Abstract
Background
Bioactive peptides derived from food are important sources for alternative medicine and possess therapeutic activity. Several biochemical methods have been achieved to isolate bioactive peptides from food, which are tedious and time consuming. In silico methods are an alternative process to reduce cost and time with respect to bioactive peptide production. In this paper, FeptideDB was used to collect bioactive peptide (BP) data from both published research articles and available bioactive peptide databases. FeptideDB was developed to assist in forecasting bioactive peptides from food by combining peptide cleavage tools and database matching. Furthermore, this application was able to predict the potential of cleaved peptides from ‘enzyme digestion module’ to identify new ACE (angiotensin converting enzyme) inhibitors using an automatic molecular docking approach.
Results
The FeptideDB web application contains tools for generating all possible peptides cleaved from input protein by various available enzymes. This database was also used for analysis and visualization to assist in bioactive peptide discovery. One module of FeptideDB has the ability to create 3-dimensional peptide structures to further predict inhibitors for the target protein, ACE (angiotensin converting enzyme).
Conclusions
FeptideDB is freely available to researchers who are interested in exploring bioactive peptides. The FeptideDB interface is easy to use, allowing users to rapidly retrieve data based on desired search criteria. FeptideDB is freely available at http://www4g.biotec.or.th/FeptideDB/. Ultimately, FeptideDB is a computational aid for assessing peptide bioactivities.
Keywords: Computer science, Information systems, Information retrieval, Database, Food science, Biochemical composition of food, Food biochemistry, Bioinformatics, Biochemistry, Bioactive peptides, In silico, In silico screening, Databases, Web tool
1. Introduction
Currently, an abundance of biological information derived from experimental in vivo and in vitro data are being interpreted and reported alongside in silico data. Given the rapid development of bioinformatics tools, a new method for data collection can be constructed. Computational approaches involving databases, online tools, and software have become popular techniques for investigating and identifying potential bioactive peptides. Amino acid sequences in food protein have been analyzed in silico to explore the release of bioactive peptides in meat (Sayd et al., 2018; Keska and Stadnik, 2016; Lafarga et al., 2015), milk (Lin et al., 2018; Nongonierma et al., 2014, 2018; Parmar et al., 2018; Nongonierma and FitzGerald, 2016a, Nongonierma and FitzGerald, 2016b; Tulipano et al., 2015), fish (Han et al., 2018; Darewicz et al., 2016), plants (Liu et al., 2018; Gangopadhyay et al., 2016; Udenigwe, 2016) and insects (Sun et al., 2017). Currently, in silico designed peptides are used as original templates for developing peptide drugs and vaccines (Guida et al., 2018; Verma et al., 2018; Droppa-Almeida et al., 2018; Saadi et al., 2017; Del Carlo et al., 2016; Nezafat et al., 2016). Interestingly, most drugs approved by the USFDA are based on peptide structures, such as Giapreza, Macrilen, Ozempic, Parsabiv, Tymlos and 60 other peptide-based drugs constructed in recent years (Usmani et al., 2017; Erak et al., 2018). Furthermore, approximately 150 types of peptides are currently used in preclinical and clinical studies (Lau and Dunn, 2018). Bioactive peptides (BPs) from food-derived proteins normally contain 2-20 amino acids. BPs exhibit numerous health benefits and signs of disease prevention, including reducing blood pressure (angiotensin I-converting enzyme (ACE) inhibitors), exerting anticoagulation effects, and mediating anti-inflammatory, antimicrobial and anti-tumor activities. Several studies have shown that BPs can be found in bovine (Fu et al., 2016), soybean (Rho et al., 2009; Fan et al., 2009), porcine (O'Keeffe et al., 2017), egg white (Rizzetti et al., 2017), milk (Tidona et al., 2009) and marine byproducts (Sable et al., 2017). In the food industry, BPs have been prepared from byproducts obtained using several methods involving both mild and strong hydrolyses. Examples of mild hydrolyses conditions include first enzymatic cleavages, using pepsin, trypsin and papain (Ryder et al., 2016), and second microbial fermentation (lactobacillus) (Chaves-López et al., 2014). In addition, there are the combination methods utilizing both enzymatic hydrolysis and microbial fermentation (Boukil et al., 2018). For peptide preparation using strong hydrolysis, high-heat and pressure treatments are applied to assist these processes (Toldrá et al., 2017). Subsequently, the food byproducts hydrolyzed will be subjected to further isolation of bioactive peptides and subsequently validated with respect to activities of the identified peptides. However, these processes are time consuming and expensive for researchers and manufacturers because they exhibit uncertainty in yield. Bioinformatics tools provide the opportunity to rapidly identify potential bioactive peptides among food proteins rather than using conventional approaches. Elucidating protein sequences is a crucial step for therapeutic product development from bioactive peptides, and molecular docking is performed to determine the therapeutic potential of novel bioactive peptides derived from food to observe protein-ligand interactions (Wu et al., 2016; Nongonierma and FitzGerald, 2016a, Nongonierma and FitzGerald, 2016b; Abdelhedi et al., 2018; Panyayai et al., 2018). Construction of a peptide database has drawn significant attention, and many such databases are now found listing instances of bioactive peptides. These include PepBank (Shtatland et al., 2007), PeptideDB (Liu et al., 2008), BIOPEP (Minkiewicz et al., 2008) with antimicrobial peptide databases, APD (Wang et al., 2016) and CAMP (Thomas et al., 2010). BIOPEP-UWM is an in silico database widely used to analyze bioactive peptides derived from food (Minkiewicz et al., 2008). In addition, using ExPASy-PeptideCutter in silico digestion tools, users are allowed to customize their enzymes to generate peptide sequences to identify bioactivities against other databases (Gasteiger et al., 2005). Extraordinary examples of bioactive peptide tools predicting probable positions of amino acid sequences include PeptideRanker (Mooney et al., 2012), PeptideLocator (Mooney et al., 2013) and AntiBP2 (Lata et al., 2010).
Therefore, the FeptideDB was constructed as a user-friendly web application to assist in bioactive peptide discovery of compounds derived from food. This application can be accessed at http://www4g.biotec.or.th/FeptideDB/. This tool is a web-based information center, providing datasets of bioactive peptides derived from food collected from published literature and databases. Moreover, this database allows the user to select suitable enzyme as in silico enzyme digestions. FeptideDB is also able to generate 3D structures of fragmented peptides from the in silico enzyme digestions to further analyze protein-ligand docking within this web application.
2. Materials and methods
2.1. Data collection and organization
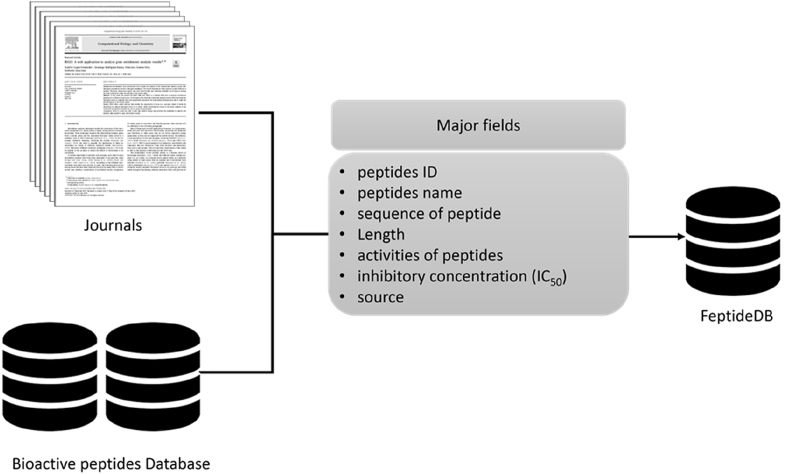
FeptideDB was designed and coded using bioactive peptides (BPs) data from both published research articles and available bioactive peptide databases as reference datasets. Datasets from the literature were manually extracted. The 12 public bioactive peptide databases that were used in this study included BIOPEP-UWM, APD, BACTIBASE (Hammami et al., 2010), CAMP, PenBase (Gueguen et al., 2006), RAPD (Li and Chen, 2008), Hmrbase (Rashid et al., 2009), PhytAMP (Hammami et al., 2009), PeptideDB, ACEpepDB (Jimsheena and Gowda, 2010), Amper (Fjell et al., 2007), and BAGEL3 (van Heel et al., 2013). Collected data were classified and compiled into relational tables using MySQL. The MySQL tables contain datasets divided into the following categories: a unique identification number (i.e., BP ID) and general BP information, including peptide name, function, sequence, inhibitory concentration (IC50) and relevant references. Then, BP information was presented in a database form, which was built on the Apache HTTP server (V 2.4) along with the MySQL server (V 5.7.10). The front-end of the database was developed using HTML and PHP (V 5.5.5), while MySQL was applied to handle the back-end. All scripts were written using Python (V 2.7) and PHP languages (see Fig. 1).
Fig. 1.
FeptideDB architecture was built on the Apache HTTP server with the MySQL server containing the peptide's name, function, sequence, inhibitory concentration (IC50) and relevant references.
2.2. System design & features
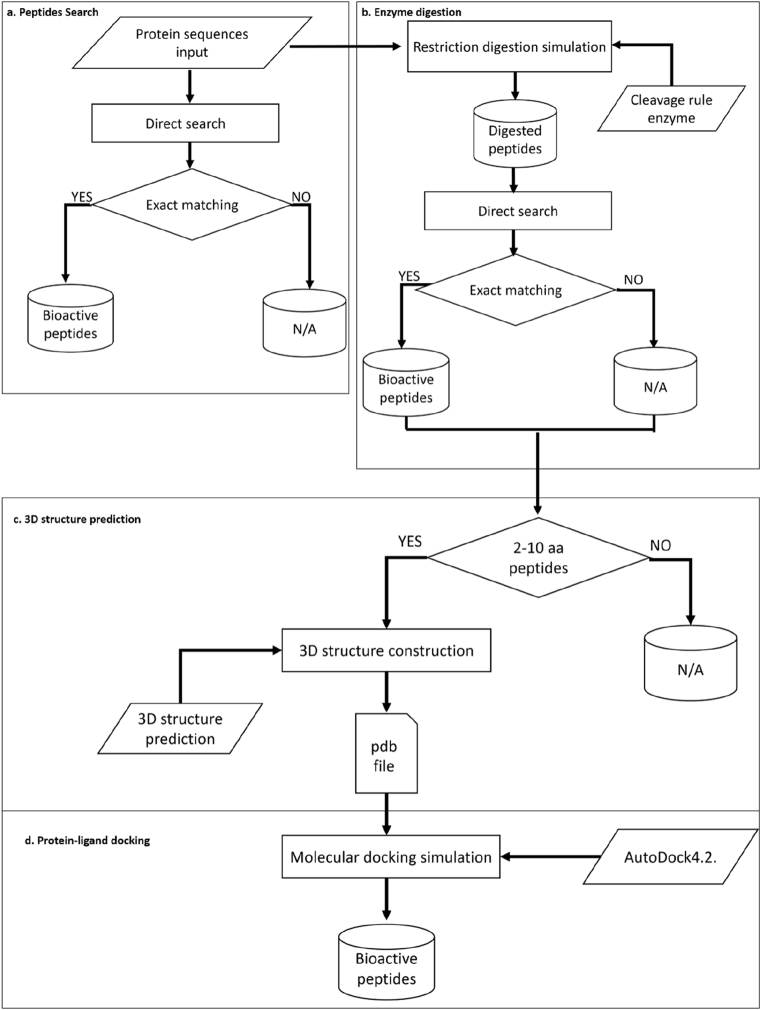
A web-based application/tool was developed with a user friendly interface to facilitate analysis of peptide data. Several tools have been integrated into this bioactive peptide database, including data extraction and analysis within the FeptideDB to be convenient. Details of FeptideDB are presented in Fig. 2 and are described as follows:
-
(a)
Bioactive peptide searches: Users can perform a search on any field of the database, e.g., sequence of peptide, activity of bioactive peptide. Then, results of predicted BPs from submitted protein sequences will be illustrated (Fig. 2a).
-
(b)
Enzyme digestion: In this in silico peptide digestion, 15 enzymes from the PeptideCutter tool (Gasteiger et al., 2005) were implemented in this function (Fig. 2b). According to these 15 restriction enzymes, users can select enzymes of preferences or choose automatic settings to obtain all feasible enzyme combinations resulting in exact matching results with the bioactive peptide database to predict biologically active peptide sequences.
-
(c)
3D structure prediction: Within this module, short cleaved peptides from the enzyme digestion step containing only 2–10 residues are automatically applied to the tLeap function in AMBER12 with AMBERff03 for creating and validating peptide structures into the PDB format (Fig. 2c) (Case et al., 2012).
-
(d)
Protein-ligand docking: This service provides the option to forecast the feasibility of modeled peptide structures from section (c) to orient and interact within the binding site of angiotensin converting enzyme (ACE-II). Protein-ligand docking was performed using AutoDock4.2 (Morris et al., 2009) with Python scripts integrated with in-house scripts (Fig. 2d). The biological activity of these modeled peptide structures against ACE-II is predicted within this module.
Fig. 2.
Illustration of the workflow of the web interface providing functions for the user, including (a) bioactive peptide searching, (b) enzyme digestion, (c) 3D structure prediction and (d) protein-ligand docking.
3. Results and discussion
3.1. FeptideDB database description
FeptideDB is a web-based application containing peptide database and analytic tools for further understanding bioactive peptides derived from food protein. This application provides a resource for BP research and supplies a platform for analysis of BPs isolated from food protein. A web interface was created to be freely accessible to users. The interface is comprised of the following sections: “Home,” “Peptides Search”, “Enzyme Digestion”, “User Guide” and “Contact us”.
3.2. Database interfaces
3.2.1. Peptide search
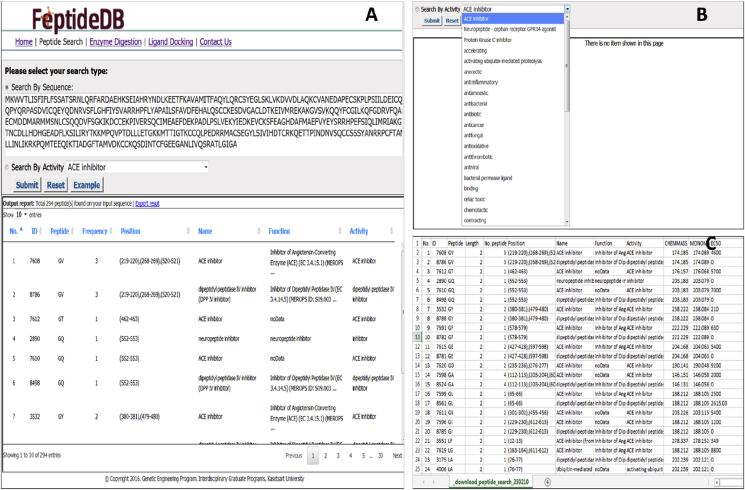
Search functionalities are designed to facilitate retrieval of useful information. FeptideDB was originated with two functions of preferences, including ‘search by sequence’ and ‘search by BP activity’ as shown in Fig. 3. There are several biological activities integrated within the latter function, such as ACE inhibition, anti-bacterial, anti-cancer, anti-oxidative activity, etc. Results from the BP search are displayed on the FeptideDB interface subsequent to the search function. This pop-up interface contains the following details: peptide sequences, position, number, name, function and activity. Moreover, these data sets are easily accessed using the ‘export file option’. Users can download results by choosing “Export results” to obtain a result_file.txt. This result_file.txt is saved in table format, which contains the following data: ID (coding item in our database), peptide sequence, length, no. peptide on protein, position, name, function, activity, CHEMMASS, MONOMASS and EC50. This file can be opened by any worksheet program.
Fig. 3.
Workflow of the peptide search function (A) Search by sequence: an example of a submitted query amino acid sequence (B) Search by BP activity: select from one of the drop down biological activities options (C) This result_file.txt is saved in table format containing the following data: ID (coding item in our database), peptide sequence, length, no. peptide on protein, position, name, function, activity, CHEMMASS, MONOMASS and EC50. This file can be opened by any worksheet program.
3.2.2. Enzyme digestion
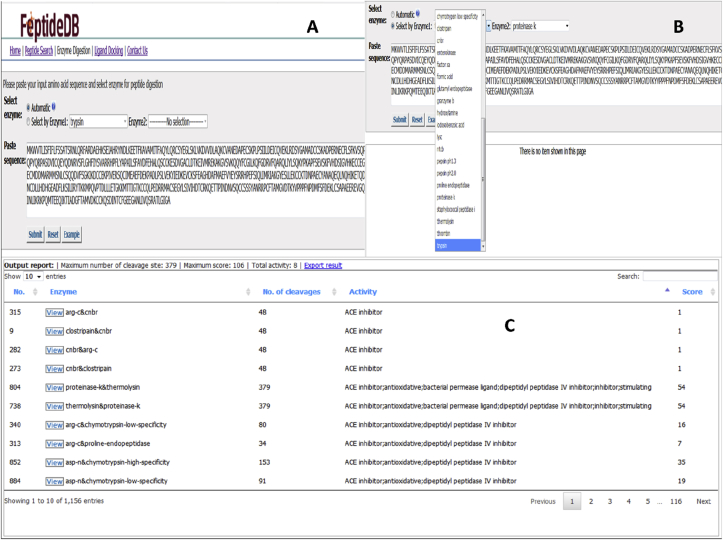
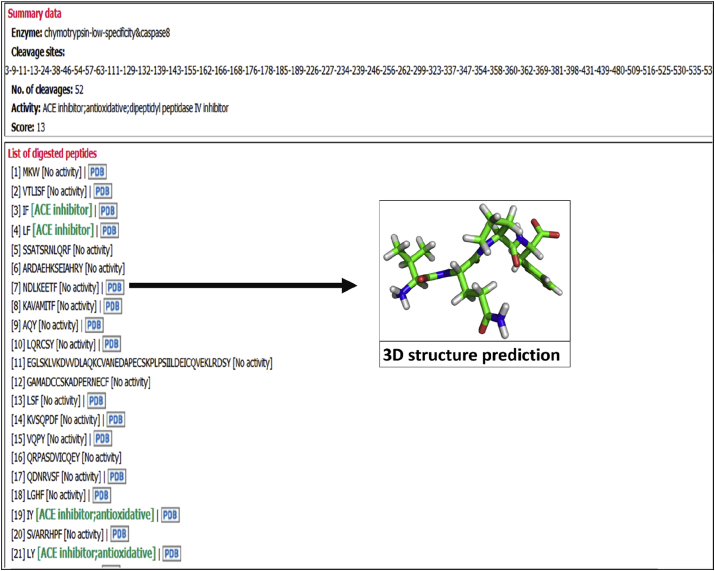
FeptideDB is an in silico enzyme digestion module to simulate BPs cleaved from query protein sequences via two cleavage options. As mentioned in the methods, 15 restriction enzymes from the PeptideCutter tool (Gasteiger et al., 2005) were implemented in this module. Query sequences are subjected to either manual or automatic restriction enzyme selection. Moreover, FeptideDB allows the automatic enzyme combination mode to increase the chance of obtaining additional potential bioactive peptides from protein sources. The feasibility of all enzyme combinations are calculated, resulting in an exact match of cleaved peptide fragments derived from food protein and BPs in the database. According to these in silico enzyme digestions, automatic mode returns results from both an individual enzyme and the combination of two enzymatic cleavage reactions. Manual enzymatic selection mode provides the opportunity for users to choose specific enzymes based on their requirements. BP fragments from the cleaved query sequence are presented with the following information: peptide fragment number, cleavage site, BP activity list and score of BP fragments by this enzyme digestion program. This displayed information can be exported as a file for download (Fig. 4). Moreover, BP fragments containing 2–10 residues are automatically generated using the FeptideDB-3D structure prediction tool (Fig. 5). This tool was written using Python code. The tLeap function in AMBER12 was applied to create and validate BP structures. These constructed structures are written out and available for download in PDB file format. Modeled BPs from the FeptideDB-3D structure prediction tool are then applied to the ACE protein-ligand docking module. This docking module provides cleaved-BPs and ACE interaction within FeptideDB. The PDB file provides user friendly understanding of new structure of BP fragments for BP bioactivity testing. A new potential ACE inhibitor from cleaved peptides can be predicted and picked up for further laboratory testing.
Fig. 4.
Enzyme digestion function: Input query sequences interface (A) an automatic option and (B) freely selecting combinations of 15 restriction enzymes choices available (C) The user can either (1) view result of each enzyme and get a new pop-up window or (2) download the summarized file by choosing “Export results” to obtain the result_file.txt.
Fig. 5.
The results of in silico enzyme digestion are displayed in the pop-up window. BP fragments containing 2–10 residues are automatically generated with the FeptideDB-3D structure prediction tool. These structures will be written out and available to download in PDB file format. Short cleaved peptides are calculated in the tLeap function of AMBER12 using AMBERff03 to create and validate peptides structure into PDB format. BP structures from this module can be further applied in the ‘Protein-ligand docking module’.
3.2.3. Protein-ligand docking
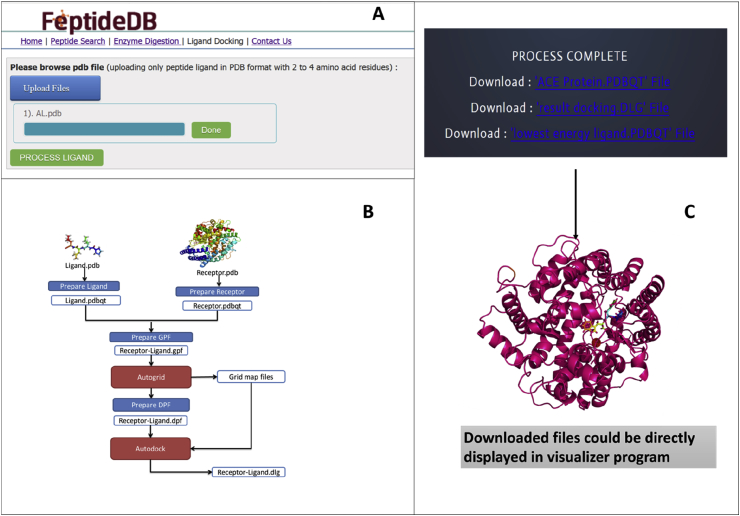
Furthermore, another module in FeptideDB predicts a new potential inhibitor for a target protein using a molecular docking program. To understand new BP sequences, angiotensin-I converting enzyme (ACE) inhibition is the first platform for extensive biological activity study. The B-AceP tool was created for ACE activity prediction of bioactive peptides derived from food protein. In this case, this module tool aimed to show the potential of our FeptideDB to perform automatic docking to generate ranked possible ACE peptide inhibitors. This module is user friendly for any scientist who is not an expert in molecular docking. The ACE protein was used as an example of this powerful technique. The enzyme digestion function creates an automatic pdb file, which uses this function, and the B-AceP tool containing the ACE protein (PDB 1O86) allows users to upload ligand pdb files. This program assists users in performing automated continuous ACE-ligand docking. The ACE-ligand docking, ligand preparation, and peptide ligand in PDB format with 2–4 amino acid residues is then uploaded. Ligands are automatically formatted for pdbqt files, which the Autodock 4.2 program requires (Morris et al., 2009) compared to another online program (Sandeep et al., 2011). The AutoDock searches for ligands with the highest binding affinity, and AutoDock runs several times to provide several docked conformations, which are analyzed for their predicted energy and the consistency of results to identify the best solution (Biesiada et al., 2011; Jamkhande et al., 2017). The grid center was set as x = 40.512, y = 37.247, and z = 43.596 to define the active site of the protein (Grid). Each ligand was run on 10 GA because this tool was create for prechecked ACE-ligand complex activity. The docked results file provides several formats, including the ACE-protein file, lowest energy ligand file and docked-log (dlg) file for users to structure visualization using a visualization program, such as AutoDockTools (ADT) (Fig 6).
Fig. 6.
The ligand docking function interface of the B-AceP program (A) The input uploading page includes only BPs with 2–4 residues in PDB format (B) Workflow of protein-ligand docking. BP structures will be automatically converted from PDB into PDBQT format. The ACE protein file (PDB Code: 1O86) is prepared as a PDBQT to perform in AutoDock4.2. Proteins and ligands were then applied for calculations and analysis in AutoDock4.2 (C) ACE protein (pdbqt file), the results of the docking file (dlg file) and files contain the lowest energy ligand file (pdbqt file) are provided for the user.
4. Conclusions
FeptideDB is a one-step web application tool that offers rapid identification and efficient analysis of bioactive peptides with a user friendly interface pipeline. It is the only automated tool that generates 3D bioactive peptides in PDB format, facilitating further use of data for molecular docking. In FeptideDB, one module for automated docking with ACE is provided for users who unfamiliar with docking programs. This module can be further applied using any protein in the future. Tools are embedded that may aid in the design of novel and more potent peptides used to guide researchers and the pharmaceutical industry in the selection of the most protein substrates. Moreover, FeptideDB emphasizes and illustrates the hypothesis for how food derived peptides reveal biological activity in 3D structural terms.
Declarations
Author contribution statement
Thitima Panyayai: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Chumpol Ngamphiw, Sissades Tongsima, Wuttichai Mhuantong, Wachira Limsripraphan: Contributed reagents, materials, analysis tools or data.
Orathai Sawatdichaikul, Kiattawee Choowongkomon: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Research and Researcher for Industries Ph.D. program (Grant No. PHD56I0051), the Center for Advanced Studies in Nanotechnology for Chemical, Food and Agricultural Industries, and the KU Institute for Advanced Studies, Kasetsart University.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The server was supported by the Project "National Infrastructure", NSTDA.
Contributor Information
Kiattawee Choowongkomon, Email: fsciktc@ku.ac.th.
Orathai Sawatdichaikul, Email: orathai.saw@ku.th.
References
- Abdelhedi O., Nasri R., Mora L., Jridi M., Toldrá F., Nasri M. In silico analysis and molecular docking study of angiotensin I-converting enzyme inhibitory peptides from smooth-hound viscera protein hydrolysates fractionated by ultrafiltration. Food Chem. 2018;239:453–463. doi: 10.1016/j.foodchem.2017.06.112. [DOI] [PubMed] [Google Scholar]
- Biesiada J., Porollo A., Velayutham P., Kouril M., Meller J. Survey of public domain software for docking simulations and virtual screening. Hum. Genomics. 2011;5:497–505. doi: 10.1186/1479-7364-5-5-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukil A., Suwal S., Chamberland J., Pouliot Y., Doyen A. Ultrafiltration performance and recovery of bioactive peptides after fractionation of tryptic hydrolysate generated from pressure-treated β-lactoglobulin. J. Membr. Sci. 2018;556:42–53. [Google Scholar]
- Case D.A., Darden T.A., Cheatham T.E., III, Simmerling C.L., Wang J., Duke R.E., Luo R., Walker R.C., Zhang W., Merz K.M., Roberts B., Hayik S., Roitberg A., Seabra G., Swails J., Götz A.W., Kolossváry I., Wong K.F., Paesani F., Vanicek J., Wolf R.M., Liu J., Wu X., Brozell S.R., Steinbrecher T., Gohlke H., Cai Q., Ye X., Wang J., Hsieh M.-J., Cui G., Roe D.R., Mathews D.H., Seetin M.G., Salomon-Ferrer R., Sagui C., Babin V., Luchko T., Gusarov S., Kovalenko A., Kollman P.A. University of California; San Francisco: 2012. AMBER 12. [Google Scholar]
- Chaves-López C., Serio A., Paparella A., Martuscelli M., Corsetti A., Tofalo R., Suzzi G. Impact of microbial cultures on proteolysis and release of bioactive peptides in fermented milk. Food Microbiol. 2014;2:117–121. doi: 10.1016/j.fm.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Darewicz M., Borawska J., Pliszka M. Carp proteins as a source of bioactive peptides-an in silico approach. Czech J. Food Sci. 2016;34:111–117. [Google Scholar]
- Del Carlo M., Capoferri D., Gladich I., Guida F., Forzato C., Navarini L., Compagnone D., Laio A., Berti F. In silico design of short peptides as sensing elements for phenolic compounds. ACS Sens. 2016;1:279–286. [Google Scholar]
- Droppa-Almeida D., Franceschi E., Padilha F.F. Immune-informatic analysis and design of peptide vaccine from multi-epitopes against corynebacterium pseudotuberculosis. Bioinf. Biol. Insights. 2018;12:1–9. doi: 10.1177/1177932218755337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erak M., Bellmann-Sickert K., Els-Heindl S., Beck-Sickinger A.G. Peptide chemistry toolbox - transforming natural peptides into peptide therapeutics. Bioorg. Med. Chem. 2018;26:2759–2765. doi: 10.1016/j.bmc.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Fan J., Hu X., Sze Sze T., Zhang Y., Tatsumi E. Isolation and characterisation of a novel angiotensin I-converting enzyme-inhibitory peptide derived from douchi, a traditional Chinese fermented soybean food. J. Sci. Food Agric. 2009;89:603–608. [Google Scholar]
- Fjell C.D., Hancock R.E., Cherkasov A. AMPer: a database and an automated discovery tool for antimicrobial peptides. Bioinformatics. 2007;23:1148–1155. doi: 10.1093/bioinformatics/btm068. [DOI] [PubMed] [Google Scholar]
- Fu Y., Young J.F., Rasmussen M.K., Dalsgaard T.K., Lametsch R., Aluko R.E., Therkildsen M. Angiotensin I-converting enzyme-inhibitory peptides from bovine collagen: insights into inhibitory mechanism and transepithelial transport. Food Res. Int. 2016;89:373–381. doi: 10.1016/j.foodres.2016.08.037. [DOI] [PubMed] [Google Scholar]
- Gangopadhyay N., Wynne K., O’Connor P., Gallagher E., Brunton N.P., Rai D.K., Hayes M. In silico and in vitro analyses of the angiotensin-I converting enzyme inhibitory activity of hydrolysates generated from crude barley (Hordeum vulgare) protein concentrates. Food Chem. 2016;203:367–374. doi: 10.1016/j.foodchem.2016.02.097. [DOI] [PubMed] [Google Scholar]
- Gasteiger E., Hoogland C., Gattiker A., Duvaud S., Wilkins M.R., Appel R.D., Bairoch A. Protein identification and analysis tools on the ExPASy server. In: Walker J.M., editor. The Proteomics Protocols Handbook. Humana Press; 2005. pp. 571–607. [Google Scholar]
- Gueguen Y., Garnier J., Robert L., Lefranc M.P., Mougenot I., de Lorgeril J., Janech M., Gross P.S., Warr G.W., Cuthbertson B., Barracco M.A., Bulet P., Aumelas A., Yang Y., Bo D., Xiang J., Tassanakajon A., Piquemal D., Bachère E. PenBase, the shrimp antimicrobial peptide penaeidin database: sequencebased classification and recommended nomenclature. Dev. Comp. Immunol. 2006;30:283–288. doi: 10.1016/j.dci.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Guida F., Battisti A., Gladich I., Buzzo M., Marangon E., Giodini L., Toffoli G., Laio A., Berti F. Peptide biosensors for anticancer drugs: design in silico to work in denaturizing environment. Biosens. Bioelectron. 2018;100:298–303. doi: 10.1016/j.bios.2017.09.012. [DOI] [PubMed] [Google Scholar]
- Hammami R., Ben Hamida J., Vergoten G., Fliss I. PhytAMP: a database dedicated to antimicrobial plant peptides. Nucleic Acids Res. 2009;37:D963–D968. doi: 10.1093/nar/gkn655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammami R., Zouhir A., Le L.C., Ben H.J., Fliss I. BACTIBASE second release: a database and tool platform for bacteriocin characterization. BMC Microbiol. 2010;10:22. doi: 10.1186/1471-2180-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Tang S., Li Y., Bao W., Wan H., Lu C., Zhou J., Li Y., Cheong L., Su X. In silico analysis and in vivo tests of the tuna dark muscle hydrolysate anti-oxidation effect. RSC Adv. 2018;8:14109–14119. doi: 10.1039/c8ra00889b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamkhande P.G., Ghante M.H., Ajgunde B.R. Software based approaches for drug designing and development: a systematic review on commonly used software and its applications. Bull. Fac. Pharm. Cairo Univ. 2017;55:203–210. [Google Scholar]
- Jimsheena V.K., Gowda L.R. Arachin derived peptides as selective angiotensin I-converting enzyme (ACE) inhibitors: structure-activity relationship. Peptides. 2010;31:1165–1176. doi: 10.1016/j.peptides.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Keska P., Stadnik J. Porcine myofibrillar proteins as potential precursors of bioactive peptides - an in silico study. Food Funct. 2016;6:2878–2885. doi: 10.1039/c5fo01631b. [DOI] [PubMed] [Google Scholar]
- Lafarga T., O'Connor P., Hayes M. In silico methods to identify meat-derived prolyl endopeptidase inhibitors. Food Chem. 2015;15:337–343. doi: 10.1016/j.foodchem.2014.11.150. [DOI] [PubMed] [Google Scholar]
- Lata S., Nitish K.M., Raghava G.P.S. Antibp2: improved version of antibacterial peptide prediction. BMC Bioinf. 2010;11:S19. doi: 10.1186/1471-2105-11-S1-S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J.L., Dunn M.K. Therapeutic peptides: historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018;26:2700–2707. doi: 10.1016/j.bmc.2017.06.052. [DOI] [PubMed] [Google Scholar]
- Li Y., Chen Z. RAPD: a database of recombinantly-produced antimicrobial peptides. FEMS Microbiol. Lett. 2008;289:126–129. doi: 10.1111/j.1574-6968.2008.01357.x. [DOI] [PubMed] [Google Scholar]
- Lin K., Zhang L.W., Han X., Xin L., Meng Z.X., Gong P.M., Cheng D.Y. Yak milk casein as potential precursor of angiotensin I-converting enzyme inhibitory peptides based on in silico proteolysis. Food Chem. 2018;254:340–347. doi: 10.1016/j.foodchem.2018.02.051. [DOI] [PubMed] [Google Scholar]
- Liu C., Fang L., Min W., Liu J., Li H. Exploration of the molecular interactions between angiotensin-I-converting enzyme (ACE) and the inhibitory peptides derived from hazelnut (Corylus heterophylla Fisch.) Food Chem. 2018;15:471–480. doi: 10.1016/j.foodchem.2017.10.095. [DOI] [PubMed] [Google Scholar]
- Liu F., Baggerman G., Schoofs L., Wets G. The construction of a bioactive peptide database in Metazoa. J. Proteome Res. 2008;7:4119–4131. doi: 10.1021/pr800037n. [DOI] [PubMed] [Google Scholar]
- Morris G.M., Huey R., Lindstrom W. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkiewicz P., Dziuba J., Iwaniak A., Dziuba M., Darewicz M. BIOPEP database and other programs for processing bioactive peptide sequences. J. AOAC Int. 2008;91:965–980. [PubMed] [Google Scholar]
- Mooney C., Haslam N.J., Holton T.A., Pollastri G., Shields D.C. PeptideLocator: prediction of bioactive peptides in protein sequences. Bioinformatics. 2013;29:1120–1126. doi: 10.1093/bioinformatics/btt103. [DOI] [PubMed] [Google Scholar]
- Mooney C., Haslam N.J., Pollastri G., Shields D.C. Towards the improved discovery and design of functional peptides: common features of diverse classes permit generalized prediction of bioactivity. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezafat N., Karimi Z., Eslami M., Mohkam M., Zandian S., Ghasemi Y. Designing an efficient multi-epitope peptide vaccine against Vibrio cholerae via combined immunoinformatics and protein interaction based approaches. Comput. Biol. Chem. 2016;62:82–95. doi: 10.1016/j.compbiolchem.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Nongonierma A.B., Dellafiora L., Paolella S., Galaverna G., Cozzini P., FitzGerald R.J. In silico approaches applied to the study of peptide analogs of ile-pro-ile in relation to their dipeptidyl peptidase IV inhibitory properties. Front. Endocrinol. 2018;9:329. doi: 10.3389/fendo.2018.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nongonierma A.B., FitzGerald R.J. Structure activity relationship modelling of milk protein-derived peptides with dipeptidyl peptidase IV (DPP-IV) inhibitory activity. Peptides. 2016;79:1–7. doi: 10.1016/j.peptides.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Nongonierma A.B., FitzGerald R.J. Learnings from quantitative structure activity relationship (QSAR) studies with respect to food protein-derived bioactive peptides: a review. RSC Adv. 2016;6 [Google Scholar]
- Nongonierma A.B., Mooney C., Shields D.C., FitzGerald R.J. In silico approaches to predict the potential of milk protein-derived peptides as dipeptidyl peptidase IV (DPP-IV) inhibitors. Peptides. 2014;57:43–51. doi: 10.1016/j.peptides.2014.04.018. [DOI] [PubMed] [Google Scholar]
- O'Keeffe M.B., Norris R., Alashi M.A., Aluko R.E., FitzGerald R.J. Peptide identification in a porcine gelatin prolyl endoproteinase hydrolysate with angiotensin converting enzyme (ACE) inhibitory and hypotensive activity. J. Funct. Foods. 2017;34:77–88. [Google Scholar]
- Panyayai T., Sangsawad P., Pacharawongsakda E., Sawatdichaikul O., Tongsima S., Choowongkomon K. The potential peptides against angiotensin-I converting enzyme through a virtual tripeptide-constructing library. Comput. Biol. Chem. 2018;77:207–213. doi: 10.1016/j.compbiolchem.2018.10.001. [DOI] [PubMed] [Google Scholar]
- Parmar H., Hati S., Sakure A. In vitro and in silico analysis of novel ACE-inhibitory bioactive peptides derived from fermented goat milk. Int. J. Pept. Res. Ther. 2018;24:441–453. [Google Scholar]
- Rashid M., Singla D., Sharma A., Kumar M., Raghava G.P. Hmrbase: a database of hormones and their receptors. BMC Genomics. 2009;10:307. doi: 10.1186/1471-2164-10-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho S.J., Lee J.S., Chung Y.I., Kim Y.W., Lee H.G. Purification and identification of an angiotensin I-converting enzyme inhibitory peptide from fermented soybean extract. Process Biochem. 2009;44:490–493. [Google Scholar]
- Rizzetti D.A., Martín Á., Corrales P., Fernandez F., Simões M.R., Peçanha F.M., Vassallo D.V., Miguel M., Wiggers G.A. Egg white-derived peptides prevent cardiovascular disorders induced by mercury in rats: role of angiotensin-converting enzyme (ACE) and NADPH oxidase. Toxicol. Lett. 2017;281:158–174. doi: 10.1016/j.toxlet.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Ryder K., Bekhit Ael-D., McConnell M., Carne A. Towards generation of bioactive peptides from meat industry waste proteins: generation of peptides using commercial microbial proteases. Food Chem. 2016;208:42–50. doi: 10.1016/j.foodchem.2016.03.121. [DOI] [PubMed] [Google Scholar]
- Saadi M., Karkhah A., Nouri H.R. Development of a multi-epitope peptide vaccine inducing robust T cell responses against brucellosis using immunoinformatics based approaches. Infect. Genet. Evol. 2017;51:227–234. doi: 10.1016/j.meegid.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Sable R., Parajuli P., Jois S. Peptides, peptidomimetics, and polypeptides from marine sources: a wealth of natural sources for pharmaceutical applications. Mar. Drugs. 2017;15:E124. doi: 10.3390/md15040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandeep G., Nagasree P., Hanisha M., Murali krishna kumar M. AUDocker LE: A GUI for virtual screening with AUTODOCK Vina. BMC Res. Note. 2011;4:445. doi: 10.1186/1756-0500-4-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayd T., Dufour C., Chambon C., Buffière C., Remond D., Santé-Lhoutellier V. Combined in vivo and in silico approaches for predicting the release of bioactive peptides from meat digestion. Food Chem. 2018;249:111–118. doi: 10.1016/j.foodchem.2018.01.013. [DOI] [PubMed] [Google Scholar]
- Shtatland T., Guettler D., Kossodo M., Pivovarov M., Weissleder R. PepBank-a database of peptides based on sequence text mining and public peptide data sources. BMC Bioinf. 2007;8:280. doi: 10.1186/1471-2105-8-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Chang Q., Liu L., Chai K., Lin G., Huo Q., Zhao Z., Zhao Z. High-throughput and rapid screening of novel ACE inhibitory peptides from sericin source and inhibition mechanism by using in silico and in vitro prescriptions. J. Agric. Food Chem. 2017;65:10020–10028. doi: 10.1021/acs.jafc.7b04043. [DOI] [PubMed] [Google Scholar]
- Thomas S., Karnik S., Barai R.S., Jayaraman V.K., Idicula-Thomas S. CAMP: a useful resource for research on antimicrobial peptides. Nucleic Acids Res. 2010;38:D774–D780. doi: 10.1093/nar/gkp1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidona F., Criscione A., Guastella A.M., Zuccaro A., Bordonaro S., Marletta D. Bioactive peptides in dairy products. Ital. J. Anim. Sci. 2009;8:315–340. [Google Scholar]
- Toldrá F., Reig M., Aristoy M.C., Mora L. Generation of bioactive peptides during food processing. Food Chem. 2017;267:395–404. doi: 10.1016/j.foodchem.2017.06.119. [DOI] [PubMed] [Google Scholar]
- Tulipano G., Faggi L., Nardone A., Cocchi D., Caroli A.M. Characterisation of the potential of b-lactoglobulin and a-lactalbumin as sources of bioactive peptides affecting incretin function: in silico and in vitro comparative studies. Int. Dairy J. 2015;48:66–72. [Google Scholar]
- Udenigwe C.C. Towards rice bran protein utilization: in silico insight on the role of oryzacystatins in biologically-active peptide production. Food Chem. 2016;191:135–138. doi: 10.1016/j.foodchem.2015.01.043. [DOI] [PubMed] [Google Scholar]
- Usmani S.S., Bedi G., Samuel J.S., Singh S., Kalra S., Kumar P., Ahuja A.A., Sharma M., Gautam A., Raghava G.P.S. THPdb: database of FDA-approved peptide and protein therapeutics. PLoS One. 2017;12 doi: 10.1371/journal.pone.0181748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heel A.J., de Jong A., Montalbán-López M., Kok J., Kuipers O.P. BAGEL3: automated identification of genes encoding bacteriocins and (non-) bactericidal posttranslationally modified peptides. Nucleic Acids Res. 2013;41(W1):448–453. doi: 10.1093/nar/gkt391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S., Sugadev R., Kumar A., Chandna S., Ganju L., Bansal A. Multi-epitope DnaK peptide vaccine against S.Typhi: an in silico approach. Vaccine. 2018;36:4014–4022. doi: 10.1016/j.vaccine.2018.05.106. [DOI] [PubMed] [Google Scholar]
- Wang G., Li X., Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016;44:D1087–D1093. doi: 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Du J., Jia J., Kuang C. Production of ACE inhibitory peptides from sweet sorghum grain protein using alcalase: hydrolysis kinetic, purification and molecular docking study. Food Chem. 2016;199:140–149. doi: 10.1016/j.foodchem.2015.12.012. [DOI] [PubMed] [Google Scholar]