Abstract
Background
Oral mucositis is a frequent and severe adverse event in patients undergoing chemoradiotherapy for head and neck cancers, especially grade 3 or 4 mucositis. Occurrence may result in drop-out from treatment, thereby reducing survival. We aimed to clarify the effectiveness and safety of rebamipide mouthwash for oral mucositis in patients with head and neck cancer receiving treatment.
Methods
We carried out a systematic review and meta-analysis of patients with head and neck cancer who were treated with rebamipide mouthwash. We searched PubMed, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL), and the World Health Organization (WHO) International Clinical Trial Registry Platform. The primary outcome was the incidence of severe oral mucositis, and secondary outcomes were time from treatment start to onset of oral mucositis, the response rate of radiotherapy, and any adverse events.
Results
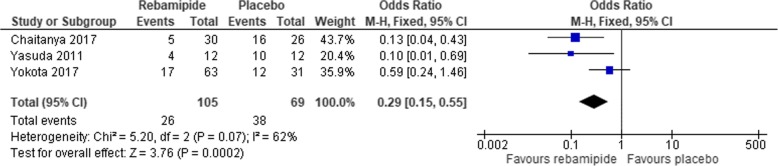
We included three studies comparing rebamipide versus placebo, all of which evaluating chemoradiotherapy induced oral mucositis. The chemotherapeutic agent was docetaxel in one study and cisplatin in the remaining two. Radiotherapy in each study consisted of 3D-conformal radiation therapy, intensity modulated radiation therapy and conventional radiation therapy, respectively. The calculated odds ratio was 0.29 [95% confidence interval (CI): 0.15 to 0.55], showing a positive association in the three studies between the incidence of grade 3–4 oral mucositis and chemotherapy for head and neck cancer. One study reported an onset of oral mucositis and the time to onset was 14.6 ± 6.4 days for the rebamipide group and 11.2 ± 4.4 days for placebo. One study reported a complete response of 8.3% for placebo and 16.7% for the rebamipide the group, and the partial response was 91.7 and 75.0%, respectively. Adverse events were reported in two studies to be 6.1 and 11.6% for placebo, and 19.4 and 26.0% in the rebamipide group, respectively.
Conclusions
Rebamipide mouthwash is effective in the prevention of severe mucositis and stomatitis. However, evaluation of adverse events in observational studies are needed.
Keywords: Meta-analysis, Rebamipide, Mouthwash, Mucositis, Chemoradiotherapy, Chemotherapy, Adverse event, Head and neck cancer
Introduction
Radiotherapy and surgery are the most effective treatments in head and neck cancer. Radiotherapy and surgery have a similar cure rate in early-stage cancer, but radiotherapy is better able to preserve organ function, with combined surgery and radiotherapy improving the prognosis in cases of advanced cancer [1]. The role of radiotherapy has broadened, as a result of developments in computer software, radiation delivery technology, and combined chemotherapy.
Oral mucositis is a severe and frequent adverse event of chemoradiotherapy for head and neck cancer, with 30–50% of patients experiencing grade 3 or 4 mucositis during treatment [2–4]. Oral mucositis results in an impaired mucosal barrier, and is associated with a longer hospitalization duration due to infection [5]. Oral mucositis is effectively treated with frequent oral rising, and several medical therapies are widely used, including mucosal coating agents, anti-inflammatory agents and topical granulocyte macrophage colony stimulating factor [5]. However, oral mucositis is still remains a critical and frequent issue for those undergoing head and neck cancer with chemoradiotherapy.
Rebamipide is a mucosal protection drug, which is used for gastiritis and gastric ulcer in a number of Asian countries [6]. Matsuda et al. first reported the efficacy of rebamipide for oral mucositis in 1994, and the subsequent rebamipide mouthwash, developed by the same authors [7], has been used for mucositis caused by Behcet’s disease [5], chemotherapy [8], and radiotherapy [9]. A pilot randomized controlled trial (RCT) in 2012 reported that rebamipide mouthwash reduced severe oral mucositis induced by radiotherapy or chemoradiotherapy [9]. Since then, there has been no further validation studies, but new trials have been published in 2017 [10, 11]. A Cochrane systematic review of oral mucositis for patients with cancer was published in 2011 [12], but the review did not include rebamipide mouthwash and has not yet been updated. Because there is a lack of a relevant systematic review on this topic, the aim of this review was to assess the effectiveness of rebamipide mouthwash in patients with oral mucositis receiving radiotherapy or chemoradiotherapy.
Methods
We carried out a systematic review and meta-analysis of patients with head and neck cancer who were treated with rebamipide. Standard guidelines for systematic review were used [13]. The protocol of this review was registered with International Prospective Register of Systematic Reviews (PROSPERO: http://www.crd.york.ac.uk/PROSPERO/) under registration number No 76566.
Eligibility criteria
We included all adult and child patients diagnosed with head and neck cancer, who were treated with radiotherapy or chemoradiotherapy and underwent therapy with rebamipide gargle, rinse or spray. Patients with both primary and recurrent head and neck cancer were included. Radiotherapy or chemoradiotherapy included preoperative, postoperative, and sole. We included RCTs and cluster-RCTs. We excluded cluster-RCTs which included only two clusters, crossover trials, and quasi-RCTs.
Search strategy
We searched the Pubmed, EMBASE, and Cochrane Central Register of Controlled Trials CENTRAL) (up to November 2018), and World Health Organization (WHO) International Clinical Trial Registry Platform databases. Medical subject headings and text words as terms in the searches were “rebamipide”, “head and neck neoplasm”, “otorhinolaryngologic neoplasms”, “radiotherapy” and “randomized control trial”.. Both published and unpublished studies in all languages prior to November 2018 were included. The search strategies and search results of each database were registered at PROSPERO.
Outcomes
The primary outcome was incidence of severe oral mucositis defined as either grade 3–4 of WHO oral toxicity scale [14], Radiation Therapy Oncology Group (RTOG) scale [15], or National Cancer Institute Common Toxicity Criteria (NCI-CTC) scale.
The secondary outcomes were time from treatment start to onset of oral mucositis, response rate (complete and partial response) of radiotherapy defined by the Response Evaluation Criteria in Solid Tumors (RECIST) [16], and any adverse events defined in each article by the authors were collected.
Study selection and data extraction
Three individual authors (SA, TF and MN) reviewed all titles and abstracts identified by electronic searches. We obtained the full text of studies that potentially met the eligibility criteria. Three authors independently assessed the eligibility of the studies from the full text. Any disagreements were resolved by discussion. We consulted another author if disagreement was not resolved by discussion. Three authors also extracted the following characteristics from the studies: patients including population (age, sex); primary site of cancer; Tumor Nodes Metastasis (TNM) classification of cancer; primary or recurrent cancer; type of radiotherapy (definitive, adjuvant, pre-operative, or postoperative); radiation technique [conventional, 3 dimensional conformal radiation therapy (3D-CRT), or intensity modulated radiation therapy (IMRT); regimens of rebamipide mouthwash.
Study quality assessment / risk of bias across studies
SA, TF and MN independently assessed the risk of bias of the included studies in following items: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. We used the Cochrane ‘Risk of bias’ tool, which involved describing each of these domains as reported in the trial and then assigning a judgement about the adequacy of each entry as ‘low’, ‘high’ or ‘unclear’ risk of bias.
Data analysis
We performed a statistical analysis of the outcomes using RevMan 5.3. We calculated odds ratios (OR) and 95% confidence interval (CI) for dichotomous outcomes, and calculated mean difference and 95% CI for continuous outcomes. For adverse eventts, we did not conduct meta-analysis and state narratively. For missing or unavailable data, we contacted the study authors. For data synthesis, we used available case analyses. A random-effect model was selected to perform statistical analyses within and between the heterogeneity of studies [17]. Chi-square test and I2 statistical analysis were used to qualitatively describe the heterogeneity and quantitatively estimate the proportion of the overall variation, respectively [17]. We conducted pre-specified subgroup analysis in primary outcome. We planned subgroup analysis of concentration of rebamipide (comparing different concentration of rebamipide) and type of radiotherapy (radiotherapy versus chemoradiotherapy), but we could not conduct subgroup analysis of type of radiotherapy because all included studies enrolled chemoradiotherapy patients. When included studies had more than two intervention groups (e.g. different concentrations of rebamipide), we split the control group into two or more groups with smaller sample sizes to make a reasonably independent comparisons.
Results
Study selection
Of the 140 potential citations, 5 articles were eligible for a full-text screening. After full-text screening, we identified three studies which met the criteria for eligibility. Figure 1 shows the process of study selection.
Fig. 1.
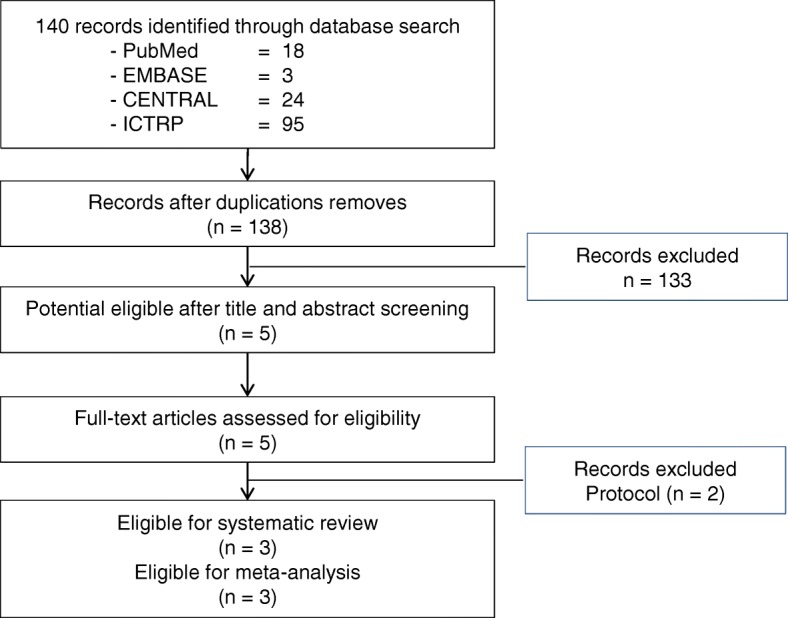
Process of study selection
Details of included studies
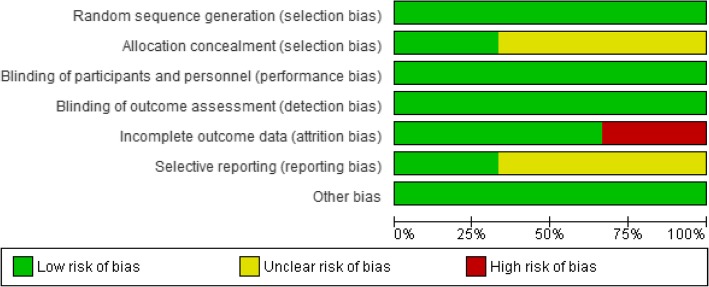
Table 1 shows the characteristics of the included studies. Three studies compared rebamipide gargle versus placebo. Two studies used the same gargle solution [9, 11], and one study used liquid [10]. All studies evaluated chemoradiotherapy-induced oral mucositis. One study used docetaxel and two studies used cisplatin. Two studies recruited participants from 2014 who underwent 3D-CRT or IMRT [10, 11]. One study recruited participants from 2005 and conventional radiotherapy was used [9], and conventional radiotherapy was undertaken preoperatively, despite this being recently uncommon [1]. Oral mucositis was assessed 4 weeks after chemoradiotherapy in one study and at the end of chemoradiotherapy in two studies. Overall, the methodological qualities of included studies were adequate, although concealment methods were unclear in two studies. One study had a 34% dropout, and the reasons included the patient’s request (n = 23), adverse events (n = 5), and physician’s judgment (n = 4) [10]. Risk of bias results are shown in Fig. 2.
Table 1.
Characteristics of included studies
| Yasuda 2011 | Chaitanya 2017 | Yokota 2017 | |
|---|---|---|---|
| Characteristics of patients | |||
| Number of patients | 24 | 60 | 94 |
| Mean age (years) | 60.4 | 51.5 | 61.0 |
| Dropout | 0 (0.0%) | 4 (6.7%) | 32 (34.0%) |
| Sex (Male/Female) | 14/10 | 59/1 | 77/17 |
| Site of cancer | Oral cavity | Head and neck squamous cell carcinoma | Head and neck cancer, primary tumor |
| TNM staging | |||
|
T1–2 Nany T3–4 Nany |
14 8a |
unclear |
58 36 |
| Type of radiation | Definitive and preoperative | Definitive and post-operative | Definitive or post-operative |
| Combination with chemotherapy | Weekly docetaxel (10 mg/m2) | Weekly CDDP (40 mg/ m2) or Tri-weekly CDDP (100 mg/ m2) | Tri-weekly CDDP (80-100 mg/ m2) |
| Radiation technique |
Conventional 2 Gy/fraction ≥40 Gy irradiation (total) |
3D-CRT or IMRT 2 Gy/fraction in 6–7 weeks Total 60–70 Gy |
3D-CRT or IMRT ≤2.2 Gy/fraction ≥60 Gy |
| Intervention of rebamipide | |||
| Type of mouthwash | Gargle | Gargle | Liquid, rinse and swallow |
| Regimens of rebamipid |
0.1% concentration 6 times daily |
0.1% concentration 6 times daily |
2 and 4% concentration 6 times daily |
| Control | Placebo | Placebo gargle | Placebo liquid |
| Timing of outcomes | At 4 weeks | At the end of chemoradiotherapy | At 57 days |
Cisplatin (CDDP)
aTNM stages were unclear in 2 patients in Yasuda 2011
Fig. 2.
Risk of bias graph: review authors’ judgement about each risk of bias item presented as percentages across all included trials
Incidence of grade 3–4 oral mucositis
Three studies evaluated incidence of grade 3–4 oral mucositis and the pooled odds ratio was 0.29 (95% CI:0.15 to 0.55). Forrest plot of incidence of grade 3–4 oral mucositis was shown in Fig. 3.
Fig. 3.
A forest plot of meta-analysis of a comparison of incidence of grade 3–4 mucositis between rebamipide and placebo
Time to onset of oral mucositis
One study evaluated onset of oral mucositis and the time to onset were 14.6 ± 6.4 days in rebamipide and 11.2 ± 4.4 days in placebo [11].
Response rate of radiotherapy
One study evaluated adverse events using Medical Dictionary for Regulatory Activities (MedDRA) system organ class and preferred terms [11]. Total numbers of adverse events were 16.1% in placebo and 19.4% in rebamipide. One study reported complete and partial radiotherapy response rates in placebo (8.3 and 91.7%) and repamipide groups (16.7 and 75.0%), respectively [9].
Any adverse events
Chaitanya et al. evaluated the pain intensity (range 1–10) was 4.2 ± 1.6 in rebamipide and 5.9 ± 2.2 in placebo. Yokota et al. evaluated any adverse events and reported that the incidence of adverse events potentially related to the study drug was 11.6% in placebo, 26% in 2% rebamipide and 13% in 4% rebamipide [10].
Discussion
Rebamipide has been used clinically for the purpose on improving mucosal lesions such as gastric ulcer treatment, erosion during gastritis, hemorrhage, redness, and edema. Rebamipide is effective for oral complications caused by cancer and its treatment. Rebamipide increased the generation of gastric mucosal prostaglandin activity [18, 19], increased the volume of mucosal mucus by the synthesis of mucous polymeric glycoprotein not involved in prostaglandin [20], directly eliminated hydroxyl radicals, and suppressed of leukocyte superoxide production [21–24].
Head and neck cancer has a higher incidence of oral complications associated with treatment than in general cancers [2–4]. Therefore, oral complications are important complications related to continuation of treatment and a decrease in patient’s quality of life (QOL). It has been shown to be useful that radiation therapy and chemotherapy are used in combination with locally advanced squamous cell carcinoma of the head and neck [25]. The incidence of mucositis is higher in concurrent radiochemotherapy than radiotherapy [26]. Management of oral complications is important.
In this meta-analysis, it was shown that rebamipide mouthwash was statistically reduced incidence of grade 3–4 oral mucositis. There was no difference in time to onset of oral mucositis in concurrent radiochemotherapy and radiotherapy alone [11].
In previous studies, There was no difference in response rate of chemoradiotherapy and incidence of adverse event in rebamipide mouthwash group and placebo [9, 10]. This may have resulted from problems in the safety profile of rebamipide mouthwash. However, time to onset of oral mucositis, response rate of radiotherapy and adverese events is not pooled, those are only outcome in each one study.
In this meta-analysis, there was a difference of 0.1 to 4% in the concentration of rebamipide mouthwash, The rebamipide concentration which shows the elimination of hydroxyl radicals and the suppressive action of leukocyte superoxide production is 10 mM to 1 mM [21, 23, 27, 28]. Since, 0.1% is 2.695 mM, it is considered to be a sufficient concentration as a direct concentration in the oral cavity. One study reported that it was a direct effect than the effect that was absorbed after gargle and reached the oral mucosa via blood stream [10]. There was no difference in effect at rebamipide concentrations of 2 and 4%, It is also considered that there was no difference because it has sufficient local concentration [10].
There was no comparison with other drugs in this meta-analysis. Palifermin and others are used for oral complications induced by cancer and its treatment. The problem is that there is no comparison and study with these another agents. The evaluation scles of oral mucositis used in each study were not iniform, therefore there was a lack of cositency.
It is shown that it is effective for pain [11], and improvement of QOL of patients is expected [29]. Opioids are used for stomatitis and mucositis in radiotherapy, but they have not been compared and studied, and this is a future subject for investigation. It is considered that the agent is retained on the damaged mucosal surface and exerts a protective action by washing in the mouth of rebamipide.
This meta-analysis had also some limitations. The sample size of each trial was small. The total number of patients were 178. The studies included in this meta-analysis used different types of radiation techniques, and these are influenced by tumor stage and site of cancer. There were variability in the chemotherapy regimens with different dosing schedules and different anticancer drugs. This may have resulted in high heterogeneity. The evaluation scales of oral mucositis used in each study were not uniform, therefore there was a lack of incosistency.
Conclusion
This meta-analysis shows that gargling treatment with rebamipide is superior to placebo for the development of mucositis and stomatitis due to chemoradiation, especially for severe cases of Grade 3 of higher. However, in order to confirm these trials, well-designed analyses are needed, and evaluation of adverse events in observational studies are also needed.
Acknowledgements
We thank Paul Williams for his proofreading.
Abbreviations
- 3D-CRT
3 dimensional conformal radiation therapy
- CDDP
Cisplatin
- CI
Confidence interval
- Cochrane CENTRAL
Cochrane Central Register of Controlled Trials
- IMRT
Intensity modulated radiation therapy
- MedDRA
Medical Dictionary for Regulatory Activities
- NCI-CTC
National Cancer Institute Common Toxicity Criteria
- PROSPERO
International Prospective Register of Systematic Reviews
- QOL
Quality of life
- RCT
Randomized cotrolled trial
- RECST
Response Evaluation Criteria in Solid Tumors
- RTOG
Radiation Therapy Oncology Group
- TNM
Tumor Nodes Metastasis
- WHO
World Health Organization
Authors’ contributions
SA, TF and MN carried out literature search, applied the inclusion criteria independently to the articles. SA, TF and MN carried out evaluation of quality, data extraction from the articles, and meta-analysis. AO, YN, TO, HT and KT helped to draft the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The dataset used for this meta-analysis are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shinsuke Akagi, Email: sa7440@kchnet.or.jp.
Takashi Fujiwara, Email: tf14817@kchnet.or.jp.
Mai Nishida, Email: mn14603@kchnet.or.jp.
Akiko Okuda, Email: ak7812@kchnet.or.jp.
Yuka Nagao, Email: ym12520@kchnet.or.jp.
Toshikatsu Okuda, Email: to7621@kchnet.or.jp.
Hidenori Tokuda, Email: ht6386@kchnet.or.jp.
Kazunobu Takayanagi, Email: ktaka@kchnet.or.jp.
References
- 1.Nutting C. Radiotherapy in head and neck cancer management: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130(S2):S66–S67. doi: 10.1017/S0022215116000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen-Tan PF, Zhang Q, Ang KK, Weber RS, Rosenthal DI, Soulieres D, et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the radiation therapy oncology group 0129 trial: long-term report of efficacy and toxicity. J Clin Oncol. 2014;32:3858–3866. doi: 10.1200/JCO.2014.55.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253–262. doi: 10.1016/S0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 5.Prescribing information: Mucosta® tablets 100mg, Ohtsuka Japan Inc, 2017 (In Japanese). Available from: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/2329021.
- 6.Matsuda T, Yamada H, Hoshi K, Ichikawa Y, Sakane T, Mizushima Y. The beneficial effect of rebamipide on recurrent oral aphthous ulcers in Behcet’s disease. J Clin Exp Med. 1994;170:773–774. [Google Scholar]
- 7.Matsuda T, Ohno S, Hirohata S, Miyanaga Y, Ujihara H, Inaba G, et al. Efficacy of rebamipide as adjunctive therapy in the treatment of recurrent oral aphthous ulcers in patients with Behçet’s disease: a randomized, double-blind, placebo-controlled study. Drugs R D. 2003;4:19–28. doi: 10.2165/00126839-200304010-00002. [DOI] [PubMed] [Google Scholar]
- 8.Nabeta I, Nakamura K, Kimura M, Kaya M, Tsuneizumi M, Nakagami K, et al. The effect of rebamipide fir prevention of mucositis associated with anthracycline chemotherapy for breast cancer. J Jpn Soc Hosp Pharm. 2010;46:1629–1634. [Google Scholar]
- 9.Yasuda T, Chiba H, Satomi T, Matsuo A, Kaneko T, Chikazu D, et al. Preventive effect of rebamipide gargle on chemoradiotherpy-induced oral mucositis in patients with oral cancer: a pilot study. J Oral Maxillofac Res. 2011;2(4):e3. doi: 10.5037/jomr.2011.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokota T, Ogawa T, Takahashi S, Okami K, Fujii T, Tanaka K, et al. Efficacy and safety of rebamipide liquid for chemoradiotherapy-induced oral mucositis in patients with head and neck cancer: a multicenter, randomized, double-blind, placebo-controlled, parallel-group phase II study. BMC Cancer. 2017;17:314. doi: 10.1186/s12885-017-3295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaitanya B, Pai KM, Yathiraj PH, Fernandes D, Chhaparwal Y. Rebamipide gargle in preventive management of chemo-radiotherapy induced oral mucositis. Oral Oncol. 2017;72:179–182. doi: 10.1016/j.oraloncology.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Worthington HV, Clarkson JE, Bryan G, Furness S, Glenny A-M, Littlewood A, et al. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2011;4:CD000978. doi: 10.1002/14651858.CD000978.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::AID-CNCR2820470134>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 15.RTOG: RTOG/EORTC Late Radiaton Morbidity Scoring Scheme. https://www.rtog.org/ResearchAssociates/AdverseEventReporting/RTOGEORTCLateRadiationMorbidityScoringSchema.aspx. Accessed 4 Mar 2019.
- 16.Mazumdar M, Smith A, Schwartz LH. A statistical simulation study finds discordance between WHO criteria and RECIST guideline. J Clin Epidemiol. 2004;57:358–365. doi: 10.1016/j.jclinepi.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011]: The Cochrane Collaboration; 2011. http://handbook.cochrane.org/. Accessed 4 Mar 2019.
- 18.Kishimoto S, Fujimura J, Machino H, Shimamoto T, Kobayashi H, Shimizu S, et al. Therapeutic effects of oral rebamipide and in combination with cimetidine on experimental gastritis in rats. Res Commun Chem Pathol Pharmacol. 1992;78:259–277. [PubMed] [Google Scholar]
- 19.Yamasaki K, Sakurai K. Role of lipid peroxidation in protection of rats by rebamipide against gastric mucosal lesions induced by stress plus indomethacin. Pathophysiology. 1994;1:251–257. doi: 10.1016/0928-4680(94)90006-X. [DOI] [Google Scholar]
- 20.Ishihara K, Komuro Y, Nishiyama N, Yamasaki K, Hotta K. Effect of rebamipide on mucus secretion by endogenous prostaglandin-independent mechanism in rat gastric mucosa. Arzneimittelforschung. 1992;42:1462–1466. [PubMed] [Google Scholar]
- 21.Ogino K, Hobara T, Ishiyama H, Yamasaki K, Kobayashi H, Izumi Y, Oka S. Antiulcer mechanism of action of rebamipide, a novel antiulcer compound, on diethyldithiocarbamate-induced antral gastric ulcers in rats. Eur J Pharmacol. 1992;212:9–13. doi: 10.1016/0014-2999(92)90065-C. [DOI] [PubMed] [Google Scholar]
- 22.Yoshikawa T, Naito Y, Nakamura S, Nishimura S, Kaneko T, Iinuma S, et al. Effect of rebamipide on lipid peroxidation and gastric mucosal injury induced by indometacin in rats. Arzneimittelforschung. 1993;43:1327–1330. [PubMed] [Google Scholar]
- 23.Naito Y, Yoshikawa T, Tanigawa T, Sakurai K, Yamasaki K, Uchida M, et al. Hydroxyl radical scavenging by rebamipide and related compounds: electron paramagnetic resonance study. Free Radic Biol Med. 1995;18:117–123. doi: 10.1016/0891-5849(94)00110-6. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki M, Miura S, Mori M, Kai A, Suzuki H, Fukumura D, et al. Rebamipide, a novel antiulcer agent, attenuates helicobacter pyloni induced gastric mucosal cell injury associated with neutrophil derived oxidants. Gut. 1994;35:1375–1378. doi: 10.1136/gut.35.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pignon JP, Bourhis J, Domenge C, Designé L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949–955. doi: 10.1016/S0140-6736(00)90011-4. [DOI] [PubMed] [Google Scholar]
- 26.Wendt TG, Grabenbauer GG, Rödel CM, Thiel HJ, Aydin H, Rohloff R, et al. Simultaneous radiochemotherapy versus radiotherapy alone in advanced head and neck cancer: a randomized multicenter study. J Clin Oncol. 1998;16:1318–1324. doi: 10.1200/JCO.1998.16.4.1318. [DOI] [PubMed] [Google Scholar]
- 27.Kim CD, Hong KW. Preventive effect of rebamipide on gastric lesions induced by ischemia-reperfusion in the rat. J Pharmacol Exp Ther. 1995;275:340–344. [PubMed] [Google Scholar]
- 28.Masamune A, Yoshida M, Sakai Y, Shimosegawa T. Rebamipide inhibits ceramide-induced Interleukin-8 production in Kato III human gastric Cancer cells. J Pharmacol Exp Thr. 2001;298:485–492. [PubMed] [Google Scholar]
- 29.Baharvand M, Hamian M, Moosavizadeh MA, Mortazavi A, Ameri A. Phenytoin mouthwash to treat cancer therapy-induced oral mucositis: a pilot studyPrimaryneuroendocrine carcinoma of breast: a rare tumor. Indian J Cancer. 2015;52:81–85. doi: 10.4103/0019-509X.175597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used for this meta-analysis are available from the corresponding author on reasonable request.