Abstract
Introduction:
Hypertension is significantly contributing to global mortality and morbidity and has been identified as the most important modifiable risk factor for early development of cardiovascular diseases (CVD).
Aim:
The aim of this study was to investigate the efficacy of different combinations of antihypertensive therapy on blood pressure, arterial stiffness and peripheral resistance in patients with essential hypertension using the brachial oscillometric ambulatory blood pressure monitor.
Methods:
This study was designed as an observational, prospective, multi centric study conducted in eight primary care centers of the Health Center of Canton Sarajevo during the period of six months. The study included 655 participants, both genders, aged between 30 and 75, who were diagnosed with hypertension according to the ESC/ESH guidelines. Participants were divided into six treatment groups based on the hypertensive drug therapy they were using; lisinopril, losartan or valsartan alone or in combination with hydrochlorothiazide (A, B and C group respectively) or combination of lisinopril, losartan or valsartan with/without hydrochlorothiazide together with amlodipine (D, E and F respectively). The participants were monitored at baseline, after 3 and 6 months (1st and 2nd follow-up). Brachial oscillometric ambulatory blood pressure monitor was used for measuring systolic (SBP), diastolic (DBP), pulse pressure (PP), pulse wave velocity (PWV) and peripheral resistance (PR).
Results:
SBP, DPB, PP, and PWV significantly decreased from baseline to 2nd follow-up in all treatment groups. The mean reductions in SBP were from -11.7 (95%CI; 9.3- 14.1) to -23.2 (95%CI; 18.3-28.1) mmHg and DBP reductions varied from -5.5 (95%CI; 3.9- 7.1) to -13.4 (95%CI; 7.7-19.1) mmHg. PWV decreased in all treatment groups (from -3.3% to -8.2%). Treatment regiment was not associated with significant differences in SBP, DBP, PP or PWV reductions or their values measured at 2nd follow-up. Peripheral resistance significantly decreased only in group C (p=0.011), group D (p=0.009) and group F (p=0.027).
Conclusion:
These data suggest that lisinopril/lisinopril + hydrochlorothiazide, losartan/losartan + hydrochlorothiazide and valsartan/valsartan + hydrochlorothiazide alone or in combination with amlodipine are equally effective and well tolerated for the reduction of both systolic and diastolic blood pressure and improve arterial stiffness in patients with essential hypertension.
Keywords: hypertension, arterial stiffness, pulse wave velocity, antihypertensive therapy
1. INTRODUCTION
Hypertension is significantly contributing to global mortality and morbidity and has been identified as the most important modifiable risk factor for early development of cardiovascular diseases (CVD) (1-3). However, in recent years, great emphasis has been placed on the role of arterial stiffness in the development of CVD (4). Arterial stiffness has long been viewed as a consequence of long-standing hypertension, but recent studies suggest that it may actually contribute to the pathogenesis of hypertension (5). Reports show that over one billion people worldwide suffer from hypertension, while research data predicts that in 2025 the number of patients will increase by additional 560 million (3, 6). The latest epidemiological data from 2016 shows that the overall mortality rate due to CVD in Bosnia and Herzegovina is 47.2% (45.6% in Federation of Bosnia and Herzegovina and 49.5% in Republika Srpska) (7, 8). CVD was the seventh leading cause of death in Federation of Bosnia and Herzegovina in 2016 (7).
Ambulatory blood pressure measurement (ABPM) is increasingly being used in clinical practice, since it provides more accurate and noninvasive measurement of blood pressure (BP) and pulse wave velocity (PWV) (9). all 45 measures differed less than 15 mmHg, 43 and 33 out of 45 differed less than 10 and 5 mmHg. As for diastolic pressures even better scores were reached when the device passed the eHs score. In phase II, data were collected in an additional 18 individuals leaving a total of 33 individuals and 99 measures. The phase counts the achieved percentages of two or three measures per individual within 15, 10 and 5 mmHg limits. Systolic pressures exceeded the required 95, 80 and 65% for 15, 10 and 5 mmHg differences with values of 98, 94 and 71%, respectively. As again for diastolic pressure the values were even better, the device passed phase II also. Thus, all phases of the European society of Hypertension procedure were passed and the results of this study can recommend the use of the mobil-o-Graph new generation ambulatory blood pressure monitor device in clinical practice. This is particularly important since the European Society of Hypertension/European Society of Cardiology (ESH/ESC) guidelines for the management of arterial hypertension suggests that measuring pulse wave velocity (PWV) can be used as a golden standard method for assessing the arterial stiffness (5,10).
The aim of pharmacological treatment of hypertension is to achieve values of systolic blood pressure (SBP) <140 mmHg and diastolic blood pressure (DBP) <90 mmHg. There are five main drug groups used as the first-line drugs: diuretics, β-blockers, calcium antagonists, angiotensin-converting enzyme (ACE) inhibitors and angio-tensin-II receptor blockers (ARBs) (6, 11) peer-reviewed journals. ”The following is a brief statement of the 2003 European Society of Hypertension (ESH. Studies suggest that these antihypertensive drugs may also improve arterial stiffness to varying degree, since the improvements in PWV has become assessment indicators for the treatment of hypertension (12). This can essentially contribute to reducing the risk of cardiovascular and renal complications as well as improvement of quality of life in patients with hypertension (4, 13, 14).
2. AIM
The aim of this study was to investigate the efficacy of different combinations of antihypertensive therapy in reduction of arterial stiffness and blood pressure using the brachial oscillometric ambulatory blood pressure monitor.
3. METHODS
This study was designed as a multi center, prospective, open-label, randomized study that was conducted in eight primary care centers of the Health Center of Canton Sarajevo over the period of six months. The study included 655 patients of both gender, 30 to 75 years old, who were diagnosed with hypertension according to the ESC/ESH guidelines (15). The participants with: hypersensitivity to components of the drugs, anamnestic angioedema, non-regulated hypertension using more than three antihypertension drugs, diagnosis of a mental disorder, presence of other chronic diseases (heart, liver or kidney failure), cancer and pregnancy were not included in the study. Patients who showed deterioration of the underlying disease, developed serious adverse reactions that required discontinuation of therapy or developed diseases that affected the course of research were further excluded from the study. The participants were randomly assigned to the treatment groups during the baseline observation. There were six treatment groups based on the hypertensive drug combination that was prescribed. The treatment groups and drug dosage are shown in the Table 1. The dosage of each drug was titrated during the baseline and the follow-ups according to the values of BP measured and ESC/ESH guidelines. Bosnalijek JSC manufactured all the drugs used in the study. The study was conducted according to the principles of the Declaration of Helsinki and was approved by the Agency for medical products and medical devices of Bosnia and Herzegovina, according to the Law on medicines of Bosnia and Herzegovina.
Table 1. Treatment groups and drug dosage in milligrams (mg).
| Group | Treatment | Dosage (mg) |
|---|---|---|
| A | lisinopril and/or lisinopril +hydrochlorothiazide | 5/10+12.5 |
| B | losartan and/or losartan + hydrochlorothiazide | 25/50+12.5 |
| C | valsartan and/or valsartan +hydrochlorothiazide | 80/80+12.5 |
| D | lisinopril and/or lisinopril+hydrochlorothiazide and amlodipine | 5/10+12.5 + 5 |
| E | losartan and/or losartan + hydrochlorothiazide and amlodipine | 25/50+12.5 + 5 |
| F | valsartan and/or valsartan +hydrochlorothiazide and amlodipine | 80/80 +12.5 + 5 |
The patients were followed during three visits: baseline and two follow-ups, with three months apart. Brachial blood pressure and pulse wave analysis was performed by Mobil-O-Graph (I.E.M., Stolberg, Germany). This device is a commercially available brachial oscillometric ambulatory blood pressure monitor and has been validated previously according to European Society of Hypertension recommendations (15) all 45 measures differed less than 15 mmHg, 43 and 33 out of 45 differed less than 10 and 5 mmHg. As for diastolic pressures even better scores were reached when the device passed the EHS score. In phase II, data were collected in an additional 18 individuals leaving a total of 33 individuals and 99 measures. The phase counts the achieved percentages of two or three measures per individual within 15, 10 and 5 mmHg limits. Systolic pressures exceeded the required 95, 80 and 65% for 15, 10 and 5 mmHg differences with values of 98, 94 and 71%, respectively. As again for diastolic pressure the values were even better, the device passed phase II also. Thus, all phases of the European Society of Hypertension procedure were passed and the results of this study can recommend the use of the Mobil-O-Graph new generation ambulatory blood pressure monitor device in clinical practice. A common cuff was centered to the left upper arm and the cuff size was chosen according to the circumference of the mid upper arm. During the first visit, general characteristics of participants were recorded, including common hypertension risk factors, (gender, age, body mass index, waist circumference, smoking and alcohol status, physical activity, blood lipid profile and blood glucose), as well as comorbidities and medications used. Using the oscillometric monitor during every visit systolic blood pressure (SBP), diastolic blood pressure (DBP), average values of pulse wave velocity (PWV), pulse pressure (PP) and mean arterial pressure (MAP) were recorded. The participants were assessed for response to treatment and adverse effects at each visit. Statistical analysis was performed by SPSS (Statistical Package for Social Sciences), version 13.0. The distribution of data from the study was tested by Kolmogorov-Smirnov test for normality and then described by measurements of central tendency and variability (median and interquartile range). A comparison of the mean values between the groups was performed by ANOVA test, followed by post hoc test (Tuckey test). ANOVA for repeated measurements was used to measure the significance of differences in variables measured at time intervals. Difference in distribution of participants with hypertension before and after the treatment was tested by McNemar test. Analysis of covariance (ANCOVA) was used evaluate the treatment effects on assessed variables adjusting for baseline measures. The level of significance was set to p<0.05.
4. RESULTS
Baseline characteristics of the participants are shown in the Table 1. Participants in the group B were significantly younger compared to participants in the group A (p=0.017); the group C (p=0.013) and the group F (p=0.028). The use of lipid-lowering medication was more common in E and F groups compared with other groups (p=0.025) (Table 2). There were no significant differences in gender distribution, anthropometric parameters, smoking and alcohol status, physical activity, lipids or blood glucose levels between the study groups (Table 2). During the study, no adverse reactions were recorded in any of the treatment groups. C-valsartan and/or valsartan +hydrochlorothiazide; D-lisinopril and/or lisinopril+ hydrochlorothiazide and amlodipine; E-losartan and/or losartan + hydrochlorothiazide and amlodipine; F-valsartan and/or valsartan +hydrochlorothiazide and amlodipine; *p<0.05 compared to A, C and F groups; **p<0.05 compared to other study groups; M-male; F-female; BMI- Body Mass Index. SBP significantly decreased from baseline to 2nd follow-up visit in all treatment groups (from -11.7 to -23.2 mmHg; p<0.001) (Table 3). Since baseline SBP value was significantly lower in group A (142.5±1.1) compared to group C (p<0.001) and group F (p<0.001), we tested different treatment effect on SBP reductions by controlling for differences in baseline systolic blood pressure and age. Reductions in SBP from baseline to 2nd follow-up were not significantly different across treatment groups once adjusted for baseline SBP and age (p=0.35).
Table 2. Baseline characteristics of enrolled patients by treatment groups. A-lisinopril and/or lisinopril +hydrochlorothiazide; B-losartan and/or losartan + hydrochlorothiazide;
| Treatment groups | ||||||||
|---|---|---|---|---|---|---|---|---|
| A N=237 |
B N=78 |
C N=180 |
D N=81 |
E N=29 |
F N=60 |
P | ||
| Age (mean ± SD) | 61.01±9.6 | 57.01±11.8* | 61.28±9.2 | 60.01±8.4 | 60.97±9.8 | 62.02±7.7 | 0.016 | |
| Gender M/F (%) | 38.1/61.9 | 38.5/61.5 | 41.7/58.3 | 38.3/61.7 | 48.3/51.7 | 38.3/61.7 | 0.9 | |
| BMI (kg/m2) | 29.6±15.3 | 28.6±5.6 | 30.3±11.3 | 28.7±4.1 | 28.1±3.5 | 30.0±4.7 | 0.8 | |
| Waist circumference (cm) | 95.3±15.3 | 95.7±18.9 | 98.6±13.9 | 95.0±15.4 | 90.2±18.2 | 100.0±13.6 | 0.07 | |
| Physical activity (%) | 60.2 | 52.6 | 52.0 | 55.8 | 48.1 | 65.5 | 0.32 | |
| Smoking (%) | Non-smoker | 54.7 | 42.1 | 54.2 | 50.6 | 44.8 | 60.0 | 0.54 |
| Ex- smoker | 22.5 | 25.0 | 20.1 | 17.3 | 24.1 | 20.0 | ||
| Smoker | 22.8 | 32.9 | 25.7 | 32.1 | 31.1 | 20.0 | ||
| Alcohol consumption (%) | 6.96 | 14.5 | 10.6 | 15.2 | 14.8 | 8.5 | 0.22 | |
| Diabetes mellitus (%) | 19.6 | 27.3 | 28.9 | 20.3 | 22.2 | 32.1 | 0.18 | |
| Antidiabetic drugs (%) | 14.9 | 18.4 | 24.3 | 19.0 | 17.2 | 23.7 | 0.11 | |
| Lipid-lowering drugs (%) | 56.4 | 51.9 | 59.0 | 64.6 | 75.9** | 75.0** | 0.025 | |
| Cholesterol (mmol/L) | 5.8±1.2 | 6.2±4.5 | 5.7±1.2 | 5.8±1.2 | 6.1±1.2 | 6.3±1.3 | 0.23 | |
| Triglycerides (mmol/L) | 2.2±1.3 | 2.2±0.9 | 2.3±1.3 | 2.2±1.1 | 2.4±1.1 | 2.5±1.2 | 0.62 | |
| Blood glucose (mmol/L) | 6.0±1.8 | 5.9±1.2 | 6.2±1.7 | 6.9±1.3 | 5.9±1.3 | 6.4±1.5 | 0.23 | |
Table 3. Systolic and diastolic blood pressure during antihypertensive treatment. SBP- systolic blood pressure; DBP-diastolic blood pressure; *-p<0.001; A-lisinopril and/or lisinopril +hydrochlorothiazide; B-losartan and/or losartan + hydrochlorothiazide; C-valsartan and/or valsartan +hydrochlorothiazide; D-lisinopril and/or lisinopril+hydrochlorothiazide and amlodipine; E-losartan and/or losartan + hydrochlorothiazide and amlodipine; F-valsartan and/or valsartan +hydrochlorothiazide and amlodipine.
| SBP (mmHg) | DBP (mmHg) | |||||||
|---|---|---|---|---|---|---|---|---|
| baseline | 2nd follow up | %Δ | ΔSBP (95%CI) mmHg | baseline | 2nd follow up | %Δ | ΔSBP (95%CI) mmHg | |
| A (N=237) | 142.5±1.1 | 130.8±1.0 | -8.8 | -11.7 (9.3-14.1)* | 87.4±0.8 | 81.8±0.7 | -6.4 | -5.5 (3.9-7.1)* |
| B(N=78) | 148.7±2.1 | 131.0±2.1 | -11.9 | -18.0 (13.6-22.4)* | 91.4±1.4 | 81.3±1.2 | -11.1 | -10.0 (6.7-13.3)* |
| C(N=180) | 151.8±1.4 | 135.3±1.2 | -10.9 | -17.3 (14.1-20.5)* | 90.4±0.9 | 83.8±0.7 | -7.3 | -6.8 (4.8-8.8)* |
| D(N=81) | 148.2±1.9 | 133.9±1.6 | -9.6 | -14.6 (10.7-17.9)* | 92.6±1.3 | 82.2±1.2 | -11.2 | -10.4 (7.6-13.2)* |
| E(N= 29) | 150.3±3.8 | 132.5±3.3 | -11.8 | -17.8 (9.6-26.0)* | 92.6±2.2 | 79.1±1.8 | -14.6 | -13.4 (7.7-19.1)* |
| F(N= 60) | 155.1±2.2 | 131.9±1.6 | -15 | -23.2 (18.3-28.1)* | 94.6±1.6 | 83.4±1.2 | -11.8 | -11.2 (7.9-14.5)* |
Baseline DBP value was significantly lower in group A compared to group D (p=0.017) and group F (p=0.001). DBP significantly decreased from baseline to 2nd follow-up visit in all treatment groups (from -6.4 to -14.6 mmHg; p<0.001) (Table 3). After controlling for differences in baseline DBP and age, no significant difference in DBP reductions from baseline to 2nd follow-up visit across the treatment groups was observed (p=0.061).
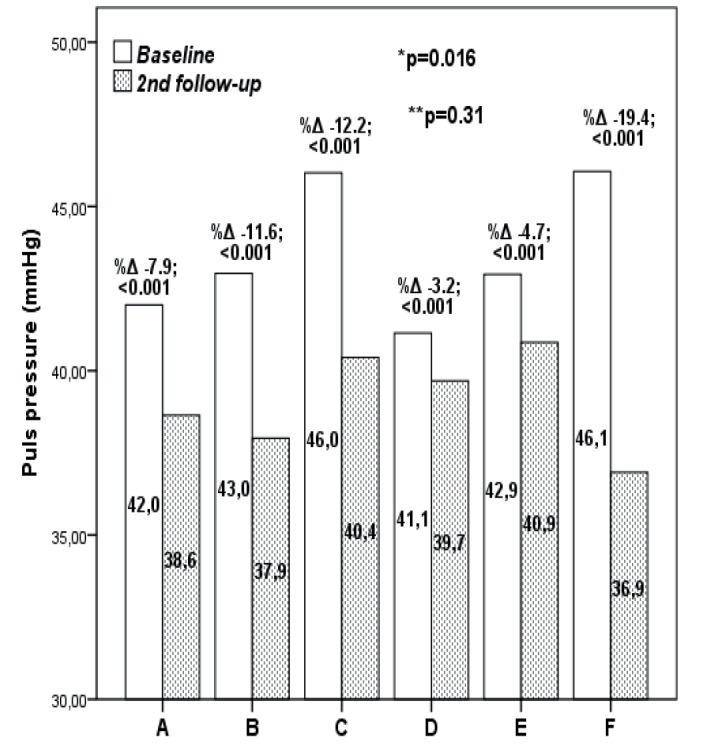
Distribution of participants with systolic and diastolic blood pressure above the recommendations (SBP>140 mmHg and DBP>90 mmHg) at baseline and 2nd follow-up is shown in the Table 3. During the antihypertensive treatment, rate of increased systolic and diastolic blood pressure significantly decreased in all study groups (p<0.001). At the 2nd follow-up, there was no significant difference in rate of increased systolic and diastolic blood pressure between the treatment groups (p=0.07) (Table 4). Also, increased diastolic blood pressure at the 2nd follow-up visit, although prevalent in 24.6% and 24.5% of participants in group C and F respectively, was not significantly higher compared to the other treatment groups (p=0.48) (Table 3).Pulse pressure significantly decreased in all treatment groups (from -3.2% in group D to -19.4% in group F)(p<0.001)(Figure 1). Pulse pressure reduction at 2nd follow-up although higher in treatment groups C and F (-12.2% and -19.4% respectively) was not significant compared to the pulse pressure reductions in other treatment groups when adjusted for age and baseline PP values (p=0.23) and no significant differences in pulse pressure values at 2nd follow-up between treatment groups was observed (p=0.31).
Table 4. Distribution of patients with increased systolic and diastolic blood pressureabove the recommendations (SBP>140 mmHg and DBP>90 mmHg) at baseline and the 2nd followup visit. A-lisinopril and/or lisinopril +hydrochlorothiazide; B-losartan and/or losartan + hydrochlorothiazide; C-valsartan and/or valsartan +hydrochlorothiazide; D-lisinopril and/or lisinopril+hydrochlorothiazide and amlodipine; E-losartan and/or losartan + hydrochlorothiazide and amlodipine; F-valsartan and/or valsartan +hydrochlorothiazide and amlodipine.
| A N=237 |
B N=78 |
C N=180 |
D N=81 |
E N=29 |
F N=60 |
p | ||
|---|---|---|---|---|---|---|---|---|
| SBP>140 mmHg | Baseline | 57.0% | 67.9% | 75.0% | 71.6% | 69.0% | 90.0% | 0.001 |
| 2nd follow-up | 23.9% | 16.0% | 33.1% | 29.6% | 31.0% | 21.1% | 0.07 | |
| DBP>90 mmHg | Baseline | 47.2% | 55.1% | 53.3% | 59.3% | 69.3% | 68.3% | 0.025 |
| 2nd follow-up | 23.0% | 14.7% | 24.5% | 23.5% | 13.8% | 24.6% | 0.48 |
Figure 1. Pulse pressure in patients on antihypertensive treatment at baseline and 2nd follow-up. *-between treatment groups at baseline; **-between treatment groups at 2ndfollowup. A-lisinopril and/or lisinopril +hydrochlorothiazide; B-losartan and/ or losartan + hydrochlorothiazide;. C-valsartan and/or valsartan +hydrochlorothiazide; D-lisinopril and/or lisinopril+hydrochlorothiazide and amlodipine; E-losartan and/or losartan + hydrochlorothiazide and amlodipine; F-valsartan and/or valsartan +hydrochlorothiazide and amlodipine.
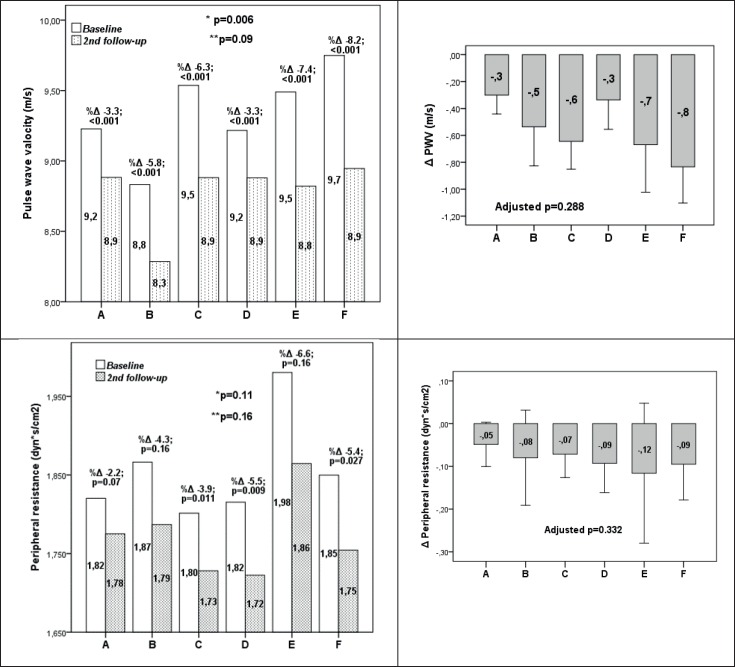
Pulse wave velocity significantly decreased in all treatment groups (from -3.3% in groups A and D to -8.2% in group F; p<0.001)(Figure 2). At baseline, participants in group F had significantly higher PWV values compared to other groups (p=0.006), but at the 2nd follow-up no significant differences in PWV values between treatment groups was observed (p=0.09) (Figure 3). Differences in PWV reduction across treatment groups were not significant when adjusted for baseline PWP values and age (p=0.288)(Figure 2). Peripheral resistance significantly decreased only in group C (p=0.011), group D (p=0.009) and group F (p=0.027). When adjusted for age and baseline peripheral resistance values no significant effect of different treatments was observed (p=0.332)(Figure 2).
Figure 2. Changes in pulse wave velocity and peripheral resistance in patients on antihypertensive treatment at baseline and 2nd follow-up. *-between treatment groups at baseline; **-between treatment groups at 2nd follow-up; adjustedp – p value adjusted for baseline PWV or peripheral resistance values and age. A-lisinopril and/or lisinopril +hydrochlorothiazide; B-losartan and/or losartan + hydrochlorothiazide;. C-valsartan and/or valsartan +hydrochlorothiazide; D-lisinopril and/or lisinopril+hydrochlorothiazide and amlodipine; E-losartan and/or losartan + hydrochlorothiazide and amlodipine; F-valsartan and/or valsartan +hydrochlorothiazide and amlodipine.
5. DISCUSSION
With this study, we aimed to investigate the effects of different combinations of antihypertensive treatment on lowering both high blood pressure and arterial stiffness.
The results of our study show that SBP significantly decreased from baseline to 2nd follow-up in all treatment groups. SBP reduction varied from -11.7 mmHg in group A to -23.2 mmHg in F group. DBP values significantly decreased from baseline to 2nd follow-up in all treatment groups from -6.4 to -14.6 mmHg. Due to the difference in baseline DBP and SBP values across treatment groups, we evaluated treatments effect by adjusting for baseline blood pressure and age. Adjusted model revealed the observed differences in SBP and DBP reductions were not statistically significant across treatment groups suggesting that the different antihypertensive treatment regimens were equally efficient in reducing both systolic and diastolic blood pressure. Adjusted systolic and diastolic blood values 6 months after therapy were not significantly different between treatment groups. During the study, no adverse reactions were recorded in any of the treatment groups. The results of our study could not show superiority of one treatment regimen over other since all of them had significant efficacy in lowering both systolic and diastolic blood pressure. Meta-analyses, which included several hundred thousand participants, has shown that a 10 mmHg reduction in SBP or a 5 mmHg reduction in DBP is associated with significant reductions in all major CV events by -20%, all-cause mortality by 10 - 15%, stroke by -35%, coronary events by -20%, and heart failure by -40% (15). In our study mean reductions in systolic and diastolic blood pressure was >10 mmHg and >5 mmHg in all treatment groups respectively. Six months after treatment was initiated, significant reduction in number of participants with systolic and diastolic hypertension was observed in all treatment groups.
Hypertension and arterial stiffness together as well as separately are one of the most important modifiable risk factors for CVD. Whether hypertension is a cause or a consequence of increased arterial stiffness is still a matter of academic discussion (16). A systematic review and meta-analysis conducted by Vlachopoulos et al. showed that the risk of cardiovascular (CV) events, CV mortality and all-cause mortality in subjects with increased PWV was almost twice as high compared with the risk of subjects with lower PWV. Although for each patient group exact values may differ slightly, for an increase in aortic PWV of 1 m/s or of 1 SD, the risk increases by more than 10% or 40%, respectively. These results highlighted the role of arterial stiffness as a potential treatment target in broader patient groups (10). Antihypertensive agents may have different effects on arterial stiffness, and thus, on central hemodynamic parameters despite having similar effects on brachial artery BP. Selecting antihypertensive agents that not only lower brachial artery BP but have a favorable impact on central BP and arterial stiffness may be an important consideration in selecting the optimal cardiovascular drug therapy (17).
In most of the conducted randomized studies, ACE inhibitors show positive effect in lowering BP as well as arterial stiffness (18). Many short to medium (less than 6 months) term studies showed a reduction of arterial stiffness when ACE inhibitors were used. These effects were obtained for most drugs in the ACE inhibitors class. These effects were attributed to the ACE inhibitors’ capability of chronically reducing remodeling of the small arteries, leading to reduction of reflection coefficients (19) progressively leading to arterial stiffening. Arterial stiffness is best characterized by measurement of pulse wave velocity (PWV). The use of lisinopril in our study also confirmed these findings. ARBs seemingly have a beneficial effect on arterial stiffness, but the results are conflicting and larger studies are needed (17). The VALUE study on valsartan long-term antihypertensive use showed that valsartan reduced central blood pressure more than the SBP and increased PP, while reducing the PWV (20) we investigated outcomes in 15 245 high-risk hypertensive subjects treated with valsartan- or amlodipine-based regimens. In this report, we analyzed outcomes in 7080 participants (46.4%. Our study also confirms these findings.
Calcium channel blockers also lower PWV and reduce wave reflections, but to a lesser degree than renin-angiotensin inhibitors. The largest amount of evidence is for the dihydropyridine calcium channel blocker amlodipine (19) progressively leading to arterial stiffening. Arterial stiffness is best characterized by measurement of pulse wave velocity (PWV). This drug was evaluated in the CAFÉ study, among other trials, where it proved to reduce central blood pressure more than peripheral blood pressure; it amplified PP and reduced PWV (19, 21) progressively leading to arterial stiffening. Arterial stiffness is best characterized by measurement of pulse wave velocity (PWV). Diuretics, in first line hydrochlorothiazide, have been shown to lower BP both as monotherapy and as an add-on agent (17). Although, many studies show positive effects of diuretics on lowering BP these studies indicate that diuretics have a rather neutral effect on central BP without any favorable effect on arterial wall composition and arterial stiffness (17,19) progressively leading to arterial stiffening. Arterial stiffness is best characterized by measurement of pulse wave velocity (PWV). Since hydrochlorothiazide was used as a single-pill combination with ACE inhibitors or ARBs, we cannot comment whether hydrochlorothiazide had positive or neutral effect on improving the arterial stiffness.
The results of our study may be limited by the relatively short follow up period which is why a large-scale study may be needed to further evaluate the long-term efficacy of these drug combinations. . Large observational and interventional studies are needed to demonstrate that targeting treatment to reduce arterial stiffness and wave reflections can reduce cardiovascular complications over and beyond BP reduction alone (16).
6. CONCLUSION
The results of our study confirm that lisinopril/lisinopril + hydrochlorothiazide, losartan/losartan + hydrochlorothiazide and valsartan/valsartan + hydrochlorothiazide drug combinations alone or in combination with amlodipine are equally effective in reducing both systolic and diastolic blood pressures and also proved to have similar effect on improving arterial stiffness.
Author’s Contribution:
Each author gave substantial contribution to the conception or design of the work and in the acquisition, analysis and interpretation of data for the work. Each author had role in drafting the work and revising it critically for important intellectual content. Each authorgave final approval of the version to be published and they agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent forms
Conflict of interest:
There are no conflicts of interest.
Financial support and sponsorship:
Nil
REFERENCES
- 1.Di Giosia P, Giorgini P, Stamerra CA, Petrarca M, Ferri C, Sahebkar A. Gender Differences in Epidemiology, Pathophysiology, and Treatment of Hypertension. [2018 Dec 15];Current Atherosclerosis Reports [Internet] 2018 Mar;20(3) doi: 10.1007/s11883-018-0716-z. http://link.springer.com/10.1007/s11883-018-0716-z. [DOI] [PubMed] [Google Scholar]
- 2.Yoon SS, Gu Q, Nwankwo T, Wright JD, Hong Y, Burt V. Trends in Blood Pressure Among Adults With Hypertension. Hypertension. 2015 Jan 1;65(1):54–61. doi: 10.1161/HYPERTENSIONAHA.114.04012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005 Jan 15;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 4.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006 Nov;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell Gary F. Arterial Stiffness and Hypertension. Hypertension. 2014 Jul 1;64(1):13–18. doi: 10.1161/HYPERTENSIONAHA.114.00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ilko A, Wudarczyk B, Czyż R, Jankowska-Polańska B. Arterial hypertension - definition, epidemiology, etiology, complications and treatment. Journal of Education, Health and Sport. 2018 Jul 1;8(8):533–541. [Google Scholar]
- 7.Statisticki-godisnjak-2016.pdf [Internet] [2018 Dec 15]. Available from: http://www.zzjzfbih.ba/wp-content/uploads/2014/04/Statisticki-godisnjak-2016.pdf .
- 8.Zdravstveno_stanje_stanovnistva_RS_2016_web.pdf [Internet] [2018 Dec 15]. Available from: http://www.phi.rs.ba/pdf/publikacije/Zdravstveno_stanje_stanovnistva_RS_2016_web.pdf.
- 9.Franssen PML, Imholz BPM. Evaluation of the Mobil-O-Graph new generation ABPM device using the ESH criteria. Blood Press Monit. 2010 Aug;15(4):229–231. doi: 10.1097/mbp.0b013e328339be38. [DOI] [PubMed] [Google Scholar]
- 10.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010 Mar 30;55(13):1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 11.Erdine S, Arı O. ESH-ESC Guidelines for the Management of Hypertension. Herz. 2006 Jun 1;31(4):331–338. doi: 10.1007/s00059-006-2829-3. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Huang B, Liu M, Li X. Effects of different types of antihypertensive agents on arterial stiffness: a systematic review and meta-analysis of randomized controlled trials. J Thorac Dis. 2015 Dec;7(12):2339–2347. doi: 10.3978/j.issn.2072-1439.2015.12.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosendorff Clive, Black Henry R, Cannon Christopher P, Gersh Bernard J, Gore Joel, Izzo Joseph L, et al. Treatment of Hypertension in the Prevention and Management of Ischemic Heart Disease. Circulation. 2007 May 29;115(21):2761–2788. doi: 10.1161/CIRCULATIONAHA.107.183885. [DOI] [PubMed] [Google Scholar]
- 14.Staessen JA, Wang J, Bianchi G, Birkenhäger WH. Essential hypertension. The Lancet. 2003 May;361(9369):1629–1641. doi: 10.1016/S0140-6736(03)13302-8. [DOI] [PubMed] [Google Scholar]
- 15.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018 Sep 1;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 16.Safar ME, Asmar R, Benetos A, Blacher J, Boutouyrie P, Lacolley P, et al. Interaction Between Hypertension and Arterial Stiffness. Hypertension [Internet] 2018. Oct, [2018 Dec 30]. Available from: http://login.research4life.org/tacsgr1www_ahajournals_org/doi/10.1161/HYPERTENSIONAHA.118.11212. [DOI] [PubMed]
- 17.Dudenbostel T, Glasser SP. Effects of Antihypertensive Drugs on Arterial Stiffness. [2018 Dec 30];Cardiol Rev [Internet] 2012 20(5) doi: 10.1097/CRD.0b013e31825d0a44. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3816577/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.London GM, Asmar RG, O’Rourke MF, Safar ME. REASON Project Investigators. Mechanism(s) of selective systolic blood pressure reduction after a low-dose combination of perindopril/indapamide in hypertensive subjects: comparison with atenolol. J Am Coll Cardiol. 2004 Jan 7;43(1):92–99. doi: 10.1016/j.jacc.2003.07.039. [DOI] [PubMed] [Google Scholar]
- 19.Janić M, Lunder M, Šabovič M. Arterial Stiffness and Cardiovascular Therapy. Biomed Res Int [Internet] 2014. [2018 Dec 30]. p. 2014. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4142148/ [DOI] [PMC free article] [PubMed]
- 20.Julius S, Weber MA, Kjeldsen SE, McInnes GT, Zanchetti A, Brunner HR, et al. The Valsartan Antihypertensive Long-Term Use Evaluation (VALUE) trial: outcomes in patients receiving monotherapy. Hypertension. 2006 Sep;48(3):385–391. doi: 10.1161/01.HYP.0000236119.96301.f2. [DOI] [PubMed] [Google Scholar]
- 21.Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113(9):1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]