Abstract
An epidemiological study of Trypanosoma evansi (T. evansi) infection in dromedaries was conducted in four wilayate (localities) of Southern Algeria: Béchar, El Bayadh, Ouargla, Tamanrasset. Between February 2014 and April 2016, 1056 camels of different ages and both sexes from 84 herds were sampled. The prevalence was determined through parasitological examination (Giemsa stained thin smear, GST), serological tests (CATT/T. evansi, ELISA/VSG RoTat 1.2, immune trypanolysis), and molecular tests (T. evansi type A specific RoTat 1.2 PCR and T. evansi type B specific EVAB PCR).
The overall prevalence was 2.4 % with GST, 32.4% with CATT/T. evansi, 23.1% with ELISA/VSG RoTat 1.2, 21.0% with immune trypanolysis (TL), 11.2 % with RoTat 1.2 PCR and 0% with EVAB PCR.
El Bayadh was the most affected wilaya with 11.8% positives in GST, 74.9% in CATT/T. evansi, 70.1% in ELISA/VSG RoTat 1.2 and 62.2% in immune trypanolysis. Only in Béchar, a non-significantly higher prevalence (13.6%) was observed with RoTat1.2 PCR than in El Bayadh (13.0%). We didn't find any evidence of the presence of T. evansi type B in the study area.
Keywords: Zoology, Algeria, Trypanosoma evansi, Surra, Dromedary camel, GST, RoTat 1.2 PCR, CATT/T. evansi, ELISA/VSG RoTat 1.2, Immune trypanolysis
1. Introduction
Trypanosoma evansi (T. evansi) causes trypanosomosis called “surra” in many countries (OIE, 2018). It affects a large number of wild and domesticated animal species in Asia, Africa and Central and South America (Luckins and Dwinger, 2004; OIE, 2018). It is especially pathogenic in camelids and equids (Desquesnes et al., 2013b). Trypanosoma evansi is a protozoan parasite transmitted mechanically by haematophagous biting flies, mainly tabanids (Atarhouch et al., 2003).
In camels, surra usually occurs in its chronic form but it may be acute with high mortality when the animal is under stress. The chronic form is characterised by reduced fertility, generalised loss of body condition, oedema, particularly of the lower parts, neuropathy and immune suppression, anaemia and eventually death (Al-Qarawi et al., 2004; Luckins, 1998; Parsani et al., 2008). In affected countries, surra is an economically important disease, which causes high mortality, low milk and meat production, poor carcass quality, reduced reproductive performance, decreased draught power and manure production (Desquesnes et al., 2013a).
Algeria covers an area of 2,381,741 km2, of which 87% is occupied by the Sahara where 344,015 camels live (Ministère de l'Agriculture, du Développement Rural et de la Pêche (MADRP), 2014).
The first case of trypanosomosis in Algeria was reported by the Sergent brothers in 1903 (Sergent and Sergent, 1905). A year later, 10% of 282 camels sampled from the South-Eastern regions of the country, showed Trypanosoma in their blood (Sergent and Sergent, 1905; Sergent and Donatien, 1921). The disease was well known by herders who associated it with the presence of biting flies. Indeed, its local name is "El Dhebab" which means "fly" in Arabic. However, no extended studies were conducted until 1999 when a parasitological survey was carried out on 1125 dromedary camels with a reported prevalence of 0.5 % in Ouargla (Marfoua, 1999). More recently, between 2005 and 2006, we sampled 1074 dromedary camels of the South of Algeria in the wilayate of El Bayadh, Ouargla, Béchar, Tindouf, Adrar and Tamanrasset. We observed 2.3% positives in Giemsa stained thin blood smears (GST) and 20.3% in CATT/T. evansi with the highest parasitological (12.3%) and serological (68.7%) prevalences in Béchar and no single positive in El Bayadh (Boushaki, 2007).
Absence of pathognomonic signs of surra necessitates laboratory analysis to confirm the infection, either by microscopy or by molecular tools to demonstrate the parasite and thus active infection or by serological tools to reveal antibodies induced by a present or past infection (Büscher, 2001, 2014; Tehseen et al., 2015). Given the low sensitivity of parasitological examinations in chronic infections, DNA amplification techniques, such as the polymerase chain reaction (PCR), are often applied as surrogate (Büscher, 2014). For surra, Trypanozoon-specific primers targeting satellite DNA or ribosomal DNA are the most sensitive (Gari et al., 2010; Masiga et al., 1992; Njiru et al., 2005) while the distinction between T. evansi type A and type B can be made with PCRs specific for the type A RoTat 1.2 gene and specific for type B minicircles (Claes et al., 2004; Njiru et al., 2006; Urakawa et al., 2001). In addition to parasitological or molecular diagnostics, serological tests are useful to provide indirect evidence of the presence of T. evansi in a susceptible population or individual. The most specific tests to detect antibodies against T. evansi type A make use of the variant surface glycoprotein (VSG) RoTat 1.2 as antigen (Bajyana Songa and Hamers, 1988; Urakawa et al., 2001). These tests comprise the direct agglutination test CATT/T. evansi (Bajyana Songa and Hamers, 1988), the ELISA/VSG RoTat 1.2 (Lejon et al., 2005; Rogé et al., 2013; Verloo et al., 2000) and the immune trypanolysis (Van Meirvenne et al., 1995; Verloo et al., 2001). Trypanosoma evansi type B typically lacks the RoTat 1.2 gene and therefore does not induce anti-RoTat 1.2 antibodies (Ngaira et al., 2005; Njiru et al., 2006). So far, T. evansi type B seems only to occur in camels in Kenya, Chad, Ethiopia and Sudan (Birhanu et al., 2015; Ngaira et al., 2005; Njiru et al., 2006; Salim et al., 2011).
In order to create awareness about surra in Algeria and to overcome the economic losses it can cause, it is necessary to acquire recent and accurate information on the epidemiology of this disease using sensitive and effective diagnostic tools. Hence, the aim of the study was to estimate the prevalence of T. evansi infection in dromedary camels in Southern Algeria, and to assess the associated risk factors.
2. Materials and methods
2.1. Ethical statement
Authorisation to conduct the survey was obtained from the Direction des Services Vétérinaires (DSV, Ministry of Agriculture, Rural Development and Fisheries). At each wilaya, the study was authorised and supervised by the respective Inspection Vétérinaire de Wilaya (IVW Béchar, El Bayadh, Ouargla, and Tamanrasset), operating under the umbrella of the Direction des Services Vétérinaires.
2.2. Study area
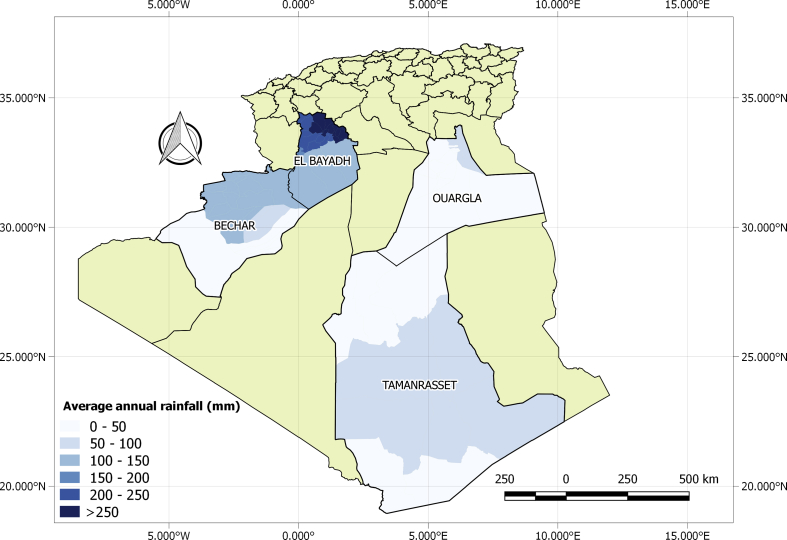
The study was conducted in four wilayate of Southern Algeria: El Bayadh, Béchar, Ouargla, Tamanrasset (Fig. 1).
Fig. 1.
Map of the study area showing the four wilayate and the average annual rainfall measured during 2014, 2015 and 2016.
El Bayadh is divided into three areas: the steppe high plains, the Saharan atlas zone, and the Pre-Saharan zone. The average temperatures range from 0 °C in January to 35.1 °C in August. The vegetation is composed mainly by degraded forests of Pinus halepensis and Ziziphus lotus in the mountains. Unsalted steppe formations are characterised by grasses like Artemisia herba alba, Stipa tenacissima and Aristida pungens. The vegetation of salty soils is characterised by Atriplex sp (Direction des Services Agricoles d'El Bayadh, 2016; Office National de la Météorologie (ONM), 2018).
Béchar is characterised by a continental desert climate. The temperature varies between 3.7 °C in January and 45 °C during July and August. The valleys are very rich in woody and perennial grasses such as Aristida pungens, and Cenchrus biflorus (Direction des Services Agricoles de Béchar, 2018; Office National de la Météorologie (ONM), 2018).
Ouargla is characterised by a Saharan climate with rare and irregular rainfall. Temperatures range from 4 °C in January to 44.6 °C in August. The plant canopy is composed of herbaceous species like Cenchrus bifloru, and Aristida pungens, shrubby vegetation with Retama raetam and Calligonum azel and woody species, mainly Acacia sp (Direction des Services Agricoles de Ouargla, 2016; Office National de la Météorologie (ONM), 2018).
Tamanrasset is divided into three regions: the north composed of plateaus and plains, the centre a region of relief, and the south with plains bordering the Sahel countries. Temperature varies between 4.7 °C in January and 47.6 °C in August. The vegetation is composed of steppe formations such as Artemisia herba alba in the north and desert formations in the south with Tamarix aphylla, Acacia sp. and Trichodesma africanum (Direction des Services Agricoles de Tamanrasset, 2016; Office National de la Météorologie (ONM), 2018).
2.3. Study characteristics
This cross-sectional study was conducted between February 2014 and April 2016 on the dromedary camel population of the selected localities. In these localities, herds were encountered in the camps, and especially in rangelands (pastures and watering wells). Ten percent of the animals in each herd were sampled randomly without stratification according to breed, sex or age. With an expected prevalence of 25% (Boushaki, 2007) and a desired precision of 10%, the calculated sample size was 1153 animals (Toma et al., 2010). The following parameters were recorded for each animal: origin, age, sex, fur colour, breeding system and presence of clinical symptoms (cachexia, conjunctival mucosae, lachrymation, oedema and lymph nodes). The breeder's knowledge of the disease, previous trypanocidal treatment, history of abortions and transhumance routes were also recorded. The age of the animals recorded was based on information from the owners. Animals were grouped into five age classes: less than one year, between one and two years, between two and four years, between 4 and 10 years, older than 10 years.
2.4. Blood sampling
For each animal, two blood samples were taken from the jugular vein: 4.5 ml on ethylene diamine tetra acetate (EDTA) and 10 ml in a dry tube. The sample on EDTA was used to measure the packed cell volume (PCV) and to extract DNA using the QIAamp DNA Blood Mini Kit 250 from Qiagen, Hilden, Germany according to the manufacturer's instructions. Extracted DNA was preserved at -20 °C till analysis. Blood taken in the dry tube was allowed to clot where after it was centrifuged for 15 min at 480 g. The obtained serum was tested with CATT/T. evansi and subsequently aliquoted into cryotubes and frozen at −20 °C for further serological tests.
2.5. Parasitological examination
Parasitological examination was performed on Giemsa stained thin smears (OIE, 2018). A smear was considered positive when at least one trypanosome was observed.
2.6. Packed cell volume
Packed cell volume was assessed after centrifugation of capillary tubes (75 mm, Hirschmann, Germany) filled with EDTA blood in a microhaematocrit centrifuge (OIE, 2018). An animal was considered anaemic when it had a PCV of less than 24% (Dioli and Stimmelmayr, 1992). For technical reasons, the PCV was carried out on only 904 samples.
2.7. CATT/T. evansi
The sera were tested for the presence of anti-T. evansi antibodies using the card agglutination test for trypanosomosis (CATT/T. evansi) (Institute of Tropical Medicine, Antwerp, Belgium) (Bajyana Songa and Hamers, 1988).
2.8. ELISA/VSG RoTat 1.2
Indirect ELISA/VSG RoTat 1.2 was carried out according to Lejon et al. (2005) and Verloo et al. (2000). Minor modifications were the coating of the native VSG RoTat 1.2 (Institute of Tropical Medicine, Antwerp, Belgium) diluted at 2 μg/ml in phosphate buffer (PB 0.01 M, pH 6.5, NaH2PO4.H2O 0.95 g/l, Na2HPO4.2H2O 0.55 g/l), the incubation of 150 μl of test sera diluted 1/100 in PBS-Blotto without shaking and reading of the absorption at 405 nm. Corrected ODs were expressed as percentage positivity (PP) of the ODcorr obtained with positive control serum included in each plate. The cut-off value was set at 30% PP, based on the bimodal distribution observed in the histogram constructed with data from the whole study cohort (Supplementary material S1).
2.9. Immune trypanolysis
Immune trypanolysis (TL) was performed at the OIE Reference Laboratory for Surra at the Insitute of Tropical Medicine, Antwerp, Belgium, according to Van Meirvenne et al. (1995) and Verloo et al. (2000).
2.10. Molecular diagnosis
Two molecular tests were carried out to detect T. evansi DNA in blood. The RoTat 1.2 PCR targets the T. evansi-specific gene coding for RoTat 1.2 VSG; the EVAB PCR targets T. evansi type B minicircle kinetoplast DNA (Birhanu et al., 2015; Urakawa et al., 2001) (Table 1). Compared to the cited references, minor changes were the activation of HotStar Taq DNA polymerase at 95 °C for 15 min and, in the EVAB PCR, the final extension for 10 min at 72 °C.
Table 1.
Details on target gene, primer sequences, amplicon lengths of the RoTat 1.2 PCR and the EVAB PCR.
| Taxon | Target gene | Primers | Primer sequences | Amplicon length | Reference |
|---|---|---|---|---|---|
| T. evansi type A | RoTat 1.2 VSG | ILO7957 | 5′- GCC ACC ACG GCG AAA GAC -3′ | 488 bp | Urakawa et al. (2001) |
| ILO8091 | 5′-TAA TCA GTG TGG TGT GC-3′ | ||||
| T. evansi type B | minicircle | EVAB-1 | 5′-ACAGTCCGAGAGATAGAG-3′ | 436 bp | Birhanu et al. (2015) |
| EVAB-2 | 5′-CTGTACTCTACATCTACCTC-3′ |
As positive control, DNA (10 ng/ml) of T. evansi type A (MCAM/ET/2013/MU/02) and type B (MCAM/ET/2013/MU/10) were included in each PCR run, together with pure water as negative control (Birhanu et al., 2016). The amplified products were visualised under UV after electrophoresis in a 2% agarose gel (Sigma, USA) at 135 V for 30 min and staining with ethidium bromide (Sigma, USA).
2.11. Data analysis
All data were recorded in Microsoft Excel. R version 3.4.1 (R Core Team, 2017) was used for statistical analysis. Percentages with 95% confidence interval (CI) were used to express prevalence. Concordance between test results was expressed in terms of indices of positive and negative agreement (Cicchetti and Feinstein, 1990) and their 95% confidence intervals were calculated (Adel et al., 2015; Graham and Bull, 1998). It was noticed that despite a high concordance between two tests, the kappa coefficient may paradoxically be low (Cicchetti and Feinstein, 1990; Feinstein and Cicchetti, 1990). Therefore, levels of agreement between diagnostic tests were indicated by indices of positive and negative agreement since these indices are not influenced by prevalence, unlike the kappa coefficient.
The t-test was used to compare between the mean PCV in the group of positive animals and negative animals according to their different diagnostic test results. Logistic regression was applied for assessing differences in prevalence of T. evansi infection according to the wilaya, the age, the sex, the fur colour, and the breeding system. P values < 0.05 were considered as significant. The map was constructed on QGIS 2.18.15.
3. Results
3.1. Study cohort
A total of 1056 dromedaries from 84 herds at 68 sites were sampled. A coverage rate of 10% was observed within all prospected herds. The number of animals sampled according to wilaya, sex and age class is represented in Table 2. About two thirds of the animals (69.9%) were adults (>4 years). Most animals were females (80.5%).
Table 2.
Number of dromedary camels sampled according to wilaya, sex and age class.
| Wilaya | Sex |
Age class (years) |
|||||
|---|---|---|---|---|---|---|---|
| Female | Male | <1 | ≥1 ≤ 2 | >2 to ≤4 | >4 and ≤10 | >10 | |
| El Bayadh | 152 | 19 | 9 | 12 | 12 | 65 | 73 |
| Ouargla | 280 | 24 | 12 | 27 | 33 | 141 | 91 |
| Béchar | 201 | 20 | 0 | 34 | 12 | 86 | 89 |
| Tamanrasset | 217 | 143 | 96 | 49 | 32 | 126 | 57 |
| Total | 850 | 206 | 117 | 122 | 89 | 418 | 310 |
Out of the 1056 animals sampled, some did not undergo all the available diagnostic tests because of technical reasons (i.e. losses, haemolysis), leading to variations in the number of samples per diagnostic test.
3.2. Mortality and morbidity
During the study period the camel owners reported 293 abortions, 60 stillbirths and 96 deaths (Table 3). The highest number of abortions, stillbirths and deaths were recorded in El Bayadh.
Table 3.
Mortality and morbidity recorded in the wilayate.
| Wilaya | Period | Abortions | Stillbirths | Deaths |
|---|---|---|---|---|
| El Bayadh | February 2014–November 2014 | 132 | 26 | 59 |
| Ouargla | February 2014–December 2015 | 30 | 10 | 10 |
| Béchar | January 2015–January 2016 | 110 | 18 | 15 |
| Tamanrasset | February 2014–April 2016 | 21 | 6 | 12 |
3.3. Clinical findings
Clinical examination revealed signs of both the acute and chronic form of disease, with lacrimation (30.2%), lymph node hypertrophy (28.4 %), rough coat (20%), cachexia (17%) and pale conjunctival mucosa (11%). Other signs were observed with lower frequency: diarrhoea, oedema, petechial haemorrhages of the oral mucous membranes, ulcerative keratitis, respiratory complications, and sterno-abdominal decubitus due to paralysis of the hind quarters. In El Bayadh, 78.9% of the parasitologically positive animals and 81.8% of the PCR positive animals showed clinical signs of surra.
3.4. Laboratory diagnostic test results
The observed overall parasitological prevalence in GST was found to be 2.4% (95% CI:1.5–3.3). Overall serological prevalence ranged from 32.4% (CI: 29.6–35.2) in CATT/T. evansi over 21.0% (CI: 18.5–23.5) with TL to 23.1% (CI: 20.5–25.7) in ELISA/VSG RoTat 1.2. Overall molecular prevalence was 11.2% (CI: 8.5–13.9) in RoTat 1.2 PCR (Table 4). No single animal was positive in EVAB PCR. Overall prevalence recorded in the other tests were statistically different from each other (p < 0.0001) except between TL and ELISA/VSG RoTat 1.2 (p = 0.28).
Table 4.
Number (n) of animals tested, number (p) and percentage (%) of positives in different tests according to wilaya, breeding system, sex, age and fur colour of the animals. CATT = CATT/T. evansi, ELISA = ELISA/VGS RoTat 1.2, TL = immune trypanolysis, PCR = RoTat 1.2 PCR
| GST |
CATT |
ELISA |
TL |
RoTat 1.2 PCR |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | p (%) | n | p (%) | n | p (%) | n | p (%) | n | p (%) | ||
| Wilaya | El Bayadh | 161 | 19 (11,8)* | 171 | 128 (74.9)* | 134 | 94 (70.1)* | 135 | 84 (62.2)* | 169 | 22 (13.0) |
| Ouargla | 221 | 2 (0,9) | 221 | 27 (12.2)* | 219 | 25 (11.4)* | 218 | 27 (12.4)* | 50 | 5 (10.0) | |
| Béchar | 304 | 4 (1,3) | 304 | 112 (36.8)* | 304 | 99 (32.6)* | 304 | 93 (30.6)* | 162 | 22 (13.6) | |
| Tamanrasset | 360 | 0 (0,0) | 360 | 75 (20.8) | 349 | 14 (4.0) | 349 | 7 (2.0) | 155 | 11 (7.1) | |
| Overall | 1046 | 25 (2.4) | 1056 | 342 (32.4) | 1006 | 232 (23.1) | 1006 | 211 (21.0) | 536 | 60 (11.2) | |
| Breeding system | sedentary | 17 | 0 (0,0) | 17 | 9 (52.9)* | 16 | 2 (12.5) | 16 | 1 (6.3) | 15 | 1 (6.7) |
| semi-intensive | 20 | 0 (0,0) | 20 | 2 (10.0) | 19 | 0 (0.0) | 18 | 1 (5.6) | 6 | 1 (16.7) | |
| transhumant | 1009 | 25 (2.5) | 1019 | 331 (32.5)* | 971 | 230 (23.7) | 972 | 209 (21.5) | 515 | 58 (11.3) | |
| Sex | female | 841 | 21 (2,5) | 850 | 288 (33.9)* | 813 | 212 (26.1)* | 813 | 199 (24.5)* | 439 | 49 (11.2) |
| male | 205 | 4 (2,0) | 206 | 54 (22.2) | 193 | 20 (10.4) | 193 | 12 (6.2) | 97 | 11 (11.3) | |
| Age class (year) | <1 | 117 | 0 (0,0) | 117 | 28 (23.9) | 115 | 9 (7.8) | 115 | 6 (5.2) | 52 | 3 (5.8) |
| ≥1 ≤ 2 | 121 | 4 (3,3) | 122 | 38 (31.1) | 117 | 27 (23.1)* | 117 | 22 (18.8)* | 67 | 7 (10.4) | |
| >2 to ≤4 | 87 | 2 (2,3) | 89 | 27 (30.3) | 85 | 16 (18.8)* | 85 | 11 (12.9) | 51 | 2 (3.9) | |
| >4 and ≤10 | 414 | 7 (1.7) | 418 | 121 (28.9) | 401 | 92 (22.9)* | 401 | 82 (20.4)* | 193 | 29 (15.0) | |
| >10 | 307 | 12 (3.9) | 310 | 128 (41.3)* | 288 | 88 (30.6)* | 288 | 90 (31.3)* | 173 | 19 (11.0) | |
| Fur colour | azeghraf | 26 | 1 (3.8) | 26 | 5 (19.2) | 26 | 4 (15.4) | 26 | 5 (19.2) | 9 | 2 (22.2) |
| baida | 211 | 3 (1.4) | 211 | 44 (20.9) | 205 | 31 (15.1) | 205 | 22 (10.7) | 105 | 13 (12.4) | |
| hamra | 334 | 9 (2.7) | 337 | 108 (32.0) | 315 | 67 (21.3) | 316 | 63 (19.9) | 157 | 16 (10.2) | |
| sefra | 252 | 3 (1.2) | 252 | 86 (34.1) | 248 | 58 (23.4) | 248 | 55 (22.2) | 132 | 12 (9.1) | |
| zerga | 223 | 9 (4.0) | 230 | 99 (43.0)* | 212 | 72 (34.0) | 211 | 66 (31.3) | 133 | 17 (12.8) | |
* = Significant difference.
Irrespective of the test used, the observed trypanosomosis prevalence was significantly higher in El Bayadh than in the other wilayate (p < 0.0001) except for RoTat 1.2 PCR where the prevalences in El Bayadh and Béchar were similar (respectively 13.0 and 13.6%). The epidemic situation in El Bayadh is reflected by the high observed prevalence in GST (11.8%, CI: 6.8–16.8) and very high serological prevalences in CATT/T. evansi (74.9%, CI: 68.4–81.4), ELISA/VSG RoTat 1.2 (70.1%, CI: 62.4–77.9) and TL (62.2%, CI: 54.0–70.4). The logistic regression shows that the animals of El Bayadh were 80 times more at risk than in Tamanrasset (OR = 80.5, CI: 37.6–200.4). Camels in Béchar were 21 times (OR = 21.5, CI: 10.5–52.0) more likely to be infected than in Tamanrasset. Camels in Ouargla were almost 7 times (OR = 6.9%, CI: 3.1–17.5) more likely to be infected than in Tamanrasset.
Regarding observed parasitological and molecular prevalences, no statistical difference was found between male and female animals. This is in contrast with observed serological prevalences. For example, according to TL, females are almost 5 times more at risk to be infected than males (OR = 4.9, CI: 2.8–9.4).
For most of the diagnostic tests, prevalence did not significantly differ between the age classes although there is a tendency to find a lower prevalence in calves (<1 year) and a higher prevalence in older animals (>10 years) (Table 4). For example, none of the calves under one year was found positive in GST but parasitological prevalence was not significantly different between the other age classes. On the other hand, TL seroprevalences in the animals between 1 and 2 years old and above 4 years old were significantly different from the calves under one year. They were respectively, four (OR = 4.2, CI: 1.7–11.8, p < 0.01), five (OR = 4. 7, CI: 2.1–12.3, p < 0.001) and eight (OR = 8.3 CI: 3.8–21.7, p < 0.0001) times more at risk to be positive than the <1 year old calves.
The cohort consisted of 1019 transhumant camels, 20 semi-intensive and 17 sedentary animals. The twenty five parasitologically positives were all transhumant. A significant difference was observed between prevalence in sedentary camels and semi-intensive ones (p < 0.01) with CATT/T. evansi (OR = 10.1, CI: 2.1–77.3). Also in the transhumant camels, the seroprevalence with CATT/T. evansi was higher than in the other breeding systems (borderline significant, p = 0.05). In the other diagnostic tests, the observed prevalence was not significantly different between breeding systems; With reference to local language, five fur colours were observed: azeghraf (bicolored), baida (white), hamra (red), sefra (yellow), and zerga (blue). Observed prevalence in the different diagnostic tests was not significantly different except in CATT/T. evansi where zerga camels were 3 times more at risk of being infected that the others (OR = 3.2, CI: 1.2–9.8).
3.5. Degree of agreement between the different diagnostic tests
A contingency table and degree of agreement between the different diagnostic tests using indices of positive and negative agreement and their 95% confidence interval are shown in Table 5.
Table 5.
Contingency table and degree of agreement, with 95% confidence interval, between the different diagnostic tests using indices of positive and negative agreement (Ppos, Pneg). CATT = CATT/T. evansi, ELISA = ELISA/VGS RoTat 1.2, TL = immune trypanolysis, PCR = RoTat 1.2 PCR.
| CATT |
ELISA |
TL |
PCR |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | ||
| GST | Pos | 20 | 5 | 19 | 2 | 18 | 3 | 16 | 9 |
| Neg | 314 | 707 | 213 | 772 | 193 | 792 | 44 | 457 | |
| Total | 334 | 712 | 232 | 774 | 211 | 795 | 60 | 466 | |
| Ppos | 0.11 (0.07–0.16) | 0.15 (0.09–0.21) | 0.16 (0.10–0.22) | 0.38 (0.23–0.51) | |||||
| Pneg | 0.82 (0.80–0.84) | 0.88 (0.87–0.90) | 0.89 (0.87–0.90) | 0.95 (0.93–0.96) | |||||
| CATT | Pos | 183 | 132 | 173 | 142 | 41 | 275 | ||
| Neg | 49 | 642 | 38 | 653 | 19 | 201 | |||
| Total | 232 | 774 | 211 | 795 | 60 | 476 | |||
| Ppos | 0.67 (0.62–0.71) | 0.66 (0.61–0.70) | 0.22 (0.16–0.28) | ||||||
| Pneg | 0.88 (0.86–0.89) | 0.88 (0.86–0.89) | 0.58 (0.54 0.62) | ||||||
| ELISA | Pos | 191 | 41 | 42 | 170 | ||||
| Neg | 20 | 753 | 15 | 263 | |||||
| Total | 211 | 794 | 57 | 433 | |||||
| Ppos | 0.86 (0.82–0.89) | 0.31 (0.24–0.38) | |||||||
| Pneg | 0.96 (0.95–0.97) | 0.74 (0.70–0.77) | |||||||
| TL | Pos | 42 | 159 | ||||||
| Neg | 15 | 275 | |||||||
| Total | 57 | 434 | |||||||
| Ppos | 0.33 (0.25–0.40) | ||||||||
| Pneg | 0.76 (0.72–0.79) | ||||||||
The indices of negative agreement are invariably higher than those of positive agreement. TL and ELISA/VSG RoTat 1.2 showed the highest concordance with the highest index of negative agreement (0.96, 95% CI: 0.95–0.97) followed by GST and RoTat 1.2 PCR (0.95, CI: 0.93–0.96). The indices of negative agreement between GST and TL, GST and ELISA/VSG RoTat 1.2, and CATT/T. evansi and TL were 0.89, 0.88, and 0.87 respectively. The lowest negative index of agreement was observed between CATT/T. evansi and RoTat 1.2 PCR (0.57). TL and ELISA/VSG RoTat 1.2 showed the highest index of positive agreement (0.86), while the lowest was seen between GST and CATT/T. evansi (0.11).
3.6. Packed cell volume
Table 6 shows the average PCV values and standard deviations (SD) according to the status of the camels in the GST, CATT/T. evansi, TL, ELISA/VGS RoTat 1.2, and RoTat 1.2 PCR. For all tests except the RoTat 1.2 PCR, positive animals had a significantly lower average PCV when compared to negative camels. It is to be noted that 13% of all camels were anaemic (PCV <24%).
Table 6.
Average packed cell volume values (in %) ± the standard deviation (SD) according to the animal status in the different diagnostic tests.
| Test | negative | positive | P |
|---|---|---|---|
| GST | 27.3 ± 4.3 | 22.2 ± 2.7 | <0.001 |
| CATT/T. evansi | 28.1 ± 3.5 | 25.0 ± 5.3 | <0.001 |
| ELISA/VSG RoTat 1.2 | 27.7 ± 3.7 | 25.0 ± 4.9 | <0.001 |
| TL | 27.7 ± 4.0 | 24.6 ± 3.7 | <0.001 |
| RoTat 1.2 PCR | 26.2 ± 5.1 | 25.2 ± 6.9 | 0.202 |
4. Discussion
This study was undertaken to investigate the epidemiological situation of T. evansi infection in dromedary camels in the South of Algeria by means of parasitological, serological and molecular diagnostic tests, including those recommended by the OIE.
The overall prevalence was found to be 2.4% with GST. This is equivalent to the parasitological prevalence estimated by Boushaki (2007) in the same region with the same technique, but is much lower than molecular prevalence (11.2%). This difference is not unexpected given the low sensitivity of the blood smear with a lower detection limit of 105trypanosomes/ml (Desquesnes et al., 2013a). More parasitologically positive animals could have been detected if more sensitive techniques like the hematocrit centrifugation technique (Woo, 1969) or the dark ground buffy coat examination (Murray et al., 1977) or mouse inoculation would have been used, which was not possible under the field conditions in all the wilayate studied. Therefore, the zero parasitological prevalence observed in Tamanrasset does not necessarily indicate the absence of surra in that wilaya since 7% of the 360 examined camels were positive in RoTat 1.2PCR and in a previous survey parasitologically positive animals were identified with GST (Boushaki, 2007).
On the other hand, overall seroprevalence estimates obtained with CATT/T. evansi (32.4%), ELISA/VSG RoTat 1.2 (23.1%), and immune trypanolysis test (21%) were much higher than parasitological and molecular prevalence estimates. This can be explained by the fact that animals with chronic infections may remain false negative in both parasitological and molecular tests when parasite loads are very low. Also, it is known that after successful cure, antibodies can remain in the circulation for several months, thus leading to serologically false positive animals in herds that are treated with trypanocides (OIE, 2018; Verloo et al., 2001). Indeed, one third of the examined camels have a history of treatment with drugs containing diminazene diaceturate (Fa.Try.Banil®, Fa.Try.Banil R.T.U.® FATRO, Italy), the only molecule with trypanocidal activity that has been authorised for over 19 years on the national Algerian market to treat domestic animals but not dromedary camel. So, it is probable that of the 151 TL positive animals that were negative in RoTat1.2 PCR, some were cured after recent trypanocidal treatment. Yet, three camels with positive GST and RoTat 1.2 PCR were negative in TL. It may be that those animals carried recent infections and that RoTat 1.2 specific antibodies were not yet detectable (Verloo et al., 2001).
On 133 animals, of which three were positive in GST, we were able to run T. evansi type B specific EVAB PCR. None of them were positive. Thus, so far we have no evidence that T. evansi type B, that has been isolated only in Eastern Africa, has already spread towards the West (Birhanu et al., 2015; Ngaira et al., 2005; Njiru et al., 2006; Salim et al., 2011).
The overall seroprevalence was higher than in Morocco, where, among 1460 dromedaries examined with CATT/T. evansi and ELISA/VSG RoTat 1.2, respectively 14.1% and 18.2% were positive (Atarhouch et al., 2003). Similarly, in Mauritania, out of 254 dromedary camels examined, 14.2% were positive with CATT/T. evansi (Dia et al., 2011).
Our results demonstrate that all wilayate in the study area are affected by T. evansi. Tamanrasset seems the least affected. Noteworthy is that in Tamanrasset, only 8 of the 25 interviewed farmers knew the disease. On the other hand, El Bayadh, which was previously free of disease (Boushaki, 2007; Marfoua, 1999), has experienced an outbreak during this investigation with high frequencies of acute cases. The outbreak is reflected by the high number of deaths (59 cases), high observed parasitological prevalence (12%) and very high serological prevalence in CATT/T. evansi (75%), ELISA/VSG RoTat 1.2 (70%) and TL (62%). This epidemic episode originated probably from the introduction of T. evansi with transhumant dromedaries from Béchar, a surra endemic wilaya bordering El Bayadh (Boushaki, 2007). In Béchar, repeated abortions over the years associated with considerable seroprevalence (30.6–36.8%) and a molecular prevalence of 13.6% are probably related to all forms and stages of the disease, from recent infection to subclinical and chronic complications. Indeed, mortality is frequent during the first two to three years of disease evolution. Some live up to four years with a subclinical infection and some resist and eventually heal themselves (Dia et al., 1997a; Röttcher et al., 1987). It should be mentioned that in 94% of the investigated farms in El Bayadh and in 63% of the farms in Béchar, treatment with Fa.Try.Banil R.T.U® is applied although diminazene diaceturate is toxic to camels and can be fatal if the dose is higher than 3.5 mg/kg (Cuisance et al., 2001; Homeida et al., 1981 in Röttcher et al., 1987). In none of the farms in Ouargla and Tamanrasset, treatment of camels with trypanocides is recorded.
Sex was found to be a risk factor associated with trypanosomosis in our study population when using serological tests only. With TL, females are five times more likely to be infected than males. This finding is in agreement with Dia et al. (1997b) but in contradiction with Boushaki (2007) and Njiru et al. (2004) where no difference was found in seroprevalence between males and females. Rather than a real biological predisposition, differences in husbandry practices applied to male and female animals should be considered as an explanatory factor (Antoine-Moussiaux and Desmecht, 2008). Seroprevalences estimated with TL and ELISA/RoTat 1.2 were also significantly higher in adult camels as compared to calves under one year old. This is in agreement with previous studies (Atarhouch et al., 2003; Dia et al., 1997c; Gutierrez et al., 2000; Ndoutamia et al., 1999) and is probably related to the chronic nature of the infection, the increasing exposure to re-infection over time, and the persistence of antibodies after curative treatment. The finding of a 20 days old calf that was positive for TL and CATT/T. evansi can be explained by the in utero passage of maternal antibodies. Vertical transmission of the parasite may be an alternative cause although usually this leads to stillbirth (Röttcher et al., 1987). Within the group of young animals aged one year, 4 of the 121 were found with trypanosomes in the blood, as was also reported by Dia et al. (1997c) but is in contrast to observations of Diall et al. (1993) and Jacquiet et al. (1994).
The breeding system in the studied wilayate is dominated by transhumance, which is a system for the rational exploitation of vegetation and water for the camel. Transhumance may favour transmission by dry season migrations to areas with bioclimatic conditions favorable to the survival of vectors.
In camels, the PCV threshold or the minimum physiologically acceptable PCV value is 24% (Dioli and Stimmelmayr, 1992). The high proportion (13%) of animals in our study cohort that had anaemia is probably related to T. evansi infection, as was observed in other studies (Birhanu et al., 2015; Boushaki, 2007; Fikru et al., 2015; Gutierrez et al., 2005). Indeed, trypanosomosis is an anaemic infection associated with intravascular hemolysis (Chaudhary and Iqbal, 2000; Gutierrez et al., 2005). In our study, we found a significantly lower PCV in test positive than in test negative animals for all diagnostic tests, except for RoTat 1.2 PCR, which is in contrast to what was observed by Birhanu et al. (2015) and Fikru et al. (2015) who reported also lower PCV values in PCR positive camels. Taking into account that anaemia may be caused by other conditions and infections, PCV has only a limited additional diagnostic value when it is applied together with serological tests.
Regarding these serological tests, we observed high positive (0.86) and negative (0.96) indices for TL and ELISA/VSG RoTat 1.2. For TL and CATT/T.evansi the positive (0.67) and negative (0.88) indices were somewhat lower. Yet, TL is a reference test and is only performed in the OIE Reference Laboratory for Surra in Antwerp, Belgium (Birhanu et al., 2015; Fikru et al., 2015; Holland et al., 2002; Tehseen et al., 2015; Verloo et al., 2000). For testing in the field or in endemic country laboratories, less demanding serological tests like CATT/T. evansi and ELISA/VSG RoTat 1.2 are available. With TL as gold standard for detection of RoTat 1.2 specific antibodies (100% sensitivity and specificity), the relative sensitivity and specificity of ELISA/VSG RoTat 1.2 with 30% PP cut-off were respectively 91% and 95%, while the relative sensitivity and specificity of CATT/T. evansi were 82%.
In the light of the results obtained, the need to implement a control strategy is obvious. Veterinary laboratories should be provided with accurate diagnostic tools in order to detect infected animals that represent potential reservoirs at an early stage. Especially during the seasons with abundant vectors, a reduction of the impact of trypanosomiasis in an endemic area may be obtained by implementing an integrated prophylactic plan based on insect trapping and treatment of all serologically positive dromedaries with an effective trypanocide such as melarsomine (Cymelarsan), irrespective of whether they are actually infected or not. Such a control plan was implemented in Morocco as part of a study programme that proved the utility of Cymelarsan to reduce surra prevalence in the pilot region (Rami et al., 2003). Care should be taken however to monitor the appearance of drug resistance when only one type of drug is used over a longer period (Luckins, 2000). Indeed, resistance of T. evansi strains to drugs like suramin and antrycide (pyrimethamine) has been reported in different countries in Africa and Asia (El Rayah et al., 1999; Zhou et al., 2004).
5. Conclusion
Our study confirms the high prevalence of surra, caused by T. evansi type A, in the dromedary population in South Algeria and its potential to spread into previously non-endemic areas like El Bayadh. There is an obvious need to implement control measures, including diagnosis, treatment and vector control, in order to reduce the incidence of the disease, not only in the study area but all over the country.
Declarations
Author contribution statement
Djamila Boushaki: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Amel Adel: Analyzed and interpreted the data; Wrote the paper.
Mamadou Lamine Dia, Nadia Kechemir Issad: Conceived and designed the experiments.
Philippe Büscher: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Hafsa Madani: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Brahim Aymard Brihoum, Hassiba Sadaoui, Nadera Bouayed: Performed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank the Veterinary Doctors of the health surveillance office (Direction des Services Vétérinaires, Ministry of Agriculture) for their collaboration. This study would not have been possible without the collaboration, during the fieldwork, of the services of the veterinary authority of the wilaya of El Bayadh, Béchar, Ouargla and Tamanrasset. We also thank the owners, contact persons and local administrators. We are grateful to the directors of the Institut National de Médecine Vétérinaire, of the central veterinary laboratory of Algiers and the regional veterinary laboratory of Laghouat, as well as all the staff. We gratefully acknowledge the technical staff of the Department of Biomedical Sciences of the Institute of Tropical Medicine, Belgium, for their assistance.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Frequency histogram of Percent Positivity values obtained in the ELISA/VSG RoTat 1.2 for the whole study cohort
References
- Adel A., Abatih E., Speybroeck N., Soukehal A., Bouguedour R., Boughalem K., Bouhbal A., Djerbal M., Saegerman C., Berkvens D. Estimation of canine Leishmania infection prevalence in six cities of the Algerian littoral zone using a Bayesian approach. PLoS One. 2015;10 doi: 10.1371/journal.pone.0117313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Qarawi A.A., Omar H.M., Abdel-Rahman H.A., El-Mougy S.A., El-Belely M.S. Trypanosomiasis-induced infertility in dromedary (Camelus dromedarius) bulls: changes in plasma steroids concentration and semen characteristics. Anim. Reprod. Sci. 2004;84:73–82. doi: 10.1016/j.anireprosci.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Antoine-Moussiaux N., Desmecht D. Epidémiologie de l'infection par Trypanosoma evansi. Ann. Med. Vet. 2008;152:191–201. [Google Scholar]
- Atarhouch T., Rami M., Bendahman M.N., Dakkak A. Camel trypanosomosis in Morocco 1: results of a first epidemiological survey. Vet. Parasitol. 2003;111:277–286. doi: 10.1016/s0304-4017(02)00382-5. [DOI] [PubMed] [Google Scholar]
- Bajyana Songa E., Hamers R. A card agglutination test (CATT) for veterinary use based on an early VAT RoTat 1/2 of Trypanosoma evansi. Ann. Soc. Belg. Med. Trop. 1988;68:233–240. [PubMed] [Google Scholar]
- Birhanu H., Fikru R., Said M., Kidane W., Gebrehiwot T., Hagos A., Alemu T., Dawit T., Berkvens D., Goddeeris B.M., Büscher P. Epidemiology of Trypanosoma evansi and Trypanosoma vivax in domestic animals from selected districts of Tigray and Afar regions, Northern Ethiopia. Parasites Vectors. 2015;8:212. doi: 10.1186/s13071-015-0818-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birhanu H., Gebrehiwot T., Goddeeris B.M., Büscher P., Van Reet N. New Trypanosoma evansi type B isolates from Ethiopian dromedary camels. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boushaki D. École Nationale Supérieure Vétérinaire d’Alger; 2007. Prévalence de la Trypanosomose Cameline en Algérie. MSc thesis. [Google Scholar]
- Büscher P. Diagnosis of human and animal African trypanosomiasis. In: Black S.J., Seed J.R., editors. The African Trypanosomes. Kluwer Academic Publishers; Boston: 2001. pp. 51–63. [Google Scholar]
- Büscher P. Diagnosis of african trypanosomiasis. In: Magez S., Radwanska M., editors. Trypanosomes and Trypanosomiasis. Springer; Wien: 2014. pp. 189–216. [Google Scholar]
- Chaudhary Z.I., Iqbal J. Incidence, biochemical and haematological alterations induced by natural trypanosomosis in racing dromedary camels. Acta Trop. 2000;77:209–213. doi: 10.1016/s0001-706x(00)00142-x. [DOI] [PubMed] [Google Scholar]
- Cicchetti D.V., Feinstein A.R. High agreement but low kappa: II. Resolving the paradoxes. J. Clin. Epidemiol. 1990;43:551–558. doi: 10.1016/0895-4356(90)90159-m. [DOI] [PubMed] [Google Scholar]
- Claes F., Radwanska M., Urakawa T., Majiwa P.A., Goddeeris B., Büscher P. Variable Surface Glycoprotein RoTat 1.2 PCR as a specific diagnostic tool for the detection of Trypanosoma evansi infections. Kinetoplastid Biol. Dis. 2004;3:1475–9292. doi: 10.1186/1475-9292-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuisance D., Itard J., Solano P., Desquesnes M., Frézil J.L., Authié E. Trypanosomoses. Méthodes de lutte. In: Lefèvre P.-C., Blancou J., Chermette R., editors. Principales Maladies Infectieuses et Parasitaires du Bétail. TEC & DOC; Paris: 2001. pp. 1695–1723. [Google Scholar]
- Desquesnes M., Dargantes A., Lai D.H., Lun Z.R., Holzmüller P., Jittapalapong S. Trypanosoma evansi and surra: a review and perspectives on transmission, epidemiology and control, impact, and zoonotic aspects. BioMed Res. Int. 2013;2013:321237. doi: 10.1155/2013/321237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desquesnes M., Holzmüller P., Lai D.H., Dargantes A., Lun Z.R., Jittaplapong S. Trypanosoma evansi and surra: a review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. BioMed Res. Int. 2013;2013:194176. doi: 10.1155/2013/194176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dia M.L., Aminetou M., Diop C., Thiam A., Jacquiet P., Mabrouk A. Autoguérison chez un chamelon expérimentalement infecté par T. evansi. Rev. Méd. Vét. 1997;8–9:713–716. [Google Scholar]
- Dia M.L., Barry Y., Ould Ahmed M., Claes F., Büscher P., Ba A. Vol. 125. AU-IBAR; Nairobi, Kenya: 2011. Nouvelles données sur la trypanosomose cameline à T. evansi en Mauritanie; pp. 391–398. (Proceedings 30th Biennial Conference of the International Scientific Council for Trypanosomiasis Research and Control (ISCTRC)). [Google Scholar]
- Dia M.L., Diop C., Aminetou M., Jacquiet P., Thiam A. Some factors affecting the prevalence of Trypanosoma evansi in camels in Mauritania. Vet. Parasitol. 1997;72:111–120. doi: 10.1016/s0304-4017(97)00054-x. [DOI] [PubMed] [Google Scholar]
- Dia M.L., Van Meirvenne N., Magnus E., Luckins A.G., Diop C., Thiam A., Jacquiet P., Hamers R. Evaluation de quatre tests de diagnostic: frottis sanguins, CATT, IFI et ELISA-AG dans l'étude de l'épidémiologie de la trypanosomose cameline à Trypanosoma evansi en Mauritanie. Rev. Elev. Med. Vet. Pays Trop. 1997;50:29–36. [Google Scholar]
- Diall O., Bocoum Z., Diarra B., Sanogo Y., Coulibaly Z., Waigalo Y. Epidémiologie de la trypamanosomose à T. evansi chez le dromadaire au Mali: résultats d’enquêtes parasitologiques et cliniques. Rev. Elev. Med. Vet. Pays Trop. 1993;46:455–461. [PubMed] [Google Scholar]
- Dioli M., Stimmelmayr R. Important camel diseases. In: Schwartz H.J., Dioli M., editors. The One-Humped Camel (C. dromedarius) in Eastern Africa. A Pictorial Guide to Diseases, Health Care, and Management. Margraf; Weikersheim,Germany: 1992. pp. 157–161. [Google Scholar]
- Direction des Services Agricoles d'El Bayadh (DSA El Bayadh) Algérie; 2016. Monographie de la wilaya d'El Bayadh. [Google Scholar]
- Direction des Services Agricoles de Béchar (DSA Béchar) Algérie; 2018. Monographie de la wilaya de Béchar. [Google Scholar]
- Direction des Services Agricoles de Ouargla (DSA Ouargla) Algérie; 2016. Monographie de la wilaya de Ouargla. [Google Scholar]
- Direction des Services Agricoles de Tamanrasset (DSA Tamanrasset) Algérie; 2016. Monographie de la wilaya de Tamanrasset. [Google Scholar]
- El Rayah I.E., Kaminsky R., Schmid C., El Malik K.H. Drug resistance in Sudanese Trypanosoma evansi. Vet. Parasitol. 1999;80:281–287. doi: 10.1016/s0304-4017(98)00221-0. [DOI] [PubMed] [Google Scholar]
- Feinstein A.R., Cicchetti D.V. High agreement but low kappa: I. The problems of two paradoxes. J. Clin. Epidemiol. 1990;43:543–549. doi: 10.1016/0895-4356(90)90158-l. [DOI] [PubMed] [Google Scholar]
- Fikru R., Andualem Y., Getachew T., Menten J., Hasker E., Merga B., Goddeeris B.M., Büscher P. Trypanosome infection in dromedary camels in Eastern Ethiopia: prevalence, relative performance of diagnostic tools and host related risk factors. Vet. Parasitol. 2015;211:175–181. doi: 10.1016/j.vetpar.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Gari F.R., Ashenafi H., Tola A., Goddeeris B.M., Claes F. Comparative diagnosis of parasitological, serological, and molecular tests in dourine-suspected horses. Trop. Anim. Health Prod. 2010;42:1649–1654. doi: 10.1007/s11250-010-9615-1. [DOI] [PubMed] [Google Scholar]
- Graham P., Bull B. Approximate standard errors and confidence intervals for indices of positive and negative agreement. J. Clin. Epidemiol. 1998;51:763–771. doi: 10.1016/s0895-4356(98)00048-1. [DOI] [PubMed] [Google Scholar]
- Gutierrez C., Corbera J.A., Juste M.C., Doreste F., Morales I. An outbreak of abortions and high neonatal mortality associated with Trypanosoma evansi infection in dromedary camels in the Canary Islands. Vet. Parasitol. 2005;130:163–168. doi: 10.1016/j.vetpar.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Gutierrez C., Juste M.C., Corbera J.A., Magnus E., Verloo D., Montoya J.A. Camel trypanosomosis in the Canary Islands: assessment of seroprevalence and infection rates using the card agglutination test (CATT/T. evansi) and parasite detection tests. Vet. Parasitol. 2000;90:155–159. doi: 10.1016/s0304-4017(00)00225-9. [DOI] [PubMed] [Google Scholar]
- Holland W.G., Thanh N.G., My L.N., Magnus E., Verloo D., Büscher P., Goddeeris B., Vercruysse J. Evaluation of whole fresh blood and dried blood on filter paper discs in serological tests for Trypanosoma evansi in experimentally infected water buffaloes. Acta Trop. 2002;81:159–165. doi: 10.1016/s0001-706x(01)00211-x. [DOI] [PubMed] [Google Scholar]
- Homeida A.M., El Amin E.A., Adam S.E.I., Mahmoud M.M. Toxicity of diminazene aceturate (Berenil) to camels. J. Comp. Path. 1981;91:355–360. doi: 10.1016/0021-9975(81)90005-0. [DOI] [PubMed] [Google Scholar]
- Jacquiet P., Dia M.L., Cheikh D., Thiam A. [Camel trypanosomiasis caused by Trypanosoma evansi (steel 1885), balbiani 1888, in islamic republic of Mauritania: results of surveys in the trarza region] Rev. Elev. Med. Vet. Pays Trop. 1994;47:59–62. [PubMed] [Google Scholar]
- Lejon V., Claes F., Verloo D., Maina M., Urakawa T., Majiwa P.A., Büscher P. Recombinant RoTat 1.2 variable surface glycoprotein as antigen for diagnosis of Trypanosoma evansi in dromedary camels. Int. J. Parasitol. 2005;35:455–460. doi: 10.1016/j.ijpara.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Luckins A.G. Epidemiology of surra: unanswered questions. J. Protozool. Res. 1998;8:106–119. [Google Scholar]
- Luckins A.G. Vol. 2. 2000. Control of non tsetse-transmitted animal trypanosomiasis drugs and drug resistance in Trypanosoma evansi; pp. 24–26. (Proceeding Workshop on Drug Delivery and Resistance in the Context of Integrated Disease Management, Nairobi, Kenya, 31 May-4 June 1999, Newsletter on Integrated Control of Pathogenic Trypanosomes and Their Vectors (ICTPV), Glascow). [Google Scholar]
- Luckins A.G., Dwinger R.H. Non-tsetse-transmitted animal trypanosomiases. In: Maudlin I., Holmes P.H., Miles M.A., editors. The Trypanosomiases. EPIDEMIOLOGY and DIAGNOSIS. Cabi Publishing; Trowbridge: 2004. pp. 269–281. [Google Scholar]
- Marfoua K. 1999. Contribution à la connaissance de la pathologie du dromadaire en Algérie. Project Report. Ministère Algérien de l'Agriculture et de la Pêche. Camel Applied Research and Development Network, The Arab Center for the Studies of Arid zones and Dry lands (CARDN/ACSAD). Regional Veterinary Laboratory of Laghouat, Algeria. CARDN/ACSAD/Camel/P 51/1999. [Google Scholar]
- Masiga D.K., Smyth A.J., Hayes P., Bromidge T.J., Gibson W.C. Sensitive detection of trypanosomes in tsetse flies by DNA amplification. Int. J. Parasitol. 1992;22:909–918. doi: 10.1016/0020-7519(92)90047-o. [DOI] [PubMed] [Google Scholar]
- Ministère de l'Agriculture, du Développement Rural et de la Pêche (MADRP) Algérie; Alger: 2014. General Agricultural Census 2014. [Google Scholar]
- Murray M., Murray P.K., McIntyre W.I. An improved parasitological technique for the diagnosis of African trypanosomiasis. Trans. R. Soc. Trop. Med. Hyg. 1977;71:325–326. doi: 10.1016/0035-9203(77)90110-9. [DOI] [PubMed] [Google Scholar]
- Ndoutamia G., Brahim B.O., Brahim A., Djimgang G., Saboun M., Doutoum A.A. La trypanosomose à Trypanosoma evansi chez les camelidés au Tchad: facteurs épidémiologiques et influence sur les paramètres hématologiques et protéoénergétiques. Rev. Med. Vet. 1999;11:899–904. [Google Scholar]
- Ngaira J.M., Olembo N.K., Njagi E.N., Ngeranwa J.J. The detection of non-RoTat 1.2 Trypanosoma evansi. Exp. Parasitol. 2005;110:30–38. doi: 10.1016/j.exppara.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Njiru Z.K., Constantine C.C., Guya S., Crowther J., Kiragu J.M., Thompson R.C., Davila A.M. The use of ITS1 rDNA PCR in detecting pathogenic African trypanosomes. Parasitol. Res. 2005;95:186–192. doi: 10.1007/s00436-004-1267-5. [DOI] [PubMed] [Google Scholar]
- Njiru Z.K., Constantine C.C., Masiga D.K., Reid S.A., Thompson R.C., Gibson W.C. Characterization of Trypanosoma evansi type B. Infect. Genet. Evol. 2006;6:292–300. doi: 10.1016/j.meegid.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Njiru Z.K., Constantine C.C., Ndung'u J.M., Robertson I., Okaye S., Thompson R.C., Reid S.A. Detection of Trypanosoma evansi in camels using PCR and CATT/T. evansi tests in Kenya. Vet. Parasitol. 2004;124:187–199. doi: 10.1016/j.vetpar.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Office National de la Météorologie (ONM) Algérie; 2018. Données climatiques des années 2014, 2015, 2016 Alger. [Google Scholar]
- OIE (World Organisation for Animal Health) 2018. Trypanosoma evansi Infection (Surra). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, Chapter 3.1.21.http://www.oie.int/fileadmin/Home/fr/Health_standards/tahm/3.01.21_TRYPANO_SURRA.pdf/ Last accession date 01 May 2019. [Google Scholar]
- Parsani H.R., Veer S., Momin R.R. Common parasitic diseases of camel. Vet. World. 2008;1:317–318. [Google Scholar]
- R. https://www.R-project.org/. (version 3.4.1. R Core Team. 2017).
- Rami M., Atarhouch T., Bendahman M.N., Azlaf R., Kechna R., Dakkak A. Camel trypanosomosis in Morocco. 2. A pilot disease control trial. Vet. Parasitol. 2003;115:223–231. doi: 10.1016/s0304-4017(03)00222-x. [DOI] [PubMed] [Google Scholar]
- Rogé S., Van Reet N., Odiwuor S., Tran T., Schildermans K., Vandamme S., Vandenberghe I., Vervecken W., Gillingwater K., Claes F., Devreese B., Guisez Y., Büscher P. Recombinant expression of trypanosome surface glycoproteins in Pichia pastoris for the diagnosis of Trypanosoma evansi infection. Vet. Parasitol. 2013;197:571–579. doi: 10.1016/j.vetpar.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Röttcher D., Schillinger D., Zweygarth E. Trypanosomiasis in the camel (Camelus dromedarius) Rev. sci. tech. Off. int. Epiz. 1987;6:463–470. doi: 10.20506/rst.6.2.301. [DOI] [PubMed] [Google Scholar]
- Salim B., Bakheit M.A., Kamau J., Nakamura I., Sugimoto C. Molecular epidemiology of camel trypanosomiasis based on ITS1 rDNA and RoTat 1.2 VSG gene in the Sudan. Parasites Vectors. 2011;4:31. doi: 10.1186/1756-3305-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent E., Donatien A. Archives des Instituts Pasteur de l'Afrique du Nord. (Alger) XXIIe Note; 1921. De l’infection latente dans la trypanosomiase des dromadaires (le Debab) pp. 179–184. [Google Scholar]
- Sergent E., Sergent E. Annales de l’Institut Pasteur d’Alger; 1905. EL-DEBAB Trypanosomiase des dromadaires de l’Afrique du Nord; pp. 17–48. [Google Scholar]
- Tehseen S., Jahan N., Qamar M.F., Desquesnes M., Shahzad M.I., Deborggraeve S., Büscher P. Parasitological, serological and molecular survey of Trypanosoma evansi infection in dromedary camels from Cholistan Desert, Pakistan. Parasites Vectors. 2015;8:415. doi: 10.1186/s13071-015-1002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma B., Dufour B., Bénet J.J., Sanaa M., Shaw A., Moutou F. troisième Ed. Association pour l'Etude de l'Epidémiologie des Maladies Animales AEEMA, Maisons-Alfort, France; 2010. Epidémiologie appliquée à la lutte collective contre les maladies animales transmissibles majeures; pp. 188–216. [Google Scholar]
- Urakawa T., Verloo D., Moens L., Büscher P., Majiwa P.A. Trypanosoma evansi: cloning and expression in Spodoptera frugiperda [correction of fugiperda] insect cells of the diagnostic antigen RoTat1.2. Exp. Parasitol. 2001;99:181–189. doi: 10.1006/expr.2001.4670. [DOI] [PubMed] [Google Scholar]
- Van Meirvenne N., Magnus E., Büscher P. Evaluation of variant specific trypanolysis tests for serodiagnosis of human infections with Trypanosoma brucei gambiense. Acta Trop. 1995;60:189–199. doi: 10.1016/0001-706x(95)00127-z. [DOI] [PubMed] [Google Scholar]
- Verloo D., Holland W., My L.N., Thanh N.G., Tam P.T., Goddeeris B., Vercruysse J., Büscher P. Comparison of serological tests for Trypanosoma evansi natural infections in water buffaloes from north Vietnam. Vet. Parasitol. 2000;92:87–96. doi: 10.1016/s0304-4017(00)00284-3. [DOI] [PubMed] [Google Scholar]
- Verloo D., Magnus E., Büscher P. General expression of RoTat 1.2 variable antigen type in Trypanosoma evansi isolates from different origin. Vet. Parasitol. 2001;97:183–189. doi: 10.1016/s0304-4017(01)00412-5. [DOI] [PubMed] [Google Scholar]
- Woo P.T.K. The haematocrit centrifuge for the detection of trypanosomes in blood. Can. J. Zool. 1969;47:921–923. doi: 10.1139/z69-150. [DOI] [PubMed] [Google Scholar]
- Zhou J., Shen J., Liao D., Zhou Y., Lin J. Resistance to drug by different isolates Trypanosoma evansi in China. Acta Trop. 2004;90:271–275. doi: 10.1016/j.actatropica.2004.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Frequency histogram of Percent Positivity values obtained in the ELISA/VSG RoTat 1.2 for the whole study cohort