Abstract
Background
Cardiac rehabilitation is beneficial for patients after ST-segment elevation myocardial infarction (STEMI). However, most institutes perform outpatient training phase (phase II) of post-MI cardiac rehabilitation after 2-4 weeks. To evaluate the possibility of performing cardiac rehabilitation with an earlier schedule after STEMI.
Methods
We conducted a series of early phase II cardiac rehabilitation starting from 5-7 days after STEMI, including the training group (n = 28) and the control group (n = 42).
Results
The results showed an improved mental component summary of the SF-36 questionnaire after 6 months in the training group. No adverse event was noticed during this early phase II training.
Conclusions
Cardiac rehabilitation after STEMI might be started earlier than previously thought for clinical use or further research.
Keywords: Acute myocardial infarction, Cardiac rehabilitation, Exercise training, Mental health
INTRODUCTION
Cardiac rehabilitation (CR) following ST-segment elevation myocardial infarction (STEMI) is reported to reduce mortality,1-3 improve exercise tolerance, and health related quality of life (HRQoL).4,5 However, most outpatient CR units started the training phase CR (phase II CR) 2 to 4 weeks after STEMI.1,4,6-8 We hypothesized that an earlier comprehensive CR program would provide more benefit for these patients. Therefore, we invited all patients with STEMI to participate in an early phase II CR program within 5 to 7 days after onset, and observed the effect of early intervention on exercise capacity and HRQoL.
METHODS
Subjects
This study was performed in a tertiary center. We started a collaborated team work of early CR program since 2007, and this study included patients who had been consulted for early CR after STEMI from 2007 till 2015 (n = 105). All these patients had received inpatient rehabilitation training and education about cardiovascular risk factors from a team consisting of physicians, nurses, nutritionists, and physical therapists. Patients with cerebrovascular diseases, severe orthopedic disorders, advanced heart failure (functional class IV), severe valvular diseases, or uncontrolled arrhythmia were excluded. Seventy patients were finally enrolled for analysis after exclusion. Informed consent was obtained from all patients before CR. The study was approved by the Institutional Review Board at the Veterans General Hospital-Kaohsiung [15-CT7-05(150423-1)].
Training protocol
All of the 70 patients were invited to participate in the CR program immediately after discharge (i.e. 5-7 days after STEMI). Twenty-eight patients agreed to participate in the CR program, while the other 42 patients refused because of the following reasons: (1) poor accessibility (n = 39) and (2) not interested in exercise training (n = 3). The two groups both underwent exercise tests and SF-36 questionnaires before discharge as baseline (t0), at 8 weeks (t1) and at 6 months follow-up (t2). The control patients were told to gradually increase activity level at home and to cease high risk lifestyle, including smoking and high fat diet, etc.
Comprehensive cardiopulmonary rehabilitation program
The patients in CR group attended 50-minute exercise sessions 3 times a week. Each session had warm-up, cycling, and cool-down components. During the first week, exercise intensity was set at 40-50% heart rate reserve as determined by the baseline exercise test, and gradually increased to 50-60% heart rate reserve in the following weeks. During all exercises, each participant was monitored by electrocardiography and pulse oximetry under the supervision of a physical therapist.
Cardiopulmonary exercise tests
All patients were tested using Metamax 3B (Cortex Biophysik Co., Germany) consisting of a bicycle ergometer, a gas analyzer and an electrocardiography monitor. Patients peddled on an upright bicycle ergometer to assess oxygen consumption at peak exercise (peakVO2) and at anaerobic threshold (AT VO2). Exercise began with an intensity of 0W workload for a 1-minute warm-up, followed by incremental loading using a ramp protocol (10 W/min) until exhaustion. The patients were tested with the Ramp Bruce protocol, following the guideline of the American College of Sports Medicine.9 All patients completed the test safely.
HRQoL
General HRQoL was measured using the Chinese (Taiwan) version of the Medical Outcome Study 36-item Short Form Survey (SF-36). The SF-36, a standardized, generic instrument measuring HRQoL, has been validated in the general Chinese-Taiwan population,10,11 and also in the heart disease populations.12-14 The SF-36 consists of 36 items divided into two categories: the physical component summary (PCS) and the mental component summary (MCS). The MCS score were formed by 4 subscales: vitality, social functioning, role-emotional, and mental health, while the PCS score by the other 4 subscales: physical functioning, role-physical, bodily pain, and general health. Both MCS and PCS scores have been standardized using a linear T-score transformation to ensure a mean of 50 and a standard deviation of 10.15
Statistical analysis
Pearson chi-square tests and independent t-tests were used to compare the between-group data at t0, t1, and t2, while the intra-group change in relation with time was compared with paired t-tests. A p value of less than 0.05 was regarded as statistical significant. All statistical analyses were conducted with SPSS® version 19.
RESULTS
The baseline characteristics of the two groups were shown in Table 1. The mean age of the CR group and the control group were 59.5 ± 11.5 (mean ± SD) and 58.4 ± 13.0 years old, respectively. We found no significant differences in all baseline characteristics.
Table 1. Clinical characteristics.
| Variable | Control group (n = 42) | CR group (n = 28) | p value |
| Age (years, mean ± SD) | 59.5 ± 11.5 | 58.4 ± 13.0 | 0.703 |
| Male/female (n) | 38/4 | 26/2 | 0.727 |
| Body weight (kgw) | 67.28 ± 7.19 | 68.53 ± 10.69 | 0.562 |
| BMI (kg/m2) | 25.05 ± 2.42 | 25.21 ± 3.41 | 0.821 |
| Anterior MI/inferior MI* | 28/14 | 17/11 | 0.611 |
| Killip II or III (n) | 25 | 14 | 0.450 |
| Peak creatine kinase (IU/L) | 2713 ± 2901 | 3262 ± 2812 | 0.446 |
| LVEF (%) | 46.5 ± 6.5 | 44.9 ± 8.6 | 0.422 |
| No. of coronary arteries with ≥ 50% stenosis (n) | 1.88 ± 0.86 | 1.93 ± 0.83 | 0.831 |
| Systemic hypertension | 50% | 54% | 0.652 |
| Diabetes mellitus | 36% | 29% | 0.601 |
| Dyslipidemia | 48% | 43% | 0.726 |
| Smoking | 57% | 54% | 0.325 |
| Medications | |||
| Aspirin | 98% | 97% | 0.770 |
| Clopidogrel | 100% | 100% | 1.000 |
| Beta-blocker | 91% | 93% | 0.727 |
| ACEI | 93% | 93% | 1.000 |
| Statin | 69% | 64% | 0.678 |
| Predischarge exercise test | |||
| Rest HR (bpm) | 74.9 ± 12.0 | 74.6 ± 11.9 | 0.903 |
| AT VO2 (ml/kg/min) | 8.65 ± 2.24 | 8.51 ± 1.51 | 0.724 |
| Peak exercise | |||
| VO2 (ml/kg/min) | 11.23 ± 3.05 | 11.45 ± 2.07 | 0.737 |
| HR (bpm) | 101.3 ± 14.3 | 103.2 ± 13.8 | 0.588 |
| SBP (mmHg) | 140.7 ± 21.9 | 141.5 ± 28.6 | 0.898 |
* Anterior MI, anterior and anteroseptal wall myocardial infarction; Inferior MI, inferior and posterior wall myocardial infarction; ACEI, angiotension-converting enzyme inhibitor; AT, anaerobic threshold; BMI, body mass index; CR, cardiopulmonary rehabilitation; HR, heart rate; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure; SD, standard deviation; VO2, oxygen uptake.
No any adverse event was found while performing early CR program. The change of MCS and PCS were shown in Figure 1. The MCS improved with time in the CR group, while worsened with time in the control group, leading to a significant difference (p < 0.05) at 6 months. Besides, one of the subscales of PCS, bodily pain, was significantly greater for the CR group (p < 0.05) at t2 (Figure 2). Otherwise, no significant between-group difference was found for peakVO2 and AT VO2 at all time.
Figure 1.
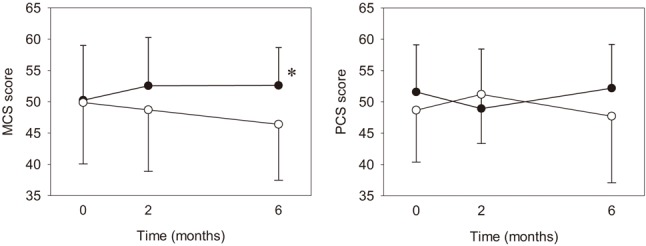
Mental component summary scores and physical component summary scores for the cardiac rehabilitation group and the control group. Solid circle, cardiac rehabilitation group; Hollow circle, control group; * p < 0.05.
Figure 2.
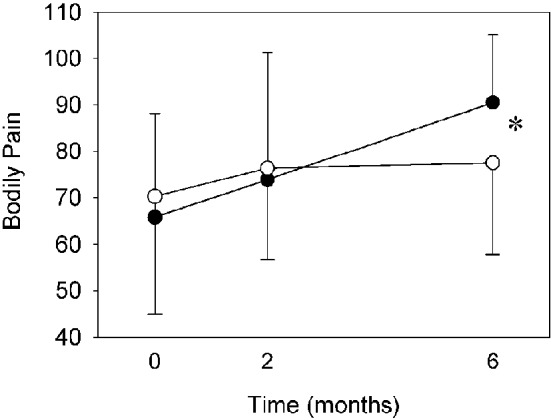
Bodily pain score for the cardiac rehabilitation group and the control group. Solid circle, cardiac rehabilitation group; Hollow circle, control group; * p < 0.05.
In the previous study,16 patients with diabetes seemed to gain less benefit from CR. As for the subgroup analysis in this study, the change of peak VO2 among trained diabetic patients [from 3.22 Metabolic Equivalent of Tasks (METs) to 4.31 METs] and trained non-diabetic patients (from 3.29 METs to 4.55 METs) showed no significant difference. The similar trend but non-significant result probably comes from a small sample size.
DISCUSSION
This study, to the best of our knowledge, represents the earliest (5-7 days after STEMI) supervised CR among current reports. No adverse event was found during such an early CR program.
Many studies had discussed the benefit of CR after STEMI.4,6,17 In these studies, the patients started CR at about 2 weeks after STEMI. However, due to better accessibility of revascularization and caring quality nowadays, patients of STEMI presented very low mortality,18,19 and required shorter hospitalization recently. We hypothesized that if the patients started the CR program earlier, they might recover better because of the following reasons: First, the patients’ misperceptions and negative beliefs could be modified early in the recovery process;20 second, the patients might be more amenable to behavioral changes during the immediate days after a major threat.20 We did not find any adverse effects caused by this early CR program. Therefore, the STEMI patients might be able to receive CR programs earlier than previously reported.
The improvement of mental health after the comprehensive CR program might be attributed to both of the exercise training and the education in our program. Exercise improves mental health through the secretion of endorphin.21 The education helped the patient monitor himself, encounter with stress, and reduce the fear of recurrence. The benefits from this 8-week training program could sustain for up to 6 months, supporting the efficacy and efficiency of the concept of early comprehensive CR.
Interestingly, although there was no significant between-group difference in PCS, the CR group had a significantly greater improvement in one of the subscales of PCS, the bodily pain, than the controls at 6 months. The improvement in this measure might be due to the education program, in which we explained to the patients about the differences of cardiac- and non-cardiac related chest pain and various symptoms they might encounter. Their awareness might reduce their worry, and then reduce the perception of bodily pain. In a study of illness-perception in STEMI patients, Petrie et al found that a less reported bodily pain might be due to a lower level of worry in their intervention group.20
The limitation of this study included the small sampling size, and the non-randomization design. The reason of non-significant improvement of peakVO2 and AT VO2 for CR group might be due to the exclusion of complicated cases. Because the patients enrolled had relatively preserved exercise capacity, the benefit from training might be limited. Since there was no adverse event caused by this early CR, more complicated patients might be included for research in the future.
CONCLUSIONS
An early ambulatory hospital-based comprehensive CR program, starting within 5 to 7 days after STEMI, was effective in improving mental health related quality of life and bodily pain in patients following STEMI.
Acknowledgments
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116:682–692. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Kureshi F, Kennedy KF, Jones PG, et al. Association between cardiac rehabilitation participation and health status outcomes after acute myocardial infarction. JAMA Cardiol. 2016;1:980–988. doi: 10.1001/jamacardio.2016.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2011;162:571–584. doi: 10.1016/j.ahj.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Izawa K, Hirano Y, Yamada S, et al. Improvement in physiological outcomes and health-related quality of life following cardiac rehabilitation in patients with acute myocardial infarction. Circ J. 2004;68:315–320. doi: 10.1253/circj.68.315. [DOI] [PubMed] [Google Scholar]
- 5.Yu CM, Lau CP, Chau J, et al. A short course of cardiac rehabilitation program is highly cost effective in improving long-term quality of life in patients with recent myocardial infarction or percutaneous coronary intervention. Arch Phys Med Rehabil. 2004;85:1915–1922. doi: 10.1016/j.apmr.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Jolliffe JA, Rees K, Taylor RS, et al. Exercise-based rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2000(4):CD001800. doi: 10.1002/14651858.CD001800. [DOI] [PubMed] [Google Scholar]
- 7.Oldridge N, Guyatt G, Jones N, et al. Effects on quality of life with comprehensive rehabilitation after acute myocardial infarction. Am J Cardiol. 1991;67:1084–1089. doi: 10.1016/0002-9149(91)90870-q. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Cao H, Jiang P, Tang H. Cardiac rehabilitation in acute myocardial infarction patients after percutaneous coronary intervention: a community-based study. Medicine. 2018;97(8) doi: 10.1097/MD.0000000000009785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson PD. Exercise prescription and proscription for patients with coronary artery disease. Circulation. 2005;112:2354–2363. doi: 10.1161/CIRCULATIONAHA.104.502591. [DOI] [PubMed] [Google Scholar]
- 10.Lu J-FR, Tseng HM, Tsai YJ. Assessment of health-related quality of life in Taiwan (I): development and psychometric testing of SF-36 Taiwan version. Taiwan J Public Health. 2003;22:501–511. [Google Scholar]
- 11.Tseng HM, Lu J-FR, Tsai YJ. Assessment of health-related quality of life in Taiwan (II): norming and validation of SF-36 Taiwan version. Taiwan J Public Health. 2003;22:512–518. [Google Scholar]
- 12.Beck CA, Joseph L, Belisle P, Pilote L. Predictors of quality of life 6 months and 1 year after acute myocardial infarction. Am Heart J. 2001;142:271–279. doi: 10.1067/mhj.2001.116758. [DOI] [PubMed] [Google Scholar]
- 13.Brown N, Melville M, Gray D, et al. Quality of life four years after acute myocardial infarction: short form 36 scores compared with a normal population. Heart. 1999;81:352–358. doi: 10.1136/hrt.81.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Failde II, Soto MM. Changes in health related quality of life 3 months after an acute coronary syndrome. BMC Public Health. 2006;6:18. doi: 10.1186/1471-2458-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ware J, Kosinski M. SF-36 Physical & Mental Health Summary Scales: A Manual for Users of Version 1. 2nd ed. Lincoln: RI: Quality Metric Incorporated; 2001. [Google Scholar]
- 16.St. Clair M, Mehta H, Sacrinty M, et al. Effects of cardiac rehabilitation in diabetic patients: both cardiac and noncardiac factors determine improvement in exercise capacity. Clin Cardiol. 2014;37:233–238. doi: 10.1002/clc.22245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasquali SK, Alexander KP, Coombs LP, et al. Effect of cardiac rehabilitation on functional outcomes after coronary revascularization. Am Heart J. 2003;145:445–451. doi: 10.1067/mhj.2003.172. [DOI] [PubMed] [Google Scholar]
- 18.Chen KC, Yin WH, Wu CC, et al. In-hospital implementation of evidence-based medications is associated with improved survival in diabetic patients with acute coronary syndrome - data from TSOC ACS-DM Registry. Acta Cardiol Sin. 2018;34:211. doi: 10.6515/ACS.201805_34(3).20180207B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sung SH, Chen TC, Cheng HM, et al. Comparison of clinical outcomes in patients undergoing coronary intervention with drug-eluting stents or bare-metal stents: a nationwide population study. Acta Cardiol Sin. 2017;33:10–19. doi: 10.6515/ACS20160608A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrie KJ, Cameron LD, Ellis CJ, et al. Changing illness perceptions after myocardial infarction: an early intervention randomized controlled trial. Psychosom Med. 2002;64:580–586. doi: 10.1097/00006842-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Harber VJ, Sutton JR. Endorphins and exercise. Sports Med. 1984;1:154–171. doi: 10.2165/00007256-198401020-00004. [DOI] [PubMed] [Google Scholar]


