Abstract
This study focused on the development of infants’ sensorimotor knowledge about the layout of their bodies. Little is known about the development of the body as a reaching space, despite the importance of this skill for many self-directed adaptive behaviors, such as removing foreign stimuli from the skin or scratching an itch. A new method was developed in which vibrating targets were placed on the heads and arms of 7- to 21-month-old infants (N = 78) to test reaching localization of targets. Manual localization improved with age, and visual localization was associated with successful reaching. Use of the ipsilateral or contralateral hand varied with body region: Infants primarily used the ipsilateral hand for head targets but the contralateral hand for arm targets, for which ipsilateral reaches were not biomechanically possible. The results of this research highlight a previously understudied form of self-knowledge involving a functional capacity to reach to tactile targets on the body surface.
Keywords: human body, infant development, perceptual motor coordination
The capacity to localize targets on the body underlies a wide range of adaptive behaviors, from removing an aversive stimulus on the skin to scratching an itch. Child-care manuals assume that infants already possess this skill when they warn that ear tugging by infants may signal an ear infection (Murkoff & Mazel, 2014). Yet despite the adaptive significance of this functional capacity and its importance as a form of self-knowledge, we know little about its developmental origins. Here, we ask how this functional capacity emerges during early development. To investigate this question, we developed a new task in which infants were prompted to localize a continuously vibrating target placed at various locations on the body (see Fig. 1).
Fig. 1.
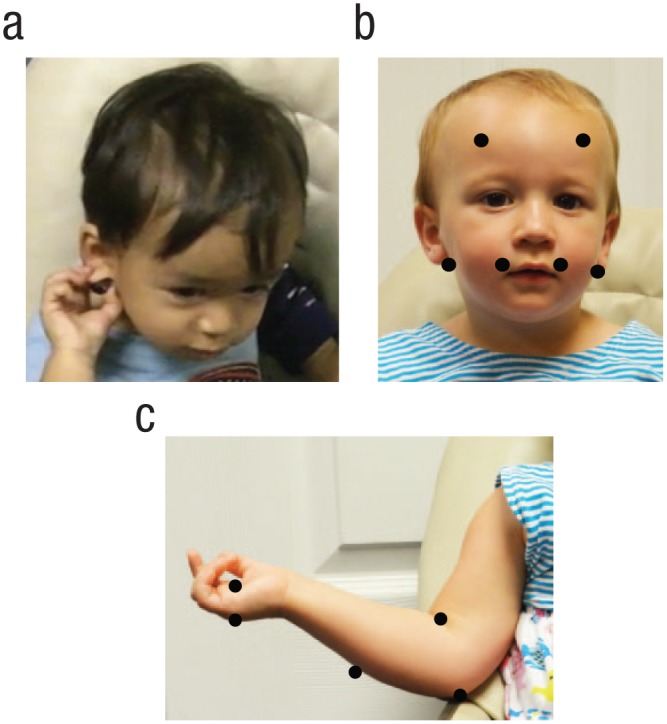
Example of an infant reaching to a target (a), target locations on head trials (b), and target locations on the arm and hand trials that were used in analyses (c). On head trials, ear targets were placed behind the earlobes.
The ability to reach to a target on the body involves an integrated set of perceptual-motor skills. As described in Penfield and Boldrey’s (1937) classic work on the human homunculus, sensation from the skin is mapped onto a neural representation of the body, which exaggerates some body parts relative to others in the somatosensory cortex but nevertheless generally preserves body topography. According to some accounts, neural information about the source of that tactile stimulation is initially skin based or anatomy based, independent of the location of the stimulus relative to that of the body in external space (Heed, Buchholz, Engel, & Röder, 2015; Longo, Azañón, & Haggard, 2010). For example, a skin-based code may identify a stimulus location on the left hand, but the left hand may occupy many different locations in space. Therefore, for localization to occur, this skin-based information must be coordinated with information that specifies the current position of the body part containing the source of the stimulation, a problem known as tactile remapping (Azañón, Camacho, Morales, & Longo, 2018; Badde & Heed, 2016). Then, to plan and execute a reach to that tactile target, individuals must relate the position of the reaching limb to the remapped location of the target on the body.
When do infants develop the ability to localize tactile targets on the body? Although hand-mouth contact has been observed in fetuses and young infants (Lew & Butterworth, 1997; Myowa-Yamakoshi & Takeshita, 2006), the origins of this broader localization ability have received little empirical attention. Instead, much developmental work on body knowledge has focused on the representation of body layout, measured behaviorally or neurally.
Behavioral evidence indicates that 3.5-month-old infants recognize discrepancies in the canonical arrangement of body parts (Bhatt, Hock, White, Jubran, & Galati, 2016; Zieber, Kangas, Hock, & Bhatt, 2015) and that knowledge about body topography and size becomes further refined in the preschool years (Brownell, Nichols, Svetlova, Zerwas, & Ramani, 2010; DeLoache, Uttal, & Rosengren, 2004). Similarly, neural evidence indicates that during the first year, infants’ brains are already organized to represent body topography. Cortical maps that preserve the topographic structure of the body distinguish among the upper limbs, lower limbs, and lips and are evident in a rudimentary form near birth as well as later in the first year (Marshall & Meltzoff, 2015; Meltzoff, Saby, & Marshall, 2019; Milh et al., 2007; Saby, Meltzoff, & Marshall, 2015). Additionally, by 8 months, infants show differences in somatosensorily evoked potentials for crossed versus uncrossed hand stimulation (Rigato, Begum Ali, van Velzen, & Bremner, 2014), suggesting that they can code limb location in relation to external space (Bremner, Mareschal, Lloyd-Fox, & Spence, 2008; Bremner & Spence, 2017), which is crucial for the tactile remapping process described above.
Critically, advances in the representation of body structure during the first year allow for the possibility that a complementary functional capacity to localize tactile targets emerges during the same period. Consistent with this possibility, research has shown that during the first half year of their life, infants already detect intermodal contingencies between visual and proprioceptive information for self-movement (Bahrick & Watson, 1985; Morgan & Rochat, 1997). Exposure to these and other intermodal contingencies (e.g., touch proprioception) for self-movement, along with advances in tactile remapping, could support the development of a more targeted capacity that enables infants to localize tactile stimuli on the body.
In the present study, we asked how a functional capacity to reach to targets on the body unfolds during early development. We created a tactile-localization task in which a continuously vibrating stimulus was placed at different points on the head and arms. We selected these two regions to compare localization to targets that were either accessible or not accessible to vision. Additionally, our method allowed us to compare localization for areas within a body region (i.e., parts of the head or parts of the arm) in contrast to current infant neuroimaging techniques, which reveal somatotopic organization between different body regions (e.g., arms, legs, and lips; Meltzoff et al., 2019). Arm trials also allowed us to examine whether infants attempt biomechanically impossible reaches to targets (e.g., an ipsilateral reach attempt to an arm target). With this method, we examined the development of tactile localization from 7 to 21 months of age. This age range was chosen because by 7 months, infants are relatively skilled at reaching, and by 21 months, most infants reach to a mark on the face when looking in mirrors, suggesting an upper limit of performance on our localization task (Amsterdam, 1972; Anderson, 1984; Bertenthal & Fischer, 1978).
Our research had three main goals. First, we examined whether the ability to localize targets emerges simultaneously across different body regions. We tested localization for head and arm regions to address this issue. Given that mechanoreceptors are especially dense in the lips and fingers and that the mouth and hands have proportionally large representations in the somatosensory cortex, at least in adults (Penfield & Boldrey, 1937; Purves et al., 2001), we hypothesized that within the head and arm regions, infants would localize tactile stimulation near the mouth and hands earliest, in comparison with locations such as the ears and elbows. Such a pattern would suggest that within different body regions, a functional capacity to localize tactile targets expands gradually during development.
Next, given the multimodal nature of early self-representation, we examined to what extent infants rely on visual information to localize targets. We hypothesized that with increasing age, infants would become able to manually localize (a) targets on the arm without looking at them and (b) targets on the head (which were not visible), suggesting that they had mastered the problem of tactile remapping by coordinating tactile and proprioceptive information.
Finally, we asked whether, on the basis of their actions, infants recognized which types of reaches to the body were biomechanically possible. Infants could reach with either the ipsilateral or contralateral arm to head targets but only with the contralateral arm to arm targets. If infants did not attempt impossible ipsilateral reaches to arm targets, this would imply that they have some knowledge about where on the body each arm can reach. Additionally, for head targets (which could be reached with either hand), we examined whether infants reached ipsilaterally or contralaterally, even though head targets were located near the midline of their body. Although infants before the end of the first year infrequently cross the midline when reaching to objects in external space (Morange & Bloch, 1996; van Hof, van der Kamp, & Savelsbergh, 2002), it is not known whether a similar pattern exists when the body is the reaching space.
Method
Participants
A total of 78 infants (41 female, 37 male; age: range = 7.00–21.63 months, M = 13.17, SD = 3.89) were included in the analyses. Five additional children were excluded because of fussiness or refusal to complete at least half of the trials in a trial block. We sampled more densely at younger ages because we anticipated that the greatest developmental change in localization would occur in the first half of the age range under study. Because this was a new topic and method, we were not able to use effect sizes to determine statistical power. Participants were recruited from day-care centers in the greater New Orleans, Louisiana, area; ads posted on Craigslist; flyers distributed at child-oriented events; and the Tulane University Department of Psychology. The sample was primarily Caucasian (55 Caucasian, 4 African American, 1 American Indian/Alaska Native, 3 Asian, 11 mixed race or ethnicity, and 4 not reported). Children were tested in day-care settings (n = 28) or the laboratory (n = 50). Written consent from the children’s parents was obtained.
Materials and procedure
The task involved localization of a continuously vibrating target attached with hypoallergenic double-sided tape to 12 different locations (6 head, 6 upper body), one at a time (see Fig. 1). We used a self-contained, continuously vibrating target that was not attached to external cables to deliver the stimulus because pilot testing indicated that infants did not reach as often to a nonvibrating target, and we did not want any cables to interfere with infant reaching. The target emitted some sound (approximately comparable with the sound of a cell phone vibrating). The target object, approximately 1.25 cm in diameter, 0.75 cm in height, and 3.5 g in weight, was custom made and consisted of a button battery (1.5 V) attached to a flat coin 3-volt DC 70-mA 12,000-rpm micro motor (also known as a pancake motor) that provided vibration of comparable strength and frequency with that of vibrating teething rings or cell phones. Black electrical tape was used to secure the battery and motor together. The device was then sealed with black liquid tape to provide a soft texture and smooth appearance. A plastic pull-tab could be removed or inserted to turn the device on or off.
The children were seated in an infant seat. If they became fussy after being placed in the infant seat, they were instead seated on their caregiver’s lap. Before testing began, the experimenter placed the vibrating target on the caregiver’s skin with the infant observing to familiarize both the infant and caregiver with the device and task.
Head and body trials were conducted in two separate blocks, the order of which was counterbalanced across participants. Each participant received 6 trials per block in random order, for 12 possible total trials. The six head locations were behind the left and right earlobes, on the left and right forehead, and on the left and right upper lip area (see Fig. 1b). The six body locations were the shoulder, crook of the elbow, elbow, forearm, palm of the hand, and top of the hand (see Fig. 1c). The side of the body was randomly chosen across the 6 trials, with infants receiving 3 trials on each side of the body (left, right). Shoulder locations were discarded because of issues with clothing interfering with the target.
The vibrating targets were attached one at a time to each area of interest. When adhering the target to the child, the experimenter simultaneously touched the same body location on the side not receiving the target. The child was then given the opportunity to localize the target manually. The testing session for each trial lasted until the child removed or contacted the target (e.g., manually localizing the target, rubbing the target off on the chair) or until approximately 40 s had elapsed. If infants did not try to reach to the target on their own, we prompted them to do so in order to keep them engaged in the task. In this case, the experimenter used the questions, “Where did it go?” and “Can you find it?” Successful target contact was reinforced with brief cheering such as, “You got it!” A video camera mounted on a tripod and positioned to focus on the child’s upper body recorded the trials.
Measures and analyses
Trials were videotaped and coded by two independent observers using Datavyu software (Datavyu Team, 2014). All trials were coded for target contact attempt (moved the arm or another body part toward the target, did not move the arm or another body part toward the target), target contact success (yes, no), and the hand used when reaching (contralateral, ipsilateral) for all attempts (both successes and failures) to localize the target. Target localization was considered successful if the child manually touched the target or removed it with something other than the hand, such as deliberately rubbing it off on the chair. A reach attempt was coded when the child made an arm movement toward the target in pursuit of the target. For three crook-of-the-elbow trials, the participant flexed the ipsilateral hand toward the target, and this motion was also counted as an attempt. For a small number of head trials (11 of 402), infants reached bilaterally. Consequently, these trials were omitted from the analyses looking at ipsilateral versus contralateral reaching. Arm trials were also coded for whether or not infants visually localized the target (yes, no).
The primary observer coded 82.1% of participants’ data. A second observer, who was blind to the hypotheses, coded the remaining participants’ data, and another observer randomly selected 20.5% of overlap with the first observer’s coding to establish interrater reliability. We adjusted for chance agreement by using Cohen’s κ. Reliability estimates were high for the outcome variables of interest (κs = 0.86–0.98, M = 0.92).
Generalized linear mixed-effects models (GLMMs) were used to examine how localization (yes, no) varied as a function of age and target location. GLMMs allow for analysis of binary dependent variables within a repeated measures design, in which different participants may have contributed different numbers of observations. We used a binomial distribution with a logit link function to accommodate our binary dependent variables (e.g., no target contact vs. target contact, ipsilateral hand vs. contralateral hand). Coefficients and standard errors for significant follow-up tests are thus reported in transformed state. To accommodate trials clustered within participants, we set participant ID as a random factor on the intercept for all GLMMs. The independent variables of interest—age and target location—were analyzed as fixed factors.
Results
After conducting preliminary analyses, we focused on whether infants became better at reaching to the targets with age and whether they performed differently in response to different target locations. Then we examined whether infants made ipsilateral versus contralateral reaches depending on the location of the target on the body. Lastly, for targets that were potentially visible (e.g., targets on the arms), we examined whether looking to the target varied with age and location and whether looking to the target was associated with success.
Preliminary analyses
A total of 764 trials were analyzed. Infants attempted to contact the target 633 times (82.85% of total trials); 613 (80.24% of total trials) of these attempts were with the hand. They successfully contacted the target 585 times (76.57% of total trials); 574 (75.13% of total trials) of these contacts were with the hand. They made no attempt to contact the target on 131 trials (17.15% of total trials). Initial analyses found no effects of block order (head first, body first), trial order (suggesting no effects of habituation to the stimuli or learning across trials), side of the head or body to which the target was adhered (left, right), or sex on target contact success. Consequently, we collapsed across left versus right target placement and did not examine effects associated with order or sex in subsequent analyses.
Successful reaching to targets increased with age (see Fig. 2)—GLMM testing age effect on all target locations: χ2(1) = 14.6, p < .001, b = 0.25, SE = 0.07. Additionally, there was no effect of body region on target localization (p > .52); infants were equally successful at localizing targets in the head regions (75.95%) and the arm regions (77.03%). In subsequent analyses, we separated trials by body region (head, arm) to compare the localization of targets within each of these regions. Sound emitted from the target did not seem to affect successful reaching to the target; infants did not perform better at locations where the target was closer to the ear and therefore louder (see below).
Fig. 2.
Successful target reaches across age, collapsed across all target locations. The line indicates predictions of the generalized linear mixed-effects model. The semitranslucent blue dots indicate raw data (0 = no successful reach, 1 = successful reach), with darker blue indicating overlapping data points.
Localization success
Head
Infants became better with age at localizing targets in the head region and were more likely to contact the target at the mouth than at the ears or forehead (see Fig. 3a). The GLMM analysis for the effects of age, target location (mouth, behind earlobe, forehead), and the Age × Target Location interaction on infants’ target contact success indicated main effects of age, Wald χ2(1) = 7.62, p < .01, and target location, Wald χ2(2) = 22.73, p < .001. The Age × Target Location interaction approached statistical significance (p = .052). Infants showed more improvement in localizing targets at the ears (z = 4.07, p < .05, b = 0.25, SE = 0.12) and forehead (z = 4.62, p < .05, b = 0.28, SE = 0.12) than the mouth, likely because they were already localizing targets at the mouth at the beginning of the age range we tested.
Fig. 3.
Successful target contact across age, separately for each (a) head location and (b) arm and hand location. Lines indicate predictions of generalized linear mixed-effects models. The semitranslucent blue dots indicate observed data (0 = no contact, 1 = target contact), with darker blue indicating overlapping data points.
Arm and hand
Infants were able to localize targets on the hand across the age range under study, but they gradually improved at reaching to the crook of the elbow and elbow locations with increasing age (see Fig. 3b). The GLMM analysis for reaching to hand and arm targets (palm, top of the hand, forearm, crook of the elbow, elbow) indicated main effects of age, Wald χ2(1) = 7.56, p < .01, and target location, Wald χ2(4) = 24.26, p < .001; the Age × Target Location interaction was also significant, Wald χ2(4) = 11.15, p < .05. Target contact remained at high levels for the palm, top of the hand, and forearm locations across the age range but improved gradually with increasing age for the crook of the elbow (z = 3.28, p < .01, b = 0.53, SE = 0.16) and elbow (z = 3.48, p < .001, b = 0.50, SE = 0.14) locations.
Ipsilateral versus contralateral reaching
The next set of analyses focused on reaches with the hand. We examined whether infants used the ipsilateral or contralateral hand when reaching to the target. (Similar results were obtained when we looked at successful reaching trials only.) Because we did not find an effect of left or right side of the head or body on target contact success, we collapsed across these factors in the following analyses. The ipsilateral and contralateral analyses below thus focused only on whether infants used the hand located on the same side of the body’s midline as the target or used the opposite hand.
Head
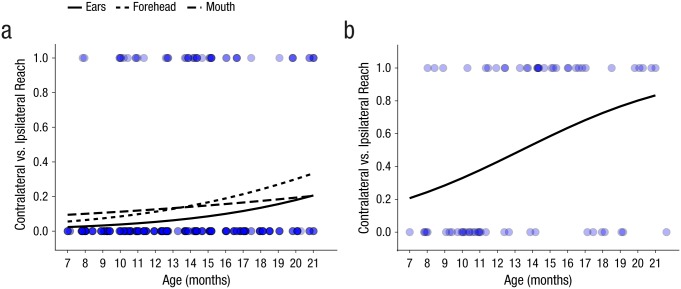
Infants made more contralateral reaches to head targets as age increased. However, ipsilateral reaching still predominated across the age range (see Fig. 4a). A GLMM testing the effects of age, target location, and the Age × Target Location interaction indicated a significant main effect of age, Wald χ2(1) = 4.41, p < .05. The main effect of target location and the Age × Target Location interaction were not statistically significant (ps > .19).
Fig. 4.
Contralateral reaches (coded 1) versus ipsilateral reaches (coded 0) across age, separately to targets at (a) each head location and (b) the palm. Lines indicate predictions of generalized linear mixed-effects models. The semitranslucent blue dots indicate observed data, with darker blue indicating overlapping data points.
Arm and hand
For most of the arm and hand locations (top of the hand, forearm, crook of the elbow, elbow), it was not biomechanically possible to contact these locations with the ipsilateral hand. Nevertheless, it is interesting to consider whether infants attempted to do so. We found no instances in which infants attempted to reach to targets on the top of the hand, forearm, or elbow with the ipsilateral hand. Three infants (unsuccessfully) attempted to reach the crook-of-the-elbow location with the ipsilateral hand. Thus, reaches made toward the arm locations (other than the palm) were almost exclusively made with the contralateral hand, suggesting that even very young infants exhibit functional knowledge of what action routes work, that is, which locations can and cannot be reached ipsilaterally.
Because the palm was the only arm or hand location that had more than a few ipsilateral reaching attempts and the only one for which infants could possibly contact the target ipsilaterally (i.e., the fingers could contact the target on the ipsilateral palm), it was the only arm or hand location that we analyzed for ipsilateral versus contralateral reaching. As infants became older, they became more likely to attempt a reach to the palm with the contralateral hand relative to first touching the target with the ipsilateral fingers, Wald χ2(1) = 5.47, p < .05, b = 0.21, SE = 0.09 (see Fig. 4b).
Looking and localization
Finally, we examined the relation between looking behavior and reaching to the target. For these analyses, we focused only on target locations that were potentially in the visual field, namely, arm and hand trials. (As noted, we found no significant differences when successful arm and hand trials that were potentially in the visual field were compared with successful head trials that were outside the visual field.) Infants might look at a target to aid in localization, and looking at the target might indicate heightened attention to the target.
First, we addressed whether visual localization varied with age or location of the target on the arm or hand. Infants looked less at the elbow location than at the hand, forearm, or crook-of-the-elbow locations, Wald χ2(4) = 23.05, p < .001. The Age × Target Location interaction and main effect of age on visual localization were not statistically significant.
Next, we examined whether looking (yes, no) at the arm targets was associated with successful target contacts. Across age, infants contacted more targets that they looked at (88.72% success for visually localized targets) than targets they did not look at (37.18% success), Wald χ2(1) = 41.74, p < .001. This effect was qualified by a significant Age × Visual Localization interaction in the GLMM, Wald χ2(1) = 7.88, p < .01 (see Fig. 5). For trials in which infants did not look at the target, they became significantly more likely to successfully contact the target with age, as indicated by a statistically significant improvement across age (z = 3.43, p < .001, b = 0.39, SE = 0.11). However, for trials in which infants looked at the target, infants contacted most targets across all ages, and follow-up tests revealed a flat slope across age.
Fig. 5.
Target contact success across age, separately for targets (a) not looked at and (b) looked at. Lines indicate predictions of generalized linear mixed-effects models. The semitranslucent blue dots indicate observed data (0 = no contact, 1 = target contact), with darker blue indicating overlapping data points.
Discussion
The present results spotlight a little recognized but embodied form of self-knowledge that involves the ability to reach to tactile targets on the body. This ability requires the integration of a complex set of body-centered spatial coding and perceptuomotor processes. Here, we report the results of a new task designed to investigate how infants combine these processes to reach to body targets. Results suggest that the ability to reach to tactile stimuli does not initially extend across the entire surface of either the head or arms but expands gradually across these regions, becoming established in the second half of infants’ first year and refined further in the second year. Infants localize targets near the mouth at a younger age than they localize targets near the ear or on the forehead, despite the relative proximity of these targets. Similarly, infants localize targets on the hand sooner than they localize targets on other areas of the arm.
To reach to a tactile stimulus on the body, individuals must engage in tactile remapping, in which they integrate tactile information about the skin-based location of the stimulus with proprioceptive or visual information about current body position in external space (Begum Ali, Spence, & Bremner, 2015; Bremner et al., 2008; Heed et al., 2010; Longo et al., 2010). They then must use this updated location information with information about the location of the reaching limb to reach to the target. Our findings suggest that infants early in the second half of their first year succeed in doing so for some body locations (mouth and hands) earlier than for others (ears, forehead, and arms). In line with these results, infants begin to code the location of some body regions with respect to external space near the start of the second half of their first year (Begum Ali et al., 2015; Rigato et al., 2014).
This gradual pattern of localization development might reflect sensory limitations. Mechanoreceptors are especially dense in the lips and fingers, and both the hands and mouth have proportionally large representations in the somatosensory cortex (Penfield & Boldrey, 1937; Purves et al., 2001; see also Meltzoff et al., 2019). Differential sensory processing, therefore, may affect infants’ tactile-remapping ability, thereby limiting their ability to code some target locations in external space. These difficulties would be consistent with the developmental patterns of localization found here. Tactile remapping alone, however, would not guarantee successful body reaches. Infants must also guide the reaching limb to targets. The present results suggest that infants begin to integrate somatosensory and motor information to support reaching to some body locations early in the second half of their first year and that this ability extends to other body locations in subsequent months.
Consistent with this developmental progression, our results showed that infants in the second year increasingly reached to arm targets without looking, suggesting that for this region, they could engage in remapping using tactile and proprioceptive information. A related progression was found for head targets other than the mouth, which were not accessible to vision. Neurophysiological work with adults suggests that these developmental changes may be related to the establishment of functional networks involving the posterior parietal cortex, a critical region for integrating motor and sensory information (Longo et al., 2010).
A striking feature of the present findings is the degree to which reaching was lateralized differently for the two body regions (head and arms). For head targets, which could be localized ipsilaterally or contralaterally, infants chiefly used the ipsilateral hand, even though targets were near the body’s midline. In contrast, infants rarely attempted ipsilateral reaches to arm targets but were as successful localizing arm targets as head ones. Thus, depending on the target’s location, infants crossed the midline and reached contralaterally, suggesting that the so-called midline barrier for infant reaching is not absolute (Provine & Westerman, 1979; van Hof et al., 2002). Moreover, the present lateralization results suggest that at the level of action, infants recognize which body parts are accessible to which arms. Infants rarely attempted biomechanically impossible ipsilateral reaches to arm targets, yet most head reaches were ipsilateral. Infant sensitivity to the body’s biomechanical properties might influence localization patterns across body regions more generally.
Conclusion
A functional capacity to localize and reach to tactile targets on the body develops gradually during infancy. Infants reach to tactile stimuli on some parts of the body (mouth and hand) before others (ears, forehead, and arms), suggesting that the body as an operative reaching space is initially fragmented. Longitudinal studies would help establish if this capacity expands across the body surface in similar ways across individual infants. Our methods and findings have implications for practice because they provide a foundation for documenting when infants become capable of localizing tactile sensations on the body before they can tell us what they feel. Tugging the ears, for instance, may signal an ear infection. Our findings also highlight two broader questions for future research. One concerns the mechanisms that give rise to this functional capacity. This capacity may derive in part from active experience, built from prenatal and postnatal patterns of self-touch and exploration (Yamada et al., 2016). The other question concerns how this functional capacity, once established, relates to other forms of self-knowledge. The capacity to localize targets on the body may contribute to an integrated sense of self. More broadly, investigation of infant body reaching has the potential to advance understanding of later developing forms of self-knowledge.
Acknowledgments
We thank B. A. Kahrs for statistical advice and M. Schlichenmeyer for coding.
Footnotes
Action Editor: Charles Hulme served as action editor for this article.
Author Contributions: This article is based in part on a dissertation submitted to Tulane University by J. E. Leed under the supervision of J. J. Lockman. J. J. Lockman conceived the study, and J. E. Leed and J. J. Lockman designed the study. Testing and data collection were performed by L. K. Chinn and J. E. Leed. J. E. Leed conducted the original data analyses, L. K. Chinn performed the data analyses described in this article, and J. E. Leed and L. K. Chinn interpreted the results under the supervision of J. J. Lockman. All the authors contributed to the writing of the manuscript and approved the final manuscript for submission.
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: This work was supported in part by National Institutes of Health Award 5R01HD067581.
Open Practices: Data and materials for this study are available from the corresponding author on request. The design and analysis plans were not preregistered.
References
- Amsterdam B. (1972). Mirror self-image reactions before age two. Developmental Psychobiology, 5, 297–305. doi: 10.1002/dev.420050403 [DOI] [PubMed] [Google Scholar]
- Anderson J. R. (1984). The development of self-recognition: A review. Developmental Psychobiology, 17, 35–49. doi: 10.1002/dev.420170104 [DOI] [PubMed] [Google Scholar]
- Azañón E., Camacho K., Morales M., Longo M. R. (2018). The sensitive period for tactile remapping does not include early infancy. Child Development, 89, 1394–1404. doi: 10.1111/cdev.12813 [DOI] [PubMed] [Google Scholar]
- Badde S., Heed T. (2016). Towards explaining spatial touch perception: Weighted integration of multiple location codes. Cognitive Neuropsychology, 33, 26–47. doi: 10.1080/02643294.2016.1168791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrick L. E., Watson J. S. (1985). Detection of intermodal proprioceptive-visual contingency as a potential basis of self-perception in infancy. Developmental Psychology, 21, 963–973. doi: 10.1037/0012-1649.21.6.963 [DOI] [Google Scholar]
- Begum Ali J., Spence C., Bremner A. J. (2015). Human infants’ ability to perceive touch in external space develops postnatally. Current Biology, 25, R978–R979. doi: 10.1016/J.CUB.2015.08.055 [DOI] [PubMed] [Google Scholar]
- Bertenthal B. I., Fischer K. W. (1978). Development of self-recognition in the infant. Developmental Psychology, 14, 44–50. doi: 10.1037/0012-1649.14.1.44 [DOI] [Google Scholar]
- Bhatt R. S., Hock A., White H., Jubran R., Galati A. (2016). The development of body structure knowledge in infancy. Child Development Perspectives, 10, 45–52. doi: 10.1111/cdep.12162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner A. J., Mareschal D., Lloyd-Fox S., Spence C. (2008). Spatial localization of touch in the first year of life: Early influence of a visual spatial code and the development of remapping across changes in limb position. Journal of Experimental Psychology: General, 137, 149–162. doi: 10.1037/0096-3445.137.1.149 [DOI] [PubMed] [Google Scholar]
- Bremner A. J., Spence C. (2017). The development of tactile perception. In Benson J. B. (Ed.), Advances in child development and behavior (Vol. 52, pp. 227–268). San Diego, CA: Academic Press. doi: 10.1016/bs.acdb.2016.12.002 [DOI] [PubMed] [Google Scholar]
- Brownell C. A., Nichols S. R., Svetlova M., Zerwas S., Ramani G. (2010). The head bone’s connected to the neck bone: When do toddlers represent their own body topography? Child Development, 81, 797–810. doi: 10.1111/j.1467-8624.2010.01434.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datavyu Team. (2014). Datavyu: A video coding tool. Databrary Project, New York University. [Google Scholar]
- DeLoache J. S., Uttal D. H., Rosengren K. S. (2004). Scale errors offer evidence for a perception-action dissociation early in life. Science, 304, 1027–1029. doi: 10.1126/science.1093567 [DOI] [PubMed] [Google Scholar]
- Heed T., Buchholz V. N., Engel A. K., Röder B. (2015). Tactile remapping: From coordinate transformation to integration in sensorimotor processing. Trends in Cognitive Sciences, 19, 251–258. doi: 10.1016/j.tics.2015.03.001 [DOI] [PubMed] [Google Scholar]
- Heed T., Holmes N. P., Zohary E., Hsiao S. S., Kubischik M., Hoffmann K., . . . Fink G. R. (2010). Touch perception: How we know where we are touched. Current Biology, 20, 604–606. doi: 10.1016/j.cub.2010.04.012 [DOI] [PubMed] [Google Scholar]
- Lew A. R., Butterworth G. (1997). The development of hand-mouth coordination in 2- to 5-month-old infants: Similarities with reaching and grasping. Infant Behavior and Development, 20, 59–69. doi: 10.1016/S0163-6383(97)90061-8 [DOI] [Google Scholar]
- Longo M. R., Azañón E., Haggard P. (2010). More than skin deep: Body representation beyond primary somatosensory cortex. Neuropsychologia, 48, 655–668. doi: 10.1016/j.neuropsychologia.2009.08.022 [DOI] [PubMed] [Google Scholar]
- Marshall P. J., Meltzoff A. N. (2015). Body maps in the infant brain. Trends in Cognitive Sciences, 19, 499–505. doi: 10.1016/j.tics.2015.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff A. N., Saby J. N., Marshall P. J. (2019). Neural representations of the body in 60-day-old human infants. Developmental Science, 22(1), Article e12698. doi: 10.1111/desc.12698 [DOI] [PubMed] [Google Scholar]
- Milh M., Kaminska A., Huon C., Lapillonne A., Ben-Ari Y., Khazipov R. (2007). Rapid cortical oscillations and early motor activity in premature human neonate. Cerebral Cortex, 17, 1582–1594. doi: 10.1093/cercor/bhl069 [DOI] [PubMed] [Google Scholar]
- Morange F., Bloch H. (1996). Lateralization of the approach movement and the prehension movement in infants from 4 to 7 months. Early Development and Parenting, 5, 81–92. doi: [DOI] [Google Scholar]
- Morgan R., Rochat P. (1997). Intermodal calibration of the body in early infancy. Ecological Psychology, 9, 1–23. doi: 10.1207/s15326969eco0901_1 [DOI] [Google Scholar]
- Murkoff H. E., Mazel S. (2014). What to expect the first year (3rd ed.). New York, NY: Workman Publishing. [Google Scholar]
- Myowa-Yamakoshi M., Takeshita H. (2006). Do human fetuses anticipate self-oriented actions? A study by four-dimensional (4D) ultrasonography. Infancy, 10, 289–301. doi: 10.1207/s15327078in1003_5 [DOI] [Google Scholar]
- Penfield W., Boldrey E. (1937). Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain, 60, 389–443. doi: 10.1093/brain/60.4.389 [DOI] [Google Scholar]
- Provine R. R., Westerman J. A. (1979). Crossing the midline: Limits of early eye-hand behavior. Child Development, 50, 437–441. doi: 10.2307/1129420 [DOI] [PubMed] [Google Scholar]
- Purves D., Augustine G. J., Fitzpatrick D., Katz L. C., LaMantia A., McNamara J. O., Williams S. M. (Eds.). (2001). Neuroscience (2nd ed.). Sunderland, England: Sinauer Associates. [Google Scholar]
- Rigato S., Begum Ali J., van Velzen J., Bremner A. J. (2014). The neural basis of somatosensory remapping develops in human infancy. Current Biology, 24, 1222–1226. doi: 10.1016/j.cub.2014.04.004 [DOI] [PubMed] [Google Scholar]
- Saby J. N., Meltzoff A. N., Marshall P. J. (2015). Neural body maps in human infants: Somatotopic responses to tactile stimulation in 7-month-olds. NeuroImage, 118, 74–78. doi: 10.1016/j.neuroimage.2015.05.097 [DOI] [PubMed] [Google Scholar]
- van Hof R., van der Kamp J., Savelsbergh G. J. R. (2002). The relation of unimanual and bimanual reaching to crossing the midline. Child Development, 73, 1353–1362. doi: 10.1111/1467-8624.00476 [DOI] [PubMed] [Google Scholar]
- Yamada Y., Kanazawa H., Iwasaki S., Tsukahara Y., Iwata O., Yamada S., Kuniyoshi Y. (2016). An embodied brain model of the human foetus. Scientific Reports, 6(1), Article 27893. doi: 10.1038/srep27893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieber N., Kangas A., Hock A., Bhatt R. S. (2015). Body structure perception in infancy. Infancy, 20, 1–17. doi: 10.1111/infa.12064 [DOI] [Google Scholar]