Abstract
Children growing up in poverty are vulnerable to negative changes in the developing brain; however, these outcomes vary widely. We tested the hypothesis that receipt of supportive parenting would offset the association between living in poverty during adolescence and the connectivity of neural networks that support cognition and emotion regulation during young adulthood. In a sample of African American youths (N = 119) living in the rural South, poverty status and receipt of supportive parenting were assessed when youths were 11 to 13 and 16 to 18 years old. At age 25, resting-state functional connectivity of the central-executive and emotion-regulation neural networks was assessed using functional MRI. The results revealed that more years spent living in poverty presaged less connectivity in both neural networks among young adults who received low levels of supportive parenting but not among those who received high levels of such parenting.
Keywords: adolescent development, environmental effects, minority groups, neural networks, poverty
In the United States, 20% of children live in poverty and another 20% live near the poverty line. For all children, exposure to poverty forecasts developmental trajectories of physical health, mental health, cognitive development, and educational attainment throughout life. Prominent among these are inverse relationships between poverty and regulation of cognition, emotion, and behavior (Blair & Raver, 2012; Farah et al., 2006; Mani, Mullainathan, Shafir, & Zhao, 2013). As interest in the effects of poverty has surged among pediatric and public health researchers, a parallel literature has developed in which scientists have theorized that growing up in poverty, where stressors are common and resources are scarce, affects brain development.
The first generation of this research has found consistent associations between growing up in low-socioeconomic-status (SES) circumstances and reductions in gray-matter volumes in children’s developing frontal-temporal networks, which are associated with emotional, social, and regulatory processing (Johnson, Riis, & Noble, 2016). In addition to conferring risk for structural metrics of brain development, growing up in low-SES circumstances may disrupt brain function in individual regions. Studies have shown, for example, that adults who grew up in such circumstances displayed large amygdala responses and low activation in both the ventrolateral and dorsolateral prefrontal cortex during an emotion-regulation task, even after adjusting for current income (Kim et al., 2013). Similar findings for brain function have been shown in neural regions that process primary and secondary rewards but only among adults reared in low-SES circumstances (Gianaros et al., 2011). Together, these findings suggest that growing up in low-SES circumstances may confer lasting effects on brain structure and function in regions subserving executive control, emotional processing, and regulation.
Although prior research has focused on individual brain regions affected by living in disadvantaged circumstances, relatively little research has been conducted on the networked interaction of multiple brain regions, which this article addresses. Recognition is growing that higher-order cognitive and emotional functions rely on networks of distributed regions rather than on single regions in isolation (Gordon et al., 2018). Recent insights about the brain’s intrinsic activity have been instrumental in identifying large-scale neural networks. Intrinsic activity refers to the spontaneous neural states that occur when a person is not engaged in a task. This resting-state activity is coordinated by a set of large-scale functional networks, each of which is composed of anatomically distributed nodes that display correlated patterns of activity (Raichle, 2015). This phenomenon is called resting-state functional connectivity (rsFC). The degree of rsFC is a temporally stable individual characteristic that reflects person-to-person variation in both the anatomical structure and functional history of a network, that is, how often the nodes are coactivated (Gratton, Sun, & Petersen, 2018). By late childhood, individual differences in rsFC are apparent (Grayson & Fair, 2017). This study examined the ways in which exposure to poverty across adolescence, a time of increased neural plasticity and reorganization (Casey, Galván, & Hare, 2005) and of heightened sensitivity to social influences such as parent–child relationships (Blakemore & Mills, 2014; Knoll, Magis-Weinberg, Speekenbrink, & Blakemore, 2015), are associated with rsFC within the central-executive network (CEN) and the emotion-regulation network (ERN) during young adulthood. To our knowledge, no studies have examined this question, despite research showing that children from low-SES families are more vulnerable to problems associated with executive functioning (e.g., academic achievement) and emotion regulation (e.g., anger and depression), which are supported by the CEN and ERN, respectively (Blair & Raver, 2012).
The CEN is a frontoparietal network, anchored in the dorsolateral prefrontal cortex and posterior parietal cortex, particularly the middle frontal gyrus and inferior parietal lobe. The CEN is crucial to working memory and to cognitive control of thought, emotion, and behavior (Sherman et al., 2014). It is related to the maintenance and manipulation of behavior in decision making (Uddin, 2015), and the strength of the CEN’s within-network connectivity is positively associated with IQ in children, adolescents, and young adults (Langeslag et al., 2013). Evidence suggests that young adults experiencing depression show hypoconnectivity within the CEN, whereby connections among key brain regions are weak or protracted in their development (Sacchet et al., 2016). Complementing the CEN is the ERN, which is anchored in the inferior gyrus, middle temporal gyrus, and precentral gyrus. The ERN plays a role in dampening neural reactivity in limbic circuitry, including the amygdala (Ochsner, Silvers, & Buhle, 2012). Impairments in emotion regulation and aberrant engagement of the ERN contribute to psychological disorders such as anxiety and major depression (Cisler, Olatunji, Feldner, & Forsyth, 2010). Thus, the first purpose of this study was to examine the hypothesis that greater exposure to poverty across adolescence would be associated 7 years later with less functional connectivity within the CEN and ERN.
Not all children and adolescents who grow up in poverty, however, experience these adverse consequences. Recent research suggests that a subset of youths who receive supportive parenting develop resistance to the consequences of poverty and environments associated with low SES. Studies show that parenting that includes high levels of sensitivity and emotional support, along with low levels of conflict, can offset many of the psychosocial disadvantages that beset children and youths in poverty (Brody, Yu, & Beach, 2016). Although supportive parenting during postnatal development sets the stage for future neural development during childhood (Luby, Belden, Harms, Tillman, & Barch, 2016) and adolescence (Bartholomeusz et al., 2014; Whittle et al., 2017), little attention has been given to the protective benefits of supportive parenting on neural networks. Informed by research showing that supportive parenting plays a significant role in the development of processes related to the CEN and ERN, such as executive control, cognitive self-regulation, and emotion regulation (Deater-Deckard, 2014; Morris, Criss, Silk, & Houltberg, 2017), we examined the hypothesis that the cumulative number of years in which African American adolescents lived in poverty would forecast less connectivity within the CEN and ERN among young adults who received low levels of supportive parenting across adolescence but not among young adults who received high levels of supportive parenting.
Finally, this study addressed three limitations in human brain-imaging research. Little is known about the role of chronicity of poverty on brain function. Poverty is typically treated as a one-time event, although it actually varies over time in the lives of many children and adolescents (Miller, Chen, Yu, & Brody, 2017). Second, the neuroscience literature is often imprecise regarding the difference between living in poverty and living in more general low-SES circumstances. What constitutes low SES varies across studies and, typically, very few participants live at or below government-defined poverty levels. Those participants who live in poverty are more likely to be minorities, whereas participants higher on the SES gradient are White (for a notable exception, see Evans & Schamberg, 2009). Finally, the vast majority of developmental neuroscience research has involved White participants, with minimal recruitment of African Americans and other racial or ethnic groups. To address these shortcomings as well as the study hypotheses, we analyzed data gathered from African American youths and their primary caregivers who had taken part in a longitudinal study that began when the youths were 11 years of age. When youths were 11 to 13 and 16 to 17 years of age, data were collected and used to calculate family poverty status and supportive parenting; rsFC data were obtained when the youths were 25 years of age to determine within-CEN and within-ERN functional connectivity.
Method
Participants
A total of 119 right-handed African Americans, age 25 years and living in rural environments, were recruited from the sample of a larger longitudinal study (Brody et al., 2013) to take part in a neuroimaging session. In the original study, 667 families were recruited randomly over 12 months from rural communities in Georgia when the participants were 11 years of age (M = 11.2, SD = 0.34). At the first assessment, the sample could be characterized as working poor; primary caregivers worked an average of 39.4 hr per week, yet 46.3% of the sample lived below federal poverty standards. When participants were 19 years of age, 500 of them were randomly selected, because of funding constraints, to participate in a collection of biological data. Equivalence analyses were executed using t tests for continuous variables and chi-square tests for categorical variables. These analyses indicated that the random subsample did not differ from the original sample on family poverty status, single-parent status, family income, parent age, parenting and parent–child relationship, parental depression, or children’s self-esteem and self-control. The age-25 data collection included 119 of the participants randomly selected from the age-19 subsample to take part in a neuroimaging session; financial constraints associated with imaging precluded inclusion of a larger sample.
All participants were screened to exclude standard MRI research contraindications (e.g., claustrophobia, pregnancy, ferrous metal implants) and left-handedness prior to enrollment. Subsequent analyses excluded 28 participants because of excessive movement (i.e., models could not be fitted because of lack of degrees of freedom resulting from volume exclusions due to intervolume movement greater than 0.2 mm or more than 25% of voxels were outliers; n = 23) and other technical problems (n = 5). Thus, the final sample for the current study was 91 (52% female; female coded as 0, male coded as 1). Power analyses revealed that a sample size of 91 would give a power of .80 to detect an effect size of .09. Independent-samples t tests comparing participants who did and did not take part in the imaging study at age 25 years revealed no differences at the baseline assessment (age 11) based on sex, parental education, income-to-needs ratio, poverty status, and number of adults in the home. The University of Georgia Institutional Review Board approved and monitored all study procedures, and all participants provided written informed consent.
Measures
Family poverty
When participants were 11 to 13 years old and 16 to 17 years old, caregivers provided data on their families’ income and size, which were used to compute families’ income-to-needs ratios (family income divided by the poverty threshold for that family size based on U.S. Census Bureau guidelines). Income-to-needs ratios of less than 1 were considered below the poverty threshold and assigned a score of 1. Poverty status at each of the five waves of assessment was summed to determine the number of years that youths lived in poverty, according to federal guidelines (M = 1.89, SD = 1.64). This operationalization of poverty is widely used and prospectively forecasts indicators of neurodevelopment, cognitive development, and emotion regulation (Blair & Raver, 2012; Farah et al., 2006; Mani et al., 2013).
Supportive parenting
Supportive parenting received by youths at 11 to 13 years old and 16 to 17 years old was assessed with two scales that have been used extensively with the target population. Each of these measures has been used in longitudinal, epidemiological research and longitudinal, randomized prevention trials with African American parents. The scales were associated across time with psychosocial variables (i.e., drug use, self-control, conduct problems) and biomarkers of physical health (Brody, 2016). The Supportive-Involved Parenting Scale (Brody et al., 2001) assessed encouragement, involvement, and communication. Cronbach’s alphas were .76 to .84 across waves. An adapted version of the Ineffective Arguing Inventory (Kurdek, 1994) was used to assess the extent to which parents and adolescents resolved rather than extended their conflicts. High scores indicated unresolved conflicts. Cronbach’s alphas were .75 to .79 across waves. Both scales were averaged across the five assessment waves and standardized (r = −.60, p < .01). The Ineffective Arguing Inventory scores were subtracted from the Supportive-Involved Parenting scores to describe parenting characterized by high levels of support and involvement and by low levels of unresolved conflict.
MRI acquisition
Imaging data were collected using a GE Signa HDxt 3-Tesla scanner (GE Healthcare, Chicago, IL) at the University of Georgia’s Bio-Imaging Research Center. Structural imaging consisted of a high-resolution T1-weighted, fast-spoiled gradient-echo scan (repetition time = 7.8 ms, echo time = 3.1 ms, flip angle = 20°, field of view = 25.6 cm, matrix = 256 × 256, 160 contiguous 1-mm axial slices, voxel size = 1 mm3). Whole-brain functional images were acquired using T2* echo-planar imaging with a single-shot gradient-echo pulse sequence (repetition time = 2,000 ms, echo time = 25 ms, flip angle = 90°, field of view = 225 × 225 mm, matrix = 64 × 64, 38 contiguous 3.5-mm axial slices, voxel size = 3.5 mm3). The resting-state paradigm consisted of two 4-min imaging runs of 120 brain volumes each. Participants were instructed to keep their eyes open, look at a fixation cross, and allow their minds to wander freely.
Image analysis
Image processing
We preprocessed fMRI data using Analysis of Functional Neuroimages (AFNI) software (Cox, 1996). Functional data sets were despiked, slice time shift corrected, and aligned to T1 data sets before being warped into Montreal Neurological Institute standardized space. The first four volumes of each run were removed to allow the scanner to reach steady state. Volumes with greater than 25% of voxels identified as outliers or intervolume movement greater than 0.2 mm along any axis were censored. Data were spatially smoothed using a 6-mm full-width half-maximum Gaussian filter and masked to exclude voxels outside the brain. Band-pass filtering was applied to remove low and high frequency noise (0.009–0.08 Hz), and motion correction was accomplished by including the six standard (de-meaned) motion parameters and their temporal derivatives as regressors of no interest. No additional nuisance regressors were included other than those described above. Global signal regression, in particular, is controversial and not recommended (Anderson et al., 2011; Murphy, Birn, Handwerker, Jones, & Bandettini, 2009; Saad et al., 2012; for a review, see Power et al., 2014). Although some other nuisance regressors such as white-matter signal may reduce noise variance incrementally, censoring and the strict framewise displacement threshold (0.2 mm) used in this study improved data quality while limiting the degrees of freedom lost in the regression model (Power et al., 2014).
Functional-connectivity analyses
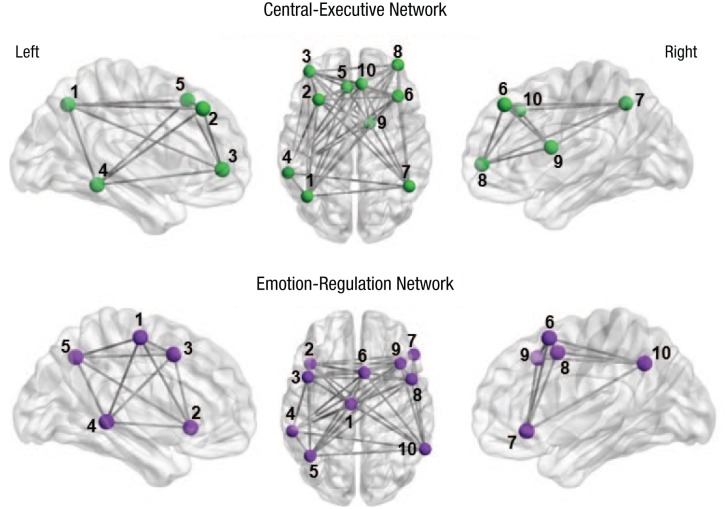
Raw time-series data for each voxel were de-meaned and converted to percentage-signal-change scores to reduce variability between scanner sessions. Region-of-interest (ROI) seed data were then calculated as the average percentage signal change for all voxels contained in a given region at each time point (i.e., each volume acquisition). We quantified rsFC using the correlation of the average time series in each ROI with the average time series in all other voxels of the other ROIs in the network over time using Pearson’s rs. To accomplish this, we converted r values to z scores using Fisher’s r-to-z transformation and averaged them per ROI. Finally, the z scores of all possible connections were averaged to compute a final value reflecting the connectivity of all possible nodes of the network. For the CEN, the ROIs were selected a priori using left and right CENs that Shirer, Ryali, Rykhlevskaia, Menon, and Greicius (2012) created. For the ERN, ROIs were selected a priori from an activation-likelihood-estimation meta-analysis by Kohn et al. (2014), who identified an ERN across 23 studies and 479 participants. For each ROI, a 5-mm sphere was placed around the coordinates at the center of mass. Table 1 presents the coordinates and labels for each ROI, and Figure 1 presents axial and sagittal views of the ROIs for the CEN and ERN networks.
Table 1.
Regions of Interest in the Central-Executive and Emotion-Regulation Networks
| Region | MNI coordinates (x, y, z) |
|---|---|
| Central-executive network | |
| 1. Left inferior parietal lobule | −42, –63, 46 |
| 2. Left middle frontal gyrus | −32, 23, 49 |
| 3. Left middle frontal gyrus | −40, 48, –01 |
| 4. Left middle temporal gyrus | −59, 42, 12 |
| 5. Left medial frontal gyrus | −07, 34, 43 |
| 6. Right middle frontal gyrus | 38, 26, 42 |
| 7. Right inferior parietal lobule | 48, –54, 47 |
| 8. Right middle frontal gyrus | 38, 54, 01 |
| 9. Right caudate | 13, 02, 14 |
| 10. Right medial frontal gyrus | 06, 37, 46 |
| Emotion-regulation network | |
| 1. Left somatomotor area | −06, 14, 58 |
| 2. Left inferior frontal gyrus | −42, 22, –06 |
| 3. Left precentral gyrus | −44, 10, 46 |
| 4. Left middle temporal gyrus | −58, –38, –02 |
| 5. Left angular gyrus | −42, –60, 44 |
| 6. Right somatomotor area | 06, 14, 58 |
| 7. Right inferior frontal gyrus | 50, 30, –08 |
| 8. Right precentral gyrus | 48, 08, 48 |
| 9. Right middle temporal gyrus | 38, 22, 44 |
| 10. Right angular gyrus | 60, –54, 40 |
Note: For each region of interest (i.e., node) within a network, we placed a 5-mm sphere around the center-of-mass coordinates for each discrete cluster, separately within the left and right hemispheres. The numbers in the Region column correspond to the nodes depicted visually in Figure 1. MNI = Montreal Neurological Institute.
Fig. 1.
Axial (center) and left- and right-hemisphere sagittal views of regions of interest for the resting-state functional connectivity networks. For each region of interest (i.e., node) within a network, we placed a 5-mm sphere around the coordinates of peak activation for each discrete cluster, separately within the left- and right-hemisphere masks. The numbers refer to regions of interest referenced in Table 1.
Results
We tested the hypothesis that supportive parenting would ameliorate the association of years exposed to family poverty during adolescence with CEN and ERN rsFC. Hierarchical multiple regression analyses were conducted and included main effects of family poverty, supportive parenting, and the interaction of family poverty and supportive parenting on rsFC. Sex was statistically controlled in all models. Interactions were interpreted by plotting the estimated levels of CEN and ERN rsFC by years in poverty and levels of supportive parenting (low = 1 SD below the mean, high = 1 SD above the mean). The results of these analyses are presented in Table 2. A main effect emerged for family poverty on ERN rsFC, and an interaction effect emerged between family poverty and supportive parenting on both CEN rsFC, F(1, 86) = 9.421, p = .003, ΔR2 = .094, and ERN rsFC, F(1, 86) = 3.981, p = .049, ΔR2 = .040. These interactions are depicted in Figures 2a and 2b. Simple-slopes analyses indicated that more time living in poverty across adolescence, from ages 11 to 17 years, was associated with less CEN rsFC (b = −0.028, 95% confidence interval, or CI = [–0.047, –0.010]; β = −0.390, 95% CI = [−0.645, −0.134]; p = .003) and less ERN rsFC (b = −0.036, 95% CI = [–0.056, −0.015]; β = –0.436, 95% CI = [−0.690, −0.182]; p = .001) among young adults who experienced low levels of supportive parenting. Poverty was not associated with rsFC of these networks among participants who experienced high levels of supportive parenting (CEN rsFC: b = 0.015, 95% CI = [−0.007, 0.038]; β = 0.212, 95% CI = [−0.099, 0.522]; p = .179; and ENR rsFC: b = −0.004, 95% CI = [−0.029, 0.021]; β = –0.047, 95% CI = [−0.356, 0.262]; p = .762). Additional analyses tested the association between participants’ current incomes at age 25 years and CEN and ERN rsFC. No significant effects were detected, and none of the findings changed when this association was included in the models as a covariate.
Table 2.
Family Poverty and Supportive Parenting as Predictors of Central-Executive and Emotion-Regulation Network Resting-State Functional Connectivity (rsFC) at Age 25 Years
| Central-executive network rsFC | Emotion-regulation network rsFC | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictor | b | 95% CI | β | 95% CI | b | 95% CI | β | 95% CI |
| Sex (male) | 0.022 | [–0.025, 0.069] | 0.094 | [–0.106, 0.294] | 0.013 | [–0.040, 0.066] | 0.049 | [–0.150, 0.248] |
| Supportive parenting (ages 11–17 years) | 0.007 | [–0.006, 0.020] | 0.107 | [–0.096, 0.309] | 0.009 | [–0.006, 0.024] | 0.121 | [–0.080, 0.323] |
| Family poverty (ages 11–17 years) | −0.006 | [–0.021, 0.009] | −0.089 | [–0.296, 0.118] | −0.020 | [–0.036, –0.003] | −0.242* | [–0.448, –0.036] |
| Supportive Parenting × Family Poverty | 0.012 | [0.004, 0.020] | 0.314** | [0.111, 0.518] | 0.009 | [0.000, 0.018] | 0.203* | [0.001, 0.406] |
Note: CI = confidence interval.
p < .05. **p < .01.
Fig. 2.
Youths’ (a) central-executive network resting-state functional connectivity (rsFC) and (b) emotion-regulation network rsFC as a function of number of years during adolescence spent in poverty and level of supportive parenting. Regression lines are shown for low (1 SD below the mean) and high (1 SD above the mean) levels of supportive parenting. Statistics in parentheses refer to simple-slopes analyses. CI = confidence interval.
Discussion
Adolescence is a period of increased neural plasticity and reorganization (Casey et al., 2005) when neural systems are particularly sensitive to social influences (Blakemore & Mills, 2014; Knoll et al., 2015). This increase in social salience may make adolescents’ neurodevelopment susceptible to both the stresses and strains of living in poverty and the stress-buffering effects of supportive parenting. Against this developmental backdrop, we tested a stress-buffering hypothesis that more years living in poverty across adolescence would interact with receipt of supportive parenting to forecast the rsFC in the CEN and ERN, large neural networks that support cognitive processes affected by poverty and critical for achievement, decision making, affective control, and mental health (Grayson & Fair, 2017). The results indicated that supportive parenting ameliorated the impact of living in poverty during adolescence on the rsFC in both the CEN and ERN years later, during emerging adulthood. These results did not change when personal income at age 25 years was included as a covariate.
To the best of our knowledge, this is the first study to present data on prospective associations between living in poverty during adolescence and rsFC in large neural networks and on the benefits of supportive parenting in buffering this process. Conceptually, these results converge with parenting effects commonly observed in animal models, wherein maternal caregiving tendencies exert lasting influences on offspring physiology, particularly in the brain, endocrine, and immune systems (Miller et al., 2011). The study is also unique because (a) it is the first to include repeated assessments across adolescence of youths’ poverty status and concurrent assessments of supportive parenting and (b) the sample was composed of African American youths, a group that has been underrepresented in neuroscience research.
These findings raise the following question: How does supportive parenting lead young people who grow up in poverty to display particular patterns of neurodevelopment? How this occurs is explicable theoretically in terms of straightforward socialization and observational learning mechanisms. When parents engage in supportive interactions with adolescents, they demonstrate cognitive control and problem-solving skills that adolescents learn through observation and modeling (Morris, Silk, Steinberg, Myers, & Robinson, 2007). This could occur directly in the context of supportive transactions between parent and adolescent or indirectly as the adolescent observes a parent interacting with other people. To the extent that adolescents have multiple opportunities over time to see planful solutions demonstrated, they will be more likely to approach problems with the belief that they can be solved with deliberate, planful, and direct action and less likely to cope with problems through avoidance or negative emotions such as anger (Brody, 2016). Consistent with this formulation is research showing that parental modeling and encouragement of self-regulatory strategies and skills, along with children’s use of them in their everyday lives, presage adolescents’ self-regulation abilities, which the CEN and ERN support. This suggests that adolescents pick up cues from their parents’ regulatory abilities that are incorporated into their own everyday behavior and into neuroregulatory systems that are at least partially contextually determined (Shonkoff et al., 2012; Telzer et al., 2014).
Conclusions drawn from the current study should be viewed in light of some limitations. First, a weakness of the study is the one-time neuroimaging assessment. A second neuroimaging assessment of the CEN and ERN would have allowed us to address a rival reverse-causality hypothesis for the study findings. This hypothesis suggests that what was observed in the present study were the effects of traitlike individual differences in youths (rsFC of the CEN and ERN) on the effects of living in poverty and supportive family environments rather than the reverse. A reverse-causation scenario might be plausible here, although it would not parsimoniously explain the interaction that we observed. If the rsFC of the CEN and ERN of the emerging adults were affecting supportive family environments, why would this be evident only among youths who spent more of their adolescent years living in poverty and not among their economically advantaged counterparts? This study is also subject to omitted-variable biases owing to the self-report assessments of income-to-needs ratios and supportive parenting. Despite the well-established predictive validity of these assessments, self-reports could be a proxy for other processes such as allelic variation and affective states that could also be involved in the prediction of large neural networks. A multiwave longitudinal study is needed that includes multiple waves of neuroimaging, along with multimethod assessments of the study constructs, to address these limitations. A study such as this could also help answer questions we were unable to address here, such as the contributions of supportive parenting to continuity or change in neural networks when adolescents grow up in rural poverty. Finally, it is not known whether these findings would generalize to urban African American youths or to members of other racial or ethnic groups. These limitations notwithstanding, the present study demonstrated that receipt of supportive parenting ameliorated the association between living in poverty and the coherence of important neural networks for youths in challenging rural circumstances.
Acknowledgments
The content of this article is the sole responsibility of the authors and does not necessarily represent the official positions of the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, or the National Institutes of Health.
Footnotes
Action Editor: Erika E. Forbes served as action editor for this article.
Author Contributions: G. H. Brody drafted the manuscript. T. Yu, R. Nusslock, A. W. Barton, G. E. Miller, E. Chen, C. Holmes, M. McCormick, and L. H. Sweet provided critical revisions. T. Yu analyzed the data under G. H. Brody’s supervision. All the authors approved the final manuscript for submission.
ORCID iDs: Gene H. Brody  https://orcid.org/0000-0002-2244-6797
https://orcid.org/0000-0002-2244-6797
Allen W. Barton  https://orcid.org/0000-0002-8888-2612
https://orcid.org/0000-0002-8888-2612
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: This research was supported by Award No. R01 HD030588 from the National Institute of Child Health and Human Development and Award No. P30 DA027827 from the National Institute on Drug Abuse to G. H. Brody.
Open Practices: Data and materials for this study have not been made publicly available, and the design and analysis plans were not preregistered. Researchers wishing to review the data should e-mail the corresponding author at gbrody@uga.edu, who will make the data available on execution of an appropriate data-sharing agreement.
References
- Anderson J. S., Druzgal T. J., Lopez-Larson M., Jeong E.-K., Desai K., Yurgelun-Todd D. (2011). Network anticorrelations, global regression, and phase-shifted soft tissue correction. Human Brain Mapping, 32, 919–934. doi: 10.1002/hbm.21079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomeusz C. F., Whittle S. L., Pilioussis E., Allott K., Rice S., Schäfer M. R., . . . Amminger G. P. (2014). Relationship between amygdala volume and emotion recognition in adolescents at ultra-high risk for psychosis. Psychiatry Research: Neuroimaging, 224, 159–167. doi: 10.1016/j.pscychresns.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Blair C., Raver C. C. (2012). Individual development and evolution: Experiential canalization of self-regulation. Developmental Psychology, 48, 647–657. doi: 10.1037/a0026472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.-J., Mills K. L. (2014). Is adolescence a sensitive period for sociocultural processing? Annual Review of Psychology, 65, 187–207. doi: 10.1037/a0026472 [DOI] [PubMed] [Google Scholar]
- Brody G. H. (2016). Family-centered prevention for rural African Americans: The Strong African American Families Program (SAAF), the Strong African American Families–Teen Program (SAAF–T), and the Adults in the Making Program (AIM). In Van Ryzin M. J., Kumpfer K. L., Fosco G. M., Greenberg M. T. (Eds.), Family-based prevention programs for children and adolescents: Theory, research, and large-scale dissemination (pp. 282–307). New York, NY: Psychology Press. [Google Scholar]
- Brody G. H., Ge X., Conger R. D., Gibbons F. X., Murry V. M., Gerrard M., Simons R. L. (2001). The influence of neighborhood disadvantage, collective socialization, and parenting on African American children’s affiliation with deviant peers. Child Development, 72, 1231–1246. doi: 10.1111/1467-8624.00344 [DOI] [PubMed] [Google Scholar]
- Brody G. H., Yu T., Beach S. R. H. (2016). Resilience to adversity and the early origins of disease. Development and Psychopathology, 28(4, Pt. 2), 1347–1365. doi: 10.1017/S0954579416000894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G. H., Yu T., Chen Y.-F., Kogan S. M., Evans G. W., Beach S. R. H., . . . Philibert R. A. (2013). Cumulative socioeconomic status risk, allostatic load, and adjustment: A prospective latent profile analysis with contextual and genetic protective factors. Developmental Psychology, 49, 913–927. doi: 10.1037/a0028847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B. J., Galván A., Hare T. A. (2005). Changes in cerebral functional organization during cognitive development. Current Opinion in Neurobiology, 15, 239–244. doi: 10.1016/j.conb.2005.03.012 [DOI] [PubMed] [Google Scholar]
- Cisler J. M., Olatunji B. O., Feldner M. T., Forsyth J. P. (2010). Emotion regulation and the anxiety disorders: An integrative review. Journal of Psychopathology and Behavioral Assessment, 32, 68–82. doi: 10.1007/s10862-009-9161-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173. doi: 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K. (2014). Family matters: Intergenerational and interpersonal processes of executive function and attentive behavior. Current Directions in Psychological Science, 23, 230–236. doi: 10.1177/0963721414531597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. W., Schamberg M. A. (2009). Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences, USA, 106, 6545–6549. doi: 10.1073/pnas.0811910106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah M. J., Shera D. M., Savage J. H., Betancourt L., Giannetta J. M., Brodsky N. L., . . . Hurt H. (2006). Childhood poverty: Specific associations with neurocognitive development. Brain Research, 1110, 166–174. doi: 10.1016/j.brainres.2006.06.072 [DOI] [PubMed] [Google Scholar]
- Gianaros P. J., Manuck S. B., Sheu L. K., Kuan D. C. H., Votruba-Drzal E., Craig A. E., Hariri A. R. (2011). Parental education predicts corticostriatal functionality in adulthood. Cerebral Cortex, 21, 896–910. doi: 10.1093/cercor/bhq160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E. M., Scheibel R. S., Zambrano-Vazquez L., Jia-Richards M., May G. J., Meyer E. C., Nelson S. M. (2018). High-fidelity measures of whole-brain functional connectivity and white matter integrity mediate relationships between traumatic brain injury and post-traumatic stress disorder symptoms. Journal of Neurotrauma, 35, 767–779. doi: 10.1089/neu.2017.5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C., Sun H., Petersen S. E. (2018). Control networks and hubs. Psychophysiology, 55, 1–18. doi: 10.1111/psyp.13032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson D. S., Fair D. A. (2017). Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. NeuroImage, 160, 15–31. doi: 10.1016/j.neuroimage.2017.01.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. B., Riis J. L., Noble K. G. (2016). State of the art review: Poverty and the developing brain. Pediatrics, 137(4), Article e20153075. doi: 10.1542/peds.2015-3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P., Evans G. W., Angstadt M., Ho S. S., Sripada C. S., Swain J. E., . . . Phane K. L. (2013). Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proceedings of the National Academy of Sciences, USA, 110, 18442–18447. doi: 10.1073/pnas.1308240110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll L. J., Magis-Weinberg L., Speekenbrink M., Blakemore S.-J. (2015). Social influence on risk perception during adolescence. Psychological Science, 26, 583–592. doi: 10.1073/pnas.1308240110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N., Eickhoff S. B., Scheller M., Laird A. R., Fox P. T., Habel U. (2014). Neural network of cognitive emotion regulation—An ALE meta-analysis and MACM analysis. NeuroImage, 87, 345–355. doi: 10.1073/pnas.1308240110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdek L. A. (1994). Conflict resolution styles in gay, lesbian, heterosexual nonparent, and heterosexual parent couples. Journal of Marriage and the Family, 56, 705–722. doi: 10.2307/352880 [DOI] [Google Scholar]
- Langeslag S. J. E., Schmidt M., Ghassabian A., Jaddoe V. W., Hofman A., Lugt A., . . . White T. J. H. (2013). Functional connectivity between parietal and frontal brain regions and intelligence in young children: The Generation R study. Human Brain Mapping, 34, 3299–3307. doi: 10.1002/hbm.22143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J. L., Belden A., Harms M. P., Tillman R., Barch D. M. (2016). Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proceedings of the National Academy of Sciences, USA, 113, 5742–5747. doi: 10.1073/pnas.1601443113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani A., Mullainathan S., Shafir E., Zhao J. (2013). Poverty impedes cognitive function. Science, 341, 976–980. doi: 10.1126/science.1238041 [DOI] [PubMed] [Google Scholar]
- Miller G. E., Chen E., Yu T., Brody G. H. (2017). Metabolic syndrome risks following the Great Recession in rural Black young adults. Journal of the American Heart Association, 6(9), Article e006052. doi: 10.1161/JAHA.117.006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. E., Lachman M. E., Chen E., Gruenewald T. L., Karlamangla A. S., Seeman T. E. (2011). Pathways to resilience: Maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychological Science, 22, 1591–1599. doi: 10.1161/JAHA.117.006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. S., Criss M. M., Silk J. S., Houltberg B. J. (2017). The impact of parenting on emotion regulation during childhood and adolescence. Child Development Perspectives, 11, 233–238. doi: 10.1111/cdep.12238 [DOI] [Google Scholar]
- Morris A. S., Silk J. S., Steinberg L., Myers S. S., Robinson L. R. (2007). The role of the family context in the development of emotion regulation. Social Development, 16, 361–388. doi: 10.1111/j.1467-9507.2007.00389.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Birn R. M., Handwerker D. A., Jones T. B., Bandettini P. A. (2009). The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? NeuroImage, 44, 893–905. doi: 10.1016/j.neuroimage.2008.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K. N., Silvers J. A., Buhle J. T. (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J. D., Mitra A., Laumann T. O., Snyder A. Z., Schlaggar B. L., Petersen S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage, 84, 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M. E. (2015). The restless brain: How intrinsic activity organizes brain function. Philosophical Transactions of the Royal Society B, 370(1668), Article 20140172. doi: 10.1098/rstb.2014.0172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad Z. S., Gotts S. J., Murphy K., Chen G., Jo H. J., Martin A., Cox R. W. (2012). Trouble at rest: How correlation patterns and group differences become distorted after global signal regression. Brain Connectivity, 2, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchet M. D., Ho T. C., Connolly C. G., Tymofiyeva O., Lewinn K. Z., Han L. K. M., Yang T. T. (2016). Large-scale hypoconnectivity between resting-state functional networks in unmedicated adolescent major depressive disorder. Neuropsychopharmacology, 41, 2951–2960. doi: 10.1038/npp.2016.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. E., Rudie J. D., Pfeifer J. H., Masten C. L., McNealy K., Dapretto M. (2014). Development of the default mode and central executive networks across early adolescence: A longitudinal study. Developmental Cognitive Neuroscience, 10, 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer W. R., Ryali S., Rykhlevskaia E. I., Menon V., Greicius M. D. (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex, 22, 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff J. P., Garner A. S., The Committee on Psychosocial Aspects of Child and Family Health, Committee on Early Childhood, Adoption, and Dependent Care, and Section on Developmental and Behavioral Pediatrics, Siegel B. S., Dobbins M. I., Earls M. F., . . . Wood D. L. (2012). The lifelong effects of early childhood adversity and toxic stress. Pediatrics, 129(1), e232–e246. doi: 10.1542/peds.2011-2663 [DOI] [PubMed] [Google Scholar]
- Telzer E. H., Qu Y., Goldenberg D., Fuligni A. J., Galván A., Lieberman M. D. (2014). Adolescents’ emotional competence is associated with parents’ neural sensitivity to emotions. Frontiers in Human Neuroscience, 8, Article 558. doi: 10.3389/fnhum.2014.00558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L. Q. (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience, 16, 55–61. [DOI] [PubMed] [Google Scholar]
- Whittle S., Vijayakumar N., Simmons J. G., Dennison M., Schwartz O., Pantelis C., . . . Allen N. B. (2017). Role of positive parenting in the association between neighborhood social disadvantage and brain development across adolescence. JAMA Psychiatry, 74, 824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]