Abstract
Diabetic kidney disease (DKD) remains the main cause for chronic kidney disease (CKD) and end-stage kidney disease (ESKD) worldwide. Both CKD and ESKD lead to major increases in risk of cardiovascular disease and death in people with diabetes. Despite optimal management of lifestyle, glucose levels and hypertension, residual risk remains high, indicating that additional therapies to mitigate the burden of the disease are desired. In past decades, new treatment options for the management of diabetes have emerged, of which some have showed promising renoprotective potential. This review discusses current understanding of the renal effects of glucagon-like peptide receptor agonists and their potential use in prevention and treatment of DKD.
Keywords: albuminuria, blood pressure, diabetic kidney disease, GLP-1 receptor agonists, incretin-based therapies
Diabetic kidney disease
Diabetic kidney disease (DKD), characterized by reduced whole-kidney glomerular filtration rate (GFR) or urinary protein leakage, is a feared complication of diabetes. Type 2 diabetes (T2D) has become the main cause for chronic kidney disease (CKD) and end-stage kidney disease (ESKD) worldwide, largely because of the pandemic increase in numbers of obesity. Approximately 33% of patients will finally need renal replacement therapy (RRT).1 Many people with DKD, however, do not reach ESKD due to competing risks of premature mortality by cardiovascular disease (CVD).2
The pathogenesis of DKD, especially in people with T2D, is complex and multifactorial, with several proposed contributing pathogenic factors. As such, chronic hyperglycemia may contribute to glomerular (and tubular) damage through induction of hyperglycemia-related inflammation and by formation of advanced glycation end products. Moreover, uncontrolled systemic hypertension may further impair glomerular structure and function. Furthermore, it is proposed that glomerular hypertension, due to an imbalance in regulation of afferent and efferent arteriolar pressure, is detrimental to glomerular function.3 Finally, obesity per se, insulin resistance, dyslipidemia and increased uric acid levels, highly prevalent in people with T2D, have been identified as risk factors in the development of DKD.4,5 Given these various pathogenic drivers, it is evident that the prevention or delay of the progression of DKD requires a multifactorial approach. This is illustrated by clinical practice guidelines that advocate lifestyle interventions including smoking cessation and weight loss, as well as dietary sodium and protein restriction. Pharmacological interventions strive to improve glycemic control, reduce blood pressure, particularly through blockers of the renin–angiotensin system (RAS), and to target dyslipidemia.2 These measures have indeed led to a better outcome for people with DKD. However, as shown in the STENO-2 trial, residual renal risk remains elevated,6 which, to a certain extent, is caused by the low number of patients able to reach the treatment goals for all individual risk factors.7 Thus, the continuous search for novel therapeutic agents that prevent the onset and progression of DKD warrants continuous investigation.
Glucagon-like peptide (GLP)-1 receptor agonists
In the last decade, a novel group of glucose-lowering agents has been developed based on the gut hormone glucagon-like peptide-1 (GLP-1).8 GLP-1 is a hormone secreted by the gastrointestinal L-cells upon food ingestion and its levels are acutely and transiently raised after a meal. GLP-1 enhances insulin secretion, while reducing glucagon release from the pancreatic islet cells with a reduction in postprandial glucose levels as the net result. On top of pancreatic effects, GLP-1 release slows down gastric emptying and small intestinal peristalsis, suppresses hepatic glucose production and induces satiety, resulting in lower food intake, all contributing to improved glycemic control and a reduction of body weight.9,10 In people with T2D, circulating levels of GLP-1 are similar to those in normoglycemic individuals, however, there is resistance to its effects.8 Interestingly, this may be overcome by raising GLP-1 to pharmacological levels, making GLP-1 an attractive target for glucose-lowering therapy.11 Because of the short half-life (2–3 min) of GLP-1 due to cleavage by the ubiquitous enzyme dipeptidyl-peptidase (DPP)-4, DPP-4-resistant GLP-1 receptor agonists were developed for clinical application.10,12
Several GLP-1 receptor agonists have been introduced for the treatment of T2D (Table 1). The compounds can be classified according to structure (based on a human GLP-1 backbone versus exendin-4-based compounds) and according to duration of action (short- versus long-acting GLP-1 receptor agonists).12 Exenatide and lixisenatide have an exendin-4 backbone and are short-acting GLP-1 receptor agonists with a half-life of 2–4 h. Several compounds based on human GLP-1 have been developed, which, through modification, are not cleaved by DPP-4 and have delayed renal elimination, resulting in half-lives up to a week (Table 1).12
Table 1.
Current GLP-1 receptor agonists and recommendations in patients with renal impairment.
| Agent | Dose | Half-life (h) | Elimination | Recommendations in patients with renal impairment |
||
|---|---|---|---|---|---|---|
| Mild | Moderate | Severe or ESKD | ||||
| Short-acting GLP-1 receptor agonists | ||||||
| Exenatide | 5–10 μg BID | 2.4 | Mainly renal | No adjustment | Conservative dose escalation | Not recommended |
| Lixisenatide | 10–20 μg QD | 3.0 | Mainly renal | No adjustment | No adjustment | Not recommended |
| Long-acting GLP-1 receptor agonists | ||||||
| Exenatide | 2 mg QW | N/A | Mainly renal | No adjustment | Not recommended | Not recommended |
| Liraglutide | 0.6 mg, 1.2 mg or 1.8 mg QD | 11.6–13.0 | Peptidases, renal and feces | No adjustment | No adjustment | Not recommended eGFR <15 ml/min |
| Albiglutide | 30–50 mg QW | ~120.0 | Peptidases and renal | No adjustment | No adjustment | Not recommended |
| Dulaglutide | 0.75–1.5 mg QW | ~112.8 | Peptidases and renal | No adjustment | No adjustment | Not recommended |
| Semaglutide | 0.5–1.0 mg QW | 165.0–184.0 | Peptidases and renal | Unknown | Unknown | Unknown |
Adapted from Muskiet et al.5
Mild renal impairment indicates creatinine clearance of 50/60–89 ml/min; moderate 30–50/60 ml/min; severe <30 ml/min or ESKD.
BID, twice daily, eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease, GLP-1, glucagon-like peptide 1; N/A, not available; QD, once daily; QW, once weekly.
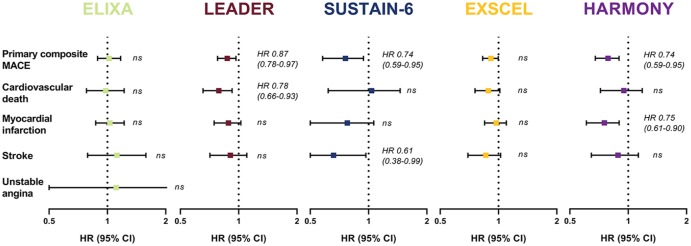
GLP-1 receptor agonists are frequently prescribed for several reasons. First, they have strong glucose-lowering potential [about 1–2% glycated hemoglobin (HbA1c) reduction], in particular in the once-weekly formulations.13 Second, they improve various components of the metabolic syndrome with significant reductions in body weight, systolic blood pressure and lipid levels, including triglycerides and low-density lipoprotein cholesterol.8,9 Third, in large cardiovascular safety trials, GLP-1 receptor agonists have been shown to reduce the primary endpoint of 3-point major adverse cardiovascular events (MACE), a composite of nonfatal myocardial infarction, nonfatal stroke and cardiovascular death (Figure 1).14 This has led to the recommendation in position statements of the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) that GLP-1 receptor agonists are the first choice for glucose lowering in people with T2D and established atherosclerotic cardiovascular disease after metformin.15
Figure 1.
Cardiovascular outcome studies with GLP-1 receptor agonists.46,47,49,51,53
Reported outcomes are hazard ratios and corresponding 95% confidence intervals. The primary Major Adverse Cardiovascular Events (MACE) composite of ELIXA was a 4-point MACE death from cardiovascular (CV) causes, nonfatal stroke, nonfatal myocardial infarction (MI), or unstable angina. EXSCEL, LEADER, HARMONY and SUSTAIN-6 had a 3-point MACE death from CV causes, nonfatal MI, or nonfatal stroke. The heterogeneity in Harmony Outomes of these trials may be due to the heterogeneity in study design (e.g. follow-up time, study population) and therefore the observed differences between agents must be interpreted cautiously. There is currently no evidence that supports the view that differences in GLP-1 backbone might explain differences in CV outcome.
CI, confidence interval; GLP-1, glucagon-like peptide 1; HR, hazard ratio; ns, nonsignificant.
Renal tubular effects of GLP-1 receptor agonists
Through their salutary effects on many identified renal risk factors like hyperglycemia, hypertension, obesity, and dyslipidemia, GLP-1 receptor agonists could favorably affect renal outcomes in people with T2D. On top of this, GLP-1 may also directly affect renal tubular function. GLP-1 infusion was shown to increase natriuresis and diuresis in rats.5,16 This was confirmed in studies in healthy volunteers,17 insulin-resistant obese males,18 and people with T2DM.19 GLP-1 receptor agonists showed similar tubular effects as native GLP-1, on acute intravenous infusion of exenatide in healthy males20 and subjects with T2D.21 In addition, a single subcutaneous injection of liraglutide in people with T2D,22 increased both absolute and fractional sodium excretion. Most studies were of very short duration and it seems that this natriuretic effect disappears after prolonged exposure.23 This loss of natriuretic effect is compatible with the GLP-1 receptor desensitization that has been observed for other regulatory systems after prolonged treatment with long-acting GPL-1 receptor agonists. Alternatively, compensation by other renal sodium transporters prevents further renal sodium loss. On the other hand, data from our group suggest sustained natriuretic effects, for up to 8 weeks of treatment, of the short-acting GLP-1 receptor agonist lixisenatide in insulin–glargine-treated patients with T2D. This may reflect preserved efficacy caused by intermittent GLP-1 receptor stimulation.24
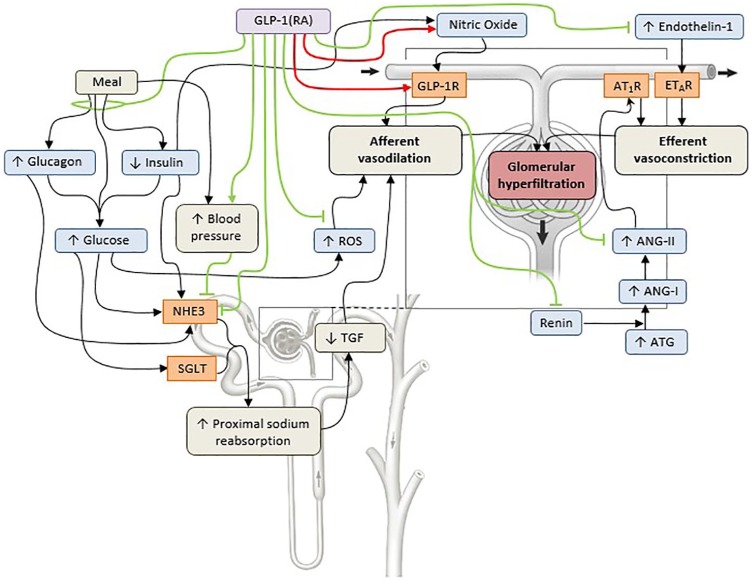
The mechanism of GLP-1 (receptor agonist)-induced sodium excretion is unclear, especially because it is debated whether a GLP-1 receptor is indeed present in the kidney. Studies suggest that GLP-1 receptors are present in afferent arterioles and juxtaglomerular cells in humans but this has also been refuted.5 The lack of an antibody with high sensitivity and specificity has hampered mapping of the GLP-1 receptor in the kidney.5 However, based on available data, the most likely involved tubular transporter mediating the natriuretic effects of GLP-1 is the sodium–hydrogen antiporter NHE3 which is located at the brush border of the proximal tubule, bound to a complex that also contains DPP-4. Indeed, pharmacological doses of GLP-1 or GLP-1 receptor agonists phosphorylate NHE3 at the protein kinase A consensus sites Ser552 and Ser605, thereby reducing its activity.25 Interestingly, phosphorylation of NHE3 could be prevented by GLP-1 receptor blockade. In humans with or without diabetes, acute GLP-1 receptor agonist infusion increases renal lithium clearance (a marker of proximal tubular sodium reabsorption)17,22 and urinary pH supporting the notion that NHE3 in the proximal tubule is involved.17,18,20–22 Notably, in a recent trial, increased NHE3 phosphorylation at site Ser552 was observed in lixisenatide- compared with the insulin–glulisine-treated individuals.24 Another factor involved in GLP-1 receptor agonist-mediated natriuresis in the acute setting could be pressure-induced natriuresis. NHE3 is rearranged and consequently deactivated in response to an increase in blood pressure.26 GLP-1 receptor agonists acutely increase blood pressure through incompletely understood mechanisms.4,5 Moreover, natriuresis could be secondary to changes in factors with a role in glucose metabolism, including insulin, glucagon, adenosine triphosphate and glucose itself, which are known to regulate NHE3 and sodium–glucose transporters (SGLTs) in the kidney, and though changes in the RAS (Figure 2).
Figure 2.
Hypothesized renal vascular and tubular effects of GLP-1 receptor agonists in diabetes.
Numerous vascular and tubular pathways are involved in fasting and postprandial renal hemodynamics in the setting of diabetes (indicated by the black lines). These pathways result in a net reduction in afferent renal arteriolar resistance (afferent vasodilation) or a net increase in efferent renal arteriolar resistance (efferent vasoconstriction), thus increasing glomerular hydraulic pressure and single-nephron glomerular filtration rate, leading to glomerular hyperfiltration.3 GLP-1RAs are associated with direct GLP-1R-mediated vasodilation and at least partly with indirect nitric-oxide-dependent afferent vasodilation (indicated by the red lines), which might enhance glomerular hyperfiltration.20 In contrast, GLP-1RAs inhibit several pathways (indicated by the green lines) that might underlie the glomerular hyperfiltration in diabetes.4,5 GLP-1 RAs induce natriuresis, possibly by inhibiting NHE3 activity, either directly or indirectly through a (postprandial) vasopressor response. NHE3 could also be inhibited through reduced levels of postprandial (a) glucagon, leading to inhibition of NHE3 in thick ascending limb27,28 and potentially in the proximal tubule;28,29 (b) insulin, leading to inhibition of NHE3 in the distal convoluted tubule;30 or (c) glucose, leading to downregulation and inhibition of NHE3 and SGLTs 1 and 2.4,5 Inhibition of sodium reabsorption in the proximal tubule increases the sodium concentration at the level of the macula densa, which consequently restores the impaired TGF.3–5 GLP-1RAs might reduce circulating levels and postreceptor actions of components of the RAS,17,19,22 although these findings could not be confirmed by others.21,23,24,31,32 GLP-1RAs might also suppress endothelin-1 expression by inhibiting the phosphorylation of nuclear factor-kB.33 Finally, GLP-1RAs activate several anti-inflammatory pathways, including reductions in oxidative stress via reactive oxygen species.34 The net effect of GLP-1RAs on renal hemodynamics is thus the result of a balance between stimulation of afferent vasodilation and inhibition of pathways that cause efferent vasoconstriction, leading to mostly neutral effects on renal hemodynamics.
ANG-I, angiotensin I; ANG-II, angiotensin II; ATG, angiotensinogen; AT1R, angiotensin-1 receptor; ETAR, endothelin receptor; GLP-1R, GLP-1 receptor; GLP-1RA, GLP-1 receptor agonist; NHE3, sodium–hydrogen exchanger isoform 3; ROS, reactive oxygen species; SGLT, sodium–glucose cotransporter; RAS, renin–angiotensin system; TGF, tubuloglomerular feedback.
Renal glomerular effects of GLP-1 receptor agonists
It has been proposed that the abovementioned tubular effects on sodium handling by GLP-1 receptor agonists could also change glomerular hemodynamics by effecting tubuloglomerular feedback (Figure 2). Blocking sodium uptake in the proximal tubule leads to more sodium delivery to the macula densa, which in turn activates tubuloglomerular feedback, resulting in afferent arteriolar vasoconstriction and a reduction in GFR. This was suggested by a study by Gutzwiller and colleagues,18 where creatinine clearance was reduced in hyperfiltering obese individuals. In addition, in an open-label, uncontrolled study, liraglutide was associated with an acute reduction in eGFR (paralleled by a reduction in albuminuria) and subsequent stabilization over time, which was reversible after a 3-week washout of the drug, suggesting a renal hemodynamic effect.35 Together with several case reports relating GLP-1 receptor agonist treatment of acute kidney injury, the renal safety of GLP-1 RA treatment was doubted.36–38
In the following years, several studies of renal hemodynamics have been performed with GLP-1 and GLP-1 receptor agonists using state-of-the-art inulin and para-aminohippuric acid (PAH) clearance techniques to measure GFR and effective renal plasma flow (ERPF), respectively. In healthy individuals, acute GLP-1 infusion did not alter GFR or ERPF,17,19 however, exenatide infusion increased GFR, ERPF and estimated glomerular hydraulic pressure in overweight, but otherwise healthy, men.20 This increment in GFR and ERPF seemed to be caused by a reduction in estimated afferent renal arteriolar resistance, which was, at least in part, dependent on nitric oxide (NO) availability.20
The situation may be different in people with T2D. Although initial reports suggested reductions of eGFR in people with T2D treated with liraglutide in an open-label study,35,39 this could not be confirmed in carefully designed subsequent studies. In randomized clinical trials of 12–30 weeks’ duration, GLP-1 receptor agonist treatment did not influence initial changes in or the slope of eGFR in people with T2DM with40 or without renal impairment.41,42
Moreover, acute administration of GLP-1,19 exenatide21 or liraglutide,22 or 12 weeks of liraglutide treatment23 did not affect fasting renal hemodynamics or estimated glomerular pressure in people with T2D and presumed single-nephron hyperfiltration (i.e. filtration fraction of ~25% in the setting of GFR > 60 ml/min/1.73 m2). Also, 12 weeks of liraglutide versus placebo in albuminuric patients with T2D did not significantly affect GFR.43 Finally, 8 weeks of lixisenatide treatment versus insulin–glulisine, did not alter postprandial renal hemodynamics in people with T2D.24
In conclusion, GLP-1 receptor agonists seem to induce glomerular hyperfiltration under physiological conditions by reducing afferent arteriolar resistance, but do not significantly alter renal hemodynamic function in people with T2D and normal kidney function, who may be more resistant to the direct, partly NO-mediated, vasodilator effect of GLP-1 receptor agonists. All in all, there is insufficient evidence to support GLP-1 receptor agonists ameliorating hyperfiltration, in analogy to SGLT2 inhibitors.44,45
Renal analyses from large cardiovascular safety trials
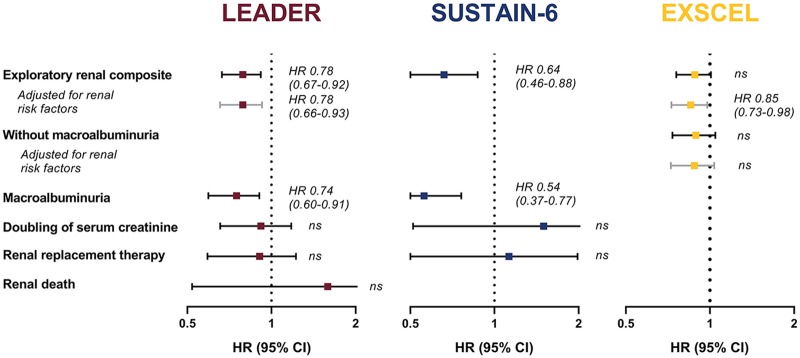
Currently, four cardiovascular safety trials of GLP-1 receptor agonists have reported renal outcomes as exploratory endpoints (Figure 3). The first safety trial reported was the ELIXA (Evaluation of LIXisenatide in Acute coronary syndrome) trial,46 which investigated the short-acting GLP-1 receptor agonist lixisenatide versus placebo in 6026 people with T2D and an acute coronary event less than 180 days before screening. In a prespecified analysis, a modest difference in percentage change of urinary albumin-to-creatinine ratio (UACR) was found in favor of lixisenatide after 108 weeks of treatment (24% versus 34%; p = 0.004). Post hoc adjustment for small differences in HbA1c levels (about 0.3%) diminished the UACR difference, which indicated some glucose dependency.46 The risk of new-onset macroalbuminuria of lixisenatide compared with placebo was reduced, with similar point estimates when adjusted for baseline characteristics such as age, blood pressure and eGFR, as well as on-trial changes in HbA1c, blood pressure and weight. Lixisenatide did not change eGFR decline; neither in the overall population nor in any UACR subgroup during the study. It should be noted that only 23% of the study population of ELIXA had a baseline eGFR < 60 ml/min/1.73 m2. The incidence of hard renal endpoints (ESKD, renal death) or surrogates (doubling of serum creatinine) was therefore too low to be investigated.
Figure 3.
Exploratory renal outcomes and their individual components in cardiovascular outcome trials.
LEADER reported a composite renal outcome that consisted of new-onset persistent macroalbuminuria, persistent doubling of serum creatinine and an estimated GFR below 45 ml/min/1.73 m2, need for renal replacement therapy, or renal death. Post hoc adjustments were made for change from baseline in HbA1c, body weight and systolic blood pressure. SUSTAIN-6 reported a composite renal outcome that consisted of new-onset persistent macroalbuminuria, persistent doubling of serum creatinine and an estimated GFR below 45 ml/min/1.73 m2 and need for renal replacement therapy. No data showing adjustments for renal risk factors are available. EXSCEL reported two composite renal outcomes. The first consisted of 40% estimated GFR decline, need for renal replacement therapy, or renal death. The second consisted of new-onset macroalbuminuria, 40% estimated GFR decline, need for renal replacement therapy, or renal death and is depicted here. Adjustment was made for renal risk factors age, sex, ethnicity, race, region, duration of diabetes, prior history of CV event, insulin use, baseline HbA1c, estimated GFR and BMI.
BMI, body mass index; CV, cardiovascular; GFR, glomerular filtration rate; HbA1c, glycated hemoglobin; HR, hazard ratio; CI, confidence interval; ns, nonsignificant.
The two subsequent safety trials were LEADER (Liraglutide Effects and Action in Diabetes: Evaluation of cardiovascular outcome Results)47,48 and SUSTAIN-6 (Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes),49 investigating respectively liraglutide and semaglutide versus placebo in 9340 and 3297 people with T2D and high cardiovascular risk. In contrast to ELIXA, these trials both reported a prespecified renal outcome of new or worsening nephropathy, which was a composite of mostly adjudicated events defined as new-onset or persistent macroalbuminuria, persistent doubling of serum creatinine and eGFR ⩽ 45 ml/min/1.73m2, need for RRT, and renal death (Figure 3).48,49 Liraglutide reduced this renal composite by 22% after 3.8 years [95% (CI) 8–33%; p = 0.003] of treatment, whereas semaglutide showed a reduction of 36% after 2.1 years (95% CI 12–54%; p = 0.005). Importantly, these reductions were predominantly driven by reductions in macroalbuminuria with reductions of, respectively, 26% and 46%, and no effect was seen on hard renal endpoints. Since the mean differences in HbA1c levels between treatment arms in LEADER and SUSTAIN-6 of ~0.6% and ~1.1% were even larger than in ELIXA, an influencing role for glucose could initially not be ruled out. In this regard, it was reassuring that the authors of the LEADER trial convincingly showed that renoprotective effects are not dependent on glucose levels, since the outcome remained more or less the same after adjustment for improvements in renal risk factors such as HbA1c, blood pressure, and body weight.50 LEADER also reported eGFR trajectories, showing a 2% slower decline in the liraglutide group than in the placebo group.48 Subgroup analyses indicated that this effect was the strongest in patients with moderate (eGFR 30–59 ml/min/1.73 m2) or severe (eGFR < 30 ml/min/1.73 m2) reduced renal function at baseline. Although the renal beneficial effects of GLP-1 receptor agonists were particularly apparent in conjunction with a decline in renal function, the number of patients with CKD greater than stage 4 was small. It is important to stress that in all three trials ELIXA, LEADER and SUSTAIN, the use of the lixisenatide, liraglutide and semaglutide, respectively, was safe; also in patients with CKD that were included in the trial.46,47,49
The following cardiovascular safety trial EXSCEL (EXenatide Study of Cardiovascular Event Lowering) investigated 14,752 people with T2D, 73% of whom had previous CVD.51 The results of the renal analysis have not been reported yet, but were presented at the ADA 2018 78th Scientific Sessions.52 As was the case with LEADER and SUSTAIN-6, hard renal outcome was unaffected by exenatide once weekly versus placebo, as the renal composite of 40% eGFR decline, RRT, and renal death did not differ between treatment arms. When new-onset macroalbuminuria was added to the composite, exenatide once weekly reduced the composite by 15% (95% CI 2–26%; p = 0.03). However, when analyzed as an individual component, the reduction of 16% in new-onset macroalbuminuria did not reach significance (95% CI 0.67–1.04; p = 0.11). eGFR levels over time also did not differ significantly between exenatide once weekly and placebo.
The recently published safety trial Harmony Outcomes (albiglutide and cardiovascular outcomes in patients with T2D and CVD)53 only reported that albiglutide does not substantially change eGFR after 16 months. The other safety trials with GLP-1 receptor agonists FREEDOM [ClinicalTrials.gov identifier: NCT01455896] and REWIND (Researching Cardiovascular Events With a Weekly Incretin in Diabetes) [ClinicalTrials.gov identifier: NCT01394952], investigating, respectively, continuous subcutaneous delivery of exenatide and dulaglutide, have not yet reported a renal outcome analysis.
Studies in people with diabetic kidney disease
The effects of GLP-1 receptor agonists in people with CKD have not been addressed in dedicated outcome studies. However, some information can be extracted from the safety trials and specific renal studies. In a post hoc, prespecified, explanatory analysis of the ELIXA trial, effects of lixisenatide on UACR reductions were analyzed according to baseline albuminuria status.54 It was shown that lixisenatide reduced UACR in people with microalbuminuria at baseline [by −21% (95% CI −42% to 0%); p = 0.0502] and to a larger extent in people with macroalbuminuria at baseline [by −39% (95% CI −69% to −10%); p = 0.007], but not in those without albuminuria. Importantly, additional corrections for baseline or on-trial HbA1c, body weight, and blood pressure did not affect the results.
In the AWARD-7 trial, the renal effects of the long-acting once-weekly GLP-1 receptor agonist dulaglutide were studied.55 AWARD-7 randomly allocated 576 people with T2D and moderate-to-severe CKD to high- and low-dose dulaglutide or active comparator-titrated insulin glargine. All participants were additionally treated with insulin lispro as the rapid-acting insulin analog. Comparison of dulaglutide versus placebo resulted in a placebo-subtracted UACR reduction of 39% (95% CI 10–69%; p = 0.007) in the population with macroalbuminuria, but not in the population without macroalbuminuria. In addition, dulaglutide resulted in a smaller eGFR decline compared with titrated insulin glargine: the cystatin C-based eGFR decline of 52-week dulaglutide high dose was 0.7 ml/min/1.73 m2 and low dose was 0.7 ml/min/1.73m2 versus 3.3 ml/min/1.73 m2 with insulin glargine (both p < 0.05).55 This effect was not influenced by the reduction in body weight.56
Whether the reduction in albuminuria and smaller decline in eGFR indicate a renoprotective effect is unclear. A reduction in albuminuria usually translates to improvements of hard renal outcomes.3,57 For the small increments in eGFR, however, this is uncertain. Reduction of glomerular pressure, as achieved by RAS blockers or SGLT2 inhibitors, is characterized by a ‘dip’ in eGFR that stabilizes over time and a reduction in albuminuria.3 For the GLP-1 receptor agonists, however, as described, dedicated studies have either shown no change in renal hemodynamics or even increases in measured GFR. In AWARD-7, the higher eGFR after treatment, is due to an initial rise in eGFR in the macroalbuminuric patients using dulaglutide.55 In LEADER, a similar rise occurred with liraglutide in the moderate and severely renal impaired patients.48 An acute elevation in eGFR could theoretically indicate an increase in glomerular pressure, which could theoretically lead to a more rapid decline in renal function.58 The rate of decline in eGFR (slope of the curve), does not seem to be altered after GLP-1 receptor agonist treatment versus placebo. In this context, it should be stressed that in these patients with CKD, no increase in adverse renal events was noted versus placebo.46,48,49,51
Conclusions and future perspectives: moving from safety to renoprotection
After the introduction of GLP-1 receptor agonists, concerns were expressed regarding their renal safety, with proposed dangers of acute kidney injury. Now, more than a decade later, it seems fair to conclude that GLP-1 receptor agonists are safe to administer to people with T2D with or without DKD. Moreover, trials in people with T2D at high risk for CVD have consistently shown reductions in albuminuria. It is important to keep in mind that these effects were independent of improvements in renal risk factors such as hyperglycemia, body weight and blood pressure. Given the doubt whether the GLP-1 receptor is expressed in the kidney, and that no glomerular/renal hemodynamic effects were demonstrated for this drug class, it remains uncertain how GLP-1 receptor agonists reduce albuminuria. Proposed mechanisms include (a) a reduction of inflammation or oxidative stress; (b) improved insulin sensitivity; (c) augmented mitochondrial function; (d) beneficial effects of systemic molecules; and (e) direct effects of GLP-1 on the tubular cell, independent of a receptor-mediated mechanism.5 Note that all these proposed mechanisms, except for the direct effects on tubular cells, could support either an effect of GLP-1 receptor agonists on glomerular permselectivity to albumin, or an effect on tubular reabsorption of albumin.
It is too early to speculate on what role GLP-1 receptor agonists will play in the future treatment of DKD. The cardiovascular safety trials with GLP-1 receptor agonists were not designed to show benefit on hard renal outcomes due to included study populations, as well as the exploratory aspect of the renal endpoints. The trials show that GLP-1 receptor agonists consistently reduce albuminuria without affecting hard renal outcome; however, one should be careful not to draw premature conclusions. Soon, the FLOW trial (Semaglutide on the Progression of Renal Impairment in Subjects With Type 2 Diabetes and Chronic Kidney Disease) [ClinicalTrials.gov identifier: NCT03819153] will be initiated. In this trial, the effects of semaglutide in patients with albuminuria and declined eGFR will be studied for 5 years. The primary endpoint is persistent eGFR decline of >50%, reaching end-stage renal disease, death from kidney disease or death from cardiovascular disease. This trial will show whether GLP-1 receptor agonists can be a useful tool for treatment of DKD.
Which other developments potentially lie ahead? First, the opportunity to combine a GLP-1 receptor agonist with other (glucose lowering) drugs that may affect renal outcomes. What first comes to mind, is the combination with SGLT2 inhibitors. Since the potential renoprotective pathways are very likely dissimilar, combination therapy with SGLT2 inhibitors might result in additive or even synergistic beneficial effects on renal outcomes.59 This hypothesis deserves future study. Second, the impressive results of novel coagonists of glucose-dependent insulinotropic peptide and GLP-1 on body weight and glucose control suggest that these agents and other coagonist formulations impacting on the gut–renal axis could have a beneficial renal outcome.
Acknowledgments
The authors were fully responsible for all content and editorial decisions and were involved at all stages of manuscript development and have approved the final version.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: DHvR is supported by a research fellowship from the Dutch Diabetes Foundation and EU Marie Curie program.
Conflict of interest statement: MJBvB, ABvdA, KH and JAJ report no conflict of interest. HJLH serves as a consultant for AbbVie, Astellas, AstraZeneca, Boehringer Ingelheim, Fresenius, Gilead, Janssen, Merck, Mundi Pharma, and Mitsubishi Tanabe. DHvR has acted as a consultant and received honoraria from Boehringer Ingelheim and Lilly, Merck, Novo Nordisk and Sanofi and has received research operating funds from Boehringer Ingelheim-Lilly Diabetes Alliance, AstraZeneca and Novo Nordisk.
ORCID iD: Michaël J.B. van Baar  https://orcid.org/0000-0003-1516-2929
https://orcid.org/0000-0003-1516-2929
Contributor Information
Michaël J. B. van Baar, Department of Internal Medicine, Amsterdam University Medical Centers, VUMC, Amsterdam, The Netherlands
Annemarie B. van der Aart, Department of Clinical Pharmacy and Pharmacology, University of Groningen, Groningen, The Netherlands Department of Clinical Pharmacy, Martini Hospital, Groningen, The Netherlands.
Klaas Hoogenberg, Department of Internal Medicine, Martini Hospital, Groningen, The Netherlands.
Jaap A. Joles, Department of Nephrology and Hypertension, University Medical Center Utrecht, Utrecht, The Netherlands
Hiddo J. L. Heerspink, Department of Clinical Pharmacy and Pharmacology, University of Groningen, Groningen, The Netherlands
Daniël H. van Raalte, Amsterdam University Medical Centers, VUMC, De Boelelaan 1117, 1081 HV, Amsterdam, The Netherlands.
References
- 1. Thomas MC, Cooper ME, Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol 2016; 12: 73–81. [DOI] [PubMed] [Google Scholar]
- 2. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013; 382: 339–352. [DOI] [PubMed] [Google Scholar]
- 3. Tonneijck L, Muskiet MH, Smits MM, et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol 2017; 28: 1023–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Muskiet MH, Smits MM, Morsink LM, et al. The gut-renal axis: do incretin-based agents confer renoprotection in diabetes? Nat Rev Nephrol 2014; 10: 88–103. [DOI] [PubMed] [Google Scholar]
- 5. Muskiet MHA, Tonneijck L, Smits MM, et al. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol 2017; 13: 605–628. [DOI] [PubMed] [Google Scholar]
- 6. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348: 383–393. [DOI] [PubMed] [Google Scholar]
- 7. Muskiet MH, Tonneijck L, Smits MM, et al. Pleiotropic effects of type 2 diabetes management strategies on renal risk factors. Lancet Diabetes Endocrinol 2015; 3: 367–381. [DOI] [PubMed] [Google Scholar]
- 8. Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab 2016; 18: 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smits MM, van Raalte DH, Tonneijck L, et al. GLP-1 based therapies: clinical implications for gastroenterologists. Gut 2016; 65: 702–711. [DOI] [PubMed] [Google Scholar]
- 10. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696–1705. [DOI] [PubMed] [Google Scholar]
- 11. Zander M, Madsbad S, Madsen JL, et al. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 2002; 359: 824–830. [DOI] [PubMed] [Google Scholar]
- 12. Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012; 8: 728–742. [DOI] [PubMed] [Google Scholar]
- 13. Shyangdan DS, Royle P, Clar C, et al. Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev 2011; CD006423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. Epub ahead of print 6 December 2017. DOI: 10.1016/S2213-8587(17)30412-6. [DOI] [PubMed] [Google Scholar]
- 15. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41: 2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang-Christensen M, Larsen PJ, Goke R, et al. Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. Am J Physiol 1996; 271: R848-56. [DOI] [PubMed] [Google Scholar]
- 17. Skov J, Dejgaard A, Frokiaer J, et al. Glucagon-like peptide-1 (GLP-1): effect on kidney hemodynamics and renin-angiotensin-aldosterone system in healthy men. J Clin Endocrinol Metab 2013; 98: E664–E671. [DOI] [PubMed] [Google Scholar]
- 18. Gutzwiller JP, Tschopp S, Bock A, et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab 2004; 89: 3055–3061. [DOI] [PubMed] [Google Scholar]
- 19. Asmar A, Simonsen L, Asmar M, et al. Renal extraction and acute effects of glucagon-like peptide-1 on central and renal hemodynamics in healthy men. Am J Physiol Endocrinol Metab 2015; 308: E641–E649. [DOI] [PubMed] [Google Scholar]
- 20. Muskiet MH, Tonneijck L, Smits MM, et al. Acute renal haemodynamic effects of glucagon-like peptide-1 receptor agonist exenatide in healthy overweight men. Diabetes Obes Metab 2016; 18: 178–185. [DOI] [PubMed] [Google Scholar]
- 21. Tonneijck L, Smits MM, Muskiet MH, et al. Acute renal effects of the GLP-1 receptor agonist exenatide in overweight type 2 diabetes patients: a randomised, double-blind, placebo-controlled trial. Diabetologia 2016; 59: 1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skov J, Pedersen M, Holst JJ, et al. Short-term effects of liraglutide on kidney function and vasoactive hormones in type 2 diabetes: a randomized clinical trial. Diabetes Obes Metab 2016; 18: 581–589. [DOI] [PubMed] [Google Scholar]
- 23. Tonneijck L, Smits MM, Muskiet MH, et al. Renal effects of DPP-4 inhibitor sitagliptin or GLP-1 receptor agonist liraglutide in overweight patients with type 2 diabetes: a 12-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2016; 39: 2042–2050. [DOI] [PubMed] [Google Scholar]
- 24. Tonneijck L, Muskiet MHA, Smits MM, et al. Postprandial renal haemodynamic effect of lixisenatide versus once-daily insulin glulisine in type 2 diabetes patients on insulin-glargine: an 8-week, randomised, open-label trial. Diabetes Obes Metab 2017; 19: 1669–1680. [DOI] [PubMed] [Google Scholar]
- 25. Farah LX, Valentini V, Pessoa TD, et al. The physiological role of glucagon-like peptide-1 in the regulation of renal function. Am J Physiol Renal Physiol 2016; 310: F123–F127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McDonough AA, Leong PK, Yang LE. Mechanisms of pressure natriuresis: how blood pressure regulates renal sodium transport. Ann N Y Acad Sci 2003; 986: 669–677. [DOI] [PubMed] [Google Scholar]
- 27. Amemiya M, Kusano E, Muto S, et al. Glucagon acutely inhibits but chronically activates Na(+)/H(+) antiporter 3 activity in OKP cells. Exp Nephrol 2002; 10: 26–33. [DOI] [PubMed] [Google Scholar]
- 28. Bankir L, Bouby N, Blondeau B, et al. Glucagon actions on the kidney revisited: possible role in potassium homeostasis. Am J Physiol Renal Physiol 2016; 311: F469–F486. [DOI] [PubMed] [Google Scholar]
- 29. Marks J, Debnam ES, Dashwood MR, et al. Detection of glucagon receptor mRNA in the rat proximal tubule: potential role for glucagon in the control of renal glucose transport. Clin Sci (Lond) 2003; 104: 253–258. [DOI] [PubMed] [Google Scholar]
- 30. Pessoa TD, Campos LC, Carraro-Lacroix L, et al. Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J Am Soc Nephrol 2014; 25: 2028–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lovshin JA, Barnie A, DeAlmeida A, et al. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care 2015; 38: 132–139. [DOI] [PubMed] [Google Scholar]
- 32. Asmar A, Simonsen L, Asmar M, et al. Glucagon-like peptide-1 does not have acute effects on central or renal hemodynamics in patients with type 2 diabetes without nephropathy. Am J Physiol Endocrinol Metab 2016; 310: E744–E753. [DOI] [PubMed] [Google Scholar]
- 33. Dai Y, Mehta JL, Chen M. Glucagon-like peptide-1 receptor agonist liraglutide inhibits endothelin-1 in endothelial cell by repressing nuclear factor-kappa B activation. Cardiovasc Drugs Ther 2013; 27: 371–380. [DOI] [PubMed] [Google Scholar]
- 34. Giacco F, Du X, Carratu A, et al. GLP-1 cleavage product reverses persistent ROS generation after transient hyperglycemia by disrupting an ROS-generating feedback loop. Diabetes 2015; 64: 3273–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Von Scholten BJ, Hansen TW, Goetze JP, et al. Glucagon-like peptide 1 receptor agonist (GLP-1 RA): long-term effect on kidney function in patients with type 2 diabetes. J Diabetes Complications 2015; 29: 670–674. [DOI] [PubMed] [Google Scholar]
- 36. Weise WJ, Sivanandy MS, Block CA, et al. Exenatide-associated ischemic renal failure. Diabetes Care 2009; 32: e22–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaakeh Y, Kanjee S, Boone K, et al. Liraglutide-induced acute kidney injury. Pharmacotherapy 2012; 32: e7–e11. [DOI] [PubMed] [Google Scholar]
- 38. Ferrer-Garcia JC, Martinez-Chanza N, Tolosa-Torrens M, et al. Exenatide and renal failure. Diabet Med 2010; 27: 728–729. [DOI] [PubMed] [Google Scholar]
- 39. Von Scholten BJ, Lajer M, Goetze JP, et al. Time course and mechanisms of the anti-hypertensive and renal effects of liraglutide treatment. Diabet Med 2015; 32: 343–352. [DOI] [PubMed] [Google Scholar]
- 40. Davies MJ, Bain SC, Atkin SL, et al. Efficacy and safety of liraglutide versus placebo as add-on to glucose-lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA-RENAL): a randomized clinical trial. Diabetes Care 2016; 39: 222–230. [DOI] [PubMed] [Google Scholar]
- 41. Tuttle KR, Heilmann C, Hoogwerf BJ, et al. Effects of exenatide on kidney function, adverse events, and clinical end points of kidney disease in type 2 diabetes. Am J Kidney Dis 2013; 62: 396–398. [DOI] [PubMed] [Google Scholar]
- 42. Tuttle KR, McKinney TD, Davidson JA, et al. Effects of once-weekly dulaglutide on kidney function in patients with type 2 diabetes in phase II and III clinical trials. Diabetes Obes Metab 2017; 19: 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Von Scholten BJ, Persson F, Rosenlund S, et al. The effect of liraglutide on renal function: a randomized clinical trial. Diabetes Obes Metab 2017; 19: 239–247. [DOI] [PubMed] [Google Scholar]
- 44. Wanner C, Heerspink HJL, Zinman B, et al. Empagliflozin and kidney function decline in patients with type 2 diabetes: a slope analysis from the EMPA-REG OUTCOME trial. J Am Soc Nephrol 2018; 29: 2755–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014; 129: 587–597. [DOI] [PubMed] [Google Scholar]
- 46. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373: 2247–2257. [DOI] [PubMed] [Google Scholar]
- 47. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mann JFE, Orsted DD, Brown-Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 2017; 377: 839–848. [DOI] [PubMed] [Google Scholar]
- 49. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–1844. [DOI] [PubMed] [Google Scholar]
- 50. Mann JFE, Orsted DD, Buse JB. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 2017; 377: 2197–2198. [DOI] [PubMed] [Google Scholar]
- 51. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017; 377: 1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bethel MA, Mentz RJ, Merrill P, et al. Renal outcomes in the EXenatide study of cardiovascular event lowering (EXSCEL) (Abstract). Diabetes 2018; 67(Suppl. 1): A522. [Google Scholar]
- 53. Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018; 392: 1519–1529. [DOI] [PubMed] [Google Scholar]
- 54. Muskiet MHA, Tonneijck L, Huang Y, et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2018; 6: 859–869. [DOI] [PubMed] [Google Scholar]
- 55. Tuttle KR, Lakshmanan MC, Gross JL, et al. Comparable glycaemic control with once weekly dulaglutide versus insulin glargine, both combined with lispro, in type 2 diabetes and chronic kidney disease (AWARD-7). Diabetologia 2017; 60: S3. [Google Scholar]
- 56. Tuttle KR, Lakshmanan MC, Rayner B, et al. Body weight and eGFR during dulaglutide treatment in type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7). Diabetes Obes Metab 2019; 21: 1493–1497. [DOI] [PubMed] [Google Scholar]
- 57. Heerspink HJL, Coresh J, Gansevoort RT, et al. Change in albuminuria as a surrogate endpoint in chronic kidney disease - Authors’ reply. Lancet Diabetes Endocrinol 2019; 7: 336–337. [DOI] [PubMed] [Google Scholar]
- 58. Van Baar MJB, Van Raalte DH, Muskiet MHA. GLP-1 receptor agonists, CKD, and eGFR trajectory. Lancet Diabetes Endocrinol 2018; 6: 764–765. [DOI] [PubMed] [Google Scholar]
- 59. Van Baar MJB, Van Ruiten CC, Muskiet MHA, et al. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care 2018; 41: 1543–1556. [DOI] [PubMed] [Google Scholar]