Abstract
Polyphenols as typical food components can influence the colloidal properties and internalization of nanomaterials (NMs) into mammalian cells. Recently, we found that 3-hydroxyflavone (H3) promoted intracellular Zn ions in ZnO nanoparticle (NP) exposed Caco-2 and HepG2 cells. However, it is unclear if H3 could affect the internalization of metal-based NMs with different morphologies. This study investigated the influence of H3 on colloidal aspects of Ag NPs and Ag nanoflakes (NFs) as well as the internalization of Ag NMs into THP-1 macrophages. 3-Hydroxyflavone at 50 μM promoted the solubility and altered hydrodynamic size, polydispersity index, and ζ potential of Ag NPs and Ag NFs, which indicated that H3 could affect the colloidal stability of Ag NMs. Only H3 but not Ag NMs significantly decreased mitochondrial activities of THP-1 macrophages. The internalization of Ag NMs was markedly increased due to the presence of H3. 3-Hydroxyflavone also exhibited antioxidative properties as it reduced intracellular reactive oxygen species and promoted the activities of ABC transporters as it reduced retention of Calcein in Ag NM-exposed THP-1 macrophages. We concluded that H3 promoted the internalization of Ag NMs into macrophages probably by altering the colloidal stability of Ag NMs and consequently NM-macrophage interactions.
Keywords: Ag nanomaterials (NMs), 3-hydroxyflavone (H3), THP-1 macrophages, colloidal stability, internalization
Introduction
Nanomaterials (NMs) in a biological microenvironment could absorb biological molecules.1 For this reason, it is necessary to consider the interactions between biological molecules and NMs to better understand the biological effects of NMs.2,3 For example, the adsorption of proteins onto NMs forms protein corona, which has been convincingly shown to affect the colloidal aspects of NMs and NM-cell interactions.4,5 Besides the well-investigated protein corona, small molecules such as lipids,6,7 vitamin C,8 and glucose9 may elicit similar effects, although this area is relatively less investigated.
Recent studies highlighted a need to investigate the interactions between phytochemicals and NMs.10 Phytochemicals are secondary metabolites derived from plants and are widely used in health-care products due to their well-documented beneficial effects to human beings.11–13 Coexposure to NMs and phytochemicals may happen in 2 situations. First, both NMs and phytochemicals could be used in food and food-related products, therefore, it is possible that human beings could be orally exposed to both of them when digesting NM-containing products.14,15 For this consideration, Martirosyan et al showed that phenolic compounds reduced the toxicity of Ag nanoparticles (NPs) to Caco-2 cells through the inhibition of oxidative stress, which highlighted a need to consider the influence of phytochemicals as food components to alter the toxic potential of NMs via oral exposure.16,17 Recently, we showed that flavones, flavonols,18 and anthocyanidins19 could protect Caco-2 cells from ZnO NP exposure, although this effect might not be completely dependent on the antioxidative properties of phytochemicals. Second, phytochemicals could be used for green synthesis of biocompatible NMs, which could lead to the contamination of phytochemicals in NMs.20–22 For this reason, previous studies showed that NMs with phytochemicals as capping agents were generally stable and biocompatible,20,23–25 and use of phytochemicals could reduce the toxicity of NMs to many different types of cells.26,27 Still, more work is needed to further understand the influence of different types of phytochemicals on the effects of NMs.
3-Hydroxyflavone (H3) is a synthetic polyphenol which has been widely used in food with relatively large amount.28 Therefore, human beings could be coexposed to H3 and NMs via for example oral exposure. Recently, we found that H3 markedly influenced the colloidal stability of ZnO NPs and consequently promoted the internalization of ZnO NPs into Caco-218 and HepG2 cells.29 However, the influence of H3 on other metal-based NMs, particularly NMs with different morphologies, is unclear. In the present study, we investigated the interactions between H3 and Ag NPs/Ag nanoflakes (NFs), so the effects of H3 on NMs with different morphologies could be studied. Ag NMs were studied, because they are among the most produced NMs all over the world,30 and have been used in food and health-care products due to their unique properties, for example, antibacterial31–33 and anticancer34 activities. Therefore, oral exposure to Ag NMs is relevant in real life, and in this situation, coexposure to Ag NMs and H3 could be possible. To investigate the effects of H3 on colloidal stability of Ag NMs, the changes of solubility, hydrodynamic size, polydispersity index (PDI), and ζ-potential brought by H3 were evaluated. To investigate whether H3 could influence the biological effects of Ag NMs, THP-1 macrophages were exposed to Ag NMs with or without the presence of H3, and cytotoxicity, Ag internalization, intracellular reactive oxygen species (ROS), and activity of ABC transporters were measured.
Materials and Methods
Cell Culture
THP-1 cells (ATCC, Manassas, Virginia) were cultured and differentiated into macrophages by the treatment of phorbol 12-myristate 13-acetate (Sigma, St. Louis, Missouri) as described.35 After differentiation, THP-1 macrophages changed from nearly spherical morphologies to elongated morphologies and became adherent onto the surfaces of cell culture dishes.35
Ag NM Preparation and Characteristics
Ag NPs (code XFJ14) and Ag NFs (code XFJ45) were purchased from Nanjing XFNANO Materials Tech Co, Ltd (Nanjing, China), and their physicochemical properties have been determined by supplier (summarized in Table 1). In this study, the morphology of XFJ14 and XFJ45 was further investigated by using transmission electron microscopy (TEM; FEI TECNAI G20, Hillsboro, OR, USA).
Table 1.
The Physicochemical Properties of Ag NMs as Measured by Supplier.
| Types of NMs | Code | Primary Size | Surface Area | Tap Density | Purity |
|---|---|---|---|---|---|
| Ag NPs | XFJ14 | ∼60-80 nm | ≥3.0 m2/g | ≥2.5 g/cm3 | >99.9 wt% |
| Ag NFs | XFJ45 | ∼5 μm | 0.80-1.45 m2/g | 2.45-3.55 g/cm3 | >99.9 wt% |
Abbreviations: NF, nanoflake; NM, nanomaterial; NP, nanoparticle.
To make the suspensions of Ag NPs and Ag NFs, 1 mg/mL NMs in MilliQ water sonicated for 2 times of 8 minutes with continuously cooling on ice using an ultrasonic processor FS-250 N (20% amplitude; Shanghai Shengxi, China). After sonication, Ag NPs and Ag NFs were diluted in full cell culture medium to desired concentrations with or without the presence of 50 μM H3 (Shanghai Yuanye Biotechnology Co, Ltd, Shanghai, China) for exposure. To indicate the changes of colloidal properties of Ag NMs due to the presence of H3, the hydrodynamic size, PDI, and ζ potential of 25 μg/mL Ag NPs and Ag NFs suspended in MilliQ water with or without the presence of 50 μM H3 was measured by Zetasizer nano ZS90 (Malvern, United Kingdom). Generally, for nonspherical NPs, the dynamic light scattering (DLS) provides an Rh (hydrodynamic radius), which is the radius of a hypothetical hard sphere.36 All the samples were analyzed for 3 times, and mean ± standard deviation (SD) was calculated.
Atomic Absorption Spectroscopy
Atomic Absorption Spectroscopy (AAS) was used to determine the solubility of Ag NMs with or without the presence of H3. The Ag standard solutions (purchased from National Institute of Metrology, Beijing, China) were prepared in water as 0.2, 0.4, 0.6, 0.8, 1.2, and 1.6 μg/mL. A 100-μg/mL Ag NPs or Ag NFs was suspended in water with or without the presence of 50 μM H3 and aged for 24 hours at 37°C in a CO2 incubator. After incubation, the suspensions were centrifuged at 16 000g for 30 minutes, and the supernatants and standard solutions were measured by an AA7000 AAS (Shimadzu Co, Ltd, Kyoto, Japan) equipped with a Zn Hollow Cathode Lamp. Experiment was done once (n = 4), and the concentrations of total soluble Ag were calculated.
Atomic Absorption Spectroscopy was also used to determine the concentrations of Ag elements in Ag NM-exposed THP-1 macrophages. Here, the cells were seeded on 6-well plates at the density of 1.2 × 106/well and exposed to 50 or 100 μg/mL Ag NMs with or without the presence of H3. After 24-hour exposure, the cells were thoroughly rinsed, removed from 6-well plates by trypsin, and lysed by 1% Triton-X. The protein concentrations were determined by using Bradford Protein Assay kits (purchased from Beyotime, Nantong, China) following manufacturer’s instructions. Ag elements in cellular lysates were dissolved in HNO3, and then the concentrations of Ag elements were measured by AAS as indicated above. Experiment was carried out once, with n = 4.
Cytotoxicity Assay
The cytotoxicity was assessed by cell counting kit-8 (CCK-8) and neutral red uptake assays using commercial kits according to the manufacturer’s instructions (Beyotime). In short, 2.4 × 105 per well THP-1 macrophages were seeded in 24-well plates and exposed to 0 (control), 6.25, 12.5, 25, 50, and 100 μg/mL Ag NMs with or without the presence of 50 μM H3. After 24-hour exposure, the CCK-8 and neutral red uptake assays were done according to manufacturer’s instructions.
Intracellular ROS
The intracellular ROS was measured by using 2′,7′-dichlorodihydrofluorescein diacetate (purchased from Sigma-Aldrich, St. Louis, Missouri). Briefly, 6 × 104 per well THP-1 macrophages were seeded on 96-well black plates and then exposed to 0 (control), 6.25, 12.5, 25, 50, and 100 μg/mL Ag NMs with or without the presence of H3 for 24 hours. After exposure, the intracellular ROS was determined as described.37
Calcein-AM Assay
The activity of ABC transporters was evaluated by using Calcein-AM assay. For this assay, 6 × 104 per well THP-1 macrophages were seeded on 96-well black plates and then exposed to 0 (control), 6.25, 12.5, 25, 50, and 100 μg/mL Ag NMs with or without the presence of H3 for 24 hours. After exposure, the cells were thoroughly rinsed with Hanks solution, and then incubated with 1 μg/mL Calcein-AM (purchased from MedChemExpress, Monmouth Junction, New Jersey) in serum-free medium for 30 minutes. After that, the cells were rinsed again with Hanks solution, and the green fluorescence was read at Ex 485 ± 20 nm and Em 528 ± 20 nm by an ELISA reader (Synergy HT; BioTek, Tacoma, Washington).
Statistics
Unless otherwise stated, the data were expressed as mean ± SD of 3 independent experiments (n = 3 for each). Two-way analysis of variance followed by TukeyHSD test was done in R version 3.3.3 to compare the difference. A P value <.05 was considered to be statistically significantly different.
Results
Characteristics of Ag NMs
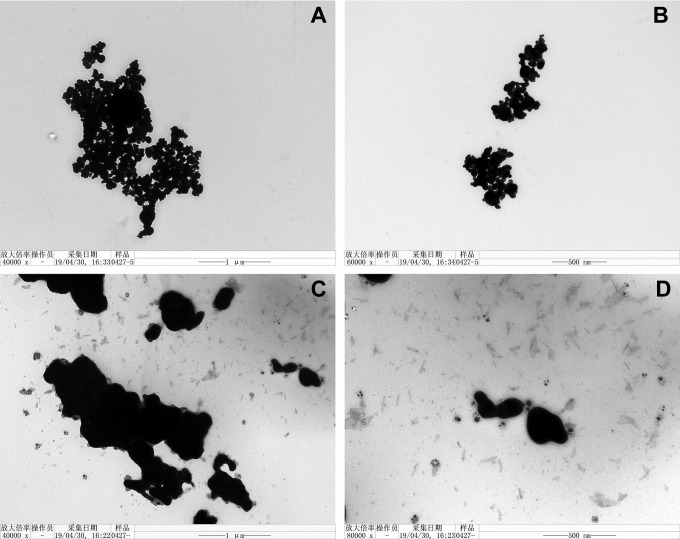
This study used high-purity Ag NMs with spherical (code XFJ14) or flake-like (code XFJ45) structures (summarized in Table 1). The representative TEM images confirmed the different morphologies of Ag NPs (Figure 1A and B) and Ag NFs (Figure 1C and D).
Figure 1.
The representative TEM images of Ag NPs (code XFJ14; A and B) and Ag NFs (code XFJ45; C and D). NF indicates nanoflake; TEM, transmission electron microscopy; NP, nanoparticle.
Changes of Colloidal Properties of Ag NMs by H3
As summarized in Table 2, Ag NPs had a relatively smaller hydrodynamic size, PDI, and absolute value of ζ potential compared with Ag NFs. The presence of H3 increased the hydrodynamic size and PDI of both Ag NPs and Ag NFs. The absolute value of ζ potential of Ag NPs increased due to the presence of H3, whereas the absolute value of ζ potential of Ag NFs decreased with the presence of H3.
Table 2.
The Average of Hydrodynamic Size, Polydispersity Index (PDI), and ζ Potential.a
| Suspensions | Hydrodynamic Size (nm) | PDI | ζ Potential (mV) |
|---|---|---|---|
| Ag NPs | 103.77 ± 1.36 | 0.410 ± 0.035 | −15.80 ± 0.40 |
| Ag NPs + H3 | 255.23 ± 186.53 | 0.483 ± 0.272 | −21.23 ± 3.98 |
| Ag NFs | 453.83 ± 42.27 | 0.689 ± 0.053 | −22.83 ± 1.50 |
| Ag NFs + H3 | 906.9 ± 431.90 | 0.869 ± 0.190 | −20.63 ± 0.75 |
Abbreviations: H3, 3-hydroxyflavone; NF, nanoflake; NP, nanoparticle; PDI, polydispersity index; SD, standard deviation.
aData were expressed as mean ± SD of 3 measurement based on a single run.
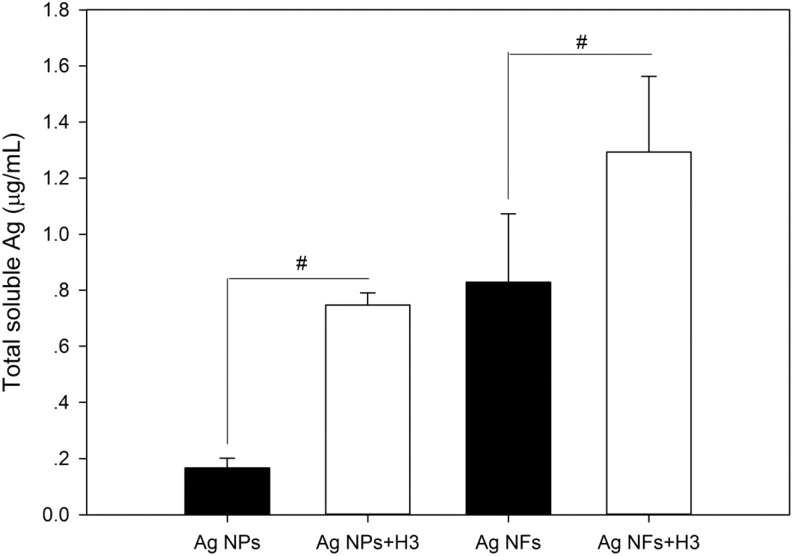
The results from AAS measurement suggest that Ag NPs and Ag NFs are partially soluble, but Ag NFs released more soluble Ag elements with the same mass concentration during 24 hours incubation. With the presence of H3, both Ag NPs (P < .01) and Ag NFs (P < .05) significantly released more soluble Ag elements in water (Figure 2).
Figure 2.
The solubility of Ag NPs (code XFJ14) and Ag NFs (code XFJ45). A 100-μg/mL Ag NPs or Ag NFs were aged for 24 hours with or without the presence of 3-hydroxyflavone (H3). After that, the suspensions were centrifuged, and the concentrations of total soluble Ag were measured by AAS. Data represent mean ± SD of 4 samples. #, P < .05, comparison between groups with or without the presence of H3. AAS indicates atomic absorption spectroscopy; NF, nanoflake; NP, nanoparticle; SD, standard deviation.
Cytotoxicity
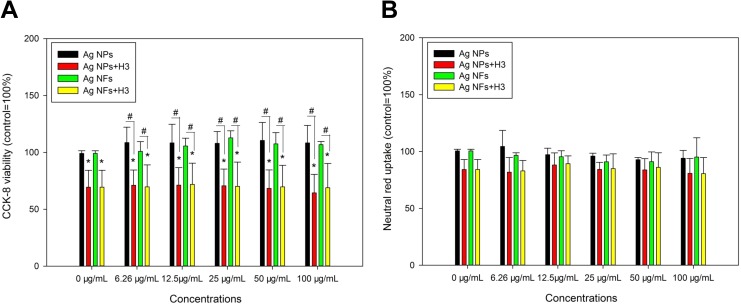
Exposure to various concentrations of Ag NPs or Ag NFs did not significantly induce cytotoxicity, that no significant decrease of CCK-8 activity (Figure 3A) or neutral red uptake (Figure 3B) was observed after Ag NM exposure. The presence of H3 significantly decreased CCK-8 viability (P < .05) but not neutral red uptake (P > .05) compared with control. With the presence of H3, all the concentrations of Ag NMs led to significantly lower CCK-8 viability compared with that induced by Ag NMs alone (P < .05; Figure 3A).
Figure 3.
The cytotoxicity of Ag NPs (code XFJ14) and Ag NFs (code XFJ45). THP-1 macrophages were exposed to various concentrations of Ag NMs with or without the presence of 3-hydroxyflavone (H3) for 24 hours. After exposure, CCK-8 (A) and neutral red uptake assays (B) were done to indicate the cytotoxicity. *, P < .05, compared with control; #, P < .05, comparison between Ag NM-exposed groups at the same mass concentrations of NMs with or without the presence of H3. CCK-8 indicates cell counting kit-8; NF, nanoflake; NM, nanomaterial; NP, nanoparticle.
Cellular Ag Elements
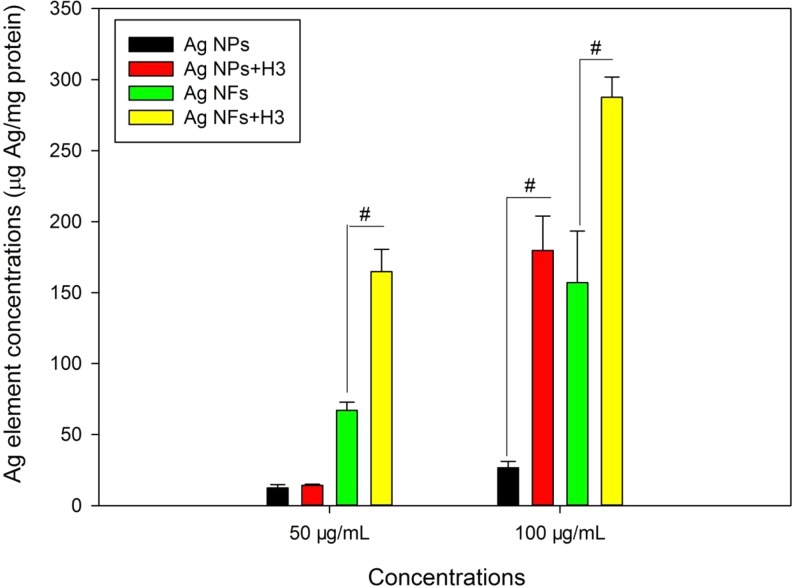
The concentrations of cellular Ag elements increased with the increase of concentrations of Ag NMs. For Ag NPs, H3 only significantly increased the level of cellular Ag elements after exposure to 100 μg/mL Ag NPs (P < .01). For Ag NFs, H3 significantly increased the concentration of cellular Ag elements after exposure to both 50 and 100 μg/mL Ag NFs (P < .01; Figure 4).
Figure 4.
The cellular Ag elements in THP-1 macrophages exposed to Ag NPs (code XFJ14) or Ag NFs (code XFJ45). THP-1 macrophages were exposed to various concentrations of Ag NMs with or without the presence of 3-hydroxyflavone (H3) for 24 hours. After exposure, the concentrations of cellular Ag elements were determined by AAS. #, P < .01, comparison between Ag NM-exposed groups at the same mass concentrations of NMs with or without the presence of H3. AAS indicates atomic absorption spectroscopy; NF, nanoflake; NM, nanomaterial; NP, nanoparticle.
Intracellular ROS
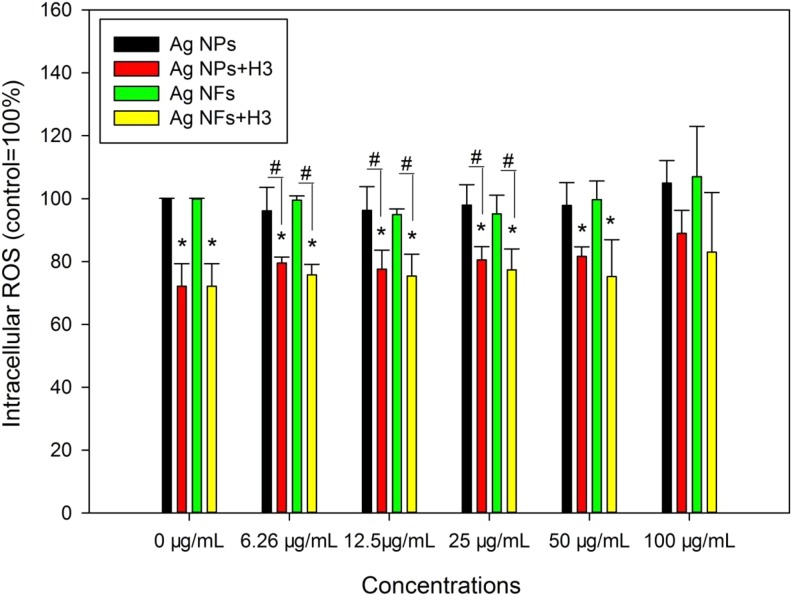
Exposure to various concentrations of Ag NMs did not significantly induce intracellular ROS (P > .05), whereas H3 significantly reduced intracellular ROS compared with control (P < .01). With the presence of H3, 6.25 (P < .05), 12.5 (P < .01), 25, and 50 (P < .05) μg/mL Ag NPs or Ag NFs significantly decreased intracellular ROS compared with control. In addition, coexposure to H3 and 6.25, 12.5, and 25 μg/mL Ag NPs or Ag NFs led to significantly lower intracellular ROS compared with that induced by Ag NPs or Ag NFs at the same concentrations (P < .05; Figure 5).
Figure 5.
The intracellular ROS in THP-1 macrophages exposed to Ag NPs (code XFJ14) or Ag NFs (code XFJ45). THP-1 macrophages were exposed to various concentrations of Ag NMs with or without the presence of 3-hydroxyflavone (H3) for 24 hours. After exposure, the intracellular ROS was measured using fluorescent probe. *, P < .05, compared with control; #, P < .05, comparison between Ag NM-exposed groups at the same mass concentrations of NMs with or without the presence of H3. NF indicates nanoflake; NM, nanomaterial; NP, nanoparticle; ROS, reactive oxygen species.
The activity of ABC Transporter
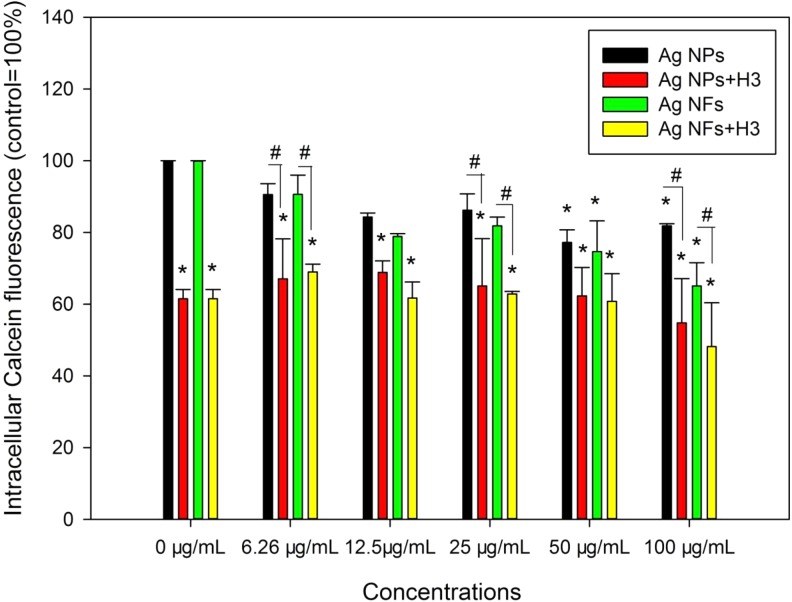
Exposure to 50 and 100 μg/mL Ag NPs or Ag NFs without the presence of H3 led to significantly lower levels of intracellular Calcein fluorescence compared with control (P < .05), whereas coexposure to H3 and all concentrations of Ag NMs significantly lowered intracellular Calcein fluorescence (P < .01). Moreover, coexposure to H3 and 6.25, 25, and 100 μg/mL Ag NPs or Ag NFs led to significantly lower intracellular Calcein fluorescence compared with that induced by Ag NPs or Ag NFs at the same concentrations (P < .05; Figure 6).
Figure 6.
The retention of Calcein in THP-1 macrophages exposed to Ag NPs (code XFJ14) or Ag NFs (code XFJ45). THP-1 macrophages were exposed to various concentrations of Ag NMs with or without the presence of 3-hydroxyflavone (H3) for 24 hours. After exposure, Calcein-AM assay was done to indicate the activity of ABC transporters. *, P < .05, compared with control; #, P < .05, comparison between Ag NM-exposed groups at the same mass concentrations of NMs with or without the presence of H3. NF indicates nanoflake; NM, nanomaterial; NP, nanoparticle.
Discussion
The present study investigated the interactions between H3 and Ag NMs. It was shown that H3 increased the solubility and changed hydrodynamic size, PDI, and ζ potential of both Ag NPs and Ag NFs (Figure 2 and Table 2), which suggests that H3 may influence the colloidal stability of Ag NMs regardless of morphologies of NMs. Previously, we already showed that H3 altered hydrodynamic size, PDI, and ζ potential of ZnO NPs in water and/or in cell culture medium.18,29 In combination with the data reported in this study, it is possible that H3 is capable to affect the colloidal aspects of different types of NMs. However, it should be noticed that the results from this study showed that H3 increased the solubility of both Ag NPs and Ag NFs (Figure 2), whereas in our previous studies H3 showed no effect on solubility of ZnO NPs.18,29 Therefore, the influence of H3 on dissolution of metal-based NPs is likely to be dependent on the types of NMs and should be investigated case by case. It has been reported by us and others before that food components, such as free fatty acids,6,38 sugars,9,39 and phytochemicals,18,40 could alter the colloidal aspects of NPs. Thus, food components are potent to affect the colloidal aspects of NMs, which should be carefully evaluated in the situations that coexposure to NMs and food components is a relevant issue.
The results from this study showed that neither Ag NPs nor Ag NFs up to 100 μg/mL were cytotoxic to THP-1 macrophages (Figure 3). This is consistent with our recent reports that XFJ14 was not cytotoxic to Caco-2 cells19 or human aortic smooth muscle cells.41 Moreover, the results from this study (Figure 5) and our previous studies19,41 showed that Ag NMs did not significantly induce intracellular ROS, which could explain the lack of cytotoxicity. 3-Hydroxyflavone-induced cytotoxicity as shown by CCK-8 assay did not further enhance the cytotoxicity of Ag NMs (Figure 3). A previous study reported that an H3-Ag NP complex was not cytotoxic to L929 mouse fibroblast cells.42 In contrast, we recently showed that H3 increased the cytotoxicity of ZnO NPs to both cancer cells and primary cells.29 It seems that the influence of H3 on cytotoxicity of NMs is dependent on the compositions of NMs.
It is interesting to notice that H3 increased the internalization of both Ag NPs and Ag NFs into THP-1 macrophages, that the levels of cellular Ag elements were significantly increased with the presence of H3 (Figure 4). Recently, we found that H3 at the same concentration used in this study increased intracellular Zn ions in ZnO NP-exposed Caco-2 cells.18 In another study, we showed that H3 increased cellular Zn elements in ZnO NP-exposed HepG2 cells.29 In combination with the data reported here, it appears that H3 is capable to increase the interactions between metal-based NPs and cells regardless of morphologies of NMs and cell types.
It has been suggested before that phytochemicals might influence the effects of NMs due to the antioxidative properties of phytochemicals.14,15 Here in this study, we also found that H3 reduced intracellular ROS in THP-1 macrophages (Figure 5), which suggests the antioxidative potential of H3. However, in our previous study, we found no association between antioxidative effects of H3 and ZnO NP-cell interactions.18
In mammalian cells, ABC transporters play an important role in the efflux of toxic drugs and NMs.43 The activity of ABC transporters could be measured by using Calcein-AM, which is converted by esterases into Calcein inside the cells and readily effluxed by ABC transporters. Thus, lower retention of intracellular Calcein could reflect higher activities of ABC transporters.44 In this study, we found that the intracellular Calcein fluorescence decreased with the increase of concentrations of Ag NMs (Figure 6), which indicates increased activities of ABC transporters after Ag NM challenge. With the presence of H3, the retention of intracellular Calcein fluorescence further decreased. It has been shown before that exposure to ZnO NPs,45,46 silicon dioxide NPs,47 and TiO2 NPs48 altered the messenger RNA and/or protein levels of nutrient transporters in intestinal cells leading to decreased uptake of nutrients. Alternatively, it also has been shown that CdTe quantum dots49 and TiO2 NPs induced ABC transporters, which could be a protective mechanism against toxic NP exposure.50 In addition, inhibition of ABC transporters has been shown as a strategy to enhance the anticancer efficacy of NMs.51,52 Therefore, it is possible that the induction of ABC transporters is a protective response to Ag NM exposure. Since H3 further reduced the retention of intracellular Calcein, the increased activities of ABC transporters after combined exposure to Ag NMs and H3 might not explain increased cellular Ag elements. One possible explanation is that H3 increases the solubility of Ag NMs leading to higher uptake of Ag elements. This possibility has been proven before by showing that digestion with the presence of food components enhanced the uptake of Ag NPs into intestinal cells compared with the digestion without the presence of food components.53
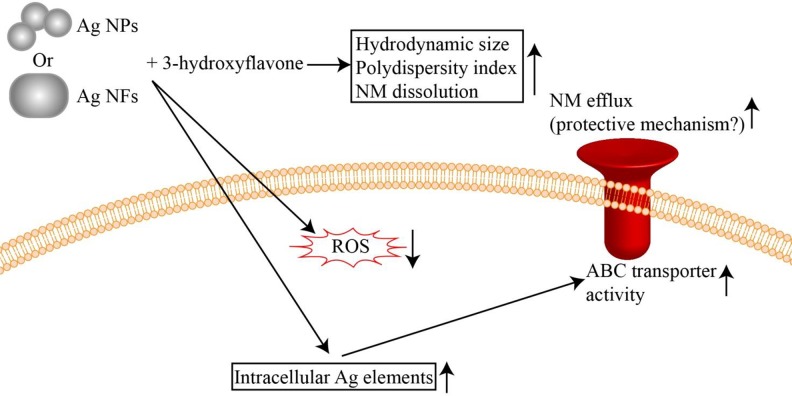
As summarized in Figure 7, the results from this study suggested that H3 could increase the dissolution of Ag NMs and consequently enhance the Ag NM-macrophage interactions. The activity of ABC transporters could then be enhanced probably as a protective mechanism to efflux toxic Ag NMs. Similar effects were observed for both Ag NPs and Ag NFs, which indicates that H3 is capable to influence the effects of Ag NMs regardless of morphologies of NMs. Therefore, if coexposure to NMs and H3 is a relevant issue, it is necessary to consider the influence of H3 on colloidal aspects and biological effects of NMs. Alternatively, it may be also plausible to use H3 to enhance the NM-cell interactions to develop targeted drug delivery systems.54–56 In perspective, we are developing nanocarriers based on H3 for drug delivery.
Figure 7.
The proposed influence of H3 on bio-effects of Ag NMs. The presence of H3 could increase the dissolution of Ag NMs and consequently enhance the Ag NM-macrophage interactions. See more in text. H3 indicates 3-hydroxyflavone; NF, nanoflake; NM, nanomaterial.
Footnotes
Authors’ Note: Yongqi Liang and Min Xie contributed equally.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yi Cao  https://orcid.org/0000-0002-9282-0051
https://orcid.org/0000-0002-9282-0051
References
- 1. Yu B, Wang X, Zhou C, et al. Insight into mechanisms of cellular uptake of lipid nanoparticles and intracellular release of small RNAs. Pharm Res. 2014;31(10):2685–2695. [DOI] [PubMed] [Google Scholar]
- 2. Au JL, Abbiati RA, Wientjes MG, Lu Z. Target site delivery and residence of nanomedicines: application of quantitative systems pharmacology. Pharmacol Rev. 2019;71(2):157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou C, Yang Z, Teng L. Nanomedicine based on nucleic acids: pharmacokinetic and pharmacodynamic perspectives. Curr Pharm Biotechnol. 2014;15(9):829–838. [DOI] [PubMed] [Google Scholar]
- 4. Cai R, Chen C. The crown and the scepter: roles of the protein corona in nanomedicine. Adv Mater. 2018;e1805740 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 5. Xiao W, Gao H. The impact of protein corona on the behavior and targeting capability of nanoparticle-based delivery system. Int J Pharm. 2018;552(1-2):328–339. [DOI] [PubMed] [Google Scholar]
- 6. Jiang M, Wu B, Sun Y, et al. Toxicity of ZnO nanoparticles (NPs) to THP-1 macrophages: interactions with saturated or unsaturated free fatty acids. Toxicol Mech Methods. 2019;29(4):291–299. [DOI] [PubMed] [Google Scholar]
- 7. Fang X, Jiang L, Gong Y, Li J, Liu L, Cao Y. The presence of oleate stabilized ZnO nanoparticles (NPs) and reduced the toxicity of aged NPs to Caco-2 and HepG2 cells. Chem Biol Interact. 2017;278:40–47. [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, Yuan L, Yao C, et al. A combined toxicity study of zinc oxide nanoparticles and vitamin C in food additives. Nanoscale. 2014;6(24):15333–15342. [DOI] [PubMed] [Google Scholar]
- 9. Chen Z, Wang Y, Zhuo L, et al. Interaction of titanium dioxide nanoparticles with glucose on young rats after oral administration. Nanomedicine. 2015;11(7):1633–1642. [DOI] [PubMed] [Google Scholar]
- 10. Cao Y, Xie Y, Liu L, et al. Influence of phytochemicals on the biocompatibility of inorganic nanoparticles: a state-of-the-art review. Phytochem Rev. 2017;16(3):555–563. [Google Scholar]
- 11. Pham NM, Do VV, Lee AH. Polyphenol-rich foods and risk of gestational diabetes: a systematic review and meta-analysis. Eur J Clin Nutr. 2019;73(5):647–656. [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Zhong J, Bai J, et al. The application of natural products in cancer therapy by targeting apoptosis pathways. Curr Drug Metab. 2018;19(9):739–749. CDM-90354. [DOI] [PubMed] [Google Scholar]
- 13. Xie J, Yang Z, Zhou C, Zhu J, Lee RJ, Teng L. Nanotechnology for the delivery of phytochemicals in cancer therapy. Biotechnol Adv. 2016;34(4):343–353. [DOI] [PubMed] [Google Scholar]
- 14. McClements DJ, Xiao H, Demokritou P. Physicochemical and colloidal aspects of food matrix effects on gastrointestinal fate of ingested inorganic nanoparticles. Adv Colloid Interface Sci. 2017;246:165–180. [DOI] [PubMed] [Google Scholar]
- 15. Cao Y, Li J, Liu F, et al. Consideration of interaction between nanoparticles and food components for the safety assessment of nanoparticles following oral exposure: a review. Environ Toxicol Pharmacol. 2016;46:206–210. [DOI] [PubMed] [Google Scholar]
- 16. Martirosyan A, Bazes A, Schneider YJ. In vitro toxicity assessment of silver nanoparticles in the presence of phenolic compounds—preventive agents against the harmful effect? Nanotoxicology. 2014;8(5):573–582. [DOI] [PubMed] [Google Scholar]
- 17. Martirosyan A, Grintzalis K, Polet M, Laloux L, Schneider YJ. Tuning the inflammatory response to silver nanoparticles via quercetin in Caco-2 (co-)cultures as model of the human intestinal mucosa. Toxicol Lett. 2016;253:36–45. [DOI] [PubMed] [Google Scholar]
- 18. Zhang C, Li Y, Liu L, Gong Y, Xie Y, Cao Y. Chemical structures of polyphenols that critically influence the toxicity of ZnO nanoparticles. J Agric Food Chem. 2018;66:1714–1722. [DOI] [PubMed] [Google Scholar]
- 19. Jiang L, Li Z, Xie Y, Liu L, Cao Y. Cyanidin chloride modestly protects Caco-2 cells from ZnO nanoparticle exposure probably through the induction of autophagy. Food Chem Toxicol. 2019;127:251–259. [DOI] [PubMed] [Google Scholar]
- 20. Khan M, Shaik MR, Adil SF, et al. Plant extracts as green reductants for the synthesis of silver nanoparticles: lessons from chemical synthesis. Dalton Trans. 2018;47(35):11988–12010. [DOI] [PubMed] [Google Scholar]
- 21. Yang X, Yang S, Chai H, et al. A novel isoquinoline derivative anticancer agent and its targeted delivery to tumor cells using transferrin-conjugated liposomes. PLoS One. 2015;10(8):e0136649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Y, Liang X, Tong R, et al. Gambogic acid-loaded polymeric micelles for improved therapeutic effect in breast cancer. J Biomed Nanotechnol. 2018;14(10):1695–1704. [DOI] [PubMed] [Google Scholar]
- 23. Rafique M, Sadaf I, Rafique MS, Tahir MB. A review on green synthesis of silver nanoparticles and their applications. Artif Cells Nanomed Biotechnol. 2017;45(7):1272–1291. [DOI] [PubMed] [Google Scholar]
- 24. Ding Q, Xu X, Yue Y, et al. Nanocellulose-mediated electroconductive self-healing hydrogels with high strength, plasticity, viscoelasticity, stretchability, and biocompatibility toward multifunctional applications. ACS Appl Mater Interfaces. 2018;10(33):27987–28002. [DOI] [PubMed] [Google Scholar]
- 25. Pan S, Xing H, Fu X, et al. The effect of photothermal therapy on osteosarcoma with polyacrylic acid-coated gold nanorods. Dose Response 2018;16(3):1559325818789841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qian P, Yan LJ, Li YQ, et al. Cyanidin ameliorates cisplatin-induced cardiotoxicity via inhibition of ROS-mediated apoptosis. Exp Ther Med. 2018;15(2):1959–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sarkar A, Sil PC. Iron oxide nanoparticles mediated cytotoxicity via PI3K/AKT pathway: role of quercetin. Food Chem Toxicol. 2014;71:106–115. [DOI] [PubMed] [Google Scholar]
- 28. Butun B, Topcu G, Ozturk T. Recent advances on 3-hydroxyflavone derivatives: structures and properties. Mini Rev Med Chem. 2018;18(2):98–103. [DOI] [PubMed] [Google Scholar]
- 29. Luo Y, Wu C, Liu L, et al. 3-Hydroxyflavone enhances the toxicity of ZnO nanoparticles in vitro. J Appl Toxicol. 2018;38(9):1206–1214. [DOI] [PubMed] [Google Scholar]
- 30. Vance ME, Kuiken T, Vejerano EP, et al. Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol. 2015;6:1769–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gaillet S, Rouanet JM. Silver nanoparticles: their potential toxic effects after oral exposure and underlying mechanisms—a review. Food Chem Toxicol. 2015;77:58–63. [DOI] [PubMed] [Google Scholar]
- 32. Zhu M, Hua D, Pan H, et al. Green electrospun and crosslinked poly(vinyl alcohol)/poly(acrylic acid) composite membranes for antibacterial effective air filtration. J Colloid Interface Sci. 2018;511:411–423. [DOI] [PubMed] [Google Scholar]
- 33. Zhu M, Xiong R, Huang C. Bio-based and photocrosslinked electrospun antibacterial nanofibrous membranes for air filtration. Carbohydr Polym. 2019;205:55–62. [DOI] [PubMed] [Google Scholar]
- 34. Sharma H, Mishra PK, Talegaonkar S, Vaidya B. Metal nanoparticles: a theranostic nanotool against cancer. Drug Discov Today. 2015;20(9):1143–1151. [DOI] [PubMed] [Google Scholar]
- 35. Yang Q, Wang M, Sun Y, Peng S, Ding Y, Cao Y. Pre-incubated with BSA-complexed free fatty acids alters ER stress/autophagic gene expression by carboxylated multi-walled carbon nanotube exposure in THP-1 macrophages. Chinese Chemical Letters. 2019;30(6):1224–1228. doi:10.1016/j.cclet.2019.03.042. [Google Scholar]
- 36. Bhattacharjee S. DLS and zeta potential—What they are and what they are not? J Control Release. 2016;235:337–351. [DOI] [PubMed] [Google Scholar]
- 37. Li Z, Liu T, Long J, et al. The toxicity of hydroxylated and carboxylated multi-walled carbon nanotubes to human endothelial cells was not exacerbated by ER stress inducer. Chinese Chem Lett. 2019;30(3):582–586. [Google Scholar]
- 38. Gong Y, Liu L, Li J, Cao Y. The presence of palmitate affected the colloidal stability of ZnO NPs but not the toxicity to Caco-2 cells. J Nanopart Res. 2017;19(10):335. [Google Scholar]
- 39. Lee JA, Kim MK, Song JH, et al. Biokinetics of food additive silica nanoparticles and their interactions with food components. Colloids Surf B Biointerfaces. 2017;150:384–392. [DOI] [PubMed] [Google Scholar]
- 40. Li Y, Zhang C, Liu L, Gong Y, Xie Y, Cao Y. The effects of baicalein or baicalin on the colloidal stability of ZnO nanoparticles (NPs) and toxicity of NPs to Caco-2 cells. Toxicol Mech Methods. 2018;28(3):167–176. [DOI] [PubMed] [Google Scholar]
- 41. Wang M, Yang Q, Long J, et al. A comparative study of toxicity of TiO2, ZnO, and Ag nanoparticles to human aortic smooth-muscle cells. Int J Nanomedicine. 2018;13:8037–8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Voicescu M, Craciunescu O, Moldovan L, Anastasescu M, Angelescu DG, Teodorescu VS. Physicochemical characterization and in vitro cytotoxic effect of 3-hydroxyflavone in a silver nanoparticles complex. J Fluoresc. 2015;25(5):1215–1223. [DOI] [PubMed] [Google Scholar]
- 43. Cao Y, Li Z, Mao L, et al. The use of proteomic technologies to study molecular mechanisms of multidrug resistance in cancer. Eur J Med Chem. 2019;162:423–434. [DOI] [PubMed] [Google Scholar]
- 44. Hollo Z, Homolya L, Davis CW, Sarkadi B. Calcein accumulation as a fluorometric functional assay of the multidrug transporter. Biochim Biophys Acta. 1994;1191(2):384–388. [DOI] [PubMed] [Google Scholar]
- 45. Abbasi-Oshaghi E, Mirzaei F, Mirzaei A. Effects of ZnO nanoparticles on intestinal function and structure in normal/high fat diet-fed rats and Caco-2 cells. Nanomedicine (Lond). 2018;13(21):2791–2816. [DOI] [PubMed] [Google Scholar]
- 46. Moreno-Olivas F, Tako E, Mahler GJ. ZnO nanoparticles affect nutrient transport in an in vitro model of the small intestine. Food Chem Toxicol. 2019;124:112–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guo Z, Martucci NJ, Liu Y, Yoo E, Tako E, Mahler GJ. Silicon dioxide nanoparticle exposure affects small intestine function in an in vitro model. Nanotoxicology. 2018;12(5):485–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guo Z, Martucci NJ, Moreno-Olivas F, Tako E, Mahler GJ. Titanium dioxide nanoparticle ingestion alters nutrient absorption in an in vitro model of the small intestine. NanoImpact. 2017;5:70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tian J, Hu J, Liu G, et al. Altered gene expression of ABC transporters, nuclear receptors and oxidative stress signaling in zebrafish embryos exposed to CdTe quantum dots. Environ Pollut. 2019;244:588–599. [DOI] [PubMed] [Google Scholar]
- 50. Dorier M, Brun E, Veronesi G, et al. Impact of anatase and rutile titanium dioxide nanoparticles on uptake carriers and efflux pumps in Caco-2 gut epithelial cells. Nanoscale. 2015;7(16):7352–7360. [DOI] [PubMed] [Google Scholar]
- 51. Li W, Zhang H, Assaraf YG, et al. Overcoming ABC transporter-mediated multidrug resistance: molecular mechanisms and novel therapeutic drug strategies. Drug Resist Updat. 2016;27:14–29. [DOI] [PubMed] [Google Scholar]
- 52. Xiao Y, Liu J, Guo M, et al. Synergistic combination chemotherapy using carrier-free celastrol and doxorubicin nanocrystals for overcoming drug resistance. Nanoscale. 2018;10(26):12639–12649. [DOI] [PubMed] [Google Scholar]
- 53. Lichtenstein D, Ebmeyer J, Knappe P, et al. Impact of food components during in vitro digestion of silver nanoparticles on cellular uptake and cytotoxicity in intestinal cells. Biol Chem. 2015;396(11):1255–1264. [DOI] [PubMed] [Google Scholar]
- 54. Yang C, Li Y, Yang Y, et al. Multidimensional theranostics for tumor fluorescence imaging, photoacoustic imaging and photothermal treatment based on manganese doped carbon dots. J Biomed Nanotechnol. 2018;14(9):1590–1600. [DOI] [PubMed] [Google Scholar]
- 55. Gao S, Tang G, Hua D, et al. Stimuli-responsive bio-based polymeric systems and their applications. J Mater Chem B. 2019;7(5):709–729. [DOI] [PubMed] [Google Scholar]
- 56. Cai L, Gu Z, Zhong J, et al. Advances in glycosylation-mediated cancer-targeted drug delivery. Drug Discov Today. 2018;23(5):1126–1138. [DOI] [PubMed] [Google Scholar]