Abstract
Background
This study aimed to investigate the protective roles and mechanisms of taurine on myocardial ischemia/reperfusion (I/R)-induced cell apoptosis, and thus provide evidence for the treatment of myocardial I/R injury and the development of related drugs.
Methods
The cardiomyocytes of neonatal rats were used to prepare the hypoxia/reoxygenation (H/R) injury model; gene transfection and small interfering RNA (siRNA) target gene silencing techniques were performed, along with methyl thiazolyl tetrazolium (MTT) assay to detect cell survival, flow cytometry to detect cell apoptosis, and Western blot to measure protein expressions.
Results
Compared with the H/R group, the apoptosis rates of cardiomyocytes in the three taurine (TAU)-protection groups were significantly decreased (p < 0.05). As the TAU concentration increased, the expression of Bcl-2 protein in H/R cardiomyocytes also gradually increased (p < 0.05), while the protein expressions of p53 up-regulated modulator of apoptosis (PUMA), C/EBP homologous protein (CHOP), Bax, glucose-regulated protein 78kD (GRP78), and caspase-3 gradually decreased (p < 0.01). TAU strongly downregulated the expression of PUMA-transfected cardiomyocytes. After targeted silencing of PUMA, the apoptosis rate was significantly decreased, while the expression of Bcl-2 protein was increased, and that of Bax protein was decreased (p < 0.05).
Conclusions
TAU significantly inhibited myocardial H/R-induced apoptosis, and the mechanism may be related to a downregulated expression of PUMA.
Keywords: Apoptosis, Ischemia/reperfusion injury, Myocardium, Taurine
INTRODUCTION
Coronary heart disease is one of the most lethal cardiovascular events worldwide.1-4 After acute myocardial infarction, timely reperfusion therapy [including thrombolysis and percutaneous transluminal coronary intervention (PCI)] and coronary flow restoration are the most effective measures to reduce the myocardial infarction size and improve the survival rate. However, during the process of blood flow restoration, new damage can be caused, namely ischemia-reperfusion (I/R) injury.5,6 Since recanalization techniques are widely used, myocardial I/R injury has become a primary factor affecting the treatment of ischemic heart diseases. Myocardial I/R injury occurs when the I/R myocardium is aggravated after the blood supply is restored, which usually occurs within the first 24 hours after reperfusion. I/R injuries often lead to diastolic dysfunction, arrhythmias, metabolic abnormalities, and structural changes,7,8 thus methods to reduce hypoxia/reoxygenation (H/R) injury have become an important medical issue. Reducing myocardial I/R injury is important to improve the clinical treatment of cardiovascular diseases. The mechanisms of myocardial I/R are complex, and are mainly related to post-reperfusion Ca2+ overload, inflammation, release of a large number of oxygen free radicals, metabolic disorders,9 and the activation of a variety of signaling pathways.10 Myocardial I/R affects heart function and also limits the efficacy of various clinical infusion therapies such as coronary thrombolysis, interventions, and bypass surgery.11-13
In recent years, several studies have reported that apoptosis is an important pathological feature of myocardial I/R injury;14,15 thus, downregulating or inhibiting myocardial cell apoptosis could significantly reduce myocardial I/R injuries. p53 up-regulated modulator of apoptosis (PUMA) is an important member of the mitochondrial apoptosis pathway.16 PUMA is a BH3-only protein with the strongest pro-apoptotic effects.17 It is significantly upregulated in intestinal I/R injuries and can mediate apoptosis through activating the mitochondrial apoptosis pathway. Knockout of PUMA has been shown to reduce apoptosis in cerebral ischemia and intestinal I/R injury.18 Taurine (TAU) is a sulfur-containing amino acid normally present in vivo, and its structure is simple. As a condition-essential amino acid in vivo, TAU has functions including anti-oxidation, scavenging free radicals, maintaining cell membrane stability and cellular osmotic pressure, regulating intracellular Ca2+ homeostasis, and acting as an anti-bacterial and anti-inflammatory agent. In addition, it can inhibit apoptosis and protect against myocardial I/R injury.19,20 Whether the cardioprotective effects of TAU are related to PUMA is still unclear. Therefore, this study aimed to elucidate the molecular mechanism by which TAU inhibits the apoptosis of myocardial cells in I/R injury and to expand the application of TAU in the prevention and treatment of I/R injury. We found that PUMA plays an important role in H/R-induced rat cardiomyocytes, but it was not clear whether TAU protected the myocardium by regulating PUMA.21 PUMA is a key molecule in myocardial I/R injury and is considered to be a new target for the prevention and treatment of ischemic heart disease.17 This study used a myocardial H/R model and techniques including exogenous PUMA gene transfection and small interfering RNA (siRNA) targeted gene silencing to observe the changes in PUMA expression and apoptosis in myocardial cells after H/R injury, with the aim of elucidating the protective roles of TAU in myocardial H/R injury and its molecular mechanisms, and to provide evidence for the targeted therapy of cardiac H/R injury and drug development. The scientific significance of this study is that TAU inhibited apoptosis and protected the myocardium by down-regulating PUMA expression. This provides a new experimental basis to widen the application of TAU in the prevention and treatment of myocardial I/R injury. This study not only elucidated the role and molecular mechanism of TAU in the prevention and treatment of myocardial I/R injury, but also provided an important basis for the prevention and treatment of TAU for other tissue and organ I/R injuries.
MATERIALS AND METHODS
Primary culture of cardiomyocytes
The separation and culture of neonatal rat cardiomyocytes were performed in our laboratory. The hearts of 1- to 3-day-old Wistar rats were removed under aseptic conditions, and residual blood was washed away with pre-chilled D-Hanks solution. After separating the attached tissues, ophthalmic scissors were used to cut the hearts into 1-mm pieces. To obtain myocardial cell suspension, the pieces were repeatedly digested with 0.08% protease while being stirred at 37 °C. The cell concentration was then adjusted to 1.0 × 10 cells/mL with 20% FBS-containing M199 medium and 0.1 mmol/L 5-bromodeoxyuridine was added. The cell suspension was separated into different flasks and cultured at 37 °C and 5% CO2. The flasks were first inverted, then flipped 90 min later for continuous culture. The culture medium was changed once every 24 h. Before drug treatment, the cells were cultured with serum-free medium for 24 h. The 4 g/L trypan blue exclusion method was used to detect myocardial cell survival. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Nanchang University.
Preparation and grouping for the rat myocardial H/R injury model
Referring to the methods established in our laboratory,17 for the third generation of the original cardiomyocytes, the normal culture medium was first replaced with 95% N2 and 5% CO2 gas mixture-saturated hypoxic culture medium. The culture plates were then placed in one 95% N2 and 5% CO2 gas mixture-saturated hypoxic well-closed container for 5 h of culture (37 °C). The culture medium was then replaced with 95% O2 and 5% CO2 gas mixture-saturated normal culture medium and cultured in a CO2 incubator (5% CO2 at 37 °C) for reoxygenation culture. The cells were divided into five groups: the normal control group (controls), H/R 2 h group, H/R 4 h group, H/R 6 h group, and H/R 12 h group. Each group was repeated three times.
Detection of myocardial cell survival and lactic dehydrogenase (LDH) activity
We used the methyl thiazolyl tetrazolium (MTT) assay to detect myocardial cell survival. The cells were cultured in 96-well culture plates at a density of 1 × 105 cells/well using 200 μL of liquid per well. Twenty μL of MTT (5 mg/mL) was added to the test samples (the control group received culture medium only). After incubating the cells at 37 °C for 4 h, the culture medium was removed, 150 μL of DMSO was added to each well, and the plates were oscillated for 10 min to fully dissolve the crystals. The absorbance of each well was detected at 490 nm on a microplate reader. Cell survival was calculated with the following formula: (absorbance of the experimental group/absorbance of the control group) × 100%. LDH activity was measured using an automatic biochemical analyzer (Beckman, USA).
Flow cytometry
The myocardial cells in each experimental group were prepared as a single cell suspension and rinsed with 4 °C pre-cooled PBS twice. Annexin V and PI dye were added according to the manufacturers’ instructions, and cell suspensions were incubated at room temperature in the dark for 15 min. Apoptosis of the cells was measured with a flow cytometer (FACS Calibur, Becton Dickinson, USA).
Ad-PUMA and siRNA transfection
Using methods established in our laboratory, myocardial cells were divided into control, Ad-GFP (adenovirus vector containing green fluorescent protein), Ad-PUMA (adenovirus vectors containing PUMA), and 3 Ad-PUMA+TAU (add TAU 1 hour before transfection) groups, each with 6 replicas. The Ad-GFP group was transfected with Ad-GFP, Ad-PUMA and 3 Ad-PUMA+TAU groups were transfected with Ad-PUMA after 24 h of myocardial cell culture, respectively, The control group was a blank control group, without any treatment. Fluorescence microscopy was performed after 72 h. Using methods established in our laboratory, the myocardial cells that were cultured for 2 days were transfected with siRNA, after which over 60% of the culture plate exhibited successful integration of siRNA. For specific siRNA concentrations and steps, we followed the protocol of LipofectamineTM2000 reagent. Twenty-four hours after transfection, we prepared the rat myocardial H/R injury model as described above.
Real-time polymerase chain reaction (RT-PCR)
For each group, the total RNA of the myocardial cells was extracted using conventional methods, and RT-PCR was used to detect the expressions of PUMA and glucose-regulated protein 78 kD (GRP78) mRNA. The following primers were used: PUMA upstream primer: 5′-AGCGGCGGAGACAAGAA-3′, downstream primer: 5′-CAAGTCCGTATCTCCATCAGTG-3′, amplified length: 323 bp; Grp78 upstream primer: 5′-CATCCAGGATCGAGCAGA-3′, downstream primer: 5′-CAAAGTAGAAGAGGGCAACC-3′, amplified length: 260 bp; glyceraldehyde phosphate dehydrogenase upstream primer: 5′-GCAAGTTCAACGGCACAG-3′, downstream primer: 5′-AGGTGGAA GAATGGGAGTTGCT-3′, amplified length: 723 bp. Five μl of the PCR amplification products were run on 1.5% agarose gel (containing 0.5 mg/L ethidium bromide), and a gel imaging system was used for photography and analysis. The results were expressed as the gray ratio of target gene product band to GAPDH product band.
Western blot
The appropriate amount of pre-cooled protein lysate was added to extract total proteins. After quantitating the protein concentration using the Bradford method, equal concentrations of proteins were used for SDS-PAGE electrophoresis. The proteins were transferred onto a polyvinylidene fluoride membrane, then incubated with the primary and secondary antibodies, respectively, followed by enhanced chemiluminescence chromogenic exposure, developing, and fixing. After scanning the films, we used gel analysis software for analysis and processing, and the results were expressed as the gray ratio of target gene product band to β-actin product band.
Statistical analysis
All experimental data were expressed as x̄ ± s, and SPSS software version 13.0 was used for the univariate analysis of variance (one-way ANOVA).
RESULTS
Impacts of H/R on cell survival and protein expression
The results of myocardial cell survival and LDH activity in the cell culture medium showed that the cardiomyocytes in the control group grew well, and that the LDH concentration in the culture medium was low (Table 1). After H/R, the myocardial cells were obviously injured, and the survival rate of each H/R group was significantly lower than that of the control group, while the LDH concentration in the medium was significantly higher, and the difference was statistically significant (p < 0.05). Figure 1 shows that the mRNA and protein expression patterns of GRP78 and PUMA in the cardiomyocytes were basically the same. When compared with the control group, the expressions of GRP78 mRNA and protein in the H/R 2-h group and those of PUMA mRNA and protein in the H/R 4-h group were significantly increased (p < 0.05). The expressions of GRP78 and PUMA reached peaks at 6 h and 12 h after H/R, suggesting that H/R might have caused an increase in the expressions of PUMA and GRP78 in the myocardial cells, and that this might be closely associated with myocardial cell injury.
Table 1. Survival and LDH activity of the cardiomyocytes (x̄ ± s, n = 3).
| Group | Survive rate (%) | LDH inmedium (U/L) |
| Control | 96.45 ± 2.18 | 141.61 ± 12.33 |
| H/R 2 h | 72.51 ± 6.31* | 756.23 ± 61.31* |
| H/R 4 h | 65.37 ± 5.23# | 892.46 ± 112.45# |
| H/R 6 h | 60.66 ± 5.48# | 1145.67 ± 154.83# |
| H/R 12 h | 56.49 ± 5.12# | 1107.88 ± 152.66# |
| H/R 24 h | 52.13 ± 5.76# | 1137.66 ± 148.73# |
* p < 0.05, # p < 0.01 vs. control.
H/R 2h, hypoxia for 5 hours/reoxygenation for 2 hours; H/R 4h, hypoxia for 5 hours/reoxygenation for 4 hours; H/R 6h, hypoxia for 5 hours/reoxygenation for 6 hours; H/R 12h, hypoxia for 5 hours/reoxygenation for 12 hours; H/R 24h, hypoxia for 5 hours/reoxygenation for 24 hours; LDH, lactic dehydrogenase.
Figure 1.
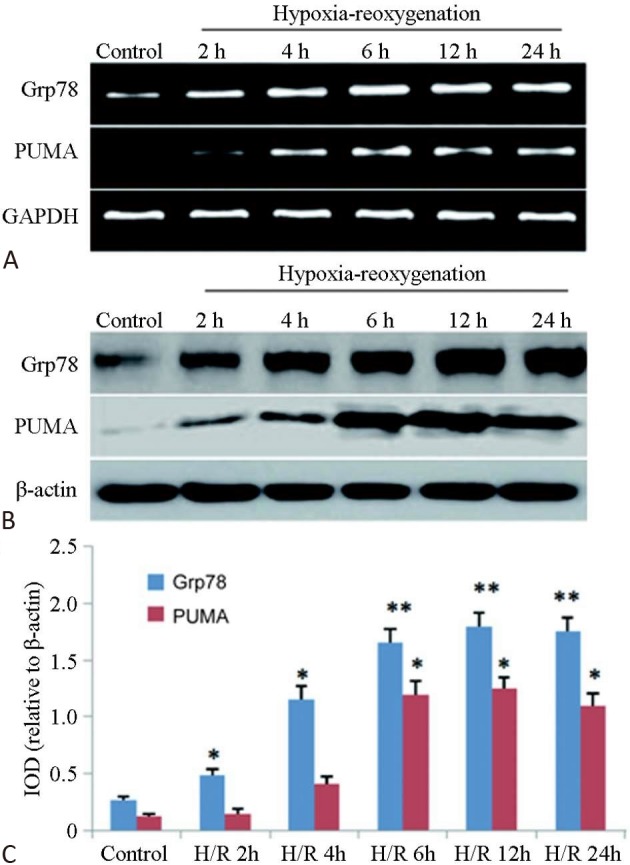
Expression changes of PUMA and GRP78 in the cardiomyocytes at different time points after H/R (n = 3). (A) RT-PCR; (B) Western blot; (C) Histogram of the protein expressions.
Impacts of TAU on H/R-induced myocardial cell apoptosis
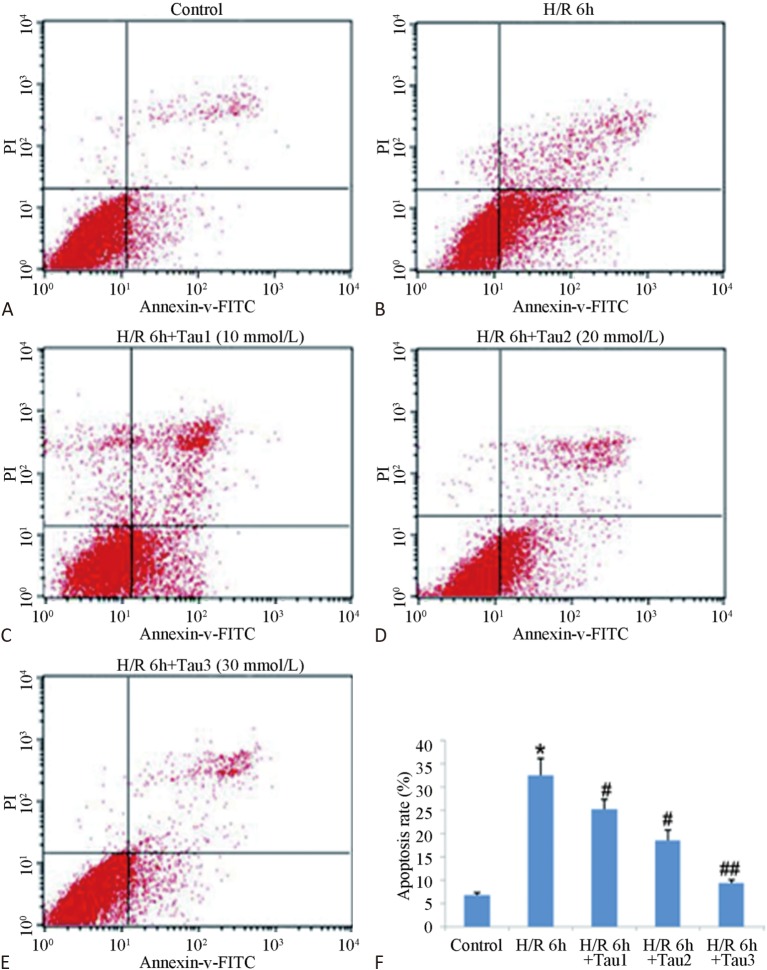
Figure 2 shows that the myocardial cell apoptosis rate of the H/R group was high (up to 38.73%) and exhibited a significant difference to the control group (p < 0.01). In addition, the 10 mmol/L TAU protection group (TAU was added an hour before myocardial cell hypoxia treatment) had an apoptosis rate of 27.05%, significantly different from the H/R group (p < 0.05). The apoptosis rate of the 20 mmol/L TAU protection group was only 9.46%, which was 75.57% lower than the I/R group, and exhibited a very significant difference compared with the I/R group (p < 0.01). The apoptosis rate of the 30 mmol/L TAU protection group was the lowest, indicating that the inhibition of TAU against H/R-induced myocardial cell apoptosis had a dose-effect relationship.
Figure 2.
Inhibition of TAU against H/R caused myocardial cell apoptosis. Note: TAU was added an hour before myocardial cell hypoxia treatment. (A) Control group; (B) H/R 6h group; (C) H/R 6h+Tau (10 mmol/L) GROUP, a final concentration of 10 mmol/L taurine was added 1 hour before hypoxia; (D) H/R 6h+Tau (20 mmol/L) GROUP, a final concentration of 20 mmol/L taurine was added 1 hour before hypoxia; (E) H/R 6h+Tau (30 mmol/L) GROUP, a final concentration of 30 mmol/L taurine was added 1 hour before hypoxia; (F) Statistical analysis of the apoptosis rate. ** p < 0.01 vs. control; # p < 0.05, ## p < 0.05 vs. H/R 6 h.
Impacts of TAU on protein expression of PUMA and C/EBP homologous protein (CHOP) in post-H/R cardiomyocytes
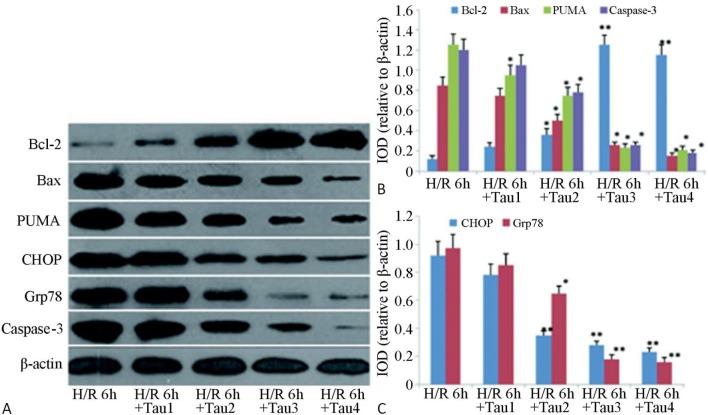
Western blot analysis showed that TAU had different degrees of impact on the protein expressions of PUMA, CHOP, Bcl-2, Bax, GRP78, and caspase-3 in post-H/R cardiomyocytes (Figure 3). As the TAU concentration increased, the pro-apoptotic protein expressions of PUMA, CHOP, Bax, GRP78, and caspase-3 in post-H/R cardiomyocytes gradually decreased (p < 0.01), while that of the anti-apoptotic protein Bcl-2 gradually increased (p < 0.05).
Figure 3.
Impacts of TAU on expressions of apoptosis-related proteins in post-H/R cardiomyocytes. Note: TAU was added an hour before myocardial cell hypoxia treatment, the final concentrations of TAU in Tau1, Tau2, Tau3, and Tau4 were 10, 20, 30 and 60 mmol/L, respectively. (A) Result of detecting bcl-2, Bax, PUMA and caspase-3 proteins by Western blot. (B) Grayscale analysis result of PUMA caspase-3 bcl-2, Bax. (C) Grayscale analysis result of GRP78 CHOP. * p < 0.05, ** p < 0.01 vs. H/R 6 h.
Inhibition effects of TAU on the overexpression of PUMA in post-H/R cardiomyocytes
Figure 4 shows that at both the RNA and protein level, the three TAU groups exhibited strong downregulation of the exogenous Ad-PUMA-induced PUMA overexpression, and that this had a significant dose-effect relationship (p < 0.05). The results showed that TAU had a good inhibitory effect on the overexpression of PUMA in cardiac cells.
Figure 4.
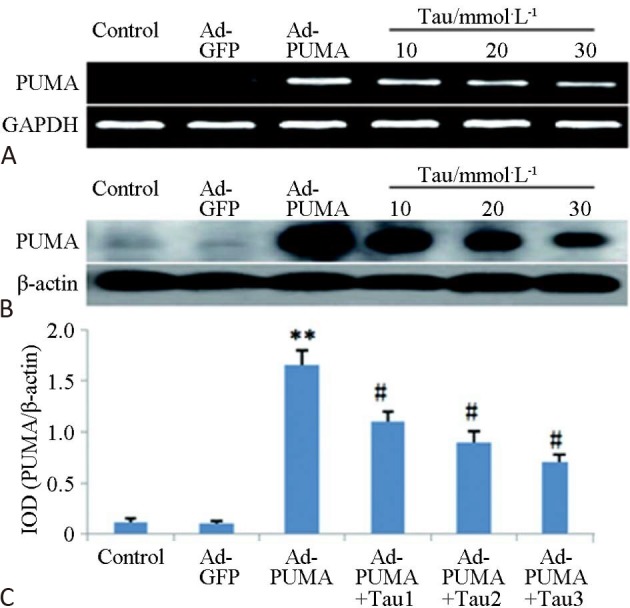
Inhibition effects of TAU on overexpression of PUMA in post-H/R cardiomyocytes. (A) RT-PCR; (B) Western blot; (C) Histogram of protein expressions. ** p < 0.01 vs control; # p <0.05 vs. H/R 6 h.
Impacts of PUMA silencing on post-H/R myocardial cell apoptosis and levels of apoptosis-associated proteins
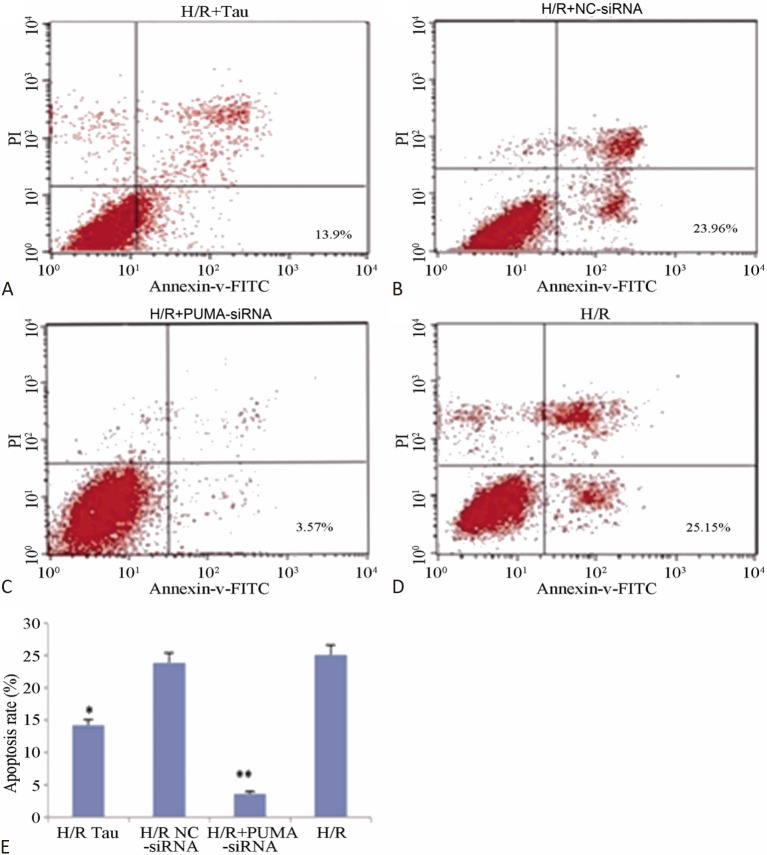
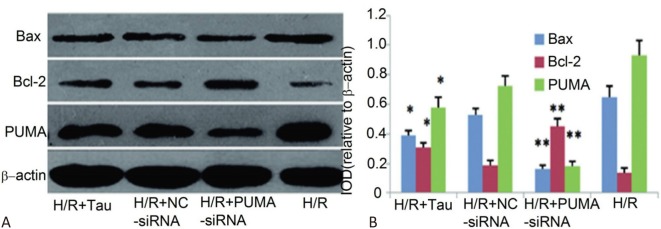
Figure 5 shows that the myocardial cell apoptosis rate of the H/R + PUMA-siRNA group was significantly reduced compared to the H/R + NC-siRNA group (p < 0.05). Figure 6 shows that the expressions of PUMA and Bax protein in the H/R + PUMA-siRNA group in the cardiomyocytes were significantly decreased compared to the H/R + NC-siRNA group (p < 0.01), while the expression of Bcl-2 protein was significantly increased (p < 0.01). The apoptosis rate and expressions of PUMA and Bax protein in the H/R + TAU group were significantly decreased compared to the H/R + NC-siRNA group (p < 0.05), while the expression of Bcl-2 protein was significantly increased (p < 0.05), indicating that PUMA plays an important role in H/R myocardial cell apoptosis, while TAU may play a role in anti-apoptosis by downregulating the expression of PUMA.
Figure 5.
Impacts of PUMA silencing on post-H/R myocardial cell apoptosis. (A) H/R+Tau group, a final concentration of 10 mmol/L taurine was added 1 hour before hypoxia; (B) H/R+NC-siRNA group; (C) H/R+PUMA-siRNA group; (D) H/R group, means hypoxia for 5 hours/reoxygenation for 6 hours; (E) Histogram of apoptosis rate in each group. * p < 0.05, ** p < 0.01 vs. H/R.
Figure 6.
Impacts of PUMA silencing on levels of apoptosis-associated proteins in post-H/R myocardial cells. (A) Result of detecting bcl-2, Bax, PUMA and caspase-3 proteins by Western blot. (B) A histogram of relative expression levels of Bax, bcl-2 and PUMA. * p < 0.05, ** p < 0.01 vs. H/R.
DISCUSSION
Apoptosis is the primary cause of myocardial cell loss caused by myocardial I/R injury, and it can lead to increased infarction size and participate in myocardial remodeling and conversion to heart failure.9,22 PUMA is known as "the center of the mitochondrial apoptosis pathway," and it plays a key role in apoptosis.16 Previous research studies have found that the effects of Ad-PUMA were better than Ad-p53 for inhibiting tumor cell proliferation and inducing apoptosis,23,24 so PUMA would appear to have great potential in tumor therapy. PUMA is also a molecular target in preventing and treating degenerative diseases caused by tissue and cell damage due to excessive apoptosis. In these diseases, the goal is to downregulate or inhibit the pro-apoptotic effects of PUMA to reduce tissue and cell damage.18,25 Toth26 found that the myocardial infarct size after I/R in PUMA deficient mice was reduced by 50% compared to normal mice. Our previous experiments showed that the expression of PUMA was significantly increased during rat myocardial cell H/R injury,21 and that it was one of the primary causes of myocardial cell H/R injury. The results of this study further confirmed that H/R leads to myocardial cell apoptosis, and that the expressions of PUMA, Bax, and caspase-3 were increased (Figure 3), while that of Bcl-2 was downregulated. We also found that targeted PUMA silencing could significantly reduce myocardial cell apoptosis (Figure 5), thus increasing the tolerance of myocardial cells towards H/R injury. Therefore, the overexpression of PUMA was a key element of H/R-induced myocardial cell apoptosis, and may be a potential new target for preventing and treating the H/R injury of cardiomyocytes.27
Numerous studies have demonstrated19,20 that TAU is abundantly expressed in the myocardium, and that it is favorable in the prevention and treatment of ischemic heart diseases including myocardial I/R injury, myocardial infarction, coronary heart disease, and heart failure.26 The main molecular mechanisms are involved in the mitochondrial apoptosis pathway and endoplasmic reticulum apoptosis pathway.16,25 For example, TAU has been shown to be able to regulate the expressions and activities of Bcl-2 and Bax, inhibit the expression of caspase-8/9,28 the formation of Apaf-1/caspase-9 apoptotic bodies,29 and regulate the Akt/Caspase-9 apoptosis pathway.30 However, whether its anti-myocardial apoptosis effect is related to PUMA has not been reported. The results of this study suggest that TAU can both inhibit H/R myocardial cell apoptosis (Figure 2) and also downregulate the increased expressions of pro-apoptotic proteins such as PUMA, Bax, and caspase-3 in post-H/R cardiomyocytes (Figure 3), as well as dose-dependently enhance the expression of anti-apoptotic protein Bcl-2. In addition, we found that TAU could downregulate the overexpression of exogenously transfected PUMA in the myocardial cells (Figure 4). These results suggest that downregulating the expression of intracellular PUMA might be an important mechanism of TAU in reducing myocardial I/R injury.
The results of this study also showed that TAU could downregulate the increased expressions of CHOP and GRP78 proteins in the H/R cardiomyocytes. GRP78 is a marker protein for endoplasmic reticulum stress, and CHOP protein is a PUMA transcription factor31 which can promote the expression of PUMA, thus contributing to the activation of Bax and caspase-3, ultimately leading to apoptosis. As for the mechanisms by which TAU downregulates PUMA expression, we hypothesize that TAU has an antioxidation effect, scavenges free radicals, and regulates intracellular Ca2+ homeostasis, thus reducing post-H/R endoplasmic reticulum stress and CHOP protein expression, as well as downregulating the expression and activity of PUMA. Therefore, we believe that the role of TAU in inhibiting I/R injury-induced myocardial cell apoptosis is via downregulating the expression and pro-apoptotic effects of PUMA. This may occur in three ways. First, reducing ERS of post-I/R myocardial cells through its antioxidation effect and by adjusting intracellular Ca2+ homeostasis19 and inhibiting the expression of transcription factor CHOP (Figure 3), thereby resulting in the downregulation of PUMA.31 Second, reducing the neutralization of PUMA towards various anti-apoptotic proteins of the Bcl-2 family, increasing the expressions of anti-apoptotic proteins such as Bcl-2 (Figure 3) and Bcl-XL inside the cardiomyocytes.19,32 Third, reducing the binding and activation of PUMA with Bax/ Bak on mitochondrial membranes, reducing mitochondrial damage and the release of apoptosis initiation factors,19,33 thereby inhibiting or reducing the pro-apoptotic effects of PUMA.
Human studies have revealed the nutritional value of TAU. Of particular note, a World Health Association study involving 50 population groups in 25 different countries reported that elevated dietary TAU consumption was associated with a decreased risk of hypertension and hypercholesterolemia.34 TAU supplementation has also been linked to a lower body mass index35 and reduced levels of inflammation markers in obese women.36 The beneficial effects of TAU were first noted experimentally and later proven epidemiologically in humans.37 Thus, the cytoprotective actions of TAU may contribute to improvements in the clinical and nutritional health of humans. Today, TAU has been approved for the treatment of congestive heart failure in Japan and shows promise in the treatment of several other diseases. It has also stimulated research into its potential therapeutic uses. Results of many clinical studies have been encouraging, suggesting a promising future for TAU therapy.38
CONCLUSIONS
In conclusion, we used PUMA exogenous gene transfection and siRNA targeted gene silencing technology to observe the role of PUMA in alleviating myocardial I/R injury with TAU. PUMA expression and its effect on promoting apoptosis two aspects, establish the PUMA is TAU against myocardial apoptosis effect of new targets, clarify the TAU on I/R PUMA expression and its effect on promoting apoptosis in myocardial cells and the influence of the molecular mechanisms.
Acknowledgments
This study was supported by National Natural Science Foundation of China (No. 81360032).
CONFLICT OF INTEREST
All authors have no conflict of interest regarding this paper.
REFERENCES
- 1.Siddall HK, Warrell CE, Yellon DM, Mocanu MM. Ischemia-reperfusion injury and cardio-protection: investigating PTEN, the phosphatase that negatively regulates PI3K, using a congenital model of PTEN haplo-insufficiency. Basic Res Cardiol. 2008;103:560–568. doi: 10.1007/s00395-008-0735-y. [DOI] [PubMed] [Google Scholar]
- 2.Zhao S, Wang Y, Zhang X, et al. Melatonin protects against hypoxia/reoxygenation-induced dysfunction of human umbilical vein endothelial cells through inhibiting reactive oxygen species generation. Acta Cardiol Sin. 2018;34:424–431. doi: 10.6515/ACS.201809_34(5).20180708A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serdar Kuyumcu M, Kuyumcu A, Yayla Ç, et al. The relationship between nesfatin-1 levels and SYNTAX score in patients with non-ST segment elevation myocardial infarction. Acta Cardiol Sin. 2018;34:386–393. doi: 10.6515/ACS.201809_34(5).20180423A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurtul A, Namik Murat S. Milking-like effect secondary to dynamic systolic compression of right coronary artery by a postinfarction left ventricular aneurysm. Acta Cardiol Sin. 2018;34:443–445. doi: 10.6515/ACS.201809_34(5).20180326C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim NH, Kang PM. Apoptosis in cardiovascular diseases: mechanism and clinical implications. Korean Circ J. 2010;40:299–305. doi: 10.4070/kcj.2010.40.7.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Li G, Wang Z, et al. Elevated expression of C3G protein in the peri-infarct myocardium of rats. Med Sci Monit Basic Res. 2013;19:1–5. doi: 10.12659/MSMBR.883709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sridharan V, Tripathi P, Sharma SK, et al. Cardiac inflammation after local irradiation is influenced by the kallikrein-kinin system. Cancer Res. 2012;72:4984–4992. doi: 10.1158/0008-5472.CAN-12-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karadeniz M, Sarak T, Duran M, et al. Hyperhomocysteinemia predicts the severity of coronary artery disease as determined by the SYNTAX score in patients with acute coronary syndrome. Acta Cardiol Sin. 2018;34:458–463. doi: 10.6515/ACS.201811_34(6).20180528B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buja LM. Myocardial ischemia and reperfusion injure. Cardiovasc Pathol. 2005;14:170–175. doi: 10.1016/j.carpath.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Tezcan O, Karahan O, Alan M, et al. Hyperbaric oxygen preconditioning provides preliminary protection against doxorubicin cardiotoxicity. Acta Cardiol Sin. 2017;33:150–155. doi: 10.6515/ACS20160404B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saleem MT, Chetty MC, Kavimani S. Putative antioxidant property of sesame oil in an oxidative stress model of myocardial injury. J Cardiovasc Dis Res. 2013;4:177–181. doi: 10.1016/j.jcdr.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui B, Zheng Y, Sun L, et al. Heart regeneration in adult mammals after myocardial damage. Acta Cardiol Sin. 2018;34:115–123. doi: 10.6515/ACS.201803_34(2).20171206A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang WT, Hsu CH, Huang TL, et al. MicroRNA-21 is associated with the severity of right ventricular dysfunction in patients with hypoxia-induced pulmonary hypertension. Acta Cardiol Sin. 2018;34:511–517. doi: 10.6515/ACS.201811_34(6).20180613A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mozaffari MS, Liu JY, Abebe W, Baban B. Mechanisms of load dependency of myocardial ischemia reperfusion injury. Am J Cardiovasc Dis. 2013;3:180–196. [PMC free article] [PubMed] [Google Scholar]
- 15.Liu XL, Li G, Wang ZH, et al. Increased expression of Dock 180 protein in the non-infarcted myocardium in rats. J Chin Med Assoc. 2013;76:164–168. doi: 10.1016/j.jcma.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Yee KS, Vousden KH. Contribution of membrane localization to the apoptotic activity of PUMA. Apoptosis. 2008;13:87–95. doi: 10.1007/s10495-007-0140-2. [DOI] [PubMed] [Google Scholar]
- 17.Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene. 2008;27:S71–S83. doi: 10.1038/onc.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu B, Qiu W, Wang P, et al. p53 independent induction of PUMA mediates intestinal apoptosis in response to ischaemia-reperfusion. Gut. 2007;56:645–654. doi: 10.1136/gut.2006.101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaffer SW, Jong CJ, Ito T, Azuma J. Effect of taurine on ischemia-reperfusion injury. Amino Acids. 2014;46:21–30. doi: 10.1007/s00726-012-1378-8. [DOI] [PubMed] [Google Scholar]
- 20.Akdemir O, Hede Y, Zhang F, et al. Effects of taurine on reperfusion injury. J Plast Reconstr Aesthet Surg. 2011;64:921–928. doi: 10.1016/j.bjps.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Tu S, Liu ZQ, Fu JJ, et al. Inhibitory effect of p53 upregulated modulator of apoptosis targeting siRNA on hypoxia/reoxygenation-induced cardiomyocyte apoptosis in rats. Cardiology. 2012;122:93–100. doi: 10.1159/000338701. [DOI] [PubMed] [Google Scholar]
- 22.Zhao ZQ, Velez DA, Wang NP, et al. Progressively developed myocardial apoptotic cell death during late phase of reperfusion. Apoptosis. 2001;6:279–290. doi: 10.1023/a:1011335525219. [DOI] [PubMed] [Google Scholar]
- 23.Yu J, Yue W, Wu B, Zhang L. PUMA sensitizes lung cancer cells to chemotherapeutic agents and irradiation. Clin Cancer Res. 2006;12:2928–2936. doi: 10.1158/1078-0432.CCR-05-2429. [DOI] [PubMed] [Google Scholar]
- 24.Sun Q, Sakaida T, Yue W, et al. Chemosensitization of head and neck cancer cells by PUMA. Mol Cancer Ther. 2007;12:3180–3188. doi: 10.1158/1535-7163.MCT-07-0265. [DOI] [PubMed] [Google Scholar]
- 25.Nickson P, Toth A, Erhardt P. PUMA is critical for neonatal cardiomyocyte apoptosis induced by endoplasmic reticulum stress. Cardiovasc Res. 2007;73:48–56. doi: 10.1016/j.cardiores.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toth A, Jeffers JR, Nickson P, et al. Targeted deletion of Puma attenuates cardiomyocyte death and improves cardiac function during ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2006;291:H52–H60. doi: 10.1152/ajpheart.01046.2005. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Guo Q, Liu X, et al. PUMA-mediated mitochondrial apoptotic disruption by hypoxic postconditioning. Apoptosis. 2015;20:1026–1032. doi: 10.1007/s10495-015-1127-z. [DOI] [PubMed] [Google Scholar]
- 28.Taranukhin AG, Taranukhina EY, Saransaari P, et al. Taurine reduces caspase-8 and caspase-9 expression induced by ischemia in the mouse hypothalamic nuclei. Amino Acids. 2008;34:169–174. doi: 10.1007/s00726-006-0405-z. [DOI] [PubMed] [Google Scholar]
- 29.Takatani T, Takahashi K, Uozumi Y, et al. Taurine inhibits apoptosis by preventing formation of the Apaf/caspase-9 apoptosome. Am J Physiol Cell Physiol. 2004;287:C949–C953. doi: 10.1152/ajpcell.00042.2004. [DOI] [PubMed] [Google Scholar]
- 30.Takatani T, Takahashi K, Uozumi Y, et al. Taurine prevents the ischemia-induced apoptosis in cultured neonatal rat cardiomycytes throuth Akt/caspase-9 pathway. Biochem Biophys Res Commun. 2004;316:484–489. doi: 10.1016/j.bbrc.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 31.Cazanave SC, Elmi NA, Akazawa Y, et al. CHOP and AP-1 cooperatively mediate PUMA expression during lipoapoptosis. Am J Physiol Gastrointest Liver Physiol. 2010;299:G236–G243. doi: 10.1152/ajpgi.00091.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li D, Li J, An Y, et al. Doxorubicin-induced apoptosis in H9c2 cardiomyocytes by NF-κB dependent PUMA upregulation. Eur Rev Med Pharmacol Sci. 2013;17:2323–2329. [PubMed] [Google Scholar]
- 33.Cheng WP, Wu GJ, Wang BW, Shyu KG. Regulation of PUMA induced by mechanical stress in rat cardiomyocytes. J Biomed Sci. 2012;19:72. doi: 10.1186/1423-0127-19-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sagara M, Murakami S, Mizushima S, et al. Taurine in 24-h urine samples is inversely related to cardiovascular risks of middle aged subjects in 50 populations of the world. Adv Exp Med Biol. 2015;803:623–636. doi: 10.1007/978-3-319-15126-7_50. [DOI] [PubMed] [Google Scholar]
- 35.Yamori Y, Taguchi T, Hamada A, et al. Taurine in health and diseases: consistent evidence from experimental and epidemiological studies. J Biomed Sci. 2010;17 Suppl 1:S6. doi: 10.1186/1423-0127-17-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosa FT, Freitas EC, Deminice R, et al. Oxidative stress and inflammation in obesity after taurine supplementation: a double-blind placebo-controlled study. Eur J Nutr. 2014;53:823–830. doi: 10.1007/s00394-013-0586-7. [DOI] [PubMed] [Google Scholar]
- 37.Schaffer S, Kim HW. Effects and mechanisms of taurine as a therapeutic agent. Biomol Ther. 2018;26:225–241. doi: 10.4062/biomolther.2017.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ginguay A, De Bandt JP, Cynober L. Indications and contraindications for infusing specific amino acids (leucine, glutamine, arginine, citrulline, and taurine) in critical illness. Curr Opin Clin Nutr Metab Care. 2016;19:161–169. doi: 10.1097/MCO.0000000000000255. [DOI] [PubMed] [Google Scholar]