deviation ratios were calculated using the method published by Zhou et al.24 The product label does not provide a clearance value for infants as young as 25 weeks postmenstrual age. PBPK, physiologically‐based pharmacokinetic; PopPK, population pharmacokinetic.
Dose‐exposure assessment
For treatment dosing, target attainment as defined by > 90% of simulated infants achieving the surrogate efficacy target of fAUC ≥ 400 mg • hour/L was not reached until the third dosing day for plasma and CSF when using the IDSA recommendation of 12 mg/kg/day (Figure 6). However, the addition of a 25 mg/kg loading dose resulted in 95% and 82% of virtual infants’ plasma and CSF exposure, respectively, exceeding the target on the first day of dosing, and target attainment was sustained throughout the simulated 2‐week dosing interval.
Figure 6.
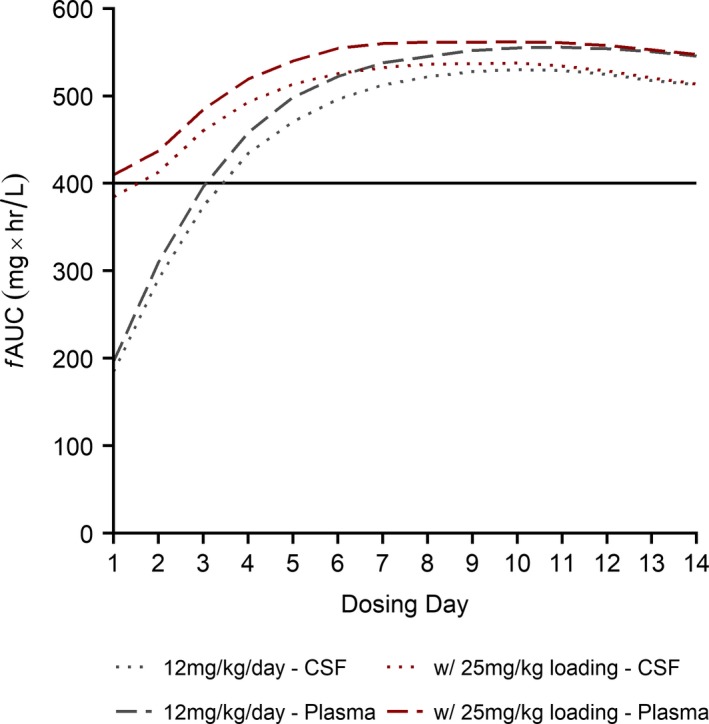
Fluconazole simulations of treatment dosing (12 mg/kg/day i.v. ± 25 mg/kg i.v. loading dose). Simulations (n = 500 virtual infants) of exposure, measured by fAUC, in preterm infants following fluconazole treatment dosing regimens over 2 weeks. Longer dashed lines represent exposure in plasma, and shorter dashed lines represent exposure in CSF. The bolded black line represents the target exposure associated with clinical efficacy. Gray lines represent exposure following dosing according to current Infectious Disease Society of America guidelines of 12 mg/kg/day i.v., and red lines represent exposure following dosing using these same guidelines plus a loading dose of 25 mg/kg i.v. All lines are the 10th percentile of simulated plasma or CSF exposure, such that 90% of simulated infants achieved this exposure or higher. A fraction unbound of 0.89 was assumed for both plasma and CSF. CSF, cerebrospinal fluid; i.v., intravenous; fAUC, area under the unbound concentration‐time curve for each dosing day; i.v., intravenous.
When evaluating target attainment based on actual MIC values measured during the prophylaxis study (0.25, 0.5, and 1 mg/L), 100% of infants reached the target exposure in plasma and CSF on the first dose for both dosing regimens evaluated previously.
Discussion
PBPK modeling shows promise in predicting drug concentrations in relevant yet inaccessible sites of action such as the brain and is often used to characterize the PKs of pediatric populations because of its ability to capture age‐dependent physiological changes.15 To date, there are limited PBPK models evaluated using human CSF or brain extracellular fluid samples, only one of which used pediatric data for model evaluation.16, 32, 33, 34 These models are advantageous in that they predict concentrations in different brain regions by incorporating various subcompartments, including brain blood, spinal CSF, and ventricular CSF spaces, and were successfully evaluated using adult data from multiple drugs.16, 32, 33, 34 A model using pediatric methotrexate data included digitized CSF and brain extracellular fluid concentrations and found that, although the model accurately characterized methotrexate's CNS distribution in rats, dogs, and human adults, it underestimated CNS concentrations in critically ill children aged 2–17 years.16 An additional PBPK model of bumetanide was developed using adult plasma data and scaled to various pediatric populations.35 It accurately predicted plasma concentrations for all infants except critically ill neonates, and it did not evaluate the model's CNS distribution predictions with CSF samples.35 Our model was developed using numerous plasma samples from preterm and term infants, many from a preterm population receiving the drug prophylactically. To our knowledge, this model is the first to use opportunistic CSF data to evaluate a PBPK model developed to characterize PKs in neonatal and preterm populations.
Preterm infants have reduced kidney function because of immature nephrons that can lead to substantial changes in PKs for fluconazole, a drug that is predominantly renally cleared.7, 36 In this model, GFR was calculated as a function of PMA using a sigmoidal hyperbolic equation and adjusted by a GFR fraction parameter.36 The GFR fraction was incorporated to account for fluconazole's tubular reabsorption, which is known to occur in adults.6, 7, 17 Whereas previous models of fluconazole in term infants through adults have used GFR fraction values of 0.15–0.18, we observed adequate model fit only after optimizing this value to 0.30.6, 10, 17 A value of less than but closer to one indicates that tubular reabsorption occurs in these infants to a lesser degree than in children or adults. This is consistent with previous findings that glomerular filtration rate and renal tubular reabsorption peak in maturation at 1–3 years PNA, and that the rate of maturation of tubular reabsorption is slower than that of glomerular filtration.36, 37, 38 Therefore, the higher GFR fraction is likely related to the younger PMA range of infants whose samples were used to optimize the GFR fraction parameter in this model when compared with populations from previous pediatric models. Simulating infants with a wide PMA range revealed that the median fraction of drug excreted unchanged in urine after five half‐lives (150 hours) was 0.9. This fraction is higher than that of adults (0.8), as might be expected given that UGT2B7 has not reached full activity in young infants.6, 20, 39 There appeared to be an increasing trend in the fraction excreted with increasing PMA, likely because of the faster rate of maturation in GFR when compared with UGT2B7 during the first year of life.39
Plasma and CSF observed concentrations were generally well captured by the model. CSF samples were within the same range as simulated concentrations albeit with some outlying CSF samples, which might be because of variations in the timing of CSF sample collection. The model performed better for observed plasma concentrations among the PPRU infants when compared with the prophylaxis study infants, possibly because of the differences between these studies in how dosing was defined in the simulations. To adjust for large variations in the standard‐of‐care dosing employed in the PPRU study, these infants were simulated using each infant's actual dosing regimen. The prophylaxis study infants received protocol‐derived dosing (6 mg/kg twice weekly), and therefore dosing was fixed in these model simulations. Exploration of the relationship of AFE and available covariates revealed only a slightly decreasing trend in AFE with serum creatinine, indicating that model misspecification may be the result of variability in renal function. There was also a potential increase in misspecification with PMA, although the clearance estimates across all PMAs modeled were consistent with those reported in a recent meta‐analysis of previous fluconazole studies in infants.40 This misspecification might reflect a difference in indication and clinical illness among infants in early vs. late prophylaxis groups. Early prophylaxis is typically initiated at birth regardless of clinical condition, whereas late prophylaxis is typically initiated at later PNA for a significant clinical illness.
Clearance in preterm and term infants can change with variation in renal function because of organ maturation and disease state. Simulated median clearance and clearance range increased with age from 24–42 weeks PMA, as shown in Figure 4. A review of fluconazole clearance in preterm infants found similar trends in the mean and variability of clearance with PMA.40 Because PMA is a function of both GA and PNA, greater variability in simulated clearance at PMA > 35 weeks may be the result of variability in the range of simulated GA.
PBPK‐derived clearance was generally within twofold of clearance derived from other sources, although there were some trends (Figure 5). PBPK‐derived clearance was higher than the PopPK‐derived values, possibly because the PopPK model included serum creatinine and PMA as covariates in determining clearance, whereas PMA was the primary driver of clearance in the PBPK model. The PBPK model simulations did not include virtual infants with abnormally high serum creatinine values (> 2 mg/dL) given the limited amount of observed data above this cutoff available for model evaluation. The simulated PBPK population included infants with physical measurements, including weight, length, and body mass index, most representative of their specific PMA. This led to a virtual population with a slightly higher average weight (0.75 kg) than the population used in the PopPK model (0.62 kg), which could further explain the discrepancy. Conversely, PBPK‐derived clearance was lower than clearance estimates in the product label (0.180 and 0.218 mL/minute/kg at 1.5 and 7.5 days PNA, respectively, for infants 26–29 weeks GA).7 The PBPK model incorporated changes in clearance as a function of both GA and PNA, whereas the product label provides discrete values for a range of ages, possibly leading to a discrepancy.
To act on pathogens in the CNS, antifungals such as fluconazole must penetrate the blood–brain, blood–CSF, and meningeal barriers to reach the key localizations of Candida meningitis, including the subcortical, periventricular, and basal ganglial cerebral areas.41 CSF concentrations have been shown to be an accurate surrogate to predicting brain interstitial fluid concentrations of drugs.42 The CSF‐to‐plasma fluconazole concentration ratio has been shown to exceed 0.8 in adults.6, 7, 8, 9 Furthermore, a recently published PopPK study in adults with cryptococcal meningitis found the mean fluconazole area under the concentration‐time curve of CSF to plasma to be 0.89.43 Although this ratio was previously unknown for the pediatric population, in this study the range of simulated plasma and simulated CSF concentrations were approximately the same (Figure 2). The CSF‐to‐plasma ratio was ~1 for one infant who had a CSF sample taken within 5 minutes of a plasma sample. No trends in model misspecification were observed in CSF concentration AFE with age. Fluconazole's ability to penetrate the CNS is expected given its structure—its high lipophilicity, low molecular weight, few heteroatoms, tertiary nitrogen, and acid dissociation constant (pKa) are all properties that have been shown to result in blood–brain barrier penetration.44, 45, 46, 47 There is no evidence to suggest that fluconazole is a substrate for common CNS transporters, further suggesting predominantly passive diffusion into the CNS.
Dosing simulations support current IDSA maintenance dosing recommendations but suggest the addition of a loading dose for treatment to reach the target exposure associated with clinical efficacy more rapidly. The incorporation of a loading dose is consistent with previous findings and is often currently used in clinical practice.12, 17, 26 The MIC of 8 mg/L used in these simulations likely provides a conservative estimate given that the actual MIC values measured during the prophylaxis study were 0.25–1 mg/L.30 A follow‐up study of Candida susceptibility to fluconazole found that only 4% of isolates were resistant, further supporting the efficacy of fluconazole when using the proposed dosing regimen.48 The dosing regimen suggested by this model is not likely to exceed safety margins in children, as the highest simulated plasma and CSF maximum concentration did not approach 80 mg/L, concentrations below which in plasma have been shown to be well tolerated in adults.49 An appropriate efficacy target for preterm infants receiving fluconazole prophylaxis is not yet established.
This study has some noteworthy limitations. First, a small number of CSF samples were used for model evaluation, and many infants only had a single CSF and/or plasma sample. A PBPK modeling approach mitigates this, as it is less dependent on large data sets than other traditional modeling approaches. This study is the first to be published on fluconazole CNS penetration in a pediatric population despite challenges in CSF sampling, including the invasive nature of sampling procedures, limited CSF volume availability, and increased risk of head bleeds for neonates.13, 14 Secondly, although most of the infants involved in these studies were receiving fluconazole prophylactically, those infants who may have had meningitis could have unknown pathophysiological differences not accounted for in the model.
In conclusion, a PBPK model was scaled to preterm and term infants to characterize fluconazole's CNS exposure. Model simulations support the use of maintenance dosing as per current IDSA dosing guidelines but suggest that the addition of a loading dose of 25 mg/kg for treatment results in more rapid target exposure attainment in the plasma and CSF of preterm infants with CNS infection. This model provides important information for fluconazole dosing in this vulnerable infant population and exemplifies the use of PBPK modeling to characterize the CNS exposure of drugs.
Funding
K.M.W. receives support from the Pediatric Critical Care and Trauma Scientist Development Program (5K12HD047349) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; 1K23HD075891). A.N.E receives support from the National Institutes of Health (NIH; 1R01‐HD076676‐01A1). S.N.S. receives support from the NICHD (5T32 GM086330). D.K.B. Jr. receives support from the NIH (2K24HD058735‐06), National Center for Advancing Translational Sciences (award UL1TR001117), NICHD contract HHSN275201000003I, and the National Institute of Allergy and Infectious Diseases contract HHSN272201500006I. C.P.H. receives salary support for research from the NICHD (1K23HD090239) and the US government for his work in pediatric and neonatal clinical pharmacology (Government Contract HHSN267200700051C; PI Benjamin under the Best Pharmaceuticals for Children Act) and other sponsors for drug development in adults and children (www.dcri.duke.edu/research/coi.jsp). M.C.‐W. receives support for research from the NIH (1R01‐HD076676‐01A1), the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the National Institute of Allergy and Infectious Diseases (HHSN272201500006I and HHSN272201300017I), the NICHD (HHSN275201000003I), the Biomedical Advanced Research and Development Authority (HHSO100201300009C), and other sponsors for drug development in adults and children (www.dcri.duke.edu/research/coi.jsp). D.G. receives support for research from the NICHD (5K23HD083465). This content is solely the responsibility of the authors and does not represent the official views of the NIH. The remaining authors have no relevant conflicts to disclose.
Conflict of Interest
The authors declared no competing interests for this work.
Author Contributions
J.G.G. and D.G. wrote the manuscript. J.G.G., K.M.W., A.E., and D.G. designed the research. J.G.G., K.M.W., A.E., K.C.W., D.K.B. Jr., P.B.S., C.P.H., M.C.‐W., S.D., A.S.R., K.S., D.L.S., N.N., and D.G. performed the research. J.G.G., A.E., S.N.S., and D.G. analyzed the data.
Supporting information
Supplementary Material S1. Supplementary information.
Supplementary Material S2. PBPK model building process and input parameters.
Figure S1. Observed dose‐normalized CSF concentration after first fluconazole dose for preterm and term infants.
Figure S2. Population simulation using original model in adults (n = 1,000) of CSF fluconazole concentration following oral dosing (800 mg daily) in adults with cryptococcal meningitis.
Figure S3. Population simulations (n = 100) of plasma fluconazole concentration following prophylactic or treatment dosing in preterm and term infants from the PPRU study.
Figure S4. Comparison of PBPK model–derived fluconazole volume of distribution to volume of distribution derived from a PopPK model.
Table S1. Parameters used in model development.
Acknowledgments
The Best Pharmaceuticals for Children Act—Pediatric Trials Network Publication Committee: Gary Furda, Duke Clinical Research Institute, Durham, NC; Daniel K. Benjamin Jr., Duke Clinical Research Institute, Durham, NC; Edmund Capparelli, University of California San Diego, San Diego, CA; Gregory L. Kearns, Arkansas Children's Hospital Research Institute, Little Rock, AR; Ian M. Paul, Penn State College of Medicine, Hershey, PA; Jan Sullivan, University of Louisville, Louisville, KY; Christoph P. Hornik, Duke Clinical Research Institute, Durham, NC; Kelly Wade, Children's Hospital of Philadelphia, Philadelphia, PA. The Eunice Kennedy Shriver National Institute of Child Health and Human Development: David Siegel, Perdita Taylor‐Zapata, Anne Zajicek, Zhaoxia Ren, Ekaterini Tsilou, Alice Pagan. The EMMES Corporation (Data Coordinating Center): Ravinder Anand and Gina Simone. Fluconazole Prophylaxis Study Clinical Trial Sites with enrolled infant(s) who provided a cerebrospinal fluid sample: University of Miami Miller School of Medicine, Miami, FL (eight infants enrolled): Shahnaz Duara (principal investigator (PI)), Karina Lifschitz (site coordinator (SC)); Arkansas Children's Hospital, Little Rock, AR (five infants enrolled): Ashley Ross (PI), Michelle Hart (SC), Howard Lee (SC); Children's Hospital of Philadelphia, Philadelphia, PA (four infants enrolled): Kelly Wade (PI), Toni Mancini (SC); University of Texas Medical Branch, Galveston, TX (four infants enrolled): Karen Shattuck (PI), Karen E. Smith (co‐PI), Kristin Pollock (SC); University of Louisville, Louisville, KY (two infants enrolled): Dan Stewart (PI), Karen Kernen (SC); Columbia University Medical Center, New York, NY (two infants enrolled): Natalie Neu (PI), Erin Humel‐Amadori (SC), Glen Bona (SC); University of Alabama‐Birmingham, Birmingham, AL (one infant enrolled): David Randolph (PI), Claire Roane (SC); University of California San Diego Medical Center, San Diego, CA (one infant enrolled): Neil Finer (PI), Wade Rich (SC); University of Texas Health Science Center at Houston, Houston, TX (one infant enrolled): Kathleen Kennedy (PI), Georgia McDavid (SC), Peggy Robichaux (SC); University of Minnesota Amplatz Children's Hospital, Minneapolis, MN (one infant enrolled): Catherine Bendel (PI), Marla Mills (SC), Nichole Birge (SC); University of Tennessee Health Science Center, Memphis, TN (one infant enrolled): Ramasubbareddy Dhanireddy (PI), Sheila Dempsey (SC); Kings County Hospital, Brooklyn, NY (one infant enrolled): Gratias Mundakel (PI), Sukhvinder Ranu (co‐investigator), Subhatra Limbu (SC). PPRU Study Clinical Trial Site with enrolled infants who provided a cerebrospinal fluid sample: Children's Hospital of Philadelphia, Philadelphia, PA (two infants enrolled): Kelly Wade (PI), Tonia Morrison (SC). The authors thank Anil Maharaj for his insightful feedback on our analyses.
Contributor Information
Daniel Gonzalez, Email: daniel.gonzalez@unc.edu.
the Best Pharmaceuticals for Children Act—Pediatric Trials Network Steering Committee:
Gary Furda, Danny Benjamin, Edmund Capparelli, Gregory L. Kearns, Ian M. Paul, Jan Sullivan, and Kelly Wade
References
- 1. Friedman, S. , Richardson, S.E. , Jacobs, S.E. & O'Brien, K. Systemic Candida infection in extremely low birth weight infants: short term morbidity and long term neurodevelopmental outcome. Pediatr. Infect. Dis. J. 19, 499–504 (2000). [DOI] [PubMed] [Google Scholar]
- 2. Fernandez, M. , Moylett, E.H. , Noyola, D.E. & Baker, C.J. Candidal meningitis in neonates: a 10‐year review. Clin. Infect. Dis. 31, 458–463 (2000). [DOI] [PubMed] [Google Scholar]
- 3. Leibovitz, E. , Iuster‐Reicher, A. , Amitai, M. & Mogilner, B. Systemic candidal infections associated with use of peripheral venous catheters in neonates: a 9‐year experience. Clin. Infect. Dis. 14, 485–491 (1992). [DOI] [PubMed] [Google Scholar]
- 4. Chesney, P.J. , Justman, R.A. & Bogdanowicz, W.M. Candida meningitis in newborn infants: a review and report of combined amphotericin B–flucytosine therapy. Johns Hopkins Med. J. 142, 155–160 (1978). [PubMed] [Google Scholar]
- 5. Hsieh, E.M. et al Medication use in the neonatal intensive care unit. Am. J. Perinatol. 31, 811–822 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Debruyne, D. Clinical pharmacokinetics of fluconazole in superficial and systemic mycoses. Clin. Pharmacokinet. 33, 52–77 (1997). [DOI] [PubMed] [Google Scholar]
- 7. DIFLUCAN® (fluconazole tablets) (fluconazole injection ‐ for intravenous infusion only) (fluconazole for oral suspension) <https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019949s051lbl.pdf> (2011). Accessed June 19, 2018.
- 8. Nau, R. , Sörgel, F. & Eiffert, H. Penetration of drugs through the blood‐cerebrospinal fluid/blood‐brain barrier for treatment of central nervous system infections. Clin. Microbiol. Rev. 23, 858–883 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kethireddy, S. & Andes, D. CNS pharmacokinetics of antifungal agents. Expert Opin. Drug Metab. Toxicol. 3, 573–581 (2007). [DOI] [PubMed] [Google Scholar]
- 10. Brammer, K.W. , Coakley, A.J. , Jezequel, S.G. & Tarbit, M.H. The disposition and metabolism of [14C]fluconazole in humans. Drug Metab. Dispos. 19, 764–767 (1991). [PubMed] [Google Scholar]
- 11. Momper, J.D. et al Population pharmacokinetics of fluconazole in premature infants with birth weights less than 750 grams. Antimicrob. Agents Chemother. 60, 5539–5545 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wade, K.C. et al Population pharmacokinetics of fluconazole in young infants. Antimicrob. Agents Chemother. 52, 4043–4049 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Testoni, D. et al Early lumbar puncture and risk of intraventricular hemorrhage in very low birth weight infants. Early Hum. Dev. 117, 1–6 (2018). [DOI] [PubMed] [Google Scholar]
- 14. Rochette, A. et al Cerebrospinal fluid volume in neonates undergoing spinal anaesthesia: a descriptive magnetic resonance imaging study. Br. J. Anaesth. 117, 214–219 (2016). [DOI] [PubMed] [Google Scholar]
- 15. Barrett, J.S. , Della Casa Alberighi, O. , Läer, S. & Meibohm, B. Physiologically based pharmacokinetic (PBPK) modeling in children. Clin. Pharmacol. Ther. 92, 40–49 (2012). [DOI] [PubMed] [Google Scholar]
- 16. Westerhout, J. , van den Berg, D.J. , Hartman, R. , Danhof, M. & de Lange, E.C.M. Prediction of methotrexate CNS distribution in different species ‐ influence of disease conditions. Eur. J. Pharm. Sci. 57, 11–24 (2014). [DOI] [PubMed] [Google Scholar]
- 17. Watt, K.M. et al Physiologically based pharmacokinetic approach to determine dosing on extracorporeal life support: fluconazole in children on ECMO. CPT Pharmacometrics Syst. Pharmacol. 7, 629–637 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benjamin, D.K. et al Effect of fluconazole prophylaxis on candidiasis and mortality in premature infants: a randomized clinical trial. JAMA 311, 1742–1749 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu, D. , Wade, K.C. , Paul, D.J. & Barrett, J.S. A rapid and sensitive LC‐MS/MS method for determination of fluconazole in human plasma and its application in infants with candida infections. Ther. Drug Monit. 31, 703–709 (2009). [DOI] [PubMed] [Google Scholar]
- 20. Wildfeuer, A. , Laufen, H. , Schmalreck, A. , Yeates, R. & Zimmerman, T. Fluconazole: Comparison of pharmacokinetics, therapy and in vitro susceptibility. Mycoses 40, 259–265 (1997). [DOI] [PubMed] [Google Scholar]
- 21. Nelder, J.A. & Mead, R. A simplex method for function minimization. Comput. J. 7, 308–313 (1965). [Google Scholar]
- 22. Bayer Technology Services . Computational systems biology software suite. PK‐Sim® and MOBI® manual. Version 7.0.0, SB Suite <http://www.systems-biology.com/products/pk-sim.html >. Accessed June 6, 2018.
- 23. Hwang, M.F. et al External evaluation of two fluconazole infant population pharmacokinetic models. Antimicrob. Agents Chemother. 61, e01352‐17 (2017). 10.1128/aac.01352-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou, W. et al Predictive performance of physiologically based pharmacokinetic and population pharmacokinetic modeling of renally cleared drugs in children. CPT Pharmacometrics Syst. Pharmacol. 5, 475–483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pappas, P.G. et al Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 62, 409–417 (2016). [DOI] [PubMed] [Google Scholar]
- 26. Watt, K.M. et al Fluconazole population pharmacokinetics and dosing for prevention and treatment of invasive candidiasis in children supported with extracorporeal membrane oxygenation. Antimicrob. Agents Chemother. 59, 3935–3943 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clancy, C.J. et al Fluconazole MIC and the fluconazole dose/MIC ratio correlate with therapeutic response among patients with candidemia. Antimicrob. Agents Chemother. 49, 3171–3177 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clancy, C.J. , Staley, B. & Nguyen, M.H. In vitro susceptibility of breakthrough Candida bloodstream isolates correlates with daily and cumulative doses of fluconazole. Antimicrob. Agents Chemother. 50, 3496–3498 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pai, M.P. , Turpin, R.S. & Garey, K.W. Association of fluconazole area under the concentration‐time curve/MIC and dose/MIC ratios with mortality in nonneutropenic patients with candidemia. Antimicrob. Agents Chemother. 51, 35–39 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Autmizguine, J. et al Effect of fluconazole prophylaxis on fluconazole Candida susceptibility in premature infants. J. Antimicrob. Chemother. 73, 3482–3487 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodgers, T. & Rowland, M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. Int. J. Drug Dev. Res. 95, 1238–1257 (2006). [DOI] [PubMed] [Google Scholar]
- 32. Yamamoto, Y. et al Prediction of human CNS pharmacokinetics using a physiologically‐based pharmacokinetic modeling approach. Eur. J. Pharm. Sci. 112, 168–179 (2018). [DOI] [PubMed] [Google Scholar]
- 33. Zakaria, Z. & Badhan, R. Development of a region‐specific physiologically based pharmacokinetic brain model to assess hippocampus and frontal cortex pharmacokinetics. Pharmaceutics 10, E14 (2018). 10.3390/pharmaceutics10010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gaohua, L. , Neuhoff, S. , Johnson, T.N. , Rostami‐Hodjegan, A. & Jamei, M. Development of a permeability‐limited model of the human brain and cerebrospinal fluid (CSF) to integrate known physiological and biological knowledge: Estimating time varying CSF drug concentrations and their variability using in vitro data. Drug Metab. Pharmacokinet. 31, 224–233 (2016). [DOI] [PubMed] [Google Scholar]
- 35. Donovan, M.D. , Abduljalil, K. , Cryan, J.F. , Boylan, G.B. & Griffin, B.T. Application of a physiologically‐based pharmacokinetic model for the prediction of bumetanide plasma and brain concentrations in the neonate. Biopharm. Drug Dispos. 39, 125–134 (2018). [DOI] [PubMed] [Google Scholar]
- 36. Rhodin, M.M. et al Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr. Nephrol. 24, 67–76 (2009). [DOI] [PubMed] [Google Scholar]
- 37. Hua, M.J. et al Urinary microalbumin and retinol‐binding protein assay for verifying children's nephron development and maturation. Clin. Chim. Acta 264, 127–132 (1997). [DOI] [PubMed] [Google Scholar]
- 38. Tayman, C. , Rayyan, M. & Allegaert, K. Neonatal pharmacology: extensive interindividual variability despite limited size. J. Pediatr. Pharmacol. Ther. 16, 170–184 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Edginton, A.N. , Schmitt, W. & Willmann, S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin. Pharmacokinet. 45, 1013–1034 (2006). [DOI] [PubMed] [Google Scholar]
- 40. Murakoso, K. , Minagawa, R. & Echizen, H. Developmental changes of fluconazole clearance in neonates and infants in relation to ontogeny of glomerular filtration rate: literature review and data analysis. J. Pharm. Heal. Care Sci. 4, 1–11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sharma, R.R. Fungal infections of the nervous system: current perspective and controversies in management. Int. J. Surg. 8, 591–601 (2010). [DOI] [PubMed] [Google Scholar]
- 42. Liu, X. et al Unbound drug concentration in brain homogenate and cerebral spinal fluid at steady state as a surrogate for unbound concentration in brain interstitial fluid. Drug Metab. Dispos. 37, 787–793 (2009). [DOI] [PubMed] [Google Scholar]
- 43. Stott, K.E. et al Population pharmacokinetics and cerebrospinal fluid penetration of fluconazole in adults with cryptococcal meningitis. Antimicrob. Agents Chemother. 62, e00885‐18 (2018). https://aac.asm.org/content/62/9/e00885-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hansch, C. , Rockwell, S.D. , Jow, P.Y.C. , Leo, A. & Steller, E.E. Substituent constants for correlation analysis. J. Med. Chem. 20, 304–306 (1977). [DOI] [PubMed] [Google Scholar]
- 45. Atkinson, F. , Cole, S. , Green, C. & , van de Waterbeemd, H. Lipophilicity and other parameters affecting brain penetration. Curr. Med. Chem. Cent. Nerv. Syst. Agents 2, 229–240 (2002). [Google Scholar]
- 46. Österberg, T. & Norinder, U. Prediction of polar surface area and drug transport processes using simple parameters and PLS statistics. J. Chem. Inf. Comput. Sci. 40, 1408–1411 (2000). [DOI] [PubMed] [Google Scholar]
- 47. Clark, D. In silico prediction of blood–brain barrier permeation. Drug Discov. Today 8, 927–933 (2003). [DOI] [PubMed] [Google Scholar]
- 48. Autmizguine, J. et al Antifungal susceptibility and clinical outcome in neonatal candidiasis. Pediatr. Infect. Dis. J. 37, 923–929 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Bellis, P. et al High‐dose fluconazole therapy in intensive care unit. Minerva Anestesiol. 69, 145–157 (2003). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1. Supplementary information.
Supplementary Material S2. PBPK model building process and input parameters.
Figure S1. Observed dose‐normalized CSF concentration after first fluconazole dose for preterm and term infants.
Figure S2. Population simulation using original model in adults (n = 1,000) of CSF fluconazole concentration following oral dosing (800 mg daily) in adults with cryptococcal meningitis.
Figure S3. Population simulations (n = 100) of plasma fluconazole concentration following prophylactic or treatment dosing in preterm and term infants from the PPRU study.
Figure S4. Comparison of PBPK model–derived fluconazole volume of distribution to volume of distribution derived from a PopPK model.
Table S1. Parameters used in model development.


