Abstract
Background
Copeptin is widely used as a predictor of an adverse prognosis in many clinical conditions. Reduced antegrade coronary flow in an infarct-related artery (IRA) is associated with adverse clinical outcomes in patients with ST-segment elevation myocardial infarction (STEMI). The aim of this study was to investigate whether copeptin level on admission was associated with IRA patency in STEMI patients.
Methods
A total of 88 patients were enrolled into the study and divided into two groups according to TIMI flow grade in the IRA before primary percutaneous coronary intervention.
Results
White blood cell count (p = 0.015), neutrophils (p = 0.047), N-terminal pro-brain natriuretic peptide (NTproBNP) (p < 0.001), copeptin (p < 0.001) and peak troponin I (p = 0.001) were significantly higher in the occluded IRA group with a significantly lower serum sodium level (p < 0.001). Age- and gender-adjusted multivariate analysis revealed that copeptin (OR = 1.970; p = 0.001), peak troponin I (1.055; p = 0.005) and NTproBNP (OR = 1.003; p = 0.010) were independent predictors of an occluded IRA. A copeptin cut-off value of > 6.8 ng/mL was found to predict an occluded IRA with a sensitivity of 80% and specificity of 100% (area under the curve: 0.917; p < 0.001). Performance ranking of the biomarkers that could predict an occluded IRA showed copeptin > peak troponin I = NTproBNP.
Conclusions
Copeptin levels were higher in the patients with an occluded IRA and STEMI. Higher levels of copeptin predicted an occluded IRA in the patients with STEMI who were admitted to the emergency department during the first three hours of chest pain.
Keywords: Copeptin, Infarct-related artery, Myocardial infarction, Patency, STEMI
INTRODUCTION
ST-segment elevation myocardial infarction (STEMI) is a life-threatening medical emergency characterized by persistent ST-segment elevation associated with typical chest pain lasting longer than 30 minutes and the consequent release of biomarkers of myocardial necrosis.1-3 Rapid and successful revascularization of an infarct-related artery (IRA) has been proven to be the most effective treatment option in patients with STEMI.1-5 However, spontaneous reperfusion (SR) of the IRA may appear only in 18 to 29% of STEMI patients, and the thrombolysis in myocardial infarction (TIMI) flow grade in advance of mechanical reperfusion has previously been demonstrated to influence mortality in patients with STEMI undergoing a primary percutaneous coronary intervention (pPCI).6-8 Previous clinical trials have shown that patients with an initially patent IRA have lower rates of heart failure and cardiogenic shock with an improved blood flow grade and smaller infarct size following pPCI.9-12 Although it is a well-known prognostic factor for patients with STEMI, limited data are available on factors related to IRA patency.
Copeptin, the C-terminal part of the arginine vasopressin (AVP) precursor peptide, is a stable and sensitive surrogate marker for AVP release.13,14 AVP and copeptin share the same precursor peptide, a 164 amino acid long preprovasopressin, which consists of a signal peptide, AVP, neurophysin II, and copeptin.15 Although the normal range of copeptin indicates the physiological AVP secretion needed to maintain plasma osmolality, the nonosmotic release of AVP is seen by a high increase in plasma copeptin in severe diseases or states, such as shock, sepsis, stroke, or cardiovascular diseases.16-18 In addition, copeptin level is elevated in all types of stress, and has been shown to be a good indicator of the stress level.19 Increased copeptin levels have also been shown to have prognostic and diagnostic value.19
In this study, we hypothesized that STEMI patients with an occluded IRA may have a higher level of ischemia leading to an increased level of acute endogenous stress, as evidenced by higher levels of copeptin. Therefore, we aimed to investigate whether copeptin level on admission was associated with IRA patency in patients with STEMI undergoing pPCI.
METHODS
Written informed consent was obtained from each patient, and the study protocol was approved by the Ethics Committee of Numune Training and Research Hospital, Ankara, Turkey. The study was conducted in accordance with the principles of the Declaration of Helsinki.
This cross-sectional, single-center study included a total of 88 consecutive patients who were admitted to our emergency department due to the first attack of STEMI between January 2016 and September 2016. The exclusion criteria were as follows: more than three hours from the onset of symptoms, a previous history of myocardial infarction (either STEMI or non-STEMI), previous PCI or coronary artery bypass grafting, the presence of decompensated heart failure, hypothalamic-neurohypophyseal system disorders, severe liver and kidney diseases, autoimmune diseases, malignancies, hematological disorders, severe valvular disease, inflammatory or infectious diseases, and using drugs affecting body fluid balance and osmotic state, such as diuretics and corticosteroids. The definite diagnosis of STEMI was made based on current guidelines.1,2
Blood samples were obtained from the antecubital vein by atraumatic puncture at the time of diagnosis before the patients were sent to the catheter laboratory. An automated blood cell counter (Beckman Coulter LH 750; Beckman Coulter Inc., USA) was used to analyze complete blood count variables. Glucose, urea, uric acid, total cholesterol, high-density lipoprotein, triglycerides, creatinine, and sodium were measured using a Beckman Coulter AU 5800 autoanalyzer (Beckman Coulter Inc., USA). Low-density lipoprotein was calculated using the Friedewald equation. Troponin I was measured on an Access 2 immunoassay device (Beckman Coulter Inc., USA) using the chemiluminescence immunoassay (CLIA) method. Blood samples taken to measure the level of copeptin were centrifuged at 2000 rpm for 20 minutes at 4 °C. Platelet poor plasma was separated and stored at -80 °C until analysis. Copeptin levels were measured using a commercially available sandwich enzyme-linked immunosorbent assay (ELISA) kit with a detection limit of 0.024 ng/mL and an interassay coefficient of variation (CV) of 12% (EASTBIOPHARM, Hangzhou Eastbiopharm Co. Ltd., China, REF: E20160426013, LOT: 20160426).
A loading dose of 600 mg clopidogrel or 180 mg ticagrelor and 300 mg acetylsalicylic acid were administered to the patients diagnosed with STEMI in the emergency setting. In addition, 5,000 IU unfractionated heparin bolus was administered before transfer for pPCI. Coronary angiography was carried out using the standard Judkins technique (Siemens Axiom Sensis XP, Germany). Each coronary artery was visualized in at least two planes perpendicular to each other. All coronary angiographic images were digitally recorded for the evaluation of IRA flow by two experienced interventional cardiologists who were blind to the study, after the recruitment had been completed. Antegrade flow of the IRA was graded visually according to the classification of the TIMI trial.20 TIMI flow was graded as follows: TIMI flow Grade 0: absent antegrade flow; TIMI flow Grade 1: partial contrast penetration beyond an occlusion with incomplete distal filling; TIMI flow Grade 2: patent epicardial artery with opacification of the entire distal artery (however, with delayed contrast filling and/or washout); TIMI flow Grade 3: patent epicardial artery with normal flow.20 According to this grading system, the study population was divided into two groups as those with TIMI 0/1 flow with an occluded IRA (n = 70) a those with TIMI 2/3 flow with a patent IRA (n = 18). All pPCI procedures were successful for revascularization of the IRA.
A complete physical examination was performed in each patient, and their medical history and risk factors for coronary artery disease were recorded. All patients underwent transthoracic echocardiography at 24 hours after pPCI using a 3.5-MHz transducer (Vivid7; GE-Vingmed Ultrasound AS, Horten, Norway). The modified Simpson’s method was used to calculate the left ventricular ejection fraction (LVEF).
Statistical analysis
Statistical analysis was performed using SPSS software version 22.0 for Windows (SPSS Inc., Chicago, IL, USA). The distribution patterns of the variables were analyzed using the Shapiro-Wilk test. Normally distributed continuous variables were presented as mean ± standard deviation, while categorical variables were presented as number and percentage. Abnormally distributed numerical variables were expressed as median and interquartile range. Pearson’s chi-square or Fisher’s exact tests were used to analyze categorical variables, and independent samples t-test or the Mann-Whitney U test were used to analyze continuous variables according to the distribution patterns. For normally distributed continuous variables, the correlation coefficients and their significance were calculated using Pearson correlation analysis. Age and gender adjustments in multivariate regression analysis were used to determine the independent predictors of an occluded IRA. Receiver operating characteristic (ROC) curve analysis was used to identify the optimal cut-off value of copeptin for the prediction of IRA patency and to detect its sensitivity and specificity. A two-sided p value of < 0.05 was considered to be statistically significant.
RESULTS
According to the baseline antegrade flow of the IRA, 88 patients were divided into two groups as the occluded IRA group (n = 70, 79.5%) and patent IRA group (n = 18, 20.5%). The baseline clinical and angiographic characteristics of the study groups are shown in Table 1. There were no statistically significant differences in age, sex, hypertension, diabetes mellitus, hyperlipidemia, smoking status, prehospital medications, heart rate, and systolic and diastolic blood pressure between the two groups (p > 0.05). However, there was a statistically significant difference in the LVEF calculated at 24 hours after the pPCI between the two groups (43.32% ± 8.56% vs. 47.61% ± 5.27%; p = 0.011). In addition, we found no statistically significant differences in the duration of chest pain (140.60 ± 20 vs. 137.94 ± 18.78 min; p = 0.618), door-to-balloon time (26.2 ± 4.4 vs. 27.5 ± 3.5 min; p = 0.247) and distribution of IRA (p = 0.695) between the two groups (Table 1).
Table 1. Baseline clinical and angiographic characteristics of the study groups.
| Variables | Baseline IRA flow | p | |
| Occluded group | Patent group | ||
| TIMI flow 0/1 (n = 70) | TIMI flow 2/3 (n = 18) | ||
| Ag e(years), mean ± SD | 62.07 ± 8.14 | 59.11 ± 10.22 | 0.196 |
| Male, n (%) | 58 (82.9) | 14 (77.8) | 0.876 |
| Hypertension, n (%) | 26 (37.1) | 6 (33.3) | 0.999 |
| Diabetes mellitus, n (%) | 23 (32.9) | 5 (27.8) | 0.680 |
| Hyperlipidemia, n (%) | 34 (48.6) | 8 (44.4) | 0.797 |
| Current smoker, n (%) | 37 (52.9) | 8 (44.4) | 0.602 |
| Systolic blood pressure, mmHg, mean ± SD | 132.81 ± 15.97 | 126.72 ± 14.93 | 0.148 |
| Diastolic blood pressure, mmHg, mean ± SD | 84.24 ± 13.06 | 79.77 ± 8.14 | 0.078 |
| Heart rate, bpm | 77.30 ± 9.31 | 75.33 ± 6.85 | 0.405 |
| Ejection fraction, % | 43.32 ± 8.56 | 47.61 ± 5.27 | 0.011 |
| Prehospital medications, n (%) | 0.999 | ||
| Aspirin | 13 (18.6) | 3 (16.7) | |
| β-Blockers | 7 (10.0) | 2 (11.1) | |
| ACE inhibitor or ARB | 18 (25.7) | 4 (22.2) | |
| Ca channel blocker | 10 (14.5) | 2 (11.1) | |
| Statin | 9 (12.9) | 2 (11.1) | |
| IRA, n (%) | 0.695 | ||
| LAD | 28 (40) | 8 (44.4) | |
| LCX | 15 (21.4) | 2 (11.1) | |
| RCA | 27 (38.6) | 8 (44.4) | |
| Duration of chest pain, min | 140.60 ± 20.37 | 137.94 ± 18.78 | 0.618 |
| Door to balloon time, min | 26.2 ± 4.4 | 27.5 ± 3.5 | 0.247 |
| Symptom to door time, min | 114.4 ± 21.8 | 110.4 ± 16.9 | 0.478 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; IRA, infarct releated artery; LAD, left anterior decending artery; LCX, left circumflex coronary artery; RCA, right coronary artery, SD, standard deviation; TIMI, thrombolysis in myocardial infarction.
Numerical parameters were expressed as mean ± SD categorical variables were expressed as numbers and percentage.
Laboratory results are shown in Table 2. On admission, white blood cell count (p = 0.015), neutrophils (p = 0.047), N-terminal pro-brain natriuretic peptide (NTproBNP) (p = 0.001), copeptin (p < 0.001), and peak troponin I (p = 0.001) were significantly higher in the occluded IRA group with a significantly lower serum sodium level (p < 0.001).
Table 2. Comparison of laboratory parameters of the study groups.
| Variables | Baseline IRA flow | p | |
| Occluded group | Patent group | ||
| TIMI flow 0/1 (n = 70) | TIMI flow 2/3 (n = 18) | ||
| Hemoglobin, g/dl | 13.64 ± 1.62 | 13.29 ± 1.44 | 0.417 |
| White blood cell count, ×109/L | 12.78 ± 2.79 | 11.03 ± 2.02 | 0.015 |
| Neutrophil, ×109/L | 10.17 ± 2.58 | 8.87 ± 1.76 | 0.047 |
| Lymphocyte, ×109/L | 1.80 (0.90-4.00) | 2.00 (1.00-3.10) | 0.157 |
| Platelet count, ×109/L | 241.36 ± 53.75 | 235.61 ± 48.39 | 0.681 |
| Glucose, mg/dL | 122.80 ± 20.70 | 119.67 ± 13.34 | 0.438 |
| Creatinine, mg/dL | 0.98 ± 0.14 | 1.00 ± 0.14 | 0.577 |
| Urea, mg/dL | 36.63 ± 10.07 | 37.28 ± 9.39 | 0.805 |
| Uric acid, mg/dL | 6.47 ± 1.53 | 6.08 ± 0.94 | 0.296 |
| Sodium, mEq/L | 132.91 ± 4.03 | 135.44 ± 2.03 | < 0.001 |
| Potassium, mEq/L | 4.06 ± 0.38 | 4.11 ± 0.32 | 0.564 |
| Total cholesterol, mg/dL | 182.21 ± 31.83 | 176.78 ± 22.45 | 0.498 |
| LDL cholesterol, mg/dL | 118.96 ± 24.30 | 117.11 ± 20.48 | 0.768 |
| HDL cholesterol, mg/dL | 37.27 ± 5.58 | 38.67 ± 5.85 | 0.351 |
| Triglyceride, mg/dL | 173.61 ± 23.16 | 171.28 ± 15.39 | 0.612 |
| Peak troponin I, ng/mL | 48.70 (16.50-87.19) | 30.48 (14.69-58.79) | 0.001 |
| N-terminal proBNP, pg/mL | 806.50 (181.32-4586.20) | 252.11 (58.65-1197.30) | 0.001 |
| Copeptin, ng/mL | 12.13 (2.20-27.24) | 3.94 (2.70-6.80) | < 0.001 |
BNP, brain natriuretic peptide; HDL, high-density lipoprotein; IRA, infarct-related artery; LDL, low-density lipoprotein; SD, standard deviation; TIMI, thrombolysis in myocardial infarction.
Numerical parameters were expressed as mean ± SD or median (min-max). Categorical variables were expressed as numbers and percentage.
In correlation analysis, a statistically significant negative correlation was found between copeptin and admission serum sodium (r = -0.786, p < 0.001) levels and LVEF (r = -0.531, p < 0.001). In addition, a statistically significant positive correlation was found between copeptin and NTproBNP (r = 0.563, p < 0.001) and peak troponin I (r = 0.611, p < 0.001) levels (Table 3). Moreover, we found a modest significant negative correlation between serum sodium and NTproBNP levels on admission (r = -0.510, p < 0.001).
Table 3. Correlation analysis between copeptin and sodium, N-terminal proBNP, peak troponin, left ventricular ejection fraction in the TIMI flow 0/1 group.
| Variables | Copeptin | |
| r | p | |
| Sodium | -0.784 | < 0.001 |
| N-terminal proBNP | 0.645 | < 0.001 |
| Peak troponin I | 0.617 | < 0.001 |
| Left ventricular ejection fraction | -0.494 | < 0.001 |
BNP, brain natriuretic peptide; R, correlation coefficient; TIMI, thrombolysis in myocardial infarction.
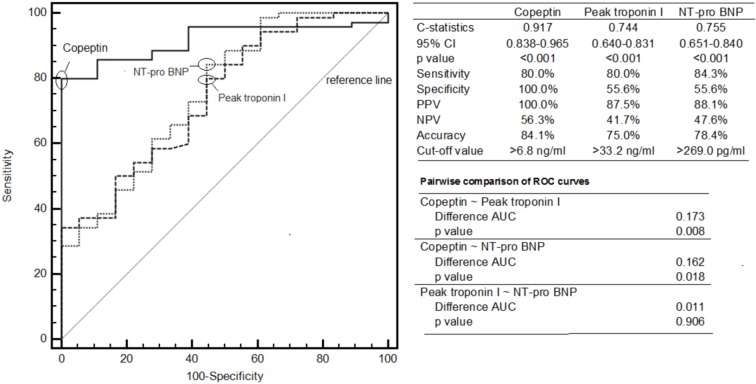
After age and gender adjustments in multivariate regression analysis, copeptin, peak troponin and NTproBNP were found to be independent predictors of an occluded IRA. The results of multivariate regression analysis are given in Table 4. In addition, a copeptin cut-off value of ≥ 6.8 ng/mL was found to predict an occluded IRA with a sensitivity of 80% and specificity of 100% [area under the curve (AUC): 0.917, 95% CI = 0.838-0.965, p < 0.001]. We then compared the predictive ability of copeptin with troponin I and NTproBNP to identify an occluded IRA using C-statistics, which showed that copeptin level had a superior diagnostic performance compared to peak troponin (AUC: 0.917 vs. 0.744, p = 0.008) and NTproBNP (AUC: 0.917 vs. 0.755, p = 0.018) to predict an occluded IRA (Figure 1).
Table 4. Univariate and multivariate logistic regression analyses of variables related to TIMI flow grade in infarct-related artery.
| Variables | Univariate model | Adjusted multivariate model* | ||||||
| OR | 95% CI | p | OR | 95% CI | p | |||
| Lower | Upper | Lower | Upper | |||||
| Copeptin | 1.958 | 1.336 | 2.869 | 0.001 | 1.970 | 1.339 | 2.897 | 0.001 |
| Peak troponin | 1.058 | 1.020 | 1.098 | 0.003 | 1.055 | 1.017 | 1.095 | 0.005 |
| N-terminal proBNP | 1.004 | 1.002 | 1.007 | 0.001 | 1.003 | 1.001 | 1.004 | 0.010 |
* Adjusted age and gender.
BNP, brain natriuretic peptide; CI, confidence interval; OR, odds ratio.
Figure 1.
Comparison of copeptin with troponin I and NT-pro BNP for the identification of occluded IRA by using C-statistics. AUC, area under the curve; BNP, brain natriuretic peptide; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value; ROC, reciever operator characteristics.
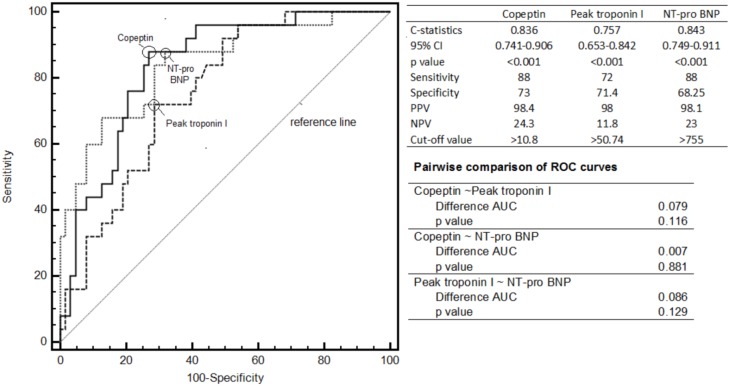
In the patent IRA group, no patient had a LVEF ≤ 40%, whereas 25 (35.7%) patients had a LVEF ≤ 40% in the occluded IRA group (p = 0.007). Copeptin, peak troponin I and NTproBNP had similar diagnostic yields to predict a reduced LVEF (Figure 2).
Figure 2.
Comparison of copeptin with troponin I and NT-pro BNP for the identification of reduced LVEF by using C-statistics. AUC, area under the curve; BNP, brain natriuretic peptide; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value; ROC, reciever operator characteristics.
DISCUSSION
To the best of our knowledge, the present study is the first to investigate the relationship between copeptin levels on admission and IRA flow rate. We found that copeptin was independently associated with antegrade flow grade of the IRA before pPCI. In addition, we found higher copeptin, peak troponin and NTproBNP levels and lower sodium level and lower LVEF in the occluded IRA group compared to the patent IRA group. We also showed that copeptin was a strong and independent predictor of an occluded IRA.
AVP, an antidiuretic hormone, is a peptide consisting of nine amino acids synthesized in magnocellular neurons in two distinct regions of the hypothalamus, supraoptic nucleus, and paraventricular nucleus.21 This neurohormone is derived from a 164 amino acid precursor protein consisting of a signal peptide, AVP moiety, the protein neurophysin 2 and copeptin, a 39 amino acid glycosylated peptide with a leucine-rich core segment.15 AVP induces vasoconstriction through the stimulation of the V1a receptor on vascular smooth muscle cells, thereby leading to adrenocorticotropic hormone (ACTH) secretion through the stimulation of the V1b receptor in the adenohypophysis and to water reabsorption through the stimulation of V2 receptors in the renal collecting duct.21
In acute life-threatening physical stress conditions such as stroke or acute myocardial infarction (AMI), the release of AVP significantly increases to maintain hemodynamic stability.22 Although AVP has been suggested to play a key role in the pathogenesis of several diseases and to have prognostic value in a number of clinical conditions, its clinical use is very limited due to technical difficulties and possible pre-analytical errors.14,23 In contrast to AVP, copeptin secreted into the circulation in equal amounts to AVP is stable in the serum or plasma at room temperature and is a simple and reliable surrogate of AVP.13,18 In recent years, due to the methodological and structural advantages over AVP, copeptin has been widely adopted as an indicator of an individual’s stress level and as a predictor of adverse prognosis in many clinical conditions, such as acute coronary syndrome, heart failure, stroke, and sepsis.16,18,24,25
Coronary artery disease is the leading cause of death worldwide, and STEMI, which requires a prompt diagnosis and urgent treatment, accounts for 25 to 40% of all AMI cases.26 Recent developments in catheter-based interventions, cardiovascular pharmacotherapy, and regional transfer networks have significantly improved the outcomes of STEMI patients.27,28 Nevertheless, post-STEMI mortality rates still remain high, being reported as 6 to 14% in-hospital and 12% at six months.2 Therefore, it is essential to establish optimal treatment strategies on admission, to perform timely discharge, and the timing of follow-up for high-risk patients.
In recent years, many theories have been suggested to explain the rapid release of AVP/copeptin after AMI. One of the most likely explanations is that AVP/copeptin is the main part of the endocrine stress response, leading to ACTH and cortisol release.24 Hence, it is not surprising that the body responds to acute and life-threatening diseases, such as AMI or stroke, by a rapid AVP/copeptin release. Another possible explanation is that direct damage to the cardiac baroreceptors or baroreceptor stimulation due to cardiac underfilling as a consequence of AMI can induce AVP/copeptin release from the posterior pituitary.22
Lamas et al.29 reported that patients with a patent IRA had a significantly improved prognosis in the setting of AMI. Vemulapalli et al.30 also showed that a longer time from symptom onset to the first device and pre-procedural TIMI 0/1 flow in the IRA were independently associated with an increased infarct size, as confirmed by cardiac magnetic resonance imaging in patients with acute anterior STEMI without cardiogenic shock.
On the other hand, Khan et al.17 were the first to report the response of circulating copeptin levels following AMI. In their Leicester Acute Myocardial Infarction Peptide (LAMP) study, they showed that plasma copeptin levels reached the highest value on the first day after AMI, and then decreased to a stable level in 980 patients. Compared to healthy controls, these values were above the normal range during the second and fifth days. The authors also examined the prognostic value of copeptin alone or in combination with NTproBNP, and they reported that copeptin levels were higher in the patients who died or were re-admitted with heart failure, compared to event-free survivors. They concluded that copeptin and NTproBNP were significant independent predictors of death or heart failure at 60 days.
In a subset of patients in the Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan (OPTIMAL) study, the prognostic value of copeptin was further investigated among patients with AMI.25 The authors reported that copeptin was a strong marker for mortality and morbidity in patients with heart failure following AMI. The predictive value of copeptin was even stronger than those of brain natriuretic peptide (BNP) and NTproBNP. Their study also demonstrated the value of serial measurements of copeptin during follow-up, which added a predictive value over a single determination at baseline.
In another study of 54 patients, Reintadler et al.31 showed that larger acute and chronic infarct sizes, as evidenced by cardiac magnetic resonance imaging, were significantly associated with copeptin levels measured on the second day of STEMI. The authors also reported that increased copeptin concentrations at baseline were related to myocardial function and remodeling at four months after STEMI. In another study, admission copeptin levels were found to be independent predictors of the final infarct size, as confirmed by cardiac magnetic resonance imaging in patient with STEMI.32
In our study, we investigated the predictive value of copeptin for IRA patency on admission. In multivariate analysis, we found that higher levels of copeptin were strong and independent predictors of TIMI 0-1 flow in the IRA (OR = 1.97, p < 0.001). Furthermore, we calculated a cut-off value for copeptin using ROC curve analysis, and showed that a copeptin level of ≥ 6.8 ng/mL could detect an occluded IRA with 80% sensitivity and 100% specificity (AUC: 0.917, p < 0.001).
In contrast to copeptin, NTproBNP and peak troponin I levels, we found significantly lower serum sodium levels on admission in the occluded IRA group. Pearson correlation analysis showed a statistically strong correlation between serum sodium and copeptin levels (r = -0.786, p < 0.001), and also a modest correlation between serum sodium and NTproBNP levels (r = -0.510, p < 0.001). We also performed partial correlation analysis to remove the effect of NTproBNP on the correlation between serum sodium and copeptin levels. The analysis revealed a strong significantly negative correlation between serum sodium and copeptin levels (r = -0.701, p < 0.001). These findings can be attributed to be the rapid release of AVP/copeptin in the early period of AMI. Elevated AVP levels may also cause increased free water reabsorption via V2 receptors from the renal collecting duct.
Moreover, in the present study, we found lower peak troponin I levels and higher LVEF in the patent IRA group compared to the occluded IRA group, although there was no significant difference in angiographic characteristics between the groups. Of note, it is well-known that elevated troponin levels are associated with the extent of myocardial necrosis and poor prognosis following myocardial infarction.33 In addition, our study results are partially consistent with previous findings demonstrating that the presence of higher antegrade flow grade in culprit vessels is related to increased procedural success, smaller acute and chronic infarct size, much more favorable short- and long-term prognoses, and improved preservation of the LVEF.10,29
Nonetheless, there are some limitations to this study. First, the sample size is relatively small, and due to the cross-sectional design of the study, no follow-up data were available. Second, we were able to measure the levels of copeptin only at the time of admission and were unable to perform serial measurements due to financial concerns of the institution. Third, although we attempted to estimate the extent of myocardial necrosis with peak troponin levels, we were unable to carry out a quantitative analysis. Further large-scale studies are required to investigate whether hyponatremia on admission can predict the flow in an IRA.
CONCLUSIONS
In conclusion, our study results showed that there was a strong relationship between the levels of copeptin and TIMI flow grade in the IRA in the patients with STEMI who were admitted to the emergency department during the first three hours of chest pain. In addition, we found that a copeptin level of ≥ 6.8 ng/mL could predict an occluded IRA with high sensitivity and specificity. We believe that further large-scale studies are needed to establish a definite conclusion on the use of copeptin in clinical practice.
CONFLICT OF INTEREST
The author(s) declare(s) that there is no conflict of interest.
REFERENCES
- 1.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary:a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the American College of Emergency Physicians and Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2013;82:E1–E27. doi: 10.1002/ccd.24776. [DOI] [PubMed] [Google Scholar]
- 2.Steg PG, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 3.Serdar Kuyumcu M, Kuyumcu A, Yayla C, et al. The relationship between nesfatin-1 levels and SYNTAX score in patients with non-ST segment elevation myocardial infarction. Acta Cardiol Sin. 2018;34:386–393. doi: 10.6515/ACS.201809_34(5).20180423A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen BF, Deng Y, Xu X, et al. Effect of selective thrombus aspiration on serum lipoprotein-associated phospholipase A2 in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention with high thrombus burden. Acta Cardiol Sin. 2018;34:233–241. doi: 10.6515/ACS.201805_34(3).20170227A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cagdas M, Karakoyun S, Yesin M, et al. The association between monocyte HDL-C ratio and SYNTAX score and SYNTAX score II in STEMI patients treated with primary PCI. Acta Cardiol Sin. 2018;34:23–30. doi: 10.6515/ACS.201801_34(1).20170823A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fefer P, Hod H, Hammerman H, et al. Relation of clinically defined spontaneous reperfusion to outcome in ST-elevation myocardial infarction. Am J Cardiol. 2009;103:149–153. doi: 10.1016/j.amjcard.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 7.Rakowski T, Dudek D, Dziewierz A, et al. Impact of infarct-related artery patency before primary PCI on outcome in patients with ST-segment elevation myocardial infarction: the HORIZONS-AMI trial. EuroIntervention. 2013;8:1307–1314. doi: 10.4244/EIJV8I11A199. [DOI] [PubMed] [Google Scholar]
- 8.Stone GW, Cox D, Garcia E, et al. Normal flow (TIMI-3) before mechanical reperfusion therapy is an independent determinant of survival in acute myocardial infarction: analysis from the primary angioplasty in myocardial infarction trials. Circulation. 2001;104:636–641. doi: 10.1161/hc3101.093701. [DOI] [PubMed] [Google Scholar]
- 9.Brodie BR, Stuckey TD, Hansen C, Muncy D. Benefit of coronary reperfusion before intervention on outcomes after primary angioplasty for acute myocardial infarction. Am J Cardiol. 2000;85:13–18. doi: 10.1016/s0002-9149(99)00598-6. [DOI] [PubMed] [Google Scholar]
- 10.De Luca G, Ernst N, Zijlstra F, et al. Preprocedural TIMI flow and mortality in patients with acute myocardial infarction treated by primary angioplasty. J Am Coll Cardiol. 2004;43:1363–1367. doi: 10.1016/j.jacc.2003.11.042. [DOI] [PubMed] [Google Scholar]
- 11.Ndrepepa G, Kastrati A, Schwaiger M, et al. Relationship between residual blood flow in the infarct-related artery and scintigraphic infarct size, myocardial salvage, and functional recovery in patients with acute myocardial infarction. J Nucl Med. 2005;46:1782–1788. [PubMed] [Google Scholar]
- 12.Zhao Q, Zhang R, Hou J, Yu B. Relationship between fragmented QRS and NT-proBNP in patients with ST elevation myocardial infarction who underwent primary percutaneous coronary intervention. Acta Cardiol Sin. 2018;34:13–22. doi: 10.6515/ACS.201801_34(1).20170903A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52:112–119. doi: 10.1373/clinchem.2005.060038. [DOI] [PubMed] [Google Scholar]
- 14.Morgenthaler NG, Struck J, Jochberger S, Dunser MW. Copeptin: clinical use of a new biomarker. Trends Endocrinol Metab. 2008;19:43–49. doi: 10.1016/j.tem.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Land H, Schutz G, Schmale H, Richter D. Nucleotide sequence of cloned cDNA encoding bovine arginine vasopressin-neurophysin II precursor. Nature. 1982;295:299–303. doi: 10.1038/295299a0. [DOI] [PubMed] [Google Scholar]
- 16.Katan M, Fluri F, Morgenthaler NG, et al. Copeptin: a novel, independent prognostic marker in patients with ischemic stroke. Ann Neurol. 2009;66:799–808. doi: 10.1002/ana.21783. [DOI] [PubMed] [Google Scholar]
- 17.Khan SQ, Dhillon OS, O'Brien RJ, et al. C-terminal provasopressin (copeptin) as a novel and prognostic marker in acute myocardial infarction: Leicester Acute Myocardial Infarction Peptide (LAMP) study. Circulation. 2007;115:2103–2110. doi: 10.1161/CIRCULATIONAHA.106.685503. [DOI] [PubMed] [Google Scholar]
- 18.Morgenthaler NG, Muller B, Struck J, et al. Copeptin, a stable peptide of the arginine vasopressin precursor, is elevated in hemorrhagic and septic shock. Shock. 2007;28:219–226. doi: 10.1097/SHK.0b013e318033e5da. [DOI] [PubMed] [Google Scholar]
- 19.Katan M, Christ-Crain M. The stress hormone copeptin: a new prognostic biomarker in acute illness. Swiss Med Wkly. 2010;140:w13101. doi: 10.4414/smw.2010.13101. [DOI] [PubMed] [Google Scholar]
- 20.The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI Study Group. N Engl J Med. 1985;312:932–936. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 21.Koshimizu TA, Nakamura K, Egashira N, et al. Vasopressin V1a and V1b receptors: from molecules to physiological systems. Physiol Rev. 2012;92:1813–1864. doi: 10.1152/physrev.00035.2011. [DOI] [PubMed] [Google Scholar]
- 22.Bolignano D, Cabassi A, Fiaccadori E, et al. Copeptin (CTproAVP), a new tool for understanding the role of vasopressin in pathophysiology. Clin Chem Lab Med. 2014;52:1447–1456. doi: 10.1515/cclm-2014-0379. [DOI] [PubMed] [Google Scholar]
- 23.Lukaszyk E, Malyszko J. Copeptin: pathophysiology and potential clinical impact. Adv Med Sci. 2015;60:335–341. doi: 10.1016/j.advms.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Katan M, Morgenthaler N, Widmer I, et al. Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuro Endocrinol Lett. 2008;29:341–346. [PubMed] [Google Scholar]
- 25.Voors AA, von Haehling S, Anker SD, et al. C-terminal provasopressin (copeptin) is a strong prognostic marker in patients with heart failure after an acute myocardial infarction: results from the OPTIMAAL study. Eur Heart J. 2009;30:1187–1194. doi: 10.1093/eurheartj/ehp098. [DOI] [PubMed] [Google Scholar]
- 26.Mehta RH, Parsons L, Rao SV, Peterson ED. Association of bleeding and in-hospital mortality in black and white patients with st-segment-elevation myocardial infarction receiving reperfusion. Circulation. 2012;125:1727–1734. doi: 10.1161/CIRCULATIONAHA.111.068668. [DOI] [PubMed] [Google Scholar]
- 27.Kaifoszova Z, Kala P, Alexander T, et al. Stent for life initiative: leading example in building STEMI systems of care in emerging countries. EuroIntervention. 2014;10 Suppl T:T87–T95. doi: 10.4244/EIJV10STA14. [DOI] [PubMed] [Google Scholar]
- 28.Nallamothu BK, Normand SL, Wang Y, et al. Relation between door-to-balloon times and mortality after primary percutaneous coronary intervention over time: a retrospective study. Lancet. 2015;385:1114–1122. doi: 10.1016/S0140-6736(14)61932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamas GA, Flaker GC, Mitchell G, et al. Effect of infarct artery patency on prognosis after acute myocardial infarction. The Survival and Ventricular Enlargement Investigators. Circulation. 1995;92:1101–1109. doi: 10.1161/01.cir.92.5.1101. [DOI] [PubMed] [Google Scholar]
- 30.Vemulapalli S, Zhou Y, Gutberlet M, et al. Importance of total ischemic time and preprocedural infarct-related artery blood flow in predicting infarct size in patients with anterior wall myocardial infarction (from the CRISP-AMI Trial). Am J Cardiol. 2013;112:911–917. doi: 10.1016/j.amjcard.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 31.Reinstadler SJ, Klug G, Feistritzer HJ, et al. Association of copeptin with myocardial infarct size and myocardial function after ST segment elevation myocardial infarction. Heart. 2013;99:1525–1529. doi: 10.1136/heartjnl-2013-303975. [DOI] [PubMed] [Google Scholar]
- 32.Ananth V, Beig JR, Tramboo NA, et al. Does plasma copeptin level at admission predict final infarct size in ST-elevation myocardial infarction. Int J Cardiol. 2016;219:326–330. doi: 10.1016/j.ijcard.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 33.Hedstrom E, Astrom-Olsson K, Ohlin H, et al. Peak CKMB and cTnT accurately estimates myocardial infarct size after reperfusion. Scand Cardiovasc J. 2007;41:44–50. doi: 10.1080/14017430601071849. [DOI] [PubMed] [Google Scholar]