Abstract
Endoscopy has a central role in the management of inflammatory bowel disease (IBD), providing crucial data for diagnostic and therapeutic decisions, treating disease-related complications, and assisting in the early detection of dysplasia and prevention of colorectal cancer in the setting of IBD. Treatment targets have significantly shifted in IBD, focusing on achieving mucosal healing, a more meaningful endpoint than clinical remission. With the emergence of novel therapies, we aim to alter the course of the disease and prevent irreversible damage to the bowel. To that end, obtaining reliable and reproducible assessments of endoscopic disease activity has become an issue of great importance. Although several guidelines include recommendations regarding endoscopic surveillance in patients with long-standing IBD, there is an open debate regarding the best examination method and the appropriate follow-up intervals. Another important issue is whether surveillance guidelines are actually implemented in real-life practice and what is the preferred surveillance method among endoscopists. Significant changes have occurred in the endoscopic world with the development of new diagnostic and therapeutic modalities and their incorporation in everyday practice. We aimed to assess the real-life application of guideline recommendations regarding endoscopy in IBD patients and to review newly emerged data which might impact these recommendations in the near future.
Keywords: colon cancer surveillance, endoscopy, inflammatory bowel disease
Introduction
The role of endoscopic examination of the bowel in the management of inflammatory bowel disease (IBD) has evolved continuously ever since the first description of the utility of sigmoidoscopy for the diagnostic work up and subsequent follow up of patients in the pivotal trial of Truelove and Witts.1 Currently, endoscopy plays a central part in the care for the IBD patient at multiple stages, providing diagnostic and prognostic data, guiding medical and surgical therapy, treating disease-related complications, and assisting in the early detection of dysplasia and prevention of colorectal cancer in the setting of IBD. The fast-paced changes in the endoscopic world have been mirrored by equally rapid paradigm shifts in the therapeutic approach to IBD, with the introduction of multiple biologic therapies altering the natural history of the disease. There has been a lag between the development of these new diagnostic and therapeutic endoscopic modalities and their incorporation in everyday practice as well as their uptake in guideline recommendations. In our paper, we aimed to assess the real-life application of guideline recommendations regarding endoscopy in IBD patients and to review newly emerged data that might affect these recommendations in the near future.
Clinical and research consequences
Diagnosing IBD
Endoscopic examination is an essential part of the diagnostic work up for all patients with suspected IBD, and the European Crohn’s and Colitis Organization (ECCO) guidelines state that ileocolonoscopy with biopsies is the preferred method to confirm the diagnosis and allow an accurate assessment of disease extent and severity.2 Despite the high diagnostic accuracy of endoscopy, which can be further enhanced by histopathological examination of biopsies adequately sampling all the examined bowel segments, a minority of cases will remain undifferentiated at the index examination, while other patients will be reclassified over time [i.e. from Crohn’s disease (CD) to ulcerative colitis (UC) and vice versa]. Establishing the right diagnosis is of the utmost importance owing to the differences in the therapeutic approach between CD and UC; ECCO guidelines currently recommend repeat endoscopy and additional biopsies as a means to enhance the diagnostic yield for cases where a definite diagnosis cannot be established after the initial evaluation. There is limited data to challenge or expand the guideline recommendations in this particular area. The first (index) ileocolonoscopy has the best accuracy for distinguishing between UC and CD of the colon, as medical therapy can change the endoscopic appearance of the bowel, especially in the setting of incomplete mucosal healing. Differentiating between the two types of IBD is usually easy if there is extracolonic involvement. At least 20–30% of CD patients3 present with lesions located proximally to the ileocecal valve, possibly out of the reach of the colonoscope and in 13% of cases the disease occurs in the upper gastrointestinal tract.4 The involvement of terminal ileum, once considered a hallmark for CD, may be present as ‘backwash ileitis’ in 10–25% of patients with established UC.5,6 In this instances, techniques such as video capsule endoscopy (CE), confocal laser endomicroscopy (CLE), or small-bowel endoscopy (SBE) could be helpful in clarifying the diagnosis. A recent metaanalysis6 showed that CE found more intestinal lesions compared with computed tomography enterography (68% versus 21%, p < 0.00001) and ileocolonoscopy (47% versus 25%, p = 0.009), but not magnetic resonance enterography (55% versus 45%, p = 0.43).
Assessing disease activity
Reliable and reproducible assessment of endoscopic disease activity has become an issue of paramount importance ever since mucosal healing has been shown to be a strong predictor of long-term patient outcome,7,8 making it an important outcome measure in many studies.9,10 The ECCO guidelines for endoscopy in IBD suggest that the Ulcerative Colitis Endoscopic Index of Severity (UCEIS), the Ulcerative Colitis Colonoscopic Index of Severity (UCCIS), or the Mayo endoscopic score could be used for the assessment of disease severity in UC, whereas the Simple Endoscopic Score for Crohn’s Disease (SESCD) or the Crohn’s Disease Endoscopic Index of Severity (CDEIS) could be used for CD; however, the guidelines recognize the fact that these instruments are rarely used outside of clinical trials.2 Several limitations of these tools have been underlined, including the lack of formal validation for most of these scores, the lack of a clear-cut definition of mucosal healing or remission, as well as the fact that applying these scores in real life is time-consuming and cumbersome for the endoscopist.
Following the FDA recommendations11 on establishing relevant endpoints, recent studies include patient-reported outcomes and clinician-reported outcomes based on endoscopy and histology assessment. However, two recent Cochrane systematic reviews of the endoscopic activity scores for UC12 and CD13 found that none of the evaluated scores were fully validated according to existing methodological norms. The reviews focused on outcomes such as reliability (intra-rater and inter-rater), validity (content, construct, criterion), responsiveness and feasibility and confirmed that the five scores endorsed by the ECCO guidelines (UCCIS, UCEIS, and Mayo score for UC, SESCD and CDEIS for CD) had been studied extensively, but required further research before full validation could be confirmed.
Although not discussed explicitly in most endoscopy guidelines, it is important to underline the fact that the assessment of disease activity in UC is dependent on the type of examination performed. Although colonoscopy with biopsies from the entire colon is usually carried out at index evaluation, many patients and physicians prefer to conduct follow-up examinations using less-invasive modalities such as sigmoidoscopy or CE.
The post hoc analysis of endoscopic examinations from the EUCALYPTUS trial of etrolizumab in UC demonstrated that using only rectosigmoidoscopy to confirm mucosal healing was associated with a risk of underestimating disease activity and overestimating treatment efficacy, especially if a nonstrict definition of MH with a Mayo endoscopic subscore of 0 or 1 was applied.14 These findings are similar to those from the older study of Kato et al.15 that demonstrated that up to 27% of patients with UC colonoscopy showed more severe lesions situated in the descending colon compared with the sigmoid or rectum.
Guiding therapy
Ileocolonoscopy is the prime tool in diagnosing IBD and should be performed before initiation of any medical therapy.16 Routine endoscopy for patients in clinical remission is not recommended by current guidelines, unless it is likely to change patient management. In patients requiring a significant change to medical therapy, endoscopic reassessment is indicated.
Treatment strategies in IBD are mainly based on the severity, distribution, and pattern of disease. ECCO guidelines state that choice of treatment is determined by extension of disease, disease course, failure and adverse events of previous treatment, severity of the most recent flare, safety of treatment, and cancer prevention.17 Even with novel therapies, there are significant unmet needs in the current management of IBD, almost half of patients having no response to therapy.18 As a consequence, personalized care of the IBD patient and guided therapy to specific clinical settings have become common practice.19 For example, in recent studies20 one of the targets in the management of CD is to change the natural history of the disease by early introduction of biologic therapy for patients with a poor prognosis (young age, extensive disease, perianal disease, needing initial treatment with steroids). In the past, therapeutic decisions were symptom-based and focused on achieving clinical remission, usually failing to change the course of disease. In contrast, use of biologics/immunosuppressants early in the course of the disease, known as the top-down approach seems to improve long-term outcomes by altering the natural course of the disease and preventing irreversible damage to the bowel.21
High postoperative recurrence rates remain an important issue in patients with CD. Identifying individuals at high risk (smoking, prior intestinal surgery, penetrating disease, perianal location, myenteric plexitis)22 of endoscopic recurrence is now the mainstay of preventing clinical recurrence of CD.23 Current ECCO guidelines recommend postoperative endoscopy 6–12 months after surgery and treatment initiation in patients with high-risk features either with thiopurines or anti-tumor necrosis factor (TNF) agents for patients with at least one risk factor for postoperative recurrence, although mesalazine or imidazole antibiotics could also constitute an option for a subset of patients.24 Thiopurine therapy is associated with reduced risk of a first surgical intervention and recent data suggest that systematic azathioprine (AZA) therapy is not superior to endoscopic-driven treatment.25 In the study by Ferrante et al., systematic AZA initiation ⩽2 weeks from surgery was not superior to endoscopic-driven initiation [therapy was only initiated in the case of endoscopic recurrence (Rutgeerts score [RS] ⩾ i2) at weeks 26 or 52 following surgery]. A reliable tool to better stratify patients at high-risk of recurrence is necessary in order to guide therapy; so far existing instruments have not met this particular need. For example, the RS, the most widely used instrument for assessing endoscopic postoperative recurrence and risk stratification is only moderately reproducible in clinical practice,26 which may lead to incorrect therapeutic decisions, as shown in the study by Marteau.23 In this study, trained endoscopists evaluated the RS on 39 videotapes of patients who had undergone resection for CD with an ileocolonic anastomosis 6 months earlier, resulting in a percentage of inappropriate therapeutic initiation of 12.8% (3.8–21.9%) when initiation was triggered by a RS ⩾ i2.
CE is useful as a complementary test in CD, especially in patients with suspected small bowel CD, but some authors suggest that CE could guide medical therapy, especially in pediatric IBD. This was shown in a study by Min et al.,27 where use of CE for assessment of symptomatic CD, UC/IBD- unclassified, or suspected IBD in pediatric patients was associated with improved outcomes (growth and mean body mass index, mean erythrocyte sedimentation rate, median Harvey–Bradshaw index improvement). However, subtle lesions can be difficult to detect with CE, a study by Rimbas et al.28 suggesting that adding virtual chromoendoscopy (VCE) techniques such as flexible spectral imaging color enhancement (FICE) to CE could improve the evaluation of true ulcerative lesions (16.5% accuracy improvement when compared with white light endoscopy).
We believe that CE might have a role as a tool in ‘the treat to target’ approach to patients (Figure 1). The concept of pan endoscopy for evaluation of CD evolution was first proven by using Pillcam colon 2 capsules. In selected populations of post-intestinal resection, patients reluctant to colonoscopy or patients with predominant small bowel disease where calprotectin levels might not reflect the disease activity, the new Crohn® capsule, which has a dedicated software that allows easy comparisons between successive examinations, might be the right tool to monitor or to assess response to treatment. Adjustment of therapy might then be made more easily based on biomarkers and capsule examination, one major drawback being the price of the investigation and the fact that it is not reimbursed. Capsule retention remains the most relevant procedure-related complication, with the risk of capsule retention increasing from 1% to 2% (non-IBD patients) up to over 10% in confirmed IBD patients.29
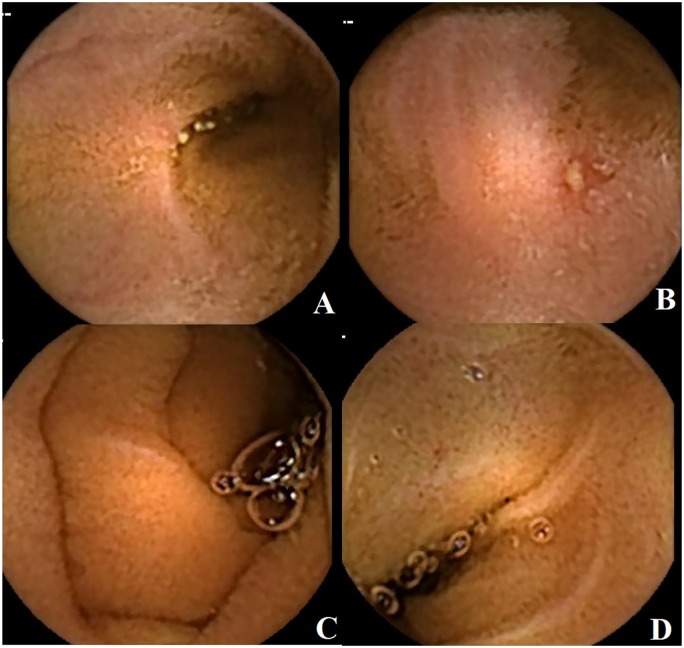
Figure 1.
Serial evaluation using capsule endoscopy in a patient showing typical small bowel lesions (A, B) with persistently normal fecal calprotectin levels. After 1 year of biological therapy, the patient was reevaluated and the examination showed mucosal healing (C, D).
Treatment targets have shifted significantly in IBD, focusing on achieving mucosal healing, a more meaningful endpoint than clinical remission, as it is associated with a reduction in complications, hospitalizations, and surgery. In the past, response was primarily based on clinical symptoms, which did not always correlate with endoscopic findings of disease activity that have been shown to predict aggressive disease and need for surgery.30 As a consequence, to optimize long-term outcomes, mucosal healing was proposed as a more relevant therapeutic endpoint.31 Currently, there is no universally validated definition of what constitutes mucosal healing in IBD, particularly in CD. Recent reports from Western countries defined mucosal healing in UC as a Mayo endoscopic subscore of 0 (inactive disease) or 1 (mild disease);16 however, in CD, consensus is lacking with respect to the definition of mucosal healing.
Cancer surveillance
Endoscopic surveillance in patients with long-standing IBD is aimed at early detection of neoplastic lesions and colorectal cancer prevention in a high-risk population. Although several guidelines include recommendations regarding endoscopic surveillance of IBD patients, there is an open debate regarding the best examination method and the appropriate follow-up intervals, reflected in the significant differences between various guidelines (Table 1). The key points of contention refer to the use of advanced imaging techniques such as classic dye chromoendoscopy (DCE) and VCE, tissue sampling (random versus targeted biopsies), and risk stratification with regard to the appropriate follow-up interval. Interpreting data from the guidelines is very difficult, especially because the rapid pace of technological development, especially the uptake of high-definition white light endoscopy (HD WLE) and the development and subsequent improvement of VCE over a relatively short period of time has introduced an inherent technological bias in the interpretation of available data in the literature.
Table 1.
Current guideline recommendations regarding endoscopic surveillance.
| Guideline | Examination method | Biopsy protocol | Follow-up intervals |
|---|---|---|---|
| ESGE: Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline – (March 2014)32 | 0.1–0.5% indigo carmine pan-colonic chromoendoscopy | Targeted biopsies | N/A |
| BSG guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (last update 2010)33 | Pan-colonic dye spraying | Targeted biopsy of abnormal areas is recommended 2–4 random biopsies from every 10 cm of the colorectum also accepted | Screening colonoscopy at 10 years - Lower risk 5 years - Intermediate risk 3 years - Higher risk 1 year Post-colectomy surveillance: - Lower risk 5 years - Higher risk 1 year |
| ECCO: Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders (February 2017)34
*Section 8. Surveillance for Colorectal Cancer in Ulcerative Colitis |
Chromoendoscopy with methylene blue or indigo carmine. High-definition endoscopy should be used if available |
Targeted biopsies preferred Alternatively, random biopsies [quadrantic biopsies every 10 cm] and targeted biopsies of any visible lesion should be performed if white light endoscopy is used. |
ECCO statement 8G
Screening colonoscopy should be offered over 8 years following the onset of symptoms - Lower risk 5 years - Intermediate risk 2–3 years - Higher risk 1 year |
| European evidence based consensus for endoscopy in inflammatory bowel disease (December 2013)19 | Pan-colonic methylene blue or indigo carmine chromoendoscopy | Targeted biopsies of any visible lesion If appropriate expertise for chromoendoscopy is not available, random biopsies (4 every 10 cm) should be performed |
|
| ASGE & AGA – SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease (2015)35 | Chromoendoscopy with methylene blue or indigo carmine. High-definition endoscopy should be used if available |
No consensus regarding random biopsies 45% agreed and 30% disagreed with performing random biopsies when using high-definition white-light colonoscopy, whereas 25% agreed and 60% disagreed with performing random biopsies when using chromoendoscopy. |
N/A |
| ASGE: The role of endoscopy in inflammatory bowel disease (2015)36 | Chromoendoscopy with pan-colonic dye spraying (0.1% methylene blue or 0.03–0.5% indigo carmine) | Pancolitis: random 4-quadrant biopsies are obtained every 10 cm from the cecum to the rectum, for a minimum of 33 specimens, Less extensive colitis: random biopsies limited to the maximally involved segments. Owing to an increased frequency of left-sided CRC in UC, consideration may be given to taking 4-quadrant biopsies every 5 cm in the left side of the colon |
All patients at 8 years Every 1–3 years - Optimal surveillance interval not defined. - Presence of risk factors merits annual surveillance - In patients with endoscopically and histologically normal mucosa on R2 surveillance colonoscopies, the surveillance interval can be lengthened. |
| If chromoendoscopy is not available or if the yield of chromoendoscopy is reduced | Random biopsies plus targeted biopsies of any suspicious appearing lesions |
CRC, colorectal cancer; UC, ulcerative colitis.
Although most guidelines16 preferentially recommend the use of classic DCE with targeted biopsies for surveillance of IBD patients, a large multicenter retrospective study failed to prove its advantage over HD WLE with targeted biopsies in detecting neoplastic lesions.37 These data are partially supported by the findings from another large retrospective study,38 which showed that targeted biopsy protocols using HD WLE, DCE, or VCE have superior diagnostic yields to random biopsy protocols, especially in standard definition WLE, but failed to show the superiority of DCE over either VCE or HD WLE. The findings of these studies need to be interpreted carefully, because the main outcome measure was not a comparison between DCE and HD WLE; however, both these large audits show that any existing advantage of DCE is hard to demonstrate in a real-life setting. Furthermore, both these studies reflect the high variability between operators and centers with respect to surveillance strategies for IBD patients.
With respect to the use of targeted biopsies versus random biopsy protocols, as shown in Table 1, most guidelines16,32,33 favor chromoendoscopy with targeted biopsies and endorse the random biopsy protocol as a second-line option, especially in case of low expertise with advanced imaging modalities. These recommendations have been recently called into question by a large trial of 1000 surveillance endoscopies in IBD, which showed that although DCE with targeted biopsies had a good diagnostic yield, adding a four-quadrant random biopsy protocol resulted in a non-negligible increase in detected lesions, amounting to 12.8% per patient with dysplasia.39 Furthermore, dysplasia detected by random biopsies was associated with a personal history of neoplasia, a tubular appearing colon and the presence of primary sclerosing cholangitis, all of which are known risk factors for CRC in the setting of IBD. This remains an area of some uncertainty, with conflicting results coming from well-designed studies. A randomized controlled trial, comparing a random-biopsy protocol to a targeted biopsy protocol with HD WLE but without DCE in all cases, showed that targeted biopsies are as effective and more cost-effective compared with random biopsies in detecting neoplasia.40 However, it should be noted that the study protocol included one random biopsy even in the targeted-biopsy arm, taken from the rectal mucosa, which is a renowned site for invisible dysplasia. By contrast, the study by Gasia et al.38 showed that targeted biopsies were superior to random biopsies in the detection of neoplasia in the setting of HD WLE, DCE, or VCE examinations, but not if standard definition WLE is used. It is interesting to note that the experts involved in the SCENIC Consensus (Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients: International Consensus Recommendations)35 did not agree on the biopsies strategy: 45% considered performing random biopsies when using high-definition white-light colonoscopy, whereas 60% disagreed with performing random biopsies when using chromoendoscopy. To further complicate matters, a recent study comparing the full spectrum endoscopy (FUSE) system to the conventional forward viewing scopes showed a significant increase in diagnostic yield for the FUSE system.41 The study used a randomized, back-to-back tandem endoscopy protocol, using either FUSE or conventional HD WLE as the first examination, followed by a switch to the other endoscopic modality coupled with DCE on the second retreat. This protocol was designed to allow comparison of detection rates both in white light and using chromoendoscopy for both treatment modalities, in an unbiased manner. Although the study is limited by the fact that it is a single-center experience with an additional potential bias being that endoscopists cannot be blinded to the study intervention, the findings however prompt further investigation into the potential role of this new technology for surveillance in IBD.
Another important issue is whether surveillance guidelines are actually implemented in real-life practice and what is the preferred surveillance method among endoscopists. A survey among French gastroenterologists working in a private practice showed that only 40% of screening colonoscopies included chromoendoscopy, with random biopsies acquired as per ECCO guideline recommendations in only 70% of cases.42 The same survey from 2014 found out that only 10% of the colonoscopy reports included a standardized assessment of endoscopic disease activity, using one of the available endoscopic scores; although this aspect is not directly linked to the question of CRC screening, it nevertheless reflects on the overall quality of colonoscopies performed for IBD patients in this setting. An Australian survey showed that gastroenterologists were more likely than surgeons to adhere to IBD surveillance guidelines and were also more likely to use advanced imaging modalities in screening for dysplasia and CRC; however, less than 50% of respondents used DCE and only less than 10% used targeted biopsies, with a majority of respondents routinely taking between 10 and 30 random biopsies (52%).43
The bottom line for evaluating the impact of any screening program is whether, overall, the benefits for the patient population outweigh the inherent risks of invasive screening maneuvers such as colonoscopy. A very long-term study of a surveillance program in a well-defined catchment area in Sweden demonstrated that although CRC incidence is increased in this target population, early detection of CRC and dysplasia resulted in a very low CRC-associated mortality, with only one death from CRC in the entire study cohort of over 300 patients.44
The impact of differences in guideline recommendations with respect to follow-up intervals has been elegantly evaluated in a retrospective analysis of a Dutch surveillance cohort45 that demonstrated the trade-off between workload (i.e. number of examinations) and detection of advanced neoplastic lesions implied by using either the BSG or AGA guidelines. Although the affect on patient-related outcomes such as CRC-related mortality or morbidity is not quantified, the study highlights the complexity involved in opting for a particular surveillance strategy.
Conclusion
Endoscopy is increasingly being used in the management of IBD patients, both for diagnostic and therapeutic purposes. Establishing the right diagnosis and performing an accurate assessment of disease extent and severity by ileocolonoscopy remains the mainstay of therapeutic decision-making in many cases. High-definition endoscopy, VCE, DCE, and CE have already been incorporated in real-life practice with the aim of better characterizing disease extent and activity and allow accurate surveillance of dysplasia in these high-risk patients. However, there is an ongoing debate regarding the best examination method and appropriate follow-up intervals for colorectal cancer screening and surveillance, which is reflected in significant variations between available guidelines. Furthermore, implementation of guideline recommendations remains suboptimal in many areas, probably reflecting a discrepancy in training and practice between referral centers, driving the research and innovations in this field and endoscopists who work outside these high-volume centers.
It is the authors’ view that, in light of current data and recommendations, a practical approach to CRC screening could incorporate a combination of dye-based HD WLE with targeted biopsies, reserving the use of VCE for the characterization of detected lesions (Figure 2). However, we acknowledge the fact that in a real-life setting, the chosen examination method and biopsy protocol is still determined by the available imaging techniques and local experience.
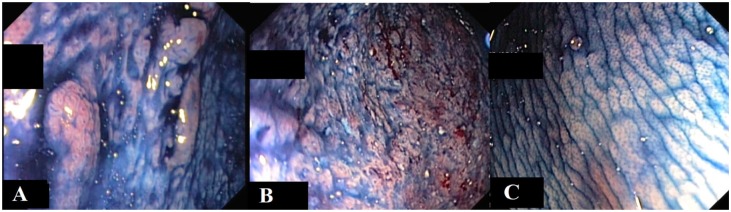
Figure 2.
Dye-based chromoendoscopy.
(A) Visible lesion with low-grade dysplasia. (B) Severe active inflammation, no dysplasia. (C) Normal colonic mucosa.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declare that there is no conflict of interest.
Ethical approval: Ethics approval was not required for this review.
Informed consent: Informed consent was not required for this review; all patients signed an informed consent regarding the anonymous use of endoscopic images.
ORCID iD: Lucian Negreanu  https://orcid.org/0000-0003-3042-0754
https://orcid.org/0000-0003-3042-0754
Contributor Information
Lucian Negreanu, University Hospital Bucharest, Gastroenterology Department, Splaiul Independentei Nr. 169, Sector 5, Bucharest, Bucuresti, Romania.
Theodor Voiosu, Colentina Clinical Hospital, Carol Davila University of Medicine and Pharmacy, Bucuresti, Romania.
Monica State, Colentina Clinical Hospital, Carol Davila University of Medicine and Pharmacy, Bucuresti, Romania.
Andrei Voiosu, Colentina Clinical Hospital, Carol Davila University of Medicine and Pharmacy, Bucuresti, Romania.
Andreea Bengus, Colentina Clinical Hospital, Carol Davila University of Medicine and Pharmacy, Bucuresti, Romania.
Bogdan Radu Mateescu, Colentina Clinical Hospital, Carol Davila University of Medicine and Pharmacy, Bucuresti, Romania.
References
- 1. Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 1955; 2: 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019; 13: 144–164. [DOI] [PubMed] [Google Scholar]
- 3. Molinie F, Gower-Rousseau C, Yzet T, et al. Opposite evolution in incidence of Crohn’s disease and ulcerative colitis in Northern France (1988-1999). Gut 2004; 53: 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wagtmans MJ, van Hogezand RA, Griffioen G, et al. Crohn’s disease of the upper gastrointestinal tract. Neth J Med 1997; 50: S2–S7. [DOI] [PubMed] [Google Scholar]
- 5. Haskell H, Andrews CW, Reddy SI, et al. Pathologic features and clinical significance of “backwash” ileitis in ulcerative colitis. Am J Surg Pathol 2005; 29: 1472–1481. [DOI] [PubMed] [Google Scholar]
- 6. Dionisio PM, Gurudu SR, Leighton JA, et al. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol 2010; 105: 1240–1248; quiz 9. [DOI] [PubMed] [Google Scholar]
- 7. Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011; 141: 1194–1201. [DOI] [PubMed] [Google Scholar]
- 8. Froslie KF, Jahnsen J, Moum BA, et al. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology 2007; 133: 412–422. [DOI] [PubMed] [Google Scholar]
- 9. Pouillon L, Ferrante M, Van Assche G, et al. Mucosal healing and long-term outcomes of patients with inflammatory bowel diseases receiving clinic-based vs trough concentration-based dosing of infliximab. Clin Gastroenterol Hepatol 2018; 16: 1276–1283.e1. [DOI] [PubMed] [Google Scholar]
- 10. Samaan MA, Pavlidis P, Digby-Bell J, et al. Golimumab: early experience and medium-term outcomes from two UK tertiary IBD centres. Frontline Gastroenterol 2018; 9: 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. FDA’s Good guidance practices regulation. Federal register/rules and regulations. 2000; 65: 182. [Google Scholar]
- 12. Mohammed VN, Samaan M, Mosli MH, et al. Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst Rev 2018; 1: Cd011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khanna R, Nelson SA, Feagan BG, et al. Endoscopic scoring indices for evaluation of disease activity in Crohn’s disease. Cochrane Database Syst Rev 2016; 8: Cd010642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Colombel JF, Ordas I, Ullman T, et al. Agreement between rectosigmoidoscopy and colonoscopy analyses of disease activity and healing in patients with ulcerative colitis. Gastroenterology 2016; 150: 389–395.e3. [DOI] [PubMed] [Google Scholar]
- 15. Kato J, Kuriyama M, Hiraoka S, et al. Is sigmoidoscopy sufficient for evaluating inflammatory status of ulcerative colitis patients? J Gastroenterol Hepatol 2011; 26: 683–687. [DOI] [PubMed] [Google Scholar]
- 16. Annese V, Daperno M, Rutter MD, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis 2013; 7: 982–1018. [DOI] [PubMed] [Google Scholar]
- 17. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis 2017; 11: 769–784. [DOI] [PubMed] [Google Scholar]
- 18. Sandborn WJ. The present and future of inflammatory bowel disease treatment. Gastroenterol Hepatol (NY) 2016; 12: 438–441. [PMC free article] [PubMed] [Google Scholar]
- 19. Kingsley MJ, Abreu MT. A personalized approach to managing inflammatory bowel disease. Gastroenterol Hepatol (NY) 2016; 12: 308–315. [PMC free article] [PubMed] [Google Scholar]
- 20. Oh EH, Oh K, Han M, et al. Early anti-TNF/immunomodulator therapy is associated with better long-term clinical outcomes in Asian patients with Crohn’s disease with poor prognostic factors. PLoS One 2017; 12: e0177479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Im JP, Ye BD, Kim YS, et al. Changing treatment paradigms for the management of inflammatory bowel disease. Korean J Intern Med 2018; 33: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gionchetti P, Dignass A, Danese S, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 2: surgical management and special situations. J Crohns Colitis 2017; 11: 135–149. [DOI] [PubMed] [Google Scholar]
- 23. Marteau P, Laharie D, Colombel JF, et al. Interobserver variation study of the rutgeerts score to assess endoscopic recurrence after surgery for Crohn’s disease. J Crohns Colitis 2016; 10: 1001–1005. [DOI] [PubMed] [Google Scholar]
- 24. Campbell JP, Vaughn BP. Optimal delivery of follow-up care after surgery for Crohn’s disease: current perspectives. Clin Exp Gastroenterol 2016; 9: 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferrante M, Papamichael K, Duricova D, et al. Systematic versus endoscopy-driven treatment with azathioprine to prevent postoperative ileal Crohn’s disease recurrence. J Crohns Colitis 2015; 9: 617–624. [DOI] [PubMed] [Google Scholar]
- 26. Chongthammakun V, Fialho A, Fialho A, et al. Correlation of the rutgeerts score and recurrence of Crohn’s disease in patients with end ileostomy. Gastroenterol Rep (Oxf) 2017; 5: 271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Min SB, Le-Carlson M, Singh N, et al. Video capsule endoscopy impacts decision making in pediatric IBD: a single tertiary care center experience. Inflamm Bowel Dis 2013; 19: 2139–2145. [DOI] [PubMed] [Google Scholar]
- 28. Rimbas M, Negreanu L, Ciobanu L, et al. Usefulness of flexible spectral imaging color enhancement (FICE) in the evaluation of subtle small bowel ulcerative mucosal lesions detected by videocapsule endoscopy. Endosc Int Open 2016; 4: E508–E514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rondonotti E. Capsule retention: prevention, diagnosis and management. Ann Transl Med 2017; 5: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Allez M, Lemann M, Bonnet J, et al. Long term outcome of patients with active Crohn’s disease exhibiting extensive and deep ulcerations at colonoscopy. Am J Gastroenterol 2002; 97: 947–953. [DOI] [PubMed] [Google Scholar]
- 31. Kakkar A, Wasan SK, Farraye FA. Targeting mucosal healing in Crohn’s disease. Gastroenterol Hepatol (NY) 2011; 7: 374–380. [PMC free article] [PubMed] [Google Scholar]
- 32. Kaminski MF, Hassan C, Bisschops R, et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy 2014; 46: 435–449. [DOI] [PubMed] [Google Scholar]
- 33. Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010; 59: 666–689. [DOI] [PubMed] [Google Scholar]
- 34. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and Ileo-anal pouch disorders. J Crohns Colitis 2017; 11: 649–670. [DOI] [PubMed] [Google Scholar]
- 35. Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc 2015; 81: 489–501.e26. [DOI] [PubMed] [Google Scholar]
- 36. Shergill AK, Lightdale JR, Bruining DH, et al. The role of endoscopy in inflammatory bowel disease. Gastrointest Endosc 2015; 81: 1101–1121.e1–13. [DOI] [PubMed] [Google Scholar]
- 37. Mooiweer E, van der Meulen-de JAE, Ponsioen CY, et al. Chromoendoscopy for surveillance in inflammatory bowel disease does not increase neoplasia detection compared with conventional colonoscopy with random biopsies: results from a large retrospective study. Am J Gastroenterol 2015; 110: 1014–1021. [DOI] [PubMed] [Google Scholar]
- 38. Gasia MF, Ghosh S, Panaccione R, et al. Targeted biopsies identify larger proportions of patients with colonic neoplasia undergoing high-definition colonoscopy, dye chromoendoscopy, or electronic virtual chromoendoscopy. Clin Gastroenterol Hepatol 2016; 14: 704–712.e4. [DOI] [PubMed] [Google Scholar]
- 39. Moussata D, Allez M, Cazals-Hatem D, et al. Are random biopsies still useful for the detection of neoplasia in patients with IBD undergoing surveillance colonoscopy with chromoendoscopy? Gut 2018; 67: 616–624. [DOI] [PubMed] [Google Scholar]
- 40. Watanabe T, Ajioka Y, Mitsuyama K, et al. Comparison of targeted vs random biopsies for surveillance of ulcerative colitis-associated colorectal cancer. Gastroenterology 2016; 151: 1122–1130. [DOI] [PubMed] [Google Scholar]
- 41. Leong RW, Ooi M, Corte C, et al. Full-spectrum endoscopy improves surveillance for dysplasia in patients with inflammatory bowel diseases. Gastroenterology 2017; 152: 1337–1344.e3. [DOI] [PubMed] [Google Scholar]
- 42. Duchesne C, Faure P, Kohler F, et al. Management of inflammatory bowel disease in France: a nationwide survey among private gastroenterologists. Dig Liver Dis 2014; 46: 675–681. [DOI] [PubMed] [Google Scholar]
- 43. Leong RW, Perry J, Campbell B, et al. Knowledge and predictors of dysplasia surveillance performance in inflammatory bowel diseases in Australia. Gastrointest Endosc 2015; 82: 708–714.e4. [DOI] [PubMed] [Google Scholar]
- 44. Rutegård M, Palmqvist R, Stenling R, et al. Efficiency of colorectal cancer surveillance in patients with ulcerative colitis: 38 years’ experience in a patient cohort from a defined population area. Scand J Surg 2016; 106: 133–138. [DOI] [PubMed] [Google Scholar]
- 45. Mooiweer E, van der Meulen AE, van Bodegraven AA, et al. Neoplasia yield and colonoscopic workload of surveillance regimes for colorectal cancer in colitis patients: a retrospective study comparing the performance of the updated AGA and BSG guidelines. Inflamm Bowel Dis 2013; 19: 2603–2610. [DOI] [PubMed] [Google Scholar]