Graphical abstract
Keywords: Citrus aurantifolia, Biochemical analysis, In vivo toxicity, Histopathological, Hepatotoxicity, Nephrotoxicity, Hematological
Abstract
Citrus aurantifolia (Christm.) Swingle (syn. C. MEDICA var. ACIDA Brandis) (family: Rutaceae) essential oil is one of the cheapest oils found in local markets. Although, it is generally accepted as non-toxic to vital organs and cells, majority of people are cynical about it usage. Herein, the present study reports the chemical composition and in vivo oral toxicity study of unripe C. aurantifolia essential oil found in Ghana. The toxicity of C. aurantifolia essential oil extract was investigated via oral administration using two methods: The acute toxicity single dose study (SDS) and the repeated dose method. The oil exhibited no acute toxicity but in the sub-chronic studies, the effects was dose and time-dependent. Chemical profile investigation of the oil showed 9 constituent of phytochemicals (Germacrene isomers (61.2%), Pineen (14%), Linalool dimmer (2.9%), Bornane (11%), Citral (2.9%), Anethole (1.5%), Anisole (1.1%), Safrole (0.3%) and Demitol (0.6%)). Histopathological studies revealed conditions such as necrosis, edema and inflammatory reaction in the liver, spleen and kidneys. Marginal upsurge of biochemical parameters above normal and elevated levels of lymphocytes (35.20–46.40 g/dL) demonstrated mild toxicity among the 100 mg/kg and 500 mg/kg dose groups at the sub-chronic stage. Low levels of hemoglobin (13.60 to 12.70 g/dL), MCV (34.20–24.0 fL), MCH (40.20–36.40 g/dL) along with high levels of liver enzymes confirmed the mild toxicity of the oil at sub-chronic stage. These results demonstrate that, despite consideration of lime essential oil as safe, it can have mild hematotoxic, nephrotoxic and hepatotoxic effects.
1. Introduction
The Search for medicinal plants and their phytochemicals as alternative for treating various diseases has generated interest among researchers. Although, C. aurantifolia’s (Lime) exact lineage is doubtful, the perception is that it was grown first in Indonesia or Southeast Asia, before transported to the Mediterranean Region and Northern Africa around 1000 CE [1]. In Ghana and other West Africa countries, lime fruits and its juice is a major medicinal recipe for local folks. The juice in combination with neem leaves extract has been used for managing malaria, diabetes, and other parasitic diseases [2]. Locally, it is a major anti-deodorant agent, and also added to local gin (“Akpeteshie”) for taste as well as masking of odour of the “Akpeteshie”. The lime juice mixed with other plant extract has been a major remedy for boil control locally. The root back is a good febrifuge. Decoction of the bark is a major remedy for gonorrhoea and related disorders. In Nigeria, the roots of the plant are chewed for headache, and eaten as a vermifuge. The leave is use as a lotion for fever and eye disease [2]. The other component of lime fruit which has not been utilized and ignored locally is its essential oil. Steam distillation and cold pressing are two main methods used to extract Lime (Citrus aurantifolia) essential oil mainly from the fruit rind. A study on aromatic volatiles in key lime oil has revealed that the freshnes and citrus aroma is as result of its major constituents such as: geranial, neural, and linalool [1]. These constituents are attributable to the immense properties (astringent, tonic, antiseptic, antiviral, antimicrobial, restorative, stimulant) of the oil [1]. Above all, they are also responsible for the health-promoting attributes [[3], [4], [5], [6], [7]]. In small doses, lime essential oil is known to serve as an appetizer and to increase one’s appetite when smelled [8]. In certain parts of Ghana, people smear the essential oil of lime on their skin to prevent mosquito bites, and some add it to their drinking water for enhanced flavour and as antioxidants [9]. One of the major uses of essential oil is aromatherapy. This treatment regimen could be used to induce relaxation, but there is lack of information or evidence to prove essential oils effectivness in treating this condition [10]. Improper use of essential oils may cause harm, including allergic reactions and skin irritation which are some of the side effects from improper use of the lime essential. Children in particular may be the most affected group via improper use of the oil [11]. D-limonene is among the major components of lime essential oil which has been reported to have certain effects on the lungs at certain concentrations [12]. One typical example, is the pumonary exposure of human volunteers to d-limonene which caused a decrease in the lung vital capacity at highest doses [13]. Research has established that renal problems caused by essential oil in humans are rare and if it should occur, it is usually associated with oral overdose in acute dosing [14]. Much have been established concerning adverse effects of volatile organic compounds (VOCs) including essential oil constituents on the lungs and other tissues [14]. If exposure is high enough, VOCs can cause sensory irritation of the airways characterized by irritation, tickling, burning, cooling, warming and stinging in the nasal cavities. Bronchial hyperactivity (BHR) is also an adverse effect associated with exposure to VOCs [15]. Long term exposure to moderate concentrations of mixtures of terpenes (an essential oil constituent) entails possible health risk [14].
Generally, lime essential oil is acknowledged as safe (GRAS) when used as a food additive and food flavouring agent by an American Food and Drug Administration (FDA) [16]. To the best of our knowledge, however, no published repeated dose toxicity study is available on the oral use of lime essential oil as medication or dietary supplement. Information on toxicity of lime essential oils when used as drug or dietary supplements is therefore scanty [16]. Additionally, not much is known about the toxic effects of the lime essential oil found in Ghana, but the originator of toxicology, Paracelsus, professed that “all substances are poisons; there is none which is not a poison, the right dose differentiates a poison from a remedy” [17,18]. This statement makes it imperative to investigate the toxicity of lime essential oil of the unripe fruits, since the unripe oil has been effective in treating several diseases locally. As several essential oils are considered “GRAS”, yet most essential oils have not been studied, especially, in concentrated internal amounts for which lime essential oil is one [19]. Ecologically, there has been few complains of pains of the heart when oil is added in drinking water. Also, burning sensation when the oil was massaged onto the skin have been reported by the users. Taking into account the rampant use of the oil all over the world, especially, Ghanaians and the fact that toxicological data lacking, we aimed to evaluate the acute, sub-acute and sub-chronic toxicity of the unripe oil extracted from slurry of entire lime fruit. Thus far, no documented toxicological assessments of unripe lime essential oil in which mainly monoterpenes are present. However, no documented fact regarding toxicity of unripe lime essential oil was found in literature. Unlike the ripe oil, the impact of unripe essential oil toxicity may be greatly be affected either positively or negatively, due to its chemical complexity. This is because, unripe lime essential oil has been found to comprise a greater number of metabolites compare to rip oil. Traditionally, the ripe oil is the most utilized oil. Preliminary investigation of unripe lime essential oil has demonstrated several potential therapeutic efficacy superior to the ripe oil. Such therapeutic efficacies are: anti-oxidant, anti-inflammatory, anti-bacterial, anti-fungal, anti-diabetic, anti-leishmanial as well as wound healing. Hypothetically, the unripe oil is expected to have no detrimental effect on vital organs.
Aim of The Study
The main intent of the present work was to investigate in vivo toxicity profile of unripe essential oil.
2. Methodology
2.1. Chemicals and materials
Reagents and Chemicals: absolute alcohol, xylene, paraffin wax, formaldehyde and eosin were acquired from Sigma Aldrich and used without further purification. Lime essential oil was extracted via steam distillation method under standard laboratory conditions following method reported in literature with slight modifications [20].
2.2. Citrus aurantifolia and extraction of unripe lime essential oil (LEO)
The unripe fruits of Citrus aurantifolia was collected from a small town called Amissakrom Ekroful in the Mfantseman District, Central Region, Ghana, with voucher specimen number CCG-4615. The fruit was identified by a Botanist (Mr Ogoe) at the School of Biological Sciences herbarium. The fruits were washed and cut into two parts, milled with a cutting mill (TECNAL, type Willye TE650) to particles size of 2.0 mm. [21]. The slurry material was then stored at room temperature (25 °C) with humidity (60%) in drum and subsequently, distilled at 80 °C [22]. Anhydrous sodium sulfate (Merck, Germany) was added to the extracted LEO untill free movement of anhydrous solid, filtered and dried LEO stored at room temperature for GC and GC/MS analysis.
2.3. Gas chromatography mass spectrometry (GC/MS) elucidation of unripe lime essential oil chemical constituents
The essential oils were analyzed using GC/MS (Shimadzu capillary GC-quadrupole MS system QP 5050A) with a fused silica capillary column DB-5 (30 m, 0.25 mm i.d, film thickness 0.25 μm). The injector and detector temperature was set at 250 °C. The essential oil dissolved in methanol was injected at initially column temparature (80 °C) for 3 min. The system temparature was increased up to 275 °C (5 °C/min). Carrier gas used was Helium (1.56 ml/min). The comparative quantity of total individual constituents of the LEO was presented as percentage peak area relative to the total peak area. Qualitative identification of the different constituents was performed via comparing relative retention times with standards values. Mass spectra of individual components were also compared with authentic reference compounds, or by retention indices (RI).
2.4. Animal
Total of 40 Sprague Dawley rats of approximately 8 weeks old and of weights between 70 and 230 g were used for the study. The Sprague Dawley rats of both sex were purchased from Noguchi Memorial Institute for Medical Research, University of Ghana, Accra and kept at the Animal House Facility of the Department of Biomedical Sciences, UCC, Cape Coast, Ghana. The females were nulliparous as well as non-pregnant and were separated from males prior to the experiment. The animals were permitted to accustom in the laboratory for 7 days. They were accommodated in polystyrene cages in which the floor was shielded with sawdust to lessen the likelihood of painful contact with a rigid surface. The animals were retained in 12 h light-dark sequence with full access to food and water. The feed was purchased from GAFCO Trading Company, Tema. Also the animals were permitted to familiarize to the animal house condition (Temperature 24–25 °C, relative humidity 60–70%). All ethical clearance in animal’s management as defined in the US National was observed. The entire animal experimentation, procedures, and techniques employed in this study were done in accordance with the National Institute of Health Quidelines for care and use of laboratory animals [23].
2.5. Maintenance and acclimatization of animal
One week acclimatization period was allowed after receiving the animals. Rats were quarantined in a room for this one week acclimatization period while daily clinical examination ensued. Only clinical healthy rats were employed for clinical examination. The rats were housed in aluminum cages (12.5 × 16 × 7.5 in.) with softwood shavings as bedding and kept in animal rooms at the laboratory. Animals were fed on food and water ad libitum. The test sample was administered by mouth using the oral gavage method and permanent board marker used to denote the rats.
2.6. Ethical approval for animal studies
The study was given ethical clearance approval by the Ethical Committee on Animals of the Department of Biomedical Sciences, School of Allied Health Sciences, University of Cape Coast, Cape Coast, Ghana in accordance with the Guide for Care and Use of Laboratory Animals, NIH, Department of Health Services Publication, USA, no. 83-23, revised 1985 [24].
2.6.1. Experimental design
The forty rats were used to determine the toxicity of lime essential oil. A day before the test, rats were weighed and grouped into four. The allowed weight variation among the animals in each group was 20%. Since no adverse effects were expected at the doses 50 mg/kg bw/d, 100 mg/kg and 500 mg/kg, controlled test was performed to analyse the repeated exposure dose in rats for 24 h, 28 and 60 days, respectively, following the guidelines 423 and 407 of the Organization for the Economic Cooperation and Development [25]. These doses were chosen for this work since no adverse effects were recorded following an akin procedure by Nǵuni et al. (2018) [26]. Calculated doses of the essential oil (base on the acute toxicity studies using graded concentrations of test substance via method developed by Chinedu et al. (See supplementary document)) [27] were orally administered to the animals as follows: Group 1, designated as low dose (50 mg/kg), Group 2: labeled as medium dose (100 mg/kg) and high dose (500 mg/kg) as group 3, with Group 4 serving as normal control. The total number of animals in each group was ten (10).
2.6.2. Preparation of essential lime oil solutions
Solutions of essential oil (50, 100 and 500 mg/kg) determined from the acute toxicity study (See supplementary document) were prepared by weighing appropriate volumes of the lime essential oil. It was then dissolved in required volumes of water to obtain the needed concentrations for each treatment groups. Since oil is immiscible with water, the oil was first dissolved in 0.1% Tween 20 a surfactant, followed by dissolution in water at ratio 1.14: 9.09: 89.00 mL of oil, Tween 20 and water respectively.
2.7. Administration of oil
Two different types of toxicity studies were conducted on the experimental rats namely the single dose study (SDS), which involves the effect of a single dose in the experimental rats. Here, rats were monitored at hourly intervals to observe behavior changes and toxicity. These signs normally present themselves in the form of apnea, dyspnea, salivation, convulsion, changes in motor activity and so on and was observed for 24 h. The repeated dose studies involve long duration studies of daily feeding, monitoring of toxicity and physiological changes. The rats in the control group were also given the same treatment except that they were given water for the duration of the experiment. After observing for 28 days for sub-acute and 60 days for sub-chronic studies, the rats were sacrificed and blood dispensed into chemistry tubes and Ethylenediaminetetraacetic acid (EDTA) tubes for biochemical and hematological indices analyses respectively. These animals were euthanized and dissected, and the spleen, liver, lungs, and kidneys collected, preserved in formalin and processed. The last phase of the study which was the sub-chronic phase ended on the sixtieth day and was carried out in the same way as the sub-acute phase.
2.8. Hematological and biochemical analysis
Experimental rats blood were analyzed for hematological and biochemical parameters following procedure reported in literature [28]. Hematological indices: red blood cells (RBCs), hemoglobin (Hb), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular, hemoglobin concentration (MCHC), monocytes (MON) neutrophils (NEU), and lymphocytes (LYM) were ascertained via an automated hematological analyzer. The blood serum was sampled into an empty vacutainer tubes after 5 min centrifuged of the whole blood. The serum was analyzed for biochemical parameters: Alanine aminotransferase (ATL), aspartate aminotransferase (AST), alkaline phosphatase (ALP) total bilirubin (TB), total protein (TP), total cholesterol (TC) triglycerides, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C) and very low-density lipoprotein cholesterol (VLDL-C) using assay kits.
2.8.1. Biochemistry analysis
Chemistry analyzers are employed to analyze the amount of certain metabolites, electrolytes, proteins, and drugs in samples of serum, plasma, urine, cerebrospinal fluid, and other body fluids [28]. In this study, the serum samples were loaded onto a tray and the test was programmed through barcode scanner. Reagents were already stored in the analyzer. The barcode scanner was used to read the test orders of the label on each test tube. Reagents were distributed into the reaction vessel after which the solution was mixed and passed through a calorimeter to measured its absorbance while it was still in its reaction vessel. Chemical concentrations were then computed and recorded accordingly.
2.8.2. Hematology analysis
The Abbot Hematology analyzer (SK 8800 Class II) was used to perform this test [29,30]. Samples were placed in a single file by hydrodynamic focusing and laminar flow and cells streamed through a flow cell for purposes of counting and analysis. White blood cells were counted and categorized by laser light-scattering data, via a multi-angle polarized scatter with separation technique. The angle of scattering as a function of cell size, refractive index nuclear shape, nuclear-cytoplasmic ratio, and granularity. Optical scatter and fluorescence technology with an argon laser were also used to count red blood cells and to separate differential leukocyte count parameters.
2.9. Histological analysis
Histological analysis was performed following literature method [31]. Firstly, the tissue went through tissue cut up where it was cut into smaller pieces to fit the tissue cassettes and into formalin. The tissue sections were set into an automated tissue processing machine (Leica TP 1020) to start the tissue processing. This consisted of four stages, continuous fixation with formalin to preserve the physical structure of the tissue and to prevent postmortem changes. Dehydration with graded alcohol, that is, from 75% to 100% (absolute) to remove all the water from the tissue and to allow tissue infiltration by nonpolar solvents were done. Clearing of alcohol from the tissue with xylene and infiltration with paraffin wax was the last stage of the tissue processing stage after which tissue embedding was carried out.
2.9.1. Tissue embedding
In this step, metallic molds were used together with molten wax to mold the tissue into blocks using the embedding center (Leica EG 110 H). The tissue blocks were then refrigerated to harden them after which microtomy was carried out. Tissue blocks were cut into thin slices using the microtome machine (Leica RM 2125 RT). The sections were cut at 5 microns, floated on a floating bath (Thermos Scientific MH 8516 × 6) at a temperature 10% below that of the paraffin wax (56 °C) and picked with the aid of microscope slide. The slides were then dried in hot air oven (Thermos scientific model 652) overnight.
2.9.2. Staining
After drying, the tissues were stained in the staining machine (Leica ST 4040) which contained three changes of xylene and several changes of alcohol. The slides of tissue were allowed to pass through the xylene and alcohol. The slides were then stained with hematoxylin for 10 to 15 min to stain the nuclei purple. They were then stained with eosin for 60 s to stain the cytoplasmic components pink. Afterward, the slides were passed through graded alcohol to take out excess water from the stained tissue.
2.9.3. Mounting
The tissue sections were mounted using DPX mountant and covered with coverslips, and subsequently examined under the microscope. Lastly, histopathological examination of the microscopic architecture of the tissues was examined on the H & E slides. Quantitative grading criteria; nil (0–5), mild (>5-24), moderate (>24-50) and severe (>50), were used to quantify the pathological lesions identified in the histological sections.
2.10. Statistical analysis
Data analysis was done via SPSS version 21.0. Data were depicted as mean ± SEM online graphs and tables. One-way-ANOVA test was employed to analyze the deviations in toxicities of different groups of animals. To compare control and treatment groups, independent samples t-test for the significance of difference were used. These were done with an alpha level of 0.05.
3. Results and discussion
3.1. Chemical composition of the unripe lime essential oil
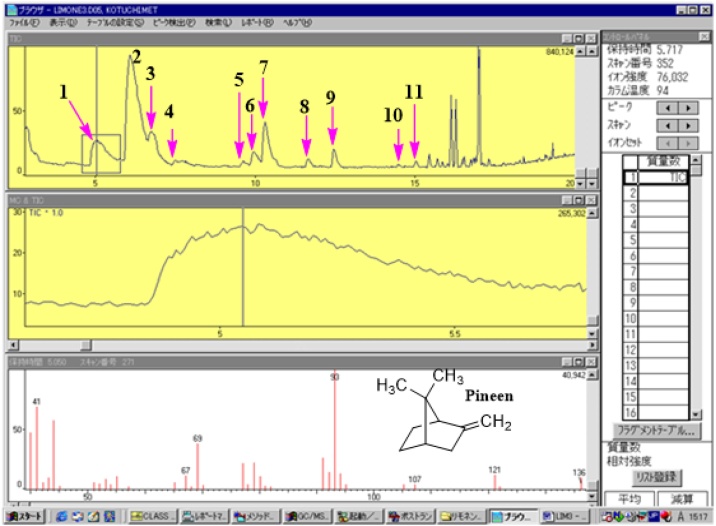
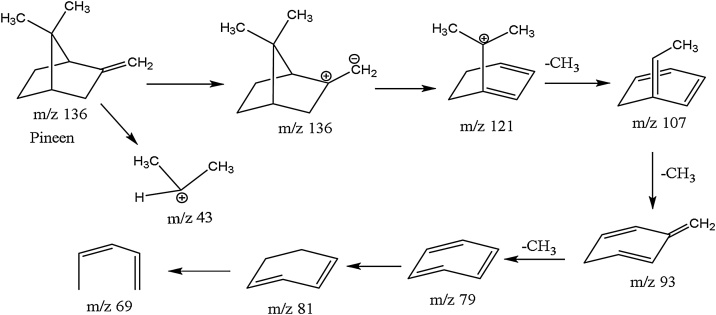
Exploiting the single ion monitoring (SIM) chromatograms created via accurate m/z representing terpenes as reported in literature [32,33], respective MS spectra of components of the essential oil were generated and the molecular formulae computed base on foundation that the isotopic fit ratios (iFit) was close to zero (Table 1). GC/MS analysis of unripe lime essential oil identified ten (10) phytochemicals as constituents (Fig. 1). Of these, two Germacrene isomers were the major components (61.2%) followed by Pineen (14%) and Bornane (11%) respectively. The rest of the components were traces as follows: Linalool dimmer (2.9%), Anisole (1.1%), Anethole (1.5%), Safrole (0.3%), Citral (2.9) and 2,6-dimethylheptan-2-ol (Demitol) (0.6%) (Table 1). Other chemical constituents were in small indefinite quantity. Table 1 represent the retention times of the major components. For example, the component labeled as 9 in the GC spectrum with m/z 152 was identified to be citral with a typical fragmentation pattern of this compound depicted in the MS spectra in Fig. 1 comparable to literature data [32,33]. The characteristics of the detected compounds were further searched and confirmed by the Dictionary of Natural Products online database (dnp.chemnetbase.com/) as well as comparison with other published data. The molecules were positively identified via individual molecular fragmentation patterns (Fig. 1, Scheme S1-5). Based on the MS fragmentation patterns, various terpenes components were tentatively identified listed in Table 1. (Fig. 1 & Figure S3). Accordingly, molecules 2, 3 and 4 (Fig. 1, Figure S1-3, Table 1) were identified as the isomers of germacrene and dimmer of linalool respectively. For instance, molecule 1 at retention time (Rt) of 5.05 min. (Fig. 1) showed a precursor ion at m/z 136 [M+H] (C10H16) and chemical break down of this molecule (Fig. 1, Scheme 1) resulted in molecular ion of m/z 121. This is predicted to be loss of methyl (−15 Da) moeity after a possible 1,2 methyl rearrangement of dimethyl derivative of pinene to a more stable derivative of β-pinene (Scheme 1) [33]. Products ions at m/z 93 corresponds to the loss of propenyl group (forty-three mass units (−43 Da) was also observed to be the bass peak with 100%. The component is tentatively recorded as β-pinene since the abundance of ion M/e = 41 is more than half of the base peak whilst that for the α-isomer is always less than one-quarter [33,34]. This molecule (m/z 136) is also distinguished from other isomers such as limonene which has it base peak at m/z 68 [34,35]. Based on respective building blocks fragmentation patterns, molecules 1-10 were positively identified and characterized similarly as presented in supplementary documents section (Figure S1–5, Scheme 1–5).
Table 1.
Percentage composition and retention time of chemical composition of unripe lime essential oil.
| Peak No. | Components | Formulae | Measured (m/z)[M+H]+ | Calculated (m/z)[M+H]+ |
Relative % | Retention Time (min) |
|---|---|---|---|---|---|---|
| 1 | Pinene | C10H16 | 136 | 136 | 14.3 | 5.05 |
| 2 | Germacrene | C15H26 | 207 | 207 | 49.0 | 6.08 |
| 3 | Germacrene isomer | C15H26 | 207 | 207 | 12.2 | 6.79 |
| 4 | Dimer linalool | 2(C10H18O) | 309 | 309 | 2.9 | 7.52 |
| 5 | Anisole | C7H8O | 110 | 110 | 1.1 | 9.64 |
| 6 | Bornane | C10H18 | 139 | 139 | 11.3 | 10.32 |
| 7 | Anethole | C10H12O | 148 | 148 | 1.5 | 11.67 |
| 8 | Citral/Geranial | C10H16O | 152 | 152 | 2.9 | 12.48 |
| 9 | Safrole | C10H10O2 | 161 | 161 | 0.3 | 14.48 |
| 10 | Damitole | C9H20O | 145 | 145 | 0.6 | 15.04 |
Fig. 1.
GC/MS of unripe lime essential oil showing MS fragment of pinene.
Scheme 1.
Fragmentation pattern of beta pinene.
3.2. Animal studies
3.2.1. General status, behaviour and clinical symptoms
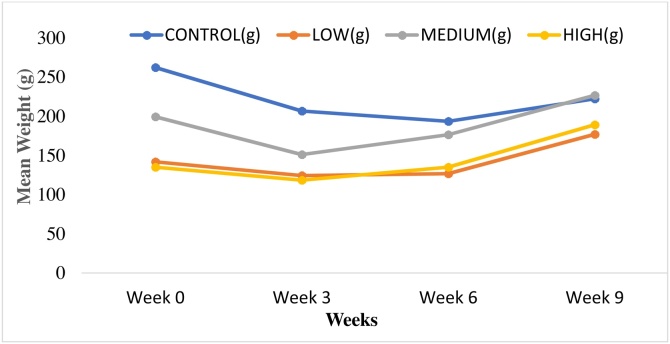
Throughout the study (60 days), the rats were examined for clinical symptoms or signs of toxicity on a daily basis. Alterations of skin and fur, mucous membranes, eyes, circulation, breadth, functions of the nervous system, salivation, diarrhea, and convulsions were some of the signs that were checked for acute toxicity but none of these were observed. However, two weeks into the test, a reduction in the appetite of the experimental animals was observed and this continued until the end of the 60 days, even though lime oil from different locations in the world have been used as appetizer. The reason could the source of the oil, thus, from unripe fruit and also location. Four cases of mortality were recorded during the study and these deaths were attributed to damaging effects of the experimentally induced disease condition created or aspirates which may have flooded the blood-air barrier due to oral gavaging process. The weights measured in four weeks (Table S2 Supplementary documents) were used to draw statistical inferences on the effects of the oil on weight. No significant variation observed between the groups’ weights under study since F critical was higher than or equal to F (Table S3). This means ingestion of the oil had no significant consequence on the weight. From the graph (Fig. 2, Table S2 supplementary document), the transient reduction in weight was observed in all the groups including the control, confirming that the reduction in weight in the low, medium and high dose groups was not as a result of test sample oil.
Fig. 2.
A graph of mean weights (g) against weeks for the control, low, medium and high dose groups.
3.3. Histopathological evaluation
3.3.1. Liver
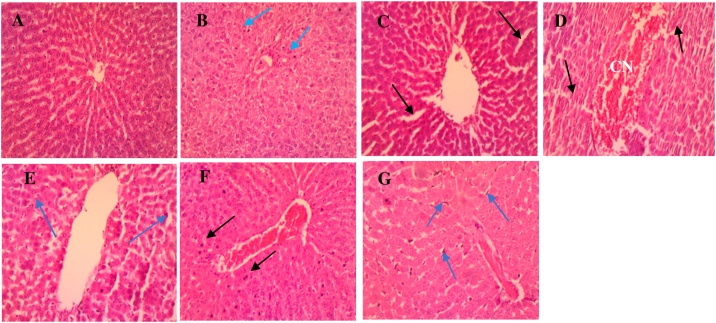
Microscopic analysis of the control group liver revealed tissue sections with normal histological structure. The parenchymal cells of the liver, hepatocytes, were tightly packed and were polygonal in shape [36]. Hepatic sinusoids, lined by endothelial cells, radiates from the central vein towards the periphery of the lobules (Fig. 3A). In the sub-acute stage, liver sections from the low dose (Fig. 3B) group showed normal cytoarchitecture with few pyknotic nuclei. This may be attributed to some constituents of the oil, germacrene D and linalool, which are known to have cytotoxic activity towards a wide range of cell lines [37,38]. Histological section from the group that had the medium dose (Fig. 3C) revealed mild signs of sinusoidal dilatation, edema and leukocytosis. For the high dose (Fig. 3D), section revealed congestion of the central vein, distortion of the tissue structure and leukocytic infiltrates. Hepatocytes of both groups (medium and high dose), however were devoid of pyknotic nuclei.
Fig. 3.
Photomicrographs of liver sections for the normal control (A), sub-acute (B–D) and sub-chronic (E–G) stage. (A) Control group: shows hepatocytes with normal cytoarchitecture devoid of injuries. (B) Low dose: shows very few pyknotic nuclei (blue arrow) of hepatocytes. (C) Medium dose: shows sinusoidal dilatation accompanied by mild oedema (black arrows), with very mild leukocytosis. (D) High dose: shows congestion (CN) within the central vein along with distortion of architecture of the tissue (black arrow) and mild leukocytosis. (E) Low dose: shows marked leukocyte infiltration (arrow heads). (F) Medium dose: CN of central vein and focal pyknotic nuclei (black arrows) present. (G) High dose: mild dilation of sinusoids, severe Kupffer cell activation (blue arrows) and CN within sinusoids and central vein (H & E × 100).
Despite the popular belief that the oil has anti-inflammatory properties, signs of inflammation were seen again in the tissue sections of all the dose groups at the sub-chronic stage of the study with the high dose group (Fig. 3G), showing the most severe pathological changes (dilation of sinusoids, severe Kupffer cell activation and congestion within sinusoids and central vein). The low dose group (Fig. 3E) also showed leukocyte infiltration which normally indicates that an infectious or toxic agent is present while the medium dose group (Fig. 3F) showed congestion of the central vein along with a few pyknotic nuclei. The severity of the pathological changes observed seemed to be time and dose dependent. For example, edema and mild leukocytosis are seen in the medium dose group at the sub-acute stage with the high dose group showing congestion, distortion of tissue architecture and mild leukocytosis. Also, mild leukocytic infiltration was observed in the sub-acute stage of the high dose group as opposed to the severe Kupffer cell activation seen in the same high dose group in the sub-chronic stage. This was corroborated by a study on trans-anethole (a constituent of lime essential oil) using rats as models where dose-related hepatic cell edema and degeneration were observed [39]. However, extracts and oils of the citrus family has been reported to be hepatoprotective in multiple studies [40,41]. Thus, from observed results, tolerable dose will be ideal for use.
3.3.2. Kidney
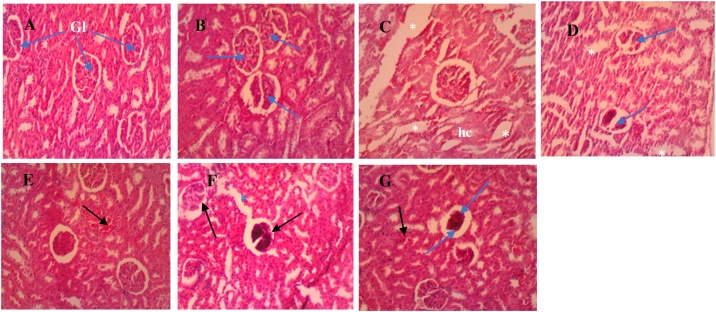
Photomicrographs of the kidney sections of the control and treated groups are presented in Fig. 4A-G. Histological section of the normal control group (Fig. 4A), showed normal renal architecture; intact representative renal corpuscles (Bowman capsule plus glomerulus), capsular space and surrounding proximal (PCT) and distal convoluted tubules (DCT). The low dose (Fig. 4B) had normal renal architecture as compared to the normal control group.
Fig. 4.
Photomicrographs of kidney sections for the normal control (A), sub-acute (B–D) and sub-chronic (E–G) stage. (A) Control: normal glomeruli (Gl) and tubules showing no injuries. (B) Low dose: shows normal histology of Gl (blue arrows) as compared to the normal group. (C) Medium dose: shows oedema (white asterisks) and hydropic changes (hc). (D) High dose: shows oedema (asterisks) and Gl necrosis (blue arrows). Also, distortion of Gl structure and the tubules, are indicative of tissue damage (E) Low dose: Normal histology as compared to the normal group, with presence of congested vessels (black arrow). (F) Medium dose: marked glomerular necrosis (black arrows) and oedema (blue asterisk) marked by dilation of the tubules. (G) High dose: shows few pyknotic cells (black arrow) of PCT and DCT, increased Bowman’s space (blue arrow head) caused by Gl atrophy and necrosis (blue arrow) (H & E × 100).
However, cases of necrosis were observed in the kidney sections for the medium and high doses (Fig. 4C and D), suggesting the presence of a toxic agent. The medium dose group (Fig. 4C) shows signs of edema and hydropic changes, with the high dose, revealing oedema and necrotizing lesions with the glomerulus, accompanied by distortion of tubules (DCT and PCT), are indicative of tissue damage.
In a previous study by Sun (2007), using experimental rats, d-limonene, microscopic evidence of compound-related nephropathy was noted. Beta pinene and D-limonene are group of organic compounds demonstrated to stimulate a unique nephropathy syndrome in rats after sub-acute or sub-chronic exposure [42,43]. This could account for the pathological lesions observed in Fig. 4C and D. Again, the pathological insults produced by the oil seem to be more severe as the dosage of the oil increases. The histological changes observed in the tissue sections at the sub-acute stage were also seen in the sub-chronic stage, but more severe in the latter (Fig. 4E-G), confirming the suspicion that the effect of the oil is dose and time-dependent. This assertion is again supported by a study on carvacrol and rosemary essential oils where it was stated that natural essential oils manifest cytotoxic effects on living cells depending on their type and concentration [44], which means the higher the dosage of the oil given, the more severe the cytotoxic effects elicited. Similarly, higher doses of essential oils from other plants elsewhere, has been reported to have nephrotoxic effect [45]. Our observation does not aggree with report by Deshmukh et al. (2017), who demonstrated that bitter orange (Citrus aurantium L.) extract of standardized 50% p-synephrine, demonstrated non-adverse effects after treating rat with 1000 mg/kg body weight/day [46]. However, it presupposes that, toxicity of Citrus aurantium extract from this study is primarily due to it’s chemical constituents with Germacrene (61.2%) as major costituent.
3.3.3. Spleen
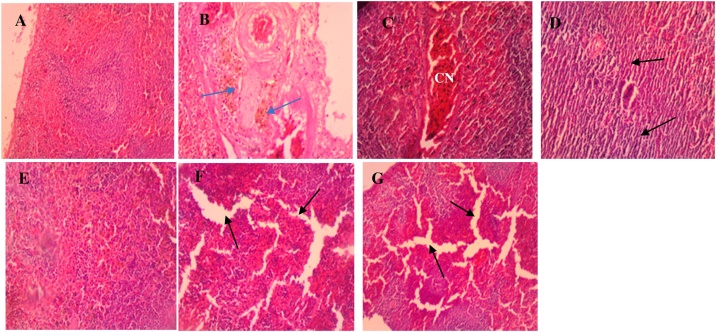
The tissue sections of the control group spleen (Fig. 5A) also showed normal cells with dense irregular collagenous connective tissue capsule covering, around the parenchyma of the dark red organ [36]. Accumulation of hemosiderin in the spleen in the low dose group in both the sub-acute (Fig. 5B) and sub-chronic (Fig. 5E) stages of the study suggests the destruction of defective or damaged red blood cells, as previously reported by other studies [47,48]. This could also be attributed to the cytotoxic effect of the major constituent of the LEO germacrene D [37].
Fig. 5.
Photomicrographs of spleen sections for the normal control (A), sub-acute (B–D) and sub-chronic (E–G) stage. (A) Control: normal tissue with the red and white pulps and the marginal zone separating them (B) Low dose: shows accumulation of hemosiderin (blue arrows) within the reticuloendothelial cells (blue arrows). (C) Medium dose: shows venous congestion (CN) with a little tissue distortion as compared to the control and low dose groups. (D) High dose: distortion of reticuloendothelial tissue and cytoarchitecture (black arrows) of spleen and atrophy of cells with marked spaces between adjacent immune cells. (E) Low dose: deposition of hemosiderin in the reticuloendothelial tissues. (F) Medium dose: shows sinusoidal dilatation and oedema (black arrows). (G) High dose: shows sinusoidal dilatation evident as oedema (black arrows), more severe than what is observed in the medium dose group (H & E × 40).
A review on the biological effects of essential oils also indicated that essential oils could act as pro-oxidants in eukaryotic cells, and produce a cytotoxic effect on living cells instead of antioxidants which protect cells [49]. It is, however, unclear why this was not observed in the medium and high dose groups. Instead, the medium and high dose groups showed dilation of sinusoids which suggests inflammation leading to distortion of reticuloendothelial cells and cytoarchitecture for both the sub-acute and sub-chronic stages of the experiment (Fig. 5F and G).
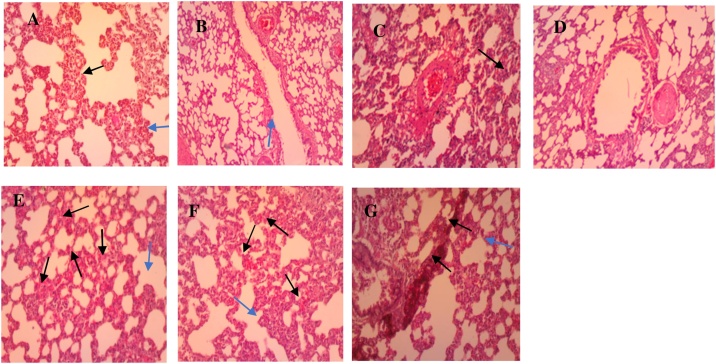
3.3.4. Lungs
Examination of control group tissue sections revealed normal histological architecture of lungs with the presence of alveolar ducts leading into alveolar sacs (Fig. 6A). The oil does not seem to have much effect on the cellular architecture of the lungs of the experimental rats (Fig. 6B-G). However, very mild influx of inflammatory cells and vascular congestion are seen in the sections. During inflammation in the lungs, the epithelial cells contrived mediators like: reactive oxygen radicals, cytokines (TNF-α, IL-1β, granulocyte / macrophage colony-stimulating factor [GM-CSF]), and platelet-activating factor to assemble inflammatory cells to the site of inflammation [50]. This requires a lot of blood to be directed to the site of inflammation which could have led to vascular congestion. The pathological conditions seen in the tissue sections in both stages of the experiment do not seem to be severe enough to classify the oil as toxic to the lungs when ingested orally. The higher dose group of the sub-chronic stage (Fig. 6G), nevertheless, showed focal areas of marked leukocytic infiltrates. And this is supported by the significantly (p < 0.001) high leukocyte count observed in this group.
Fig. 6.
Photomicrographs of lungs sections for the normal control (A), sub-acute (B–D) and sub-chronic (E–G) stage. (A) Control: shows normal alveolar duct with its walls consisting of adjacent alveoli (blue arrow) separated by interalveolar septa (black arrow). (B) Low dose: has normal histology as compared to the normal control group. (C) Medium dose: mild leukocytosis (blue arrow) with thickened aveoli septa (black arrow). (D) High dose: shows the presence of few inflammatory cells. (E) Low dose: slightly thickened aveoli septa (blue arrow), accompanied by focal inter-aveoli congestion (black arrows) (F) Medium dose: moderately thickened aveoli septa (blue arrow) accompanied by mild inter-aveoli congestion (black arrows) (G) High dose: shows marked leukocytic infiltrate within sections (H&E × 100).
A study conducted on the dermal toxicity of linalool, another constituent of lime essential oil, and its esters, yielded some results that could further help to understand the effects observed in the tissues. Linalool was applied to the skin of Wistar rats at dose levels of 125, 250, 500, 1000, 2000 and 4000 mg/kg/day for 29 consecutive days as a range-finding study for a 90-day study [51]. In the aforementioned study, some of the histopathological changes observed were erythema and edema which are signs of inflammation. This means linalool could also have contributed to the inflammatory reaction seen in the liver, kidney, and lungs.
3.4. Serum biochemical parameters
From Table 2, it was observed that when the lime essential oil was administered to the rats for sub-acute period, slight decrease in the total cholesterol levels except for the HDL which appears to have increased significantly among the low to medium dose group rats. The increase in the HDL levels might be the reason for the decrease levels of total cholesterol. When the lime essential oil’s dose was increased (among the high dose group rats), substantial increment of the total cholesterol levels was discovered except LDL and VLDL levels which reduced drastically relative to control group at both sub-acute stages (Table 2). Drastic reduction in HDL levels will mean less cholesterol is transported for metabolism by the liver. Hence, more cholesterol will be present in the blood [52,53]. In Table 2, it is also observed that the liver enzymes namely AST and ALT increased in all the various dose groups administered rats in the sub-acute stage as compared to the normal control. This result might indicate injury to the liver. This is because ALT is more specific for hepatic injury since it is found mainly in the hepatocytes. Comparing the liver enzyme data among low and medium dose groups with the high dose group, high dose resulted in an increased levels of the liver enzymes. This could be as a result of the mild derangements impacted on the hepatocytes in this group. In addition, the amount of conjugated bilirubin increased and that of unconjugated bilirubin decreased in all the groups relative to the normal control. The high levels of the conjugated bilirubin might be as a result of increased levels of liver enzymes within the liver cells that process the bilirubin. This could also be due to hepatocytic cell damage seen in the histological analysis, affecting liver function. These may facilitate bilirubin spill into the blood stream [54]. Comparing levels of bilirubin recorded in the low and medium dose groups to those recorded in the high dose, the high dose group had higher levels of conjugated bilirubin and very low levels of unconjugated bilirubin.
Table 2.
Serum biochemical parameters in rats treated orally with unadulterated Lime Essential oil (daily dose) at the sub-acute stage (n = 10).
| Biochemical Parameters (+SEM) | Determined Dosages (mg/kg bw) |
||||
|---|---|---|---|---|---|
| Normal control | Low (50) | Medium (100) | High (500) | aReference range | |
| Cholesterol (mmol/L) | 1.61 + 0.12 | 1.58+0.01 ns | 1.58+0.01 ns | 1.79 +0.01 ns | 1.20-2.38 |
| Triglyceride (mmol/L) | 0.86+0.01 | 0.67+0.01 ns | 0.62+0.01 ns | 0.78+0.01 ns | 0.35–1.4 |
| HDL (mg/dL) | 22.00+1.16 | 33.00+0.29*** | 33.00+1.16*** | 47.00+1.16*** | 20.0-23.0 |
| LDL (g/dL) | 25.00+0.58 | 16.00+1.16*** | 17.00+0.58*** | 18.00+0.57** | 23.5-27.5 |
| VLDL (mg/L) | 15.00+1.73 | 12.00+1.16 ns | 11.00+0.58 ns | 14.00+0.29ns | 15.5-20.5 |
| Dir. Bili. (mg/dl) | 0.01+ 0.00 | 0.12+0.01 ns | 0.15+0.01 ns | 0.26+0.01 ns | 0.05-0.10 |
| Indir. Bil (mg/dL) | 0.75+ 0.03 | 0.72+0.06 ns | 0.71+0.01 ns | 0.48+0.01ns | 0.74-0.80 |
| AST (μ/L) | 12.50+0.29 | 190.0+0.58*** | 175.00+2.89*** | 435.00+1.16*** | 39.0-111 |
| ALT (μ/L) | 66.00+0.58 | 100.00+5.77*** | 434.00+1.16*** | 118.00+0.58*** | 20.0-61.0 |
| ALP (μ/L) | 354.00+0.58 | 624.00+0.58*** | 282.00+1.16*** | 524.00+0.58*** | 39.0-216.0 |
Abbreviations: AST = aspartate aminotransferase, ALT = alanine aminotransferase, ALP = Alkaline Phosphatase Level, HDL= High density lipoprotein, LDL = Low density lipoprotein, VLDL= very low-density lipoprotein. Note: Significant difference between Control group and Experimental group: ns implies non-significant difference; (*) = P<0.05, (**) = P=<0.01, and (***) = P<0.001[63]a.
Higher levels of blood conjugated bilirubin might be due to severe damage to the liver cells and enzymes, as a result of reduced number of viable hepatocytes [54]. In Table 3, there is a general increase in the total cholesterol at the sub-chronic stage, excluding the HDL levels which decreases drastically among the medium and high dosed groups. The decrease in the HDL levels in the medium to high dose rats signifies that the liver might be impaired and hence produces it in low quantities (Table 3). Similarly, VLDL which is tagged as bad cholesterol reduced successively in low to high dosage groups. High VLDL cholesterol level has been reported to be connected with peril of cardiovascular disease and stroke. Mostly, VLDL cholesterol level is often not targeted for high blood cholesterol treatment, but rather therapeutic remedy target LDL cholesterol level [55,56]. However, any drug that may target both LDL and VLDL will be a good therapy for dealing with high cholesterol levels in the blood.
Table 3.
Serum biochemical parameters in rats treated orally with unadulterated Lime Essential oil (daily dose) at the sub-chronic stage (standard error of the mean = SEM) (n = 10).
| Biochemical Data | Determined Dosages (mg/kg/bw) |
||||
|---|---|---|---|---|---|
| Normal control | Low (50) | Medium (100) | High (500) | bReference range | |
| Cholesterol (mmol/L) | 1.17+0.01 | 1.61+0.01 ns | 1.42+0.01 ns | 1.37+0.01 ns | 1.10-1.20 |
| Triglyceride (mmol/L) | 0.41+ 0.01 | 0.78+0.01 ns | 0.66+ 0.01 ns | 0.88+0.02 ns | 0.35-0.45 |
| HDL (mg/Dl) | 27.00+0.58 | 45.0+0.58*** | 18.00+0.58 *** | 14.00+0.58*** | 20.0-30.0 |
| LDL (g/Dl) | 11.00+0.58 | 3.00+0.05*** | 25.00+0.29*** | 23.00+0.58*** | 10.0-15.0 |
| VLDL (mg/L) | 7.00+ 0.58 | 14.0+0.58*** | 12.0+0.06*** | 16.00+0.58*** | 5.0-10.0 |
| Dir. Bil. (mg/dl) | 0.16+0.02 | 0.10+0.29 ns | 0.14+0.01 ns | 0.13+0.01 ns | 0.15-0.20 |
| Indir. Bil. (mg/dl) | 0.27+ 0.01 | 0.73+0.01 ns | 0.67+0.01 ns | 0.73+0.02 ns | 0.20-0.30 |
| AST (μ/L) | 120.00+2.89 | 319.0+0.58*** | 159.0+0.58*** | 124.0+1.16 ** | 100-130.0 |
| ALT(μ//L) | 96.00 +0.58 | 107.0+1.15*** | 434.00+0.58*** | 80.0+0.58*** | 90.0-100.0 |
| ALP (μ//L) | 669.00+0.58 | 584.0+1.73*** | 933.00+0.58*** | 668.0+0.58 ns | 600-700.0 |
Note: Significant difference between Control group and Experimental group: ns implies non-significant difference; (*) = P < 0.05, (**) = P = <0.01, and (***) = P<0.001 [64]b.
Similarly, liver enzymes increased across the different doses administered except ALT which appears to decrease in the high dose administered rats in respect of the control group (Table 3). This result might indicate severe damage to the liver as a result of depletion of ALT levels in the liver. The resolution above agrees with Al-seeni et al. who discovered decline in liver enzymes after tartrazine was administered to rats [57]. Comparing the levels of AST among the low, medium and high dose groups administered rats, it could be observed from the results that, the levels of AST decrease as the dose increases, while the levels of ALT drastically increased among low and medium dose administered rats, except in the high dose rats which recorded decreased levels (Table 3). Finally, there were increasing levels of unconjugated bilirubin and decrease levels of conjugated bilirubin among all the various groups of rats as compared to the control group. This reason might be as a result of increased rate of red blood cells breakdown; consequently, there is much unconjugated bilirubin in the blood waiting to be conjugated by the liver. Again, injured hepatocytes revealed during histopathological analysis, are unable to keep pace with the processing of the unconjugated bilirubin. This increased amount of bilirubin in the plasma, accounting for the increasing levels of unconjugated bilirubin. The situation could also be attributed to an interrupted regulation of hepatobiliary conveyance systems at the sinusoidal and canalicular membrane of hepatocytes by the individual components of the unripe lime essential oil [58].
3.5. Analysis of hematological parameters
Table 4 depicts the hematological data of the sub-acute studies. The levels of RBCs increased significantly in all the groups at the sub-acute stage. The same results can be observed in the medium and high dose groups at the sub-chronic stage of the study (Table 5) compared to the control and reference range. The trend of hematological result is attributed to uncontrolled erythropoiesis activities in the bone marrow due to low oxygen levels, in this case, more RBCs is produced to compensate for any condition that results in low oxygen levels [59]. This also reflects a corresponding increase in the hematocrit levels since hematocrit determines the percentage of RBCs in blood by volume that is composed of RBCs. Surprisingly, the levels of RBC from the low dose group at the sub-chronic stage (Table 5) were normal. Hemoglobin levels decreased in all groups but highly significant (p < 0.001) among the medium (100 mg/kg) dosed groups (both sub-acute and sub-chronic stage). The decreased levels of hemoglobin might have resulted from the inability to utilize iron to make hemoglobin, as a result there was a decreased level of MCV due to small average RBCs size which might be a deficiency of iron, and this phenomenon is supported by mild to moderate hemosiderin deposit within the spleen of these groups. This has been reported in previous studies [48].
Table 4.
Hematological parameters in rats treated orally with unadulterated Lime Essential oil at sub-acute (+SEM) (n = 10).
| Hematological Parameters | Determined Dosages (mg/kg BW) |
||||
|---|---|---|---|---|---|
| Normal control | Low(50) | Medium (100) | High (500) | cReference range | |
| RBCs (M/u) | 3.97+ 0.01 | 6.68+0.02*** | 6.95+0.01*** | 7.55+0.03*** | 4.3–6.46 |
| Hematocrit (%) | 33.80+0.06 | 38.70+0.06*** | 38.10+0.06*** | 39.80+0.12 *** | 39–47 |
| MCV (fL) | 85.30+0.06 | 57.90+0.26*** | 55.90+0.29*** | 52.70+0.12*** | 70–88 |
| Hemoglobin(g/dL) | 13.60+0.17 | 12.80+0.12 ns | 7.70+0.12*** | 13.30+0.06 ns | 12.3–17.5 |
| MCH (pg) | 34.20+0.12 | 19.30+0.12*** | 19.50 +0.06*** | 17.60+0.06*** | 25-31 |
| MCHC (g/dL) | 40.20+0.12 | 33.30+0.17*** | 34.8+0.06*** | 33.40+0.23*** | 32-39 |
| Platelet (105/μL) | 6.13+0.56 | 7.52+ 1.16*** | 6.32+1.16*** | 9.67+0.58*** | 8.00–9.00 |
| Lymphocytes (%) | 35.20+0.06 | 75.50+0.29*** | 66.00+0.29*** | 79.50+0.29*** | 63–93 |
| MID (%) | 15.90+0.12 | 2.70+ 0.06*** | 1.10+0.06*** | 0.80+0.03*** | 13.0-16.0 |
RBC = red blood cells, MCV = mean corpuscular volume, MCH = mean corpuscular hemoglobin, MCHC = mean corpuscular hemoglobin concentration, MID = multi-infarct dementia. Note: Significant difference between Control group and Experimental group: ns implies non-significant difference; (*) = P < 0.05, (**) = P=<0.01, and (***) = P<0.001 [65,66]c.
Table 5.
Hematological parameters in rats treated orally with unadulterated Lime Essential oil at the sub-chronic stage (+SEM) (n = 10).
| Hematological Parameters | Determined Dosages (mg/kg bw) |
||||
|---|---|---|---|---|---|
| Normal control | Low (30) | Medium (100) | High (500) | dReference rage | |
| RBCs (M/u) | 3.97+0.01 | 3.83+0.01 ns | 4.94+0.01 ns | 5.25+0.03 ns | 4.3–6.46 |
| Hematocrit (%) | 33.80+0.35 | 15.80+0.12*** | 40.80+0.06*** | 34.80+0.12 ns | 39–47 |
| MCV (fL) | 85.30+0.06 | 91.80+0.58*** | 82.70+0.06 ** | 66.40+0.058*** | 70–88 |
| Hemoglobin(g/dL) | 13.60+0.06 | 10.00 + 0.58*** | 10.00+0.12*** | 12.70+0.058 ns | 12.3–17.5 |
| MCH (pg) | 34.20+0.12 | 57.80+0.17*** | 20.20+0.12*** | 24.10+0.06*** | 25-31 |
| MCHC (g/dL) | 40.20+0.12 | 63.20+0.06*** | 24.50+0.29*** | 36.40+0.23*** | 32-39 |
| Platelet | 613.00+1.73 | 1034+0.58*** | 791.00+0.58** | 139.00+1.16*** | 8.00–9.00 |
| Lymphocytes (%) | 35.20+0.06 | 41.90+0.06*** | – | 46.40+0.23*** | 63–93 |
| MID (%) | 15.90+0.23 | 15.60+0.12 ns | – | 3.00+0.289*** | 13.0-16.0 |
In Table 4 (all dose groups) and Table 5 (medium and high dose groups), it can also be observed that the levels of MCH, MCHC, and MCV reduced significantly (p < 0.001) among all the various dose groups compensating the smaller RBCs size due to low levels of hemoglobin found in each cell, while the corresponding increase in the sub-chronic stage of low dose group might result from the normal levels of the RBCs. The platelet and lymphocyte levels increased drastically (p < 0.001) in both sub-acute and sub-chronic stages (Table 4, Table 5), except in the high dose group which appears to show a decreased level. This might be as a result of inflammation in the liver leading to bone marrow production of more platelets and lymphocytes to heal wounds and fight against etiological agents respectively, as reported earlier [60,61]. While the decrease levels of the platelets in sub-chronic high dose group might be due to damage to the liver, it reduces the levels of globulin produced to assist in blood clotting [62]. The inflammation in the liver is also confirmed by the decrease levels of MID at the sub-acute stage (Table 4) and also by histopathology studies (Fig. 4).
4. Conclusion
On the whole, it seems the consumption of lime essential oil leads to cellular injury (necrosis) and trigger inflammatory response in tissues. These effects are strongly attributed to β-pinene, trans-anethole, germacrene D and linalool which are the main components of the oil identified by GC/MS. The present results means continuous exposure to the oil could lead to organ malfunction. However, the oil does not seem to elicit any signs of acute toxicity or to have any significant effect on weight. From the results obtained, it is safe to conclude that the oil has some form of toxic effects on some of the vital organs at the sub-chronic stage. Considering the pathological effects seen in the various tissues and the fact that these effects became more severe as the dosage and time of exposure to the oil increased, further work will be crucial to explain this concept. From the results, it is recommended that mechanistic tests be carried out to determine exactly how the oil causes the changes seen in the organs. Other routes of administration should be considered since the oil is used in a lot of ways.
Declaration of Competing Interest
The authors have no conflict of interest to declare.
Acknowledgments
The authors are grateful to University of Cape Coast for support and to Mr. K. Hayakawa and Dr. Y. Wakikawa of Advanced Instrumental Analysis Center at Shizuoka Institute of Science and Technology for their technical GC/MS support.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.toxrep.2019.06.020.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Narang N., Jiraungkoorskul W. Anticancer activity of key lime. Citrus aurantifolia Pharmacogn. Rev. 2016;10:118–122. doi: 10.4103/0973-7847.194043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ivine F.R. Oxford University Press; Oxford: 1961. Woody Plants of Ghana; p. 493. [Google Scholar]
- 3.Perry N., Perry E. Aromatherapy in the management of psychiatric disorders clinical and neuropharmacological perspectives. CNS Drugs. 2006;20:257–280. doi: 10.2165/00023210-200620040-00001. [DOI] [PubMed] [Google Scholar]
- 4.Jimbo D., Kimura Y., Taniguchi M., Inoue M., Urakami K. Effect of aromatherapy on patients with Alzheimer’s disease. Psychogeriatrics. 2009;9:173–179. doi: 10.1111/j.1479-8301.2009.00299.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith C.A., Collins C.T., Crowther C.A. Aromatherapy for pain management in labour. Cochrane Database Syst. Rev. 2011 doi: 10.1002/14651858.CD009215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai T.K., Cheung M.C., Lo C.K., Ng K.L., Fung Y.H., Tong M., Yau C.C. Effectiveness of aroma massage on advanced cancer patients with constipation: a pilot study. Complement. Ther. Clin. Pract. 2011;17:37–43. doi: 10.1016/j.ctcp.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Shiina Y., Funabashi N., Lee K., Toyoda T., Sekine T., Honjo S., Hasegawa R., Kawata T., Wakatsuki Y., Hayashi S., Murakami S., Koike K., Daimon M., Komuro I. Relaxation effects of lavender aromatherapy improve coronary flow velocity reserve in healthy men evaluated by transthoracic Doppler echocardiography. Int. J. Cardiol. 2008;129:193–197. doi: 10.1016/j.ijcard.2007.06.064. [DOI] [PubMed] [Google Scholar]
- 8.Patil K. 2019. Health Benefits of Lime Essential Oil Organic Information Services Pvt Ltd (OIS) p. 11.https://www.organicfacts.net/health-benefits/essential-oils/lime-essential-oil.html [Google Scholar]
- 9.Sustainable Baby Steps (SBS) 2017. An Introductory Guide to 1000’s of Uses for Essential Oils.http://www.sustainablebabyfacts.com Retrieved March 10, 2017 from. [Google Scholar]
- 10.Lee M.S., Choi J.C. Aromatherapy for health care: an overview of systematic reviews. Maturitas. 2012;3:257–260. doi: 10.1016/j.maturitas.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 11.Posadzki P., Alotaibi A., Ernst E. Adverse effects of aromatherapy: a systematic review of case reports and case series. Int. J. Risk Saf. Med. 2012;24:147–161. doi: 10.3233/JRS-2012-0568. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y.W., Kim M.J., Chung B.Y., Bang du Y., Lim S.K., Choi S.M., Lim D.S., Cho M.C., Yoon K., Kim H.S., Kim K.B., Kim Y.S., Kwack S.J., Lee B.M. Safety evaluation and risk assessment of d-Limonene. J. Toxicol. Environ. Health B Crit. Rev. 2013;16:17–38. doi: 10.1080/10937404.2013.769418. [DOI] [PubMed] [Google Scholar]
- 13.Falk-Filipsson A., Lof A., Hagberg M., Hjelm E.W., Wang Z. D-limonene exposure to humans by inhalation: uptake, distribution, elimination, and effects on the pulmonary. Funct. J. Toxicol. Environ. Health. 1993:77–88. doi: 10.1080/15287399309531702. [DOI] [PubMed] [Google Scholar]
- 14.Tisserand R., Young R. A Guide for Health Care ProfessionalsChurchill Livingstone. 2nd ed. 2014. Essential oil safety; pp. 97–110. Chapt.6. [Google Scholar]
- 15.Elliot L., Matthew P., Stephanie J. Volatile organic compounds and pulmonary function in the third national health and nutrition examination survey, 1988-1994. Natl. Inst. Environ. Health Sci. 2006;144(8):1210–1214. doi: 10.1289/ehp.9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christina B., Monice F. 2013. Safety Assessment of Citrus-derived Ingredients as Used in Cosmetics Cosmetic Ingredient Review. Washington, DC. [Google Scholar]
- 17.Trautmann N. The dose makes the poison or does it? Bioscience. 2005 [Google Scholar]
- 18.Frank P., Ottoboni M.A. 3rd ed. John Wiley & Sons, Inc.; New York: 2011. The Dose Makes the Poison: A Plain-Language Guide Toxicology. [Google Scholar]
- 19.Wellness Mama Risks and Uses of Essential Oils . 2017. what you need to know about safety Wellness Mama.https://wellnessmama.com/26519/essential-oils-risks/ [Google Scholar]
- 20.Njoku V.I., Evbuomwan B.O. Analysis and comparative study of essential oil extracted from Nigerian orange, lemon and lime peels. Diabetology. 2014;1:006–014. [Google Scholar]
- 21.Kanaze F.I., Termentzi A., Gabrieli C., Niopas I., Georgarakis M., Kokkalou E. The phytochemical analysis and antioxidant activity assessment of orange peel (Citrus sinensis) cultivated in Greece-Crete indicates a new commercial source of hesperidin Biomed. Chromatography. 2008;23:239–249. doi: 10.1002/bmc.1090. Sumise. [DOI] [PubMed] [Google Scholar]
- 22.Suade I. 2015. Akpeteshie Distillery in Ada Foah Ghana.http://www.ezime-guesthouse.com/index.php?cont=activities_cont&photo=akpeteshidistillery_afrivi [Google Scholar]
- 23.Institute for Laboratory Animal Research (ILAR) 8th edition. The National Academies Press; Washington D.C: 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 24.Garber J.C., Barbee R.W., Bielitzki J.T., Clayton L.A., Donovan J.C., Hendriksen C.F.M., Kohn D.F., Lipman N.S., Melcher, Quimby J.F.W. Vol. 8. The National Academic Press; Washington, DC: 2010. p. 220. (Guide for the Care and Use of Laboratory Animals). [Google Scholar]
- 25.OECD/OCDE . 2008. 407 Guidelines for the Testing of Chemicals: Repeated Dose 28 -Day Oral Toxicity Study in Rodents.https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecdtg407-2008.pd [Google Scholar]
- 26.Ng’unia T., Klaasen J.A., Fielding B.C. 2018. Acute Toxicity Studies of the South African Medicinal Plant Galenia africana Toxicology Reports 5; pp. 813–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chinedu E., Arome D., Ameh F.S. A new method for determining acute toxicity in animal models. Toxicol. Int. 2013;20(3):224–226. doi: 10.4103/0971-6580.121674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girling S.J., Campbell-Palmer R., Pizzi R., Fraser M.A., Cracknell J., Arnemo J., Rosell F. Haematology and serum biochemistry parameters and variations in the eurasian beaver (Castor fiber) PLoS One. 2015;10:e0128775. doi: 10.1371/journal.pone.0128775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organisation . WHO; 2003. Guidelines on Standard Operating Procedures for Haematology. [Google Scholar]
- 30.World Health Organization . 2nd ed. WHO; 2000. Manual of Basic Techniques for a Health Laboratory. [Google Scholar]
- 31.Tosta T.A.A., Neves L.A., do Nascimento M.Z. Vol. 9. 2017. pp. 35–43. (Informatics in Medicine Unlocked Informatics in Medicine Unlocked). [Google Scholar]
- 32.Kupska M., Wasilewski T., Jedrkiewicz R., Gromadzka J., Namiesnik J. Determination of terpene profiles in potential superfruits. Int. J. Food Prop. 2016;19:2726–2738. [Google Scholar]
- 33.National institute of standard technology (NIST) 2016. Material Measurement Laboratory NIST Chemistry WebBook.http://webbook.nist.gov/cgi/cbook.cgi?ID=C94597&Mask=200 NIST MS number 229569. [Google Scholar]
- 34.Djenane D. Chemical profile, antibacterial and antioxidant activity of algerian Citrus Essential oils and their application in Sardina pilchardus. Foods. 2015;4:208–228. doi: 10.3390/foods4020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yermakov A.I., Khlaifat A.L., Qutob H., Abramovich R.A., Khomyakov Y.Y. Characteristics of the GC-MS mass spectra of terpenoids (C10H16) Chem. Sci. J. 2010;2010:1–10. [Google Scholar]
- 36.Asante D.B., Effah-Yeboah E., Barnes P., Abban H.A., Ameyaw E.O., Boampong J.N., Ofori E.G., Dadzie J.B. Antidiabetic effect of young and old ethanolic leaf extracts of Vernonia amygdalina: a comparative study. J. Diabetes Res. 2016:1–13. doi: 10.1155/2016/8252741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Essien E.E., Newby J.M., Walker T.M., Ogunwande I.A., Setzer W.N., Ekundayo O. Essential oil constituents, anticancer and antimicrobial activity of Ficus mucoso and Casuarina equisetifolia leaves. Am. J. Essent. Oils Nat. Prod. 2016:1–6. [Google Scholar]
- 38.Prashar A., Locke I.C., Evans C.S. 2004. Cytotoxicity of Lavender Oil and Its Major Components to Human Skin Cells Cell Pro-life; p. 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shelanski M.V. 1958. Unpublished Report of Industrial Biology Research and Testing Laboratories Submitted to WHO. [Google Scholar]
- 40.Dosoky N.S., Setzer W.N. Biological activities and safety of Citrus spp. essential oils. Int. J. Mol. Sci. 2018;19:1966. doi: 10.3390/ijms19071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou T., Yu-Jie Z., Dong-Ping X., Fang W., Yue Z., Jie Z., Ya L., Jiao-Jiao Z., Hua-Bin L. Protective effects of lemon juice on alcohol-induced liver injury in mice. Biomed. Res. Int. 2017;2017:1–8. doi: 10.1155/2017/7463571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun J. 2007. D-Limonene: Safety and Clinical Applications Alternative Medical Review; pp. 259–265. [PubMed] [Google Scholar]
- 43.Clayton G.D., Clayton F.E., editors. 3rd ed. Vol. 2A, 2B, 2C. John Wiley Sons; New York: 1981. p. 3243. (Patty’s Industrial Hygiene and Toxicology: Toxicology). 1982. [Google Scholar]
- 44.Melušová M., Jantová S., Horváthová E. Carvacrol and Rosemary Oil at higher concentrations induce apoptosis in human hepatoma HepG2 cells. Interdiscip. Toxicol. 2014;7:189–194. doi: 10.2478/intox-2014-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liaqat I., Naila R., Qurat-ul-Ain S., Hafiz M.T., Muhammad A., Najma A. Toxicological evaluation of essential oils from some plants of Rutaceae family. Evid. Based Complement Altern. Med. 2018;2018:1–8. doi: 10.1155/2018/4394687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deshmukh N.S., Stohs S.J., Magar C.C., Kale A., Sowmya B. 2017. Bitter Orange (Citrus Aurantium L.) Extract Subchronic 90-day Safety Study in Rats Toxicology Reports 4; pp. 598–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y., Enshuang G., Xin Z., Bing W., Lei Y., Liming Z., Jun H., Xueyan F. A sub-chronic toxicity study of ethanol root extract of baked Aconitum flavum in rats. Revista Brasileira de Farmacognosia. 2016;26:438–445. [Google Scholar]
- 48.Sulistyo H., Kurniawan D.W., Rujito L. Biochemical and histopathological effects of green tea nanoparticles in ironized mouse model. Res. Pharm. Sci. 2017;12:99. doi: 10.4103/1735-5362.202448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils – a review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 50.Moldoveanu B., Otmishi P., Jani P., Walker J., Sarmiento X., Guardiola J., Saad M., Yu J. Inflammatory mechanisms in the lungs. J. Inflamm. Res. 2009;2:1–5. [PMC free article] [PubMed] [Google Scholar]
- 51.Bickers D., Calow P., Greim H., Hanifin J.M., Rogers A.E., J.H Saurat, Sipes I.G., Smith R.L., Tagami H. A toxicologic and dermatologic assessment of Linalool and related esters when used as fragrance ingredients. Food Chem. Toxicol. 2003;41:919–942. doi: 10.1016/s0278-6915(03)00016-4. [DOI] [PubMed] [Google Scholar]
- 52.2014. Doc’s Opinion HDL Cholesterol- The Good Cholesterol Explained.www.docsopinion.com Retrieved June 9, 2017, from. [Google Scholar]
- 53.Fielding C.J., Reaven G.M., Fielding P.E. Human noninsulin-dependent diabetes: identification of a defect in plasma cholesterol transport normalized in vivo by insulin and in vitro by selective immunoadsorption of apolipoprotein E. Proc. Natl. Acad. Sci. U. S. A. 1982;79:6365–6369. doi: 10.1073/pnas.79.20.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.University of Rochester Medical Centre (URMC) 2017. What Are Platelets?http://www.urmc.rochester.edu Retrieved June 9, 2017. [Google Scholar]
- 55.Semenkovich C.F. Disorders of lipid metabolism. In: Goldman L., Schafer A.I., editors. Vol. 24. Elsevier Saunders; PA: 2011. p. 213. (Goldman’s Cecil Medicine Philadelphia). chap. [Google Scholar]
- 56.Stone N.J., Robinson J.G., Lichtenstein A.H., Goff D.C., Jr, Lloyd-Jones D.M., Smith S.C., Jr, Blum C., Schwartz J.S. ACC/AHA Cholesterol Guideline Panel Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 American College of Cardiology / American Heart Association cholesterol guideline. Ann. Intern. Med. 2013;160(2014):339–343. doi: 10.7326/M14-0126. [DOI] [PubMed] [Google Scholar]
- 57.Al-Seeni M.N., Rabey H.A.E., Al-Hamed A.M., Zamazami M.A. Nigella sativa oil protects against tartrazine toxicity in male rats. Toxicol. Rep. 2018;5:146–155. doi: 10.1016/j.toxrep.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Docea A.O., Gofita E., Goumenou M., Calina D., Rogoveanu O., Varut M., Olaru C., Kerasioti E., Fountoucidou P., Taitzoglou I., Zlatian O., Rakitskii V.N., Hernandez A.F., Kouretas D., Tsatsakis A. Six months exposure to a real life mixture of 13 chemicals’ below individual NOAELs induced non monotonic sex-dependent biochemical and redox status changes in rats. Food Chem. Toxicol. 2018 doi: 10.1016/j.fct.2018.03.052. [DOI] [PubMed] [Google Scholar]
- 59.Sherwood L., Klansman H., Yancey P. Animal physiology. Brooks/Cole Cengage Learn. 2005;12:54–60. [Google Scholar]
- 60.Robinson M.W., Harmon C., O’Farrelly C. Liver immunology and its role in inflammation and homeostasis’. Cell. Mol. Immunol. 2016;13:267–276. doi: 10.1038/cmi.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kubes P., Craig J. Vol. 36. 2018. pp. 247–277. (Immune Responses in the Liver Annual Review of Immunology). [DOI] [PubMed] [Google Scholar]
- 62.2017. Mayo Clinic Symptoms: High White Blood Cell Count.http://www.mayoclinic.org Retrieved June 9, 2017, from. [Google Scholar]
- 63.Charles River Laboratories (CRL) Baseline hematology and clinical chemistry values for Charles River Wistar rats (CR:(W)BR) as a function of sex and age. Charles River Technol. Bull. 1982;1:2. [Google Scholar]
- 64.Charles B.C., Mary L.A. Clinical laboratory parameters for crl:CD(SD) Rats Charles River Lab. 2006;65:3–14. [Google Scholar]
- 65.Giknis M.L.A., Clifford C.B. 2008. Clinical Laboratory Parameters for Crl:w/(Han) Charles River Accelerated Drug Development Exactly.http://www.criver.com/files/pdfs/rms/wistarhan/rm_rm_r_wistar_han_clin_lab_parameters_08.aspx [Google Scholar]
- 66.Baseline C.R.L. 1984. Baseline Hematology and Clinical Chemistry Values for Charles River Fischer344 Rats-CDF庐 (F-344)CrlBRas a Function of Sex and Age.https://www.criver.com/sites/default/files/resources/BaselineHematologyandClinicalChemistryValuesforCharlesRiverFischer344Rats-CDF%C2%AEF-344CrlBRasaFunctionofSexandAge.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.