Abstract
Obesity is thought to cause ill health because of the biological strain that excess fat has on physiological function. We tested an alternative explanation in a population-based sample of 3,609 older English adults—that the pervasive discrimination experienced by individuals with excess weight may in part explain why obesity is associated with subsequent multisystem physiological dysregulation, measured via clinical indicators of cardiovascular, metabolic, and immune function. We found that both obesity and perceived weight discrimination predicted an increase in physiological dysregulation from baseline to follow-up 4 years later. Perceived discrimination because of body weight experienced by individuals with obesity explained more than one quarter of the prospective association between obesity and a deterioration in biomarkers of health status. These findings highlight the possibility that the stigma experienced by individuals with obesity may play an important role in explaining the obesity-related disease burden.
Keywords: obesity, weight discrimination, obesity stigma, dysregulation, allostasis, longitudinal research
The prevalence of obesity continues to rise globally and has been paralleled by dramatic increases in related health comorbidities, disability, and reduced quality of life (GBD 2015 Obesity Collaborators, 2017). Relations between obesity and critical health outcomes, including diabetes, hypertension, cardiovascular disease, and premature mortality, are well documented (e.g., Guh et al., 2009). Until recently (Hunger & Major, 2015; Robinson, Sutin, & Daly, 2017; Tomiyama et al., 2018), the health-damaging effects of obesity have been assumed to result from the direct impact of excess adipose tissue on a series of biological processes, including insulin resistance, glucose intolerance, raised blood pressure, low-grade inflammation, and dyslipidemia (Kahn, Hull, & Utzschneider, 2006; Van Gaal, Mertens, & De Block, 2006). However, there are other plausible mechanisms that may explain why obesity is associated with deterioration of physiological functioning and biological ill health, one of which we focus on here: the widespread discrimination and stigma experienced by individuals living with obesity.
Negative attitudes toward persons with obesity are widespread in contemporary society, as evidenced by disparaging media portrayals (e.g., unpopular, unattractive) and negative beliefs (e.g., incompetent, lazy) held by the public and health-care professionals (Pearl, 2018; Puhl & Heuer, 2009). Such weight-biased stereotypes may perpetuate the social devaluation, denigration, and unjust treatment of people with obesity identified within educational, employment, and health-care settings (Pearl, 2018). Perhaps because of this, in recent years there has been a marked increase in individuals reporting unfair treatment based on their body weight, otherwise known as perceived weight discrimination (Andreyeva, Puhl, & Brownell, 2008). This is particularly concerning because weight-based discrimination has been conceptualized as a pervasive social stressor that may connect obesity to poor mental and physical health (Daly, Robinson, & Sutin, 2017; Hunger & Major, 2015; Tomiyama, 2014).
Abundant evidence has linked weight-based discrimination to a host of significant psychosocial consequences, including increased risk of loneliness, diminished self-worth, depression, and anxiety (Hatzenbuehler, Keyes, & Hasin, 2009; Robinson et al., 2017; Sutin, Stephan, et al., 2016). In addition to these damaging mental-health effects, recent findings have tied weight discrimination to unfavorable physical health outcomes, including weight gain, declines in subjective health and mobility, greater disease burden, and premature mortality (Schafer & Ferraro, 2011; Sutin, Stephan, & Terracciano, 2015). Prominent theoretical models have suggested that heightened psychological and physiological stress responses to perceived weight discrimination may explain why these experiences are so closely related to poor physical health, but formal testing of this proposition has been limited (Pascoe & Smart Richman, 2009; Tomiyama, 2014).
Experimental studies have shown that exposure to weight-stigmatizing content can induce acute increases in autonomic system and hypothalamic-pituitary-adrenal axis activation, as gauged by raised blood pressure (Major, Eliezer, & Rieck, 2012) and increased cortisol reactivity (Himmelstein, Incollingo Belsky, & Tomiyama, 2015; Schvey, Puhl, & Brownell, 2014). Short-term laboratory studies also suggest that the stigma and stress attached to feeling overweight may compromise self-regulation, leading to increased caloric intake (Vartanian & Porter, 2016). Observational studies have also linked experiences of weight-based discrimination to overeating and unhealthy eating (e.g., Sutin, Robinson, Daly, & Terracciano, 2016) and elevated hair cortisol levels (Jackson, Kirschbaum, & Steptoe, 2016), an indicator of prolonged stress exposure. Taken together, these studies provide initial evidence to suggest that weight stigma may impact cardiovascular and neuroendocrine function and appetite regulation, potentially generating pronounced downstream physiological effects across multiple bodily systems (Epel, Lapidus, McEwen, & Brownell, 2001; Tomiyama, 2014). Indeed, recent evidence has linked sustained unfair treatment based on body weight to increased physiological dysregulation, as gauged by raised inflammation levels (Sutin, Stephan, Luchetti, & Terracciano, 2014), impaired glycemic control (Potter et al., 2015), and lipid and metabolic dysregulation (Vadiveloo & Mattei, 2017).
Despite this evidence, it is not yet known whether the widespread experience of weight-based discrimination among people with obesity explains why obesity produces long-term changes in objective indicators of human health. To date, studies examining the negative health effects of unfair treatment based on body weight have tended to statistically adjust for body mass index (BMI), thus emphasizing that weight-based discrimination may have adverse effects over and above those of obesity. A small number of studies have demonstrated that weight-based discrimination and concerns about weight mediate the cross-sectional association between body weight and subjective health (e.g., Hunger & Major, 2015; Jackson, Beeken, & Wardle, 2015). However, such studies rely on self-reported measures, which are prone to bias, and their cross-sectional nature cannot rule out the distinct possibility of reverse causality.
The aim of the current study was therefore, first, to examine the relationship over 4 years between obesity and longitudinal increases in biological ill health in the form of physiological dysregulation. We operationalized dysregulation using a combination of objective clinical indicators of cardiovascular, inflammatory, and lipid and metabolic dysregulation considered indicative of allostatic load (Hampson, Goldberg, Vogt, Hillier, & Dubanoski, 2009; Juster, McEwen, & Lupien, 2010). Allostatic-load theory suggests that such dysregulation measures can capture the downstream biological toll of repeated attempts to adapt to a chronic stressor, such as weight discrimination, which over time may produce an accumulation of wear and tear across multiple physiological systems (Seeman, McEwen, Rowe, & Singer, 2001). More importantly, we also aimed to use our integrative measure of biological risk to test whether the physiological consequences of obesity may be, at least in part, attributable to the stress of weight discrimination. Specifically, for the first time, we tested whether perceived weight-based discrimination forecasts longitudinal changes in physiological dysregulation and then examined whether these discriminatory experiences mediate the prospective association between obesity and dysregulation in a large population-based study of older English adults.
Method
Participants
This study drew on data from the English Longitudinal Study of Ageing (ELSA), an ongoing, prospective observational study to analyze the health and aging of older (≥ 50 years), noninstitutionalized men and women in England. The ELSA cohort was recruited from three waves of the Health Survey for England (1998, 1999, and 2001), an annual, nationally representative, cross-sectional household survey based on a stratified random sample of English households. Initial participants were recruited if they were born before March 1, 1952, and were interviewed as part of Wave 1 in 2002–2003. The sociodemographic characteristics of the ELSA sample have been shown to align broadly with the results of the national census. Further information on the ELSA measures and sampling procedures is detailed elsewhere (Steptoe, Breeze, Banks, & Nazroo, 2012). Since 2002, participants have been followed up with face-to-face interviews in participants’ own homes every 2 years and clinical assessments, including blood-sample analysis, in alternate ELSA waves every 4 years. Refreshment samples were added as part of each wave since 2006 in order to maintain sample size and representativeness. Participants provided informed consent to take part in ELSA, and ethical approval was obtained from the London Multicentre Research Ethics Committee.
For the purposes of the current study, the baseline sample is individuals with available height and weight measurements from the Wave 4 clinical assessment (2008–2009). We examined longitudinal change in physiological dysregulation over 4 years from baseline (Wave 4) to follow-up (Wave 6: 2012–2013). Experiences of weight discrimination were assessed as part of the Wave 5 (2010–2011) interview. We restricted our sample to individuals with available data on baseline BMI, weight discrimination, and physiological dysregulation at baseline and follow-up (for sample characteristics, see Table 1). Of the 6,121 participants with baseline (Wave 4) BMI and dysregulation data, 848 did not have information on weight-based discrimination from Wave 5, 1,577 were excluded because they did not have information on physiological dysregulation levels at follow-up, and 87 were missing additional covariate or survey weight data. There was no difference in gender or obesity between individuals with baseline but not follow-up data. However, participants included in the final analytic sample (N = 3,609) were more likely to be younger, to be White, and to have greater household wealth and were less likely to have been diagnosed with lung disease or to have high levels of depressive symptoms (all ps < .01). Individuals included in the sample also had lower levels of baseline physiological dysregulation.
Table 1.
Basic Demographic Characteristics and Descriptive Statistics
| Variable | Full sample (N = 3,609) |
Participants with obesity (n = 1,122) |
Participants without obesity (n = 2,487) |
|---|---|---|---|
| Age (years) | M = 64.78 (SD = 9.24) | M = 64.26 (SD = 8.94) | M = 65.03 (SD = 9.36) |
| Female | 52.7% | 56.8% | 50.8% |
| White | 96.2% | 96.3% | 96.2% |
| Hold a degree | 16.0% | 12.2% | 17.8% |
| Wealth quintilea | M = 3.07 (SD = 1.37) | M = 2.77 (SD = 1.31) | M = 3.21 (SD = 1.37) |
| BMI baseline (kg/m2) | M = 28.22 (SD = 5.05) | M = 34.07 (SD = 4.10) | M = 25.60 (SD = 2.68) |
| Perceive weight discrimination | 4.6% | 13.0% | 0.8% |
Note: BMI = body mass index.
Net household wealth ranged from 1 (lowest quintile) to 5 (highest quintile).
We used inverse probability weighting to account for potential bias due to selective attrition. Specifically, we used all baseline covariate data to estimate each participant’s predicted probability of retention at follow-up from a logistic regression model. We then calculated weights that were inversely proportional to the probability of retention. Applying these weights to our analyses provided a correction for the baseline differences observed between participants lost to follow-up and those retained in the analytic sample. For example, those with baseline characteristics associated with a reduced likelihood of retention (e.g., low wealth) were assigned larger weights to compensate for the underrepresentation of this group in the final sample. These weights were combined with the ELSA Wave 4 clinical assessment weights to account for baseline nonresponse and more accurately represent the characteristics of the population.
Measures
Anthropometry
Objective anthropometric measurements were taken by trained nurses as part of the Wave 4 clinical assessment. Weight without shoes and in light clothing was recorded to the nearest 0.1 kg using Tanita THD-305 portable electronic scales (Tanita Corporation, Arlington Heights, IL). A portable stadiometer was used to measure standing height without shoes to the nearest millimeter. Each participant’s BMI was calculated as weight (in kilograms) divided by height (in meters squared), and obesity was defined as a BMI greater than or equal to 30 kg/m2.
Perceived weight discrimination
Weight-based discrimination was measured using a version of the Perceived Everyday Experiences With Discrimination Scale (Williams, Yu, Jackson, & Anderson, 1997), administered in ELSA. Participants were first asked, “In your day-to-day life, how often have any of the following things happened to you?” Participants then rated how frequently they encountered a set of five discriminatory experiences (e.g., “you are treated with less respect or courtesy,” “you are threatened or harassed”) on a scale from 1 (almost every day) to 6 (never). Participants then identified the reasons that they were treated unfairly from a list of options that included weight. Perceived weight discrimination was defined as reporting any form of discrimination and attributing this to weight.
Physiological-dysregulation index
As part of the Wave 4 and Wave 6 follow-up assessments, participants were visited in their homes by a trained nurse who collected biomarker data. In line with prior research (e.g., Daly et al., 2017; Hampson et al., 2009), we drew on biomarkers of cardiovascular, immune, and metabolic system functioning to generate a composite index of physiological dysregulation indicative of allostatic load (Seeman et al., 2001). Cardiovascular measures were systolic and diastolic blood pressure and resting pulse rate. Inflammation levels were assessed using C-reactive protein and white-blood-cell count. Lipid and metabolic dysregulation were assessed with the ratio of total blood cholesterol to high-density lipoprotein (HDL) cholesterol, triglyceride levels, and glucose and glycated hemoglobin levels (hemoglobin A1c).
As part of the clinical assessment, nurses followed standardized procedures to assess participants’ resting blood pressure and pulse rate from the right arm using an Omron HEM-907 oscillometric blood pressure monitor (Omron Corporation, Kyoto, Japan). Three readings were taken, and average levels from the last two readings were used in the current analysis. Blood samples were drawn from participants who gave consent, except for participants with clotting or bleeding disorders, those with a history of fits or convulsions, or those on anticoagulant medications (e.g., Warfarin). Blood samples were analyzed following the technical specifications and quality-control guidelines outlined in the Health Survey of England technical report (Craig, Deverill, & Pickering, 2006).
We employed the z-score method (Hampson et al., 2009; Juster et al., 2010) to assess physiological-dysregulation levels. This involved averaging the computed z scores for each biomarker in order to place all biomarkers onto a common scale and generate a single continuous summary score. Although z scores have been used in a broad set of studies, they appear less frequently than traditional count-based scores in the allostatic-load literature (Hampson et al., 2009; Juster et al., 2010; Seplaki, Goldman, Glei, & Weinstein, 2005). Count-based scores are calculated by summing the number of biomarkers for which an individual scores above a high-risk percentile threshold of biomarker values (e.g., upper 25th percentile) at a given time point. Such count-based formulations are insensitive to mean-level changes in dysregulation levels over time. In predictive models, z scores have shown performance that is equivalent to or superior than count-based scores (Seplaki et al., 2005), and z scores can be integrated into analyses examining longitudinal change. We therefore combined biomarker z scores to produce a summary score that captured the full continuum of biological risk associated with multisystem physiological dysregulation.
To conduct longitudinal analyses, one must calculate biomarker z scores across both time points together to ensure that changes over time are retained. We followed this approach, and to maximize the sample size, we included ELSA participants if they provided data on at least three quarters of the biological measures, and remaining missing observations (< 6% of cases) were replaced with mean imputation. Individual-biomarker z scores were then averaged to generate a normally distributed physiological-dysregulation index, which was restandardized across both time points to retain longitudinal changes and produce an index with a mean of 0 and a standard deviation of 1.
In keeping with the allostatic-load literature (Juster et al., 2010), we refer to our outcome measure as an index rather than a scale. This index taps a broad set of biomarkers to assess cumulative dysregulation across multiple biological systems (Seeman et al., 2001). Individual biomarkers are not assumed to be empirically correlated when a cumulative index is constructed (Streiner, 2003). We observed an average correlation of .51 between the individual biomarkers and the overall index (see Table S1 in the Supplemental Material available online), and the test-retest reliability for the physiological-dysregulation index was .68, r(3607) = .68, p < .01.
Covariates
We included controls for age, sex, ethnicity (White vs. non-White), whether the participant held a degree-level qualification, and overall household wealth (net nonpension wealth, adjusted to account for household size and converted to quintiles to remove skewness). We also adjusted for whether participants were taking medication to treat high blood pressure or diabetes, injecting insulin, or receiving treatment with cholesterol-reducing agents (e.g., statins) and diagnosis of a range of health conditions at baseline. Specifically, we included 19 variables assessing whether participants had been diagnosed with each of the following health conditions: high blood pressure, angina, congestive heart failure, myocardial infarction, arrhythmia, heart murmur, diabetes or high blood glucose, stroke, high cholesterol, lung disease, asthma, arthritis, osteoporosis, cancer, psychiatric condition, glaucoma, diabetic retinopathy, macular degeneration, and cataracts. Finally, we included a control for depressive symptoms assessed using a validated eight-item version of the Center for Epidemiology Depression Scale (CES-D; Turvey, Wallace, & Herzog, 1999). The CES-D uses a yes/no response format to assess feelings during the past week (e.g., “felt sad,” “could not get going”), and responses are summed and averaged to produce a mean score ranging from 0 to 8 (M = 1.39, SD = 1.94; Cronbach’s α = .82), with higher scores indicating more depressive symptoms.
Analytic strategy
Our analyses aimed to (a) estimate the association between obesity and subsequent dysregulation, (b) test whether perceived weight discrimination predicted physiological dysregulation over and above initial obesity, and (c) assess whether our measure of perceived weight discrimination may mediate part of the association between obesity and physiological dysregulation. Because we estimate three key associations, we used a reduced critical value of .01. We controlled for demographic characteristics, medication usage, health conditions, and depressive symptoms in all models. In a preliminary test of our study predictions, we first examined the predicted associations without including baseline dysregulation as a covariate. Next, we turned our attention to the relationships among obesity, weight discrimination, and changes in dysregulation over time to provide a more stringent test of our main study hypotheses.
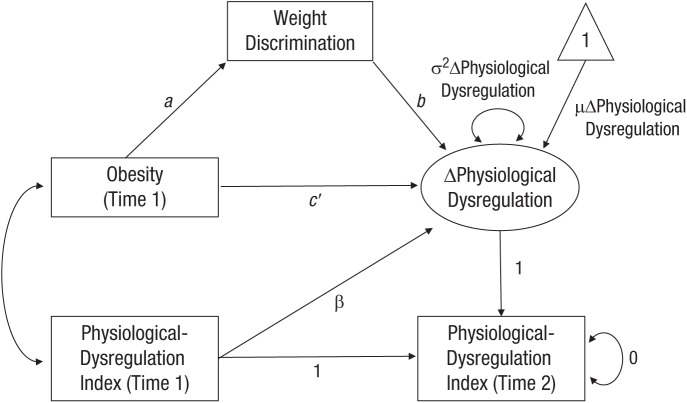
The latent-change-score (LCS) approach allows change to be explicitly modeled within a two-wave time series. Thus, in the current study, it was possible to identify increases or decreases in dysregulation levels from baseline to follow-up and to estimate whether obesity, weight discrimination, or both predicted this change. In the LCS model, change in physiological dysregulation was rendered explicit by constraining baseline (Time 1) and follow-up (Time 2) dysregulation to be identical (i.e., by fixing the autoregression path to unity) and including an LCS with a factor loading fixed to 1 that captures the residual variance in dysregulation at follow-up (for the model constraints imposed, see Fig. 1). Finally, we include a standardized regression parameter that accounted for the association between initial physiological dysregulation and the change score.
Fig. 1.
Latent-change-score model of the mediation channel from obesity to longitudinal changes in physiological dysregulation through perceived weight discrimination. Physiological-dysregulation-index variables at baseline (Time 1) and follow-up (Time 2) were constrained to be equal by fixing the autoregressive path to unity. The latent-change-score factor loading was fixed to 1 to capture the residual variance in dysregulation at follow-up. The rate of change in dysregulation is denoted by µΔPhysiological Dysregulation and variance in change by σ2ΔPhysiological Dysregulation. Covariates are omitted from the path diagram.
Having tested whether an association exists between obesity and longitudinal change in dysregulation (total effect; path c), we conducted a series of tests to establish that the preconditions for successful mediation were present, as illustrated in Figure 1. First, we tested whether the independent variable (obesity) was associated with the mediator (perceived weight discrimination; path a) in a model that included baseline physiological dysregulation and covariates and, second, whether the mediator predicted longitudinal change in physiological dysregulation over the study period (path b) in a fully adjusted model. When the conditions for mediation were present, a formal test of the significance levels of the potential indirect effect was conducted. To avoid biased standard errors and statistical-significance levels due to unspecified nonconstant error variance, we estimated robust standard errors in all regression models.
We also reestimated our LCS models in a series of robustness tests. First, we tested whether the same pattern of results was observed when (a) a continuous BMI measure was examined rather than a dichotomous obesity indicator or (b) waist circumference was used as an alternative measure of adiposity. We also tested whether the main study findings were moderated by participant gender. Next, we tested whether the association between weight discrimination and subsequent dysregulation was observed when more comprehensive controls for initial adiposity were included (i.e., adjusting for baseline waist circumference in addition to baseline BMI). Finally, we assessed the link between perceived weight discrimination and changes in dysregulation levels for each biological indicator individually.
Results
Descriptive statistics for basic demographic and baseline characteristics are shown in Table 1. The mean BMI of the sample was 28.22 kg/m2 (SD = 5.05), and the prevalence of obesity was 31% at baseline. In total, 13% of participants in the obese group reported weight discrimination, compared with 0.8% in the remainder of the sample (odds ratio = 18.90, 95% confidence interval, or CI = [9.92, 35.99], p < .01). Table S2 in the Supplemental Material presents data on the prevalence of chronic conditions and medication usage. A substantial portion of the sample had been diagnosed with high blood pressure (39.4%), high cholesterol (35%), and arthritis (36.6%) and were taking blood pressure medication (29%) and cholesterol-lowering medication (20.6%). Table S3 in the Supplemental Material details the correlations between all variables included in our main analyses. Age was positively correlated with physiological dysregulation, whereas being female, being White, and holding a degree were negatively associated with dysregulation. Household wealth was negatively correlated with physiological dysregulation at baseline and follow-up (average r = −.20). Obesity at baseline was positively correlated with physiological dysregulation at both time points (average r = .30), as was weight discrimination (average r = .16) and depressive symptoms (average r = .13). The presence of diabetes (r = .26) and use of diabetes medication (r = .24) were positively linked to dysregulation across waves.
In an initial regression model that did not adjust for baseline dysregulation levels, we found that obesity predicted physiological dysregulation at follow-up (d = 0.45, 95% CI = [0.37, 0.53], p < .01). Perceived weight discrimination also predicted raised levels of dysregulation at follow-up (d = 0.34, 95% CI = [0.13, 0.54], p < .01) in this model. Further, the inclusion of weight discrimination reduced the association between obesity and dysregulation by 9% (d = 0.41, 95% CI = [0.33, 0.50], p < .01). Mediation analyses confirmed the presence of a statistically significant indirect effect of weight discrimination, which explained 9% of the overall association between obesity and physiological dysregulation (d = 0.04, 95% CI = [0.02, 0.07], p < .01). To build on this initial evidence, we next examined the interlinkages among obesity, weight discrimination, and changes in dysregulation over time.
LCS model
There was a heterogeneous pattern of longitudinal change across the biomarkers examined, as detailed in Table S1. The majority of biomarkers did not change markedly over the study period. In contrast, glucose increased notably (from 4.9 to 5.4 mmol/L), and there was evidence of a decline in triglycerides (from 1.8 to 1.5 mmol/L) and in the ratio of total cholesterol to HDL cholesterol (from 3.8 to 3.5). After converting all biomarkers to z scores and producing a combined index, we found that, on average, the unconditional mean-level change in physiological dysregulation was positive (d = 0.03) but not significantly different from zero.
Obesity at baseline forecasted an increase of 0.09 standard deviations in physiological dysregulation from baseline to follow-up (d = 0.09, 95% CI = [0.02, 0.16], p < .01). We also found that perceived weight discrimination predicted an increase of 0.23 standard deviations in physiological dysregulation over the study period (d = 0.23, 95% CI = [0.07, 0.40], p < .01). We therefore found evidence for a longitudinal association between obesity and subsequent physiological dysregulation (total effect; path c), that obesity was associated with high levels of weight discrimination (mediation path a), and that weight discrimination predicted increased physiological dysregulation over time (mediation path b), as illustrated in Figure 1. Further, the link between obesity and increased dysregulation was no longer statistically significant after the inclusion of weight discrimination in the regression model (total effect: d = 0.09, 95% CI = [0.02, 0.16], p < .01, direct effect: d = 0.07, 95% CI = [0.00, 0.13], p > .01).
Formal mediation analysis revealed a significant indirect effect of obesity on physiological dysregulation through weight discrimination (d = 0.02, 95% CI = [0.01, 0.04], p < .01), as shown in Table 2. An examination of the effect ratio showed that the indirect effect of weight discrimination could account for 27% of the total effect of obesity on longitudinal changes in physiological dysregulation.
Table 2.
Results From the Mediation Model of the Indirect Effect of Obesity on Longitudinal Changes in Physiological Dysregulation, as Mediated by Weight Discrimination (N = 3,609)
| Path | Point estimate | 95% CI | Effect ratio |
|---|---|---|---|
| Obesity → discrimination (IV to mediator, path a) |
OR = 18.90* | [9.92, 35.99] | |
| Discrimination → dysregulation (mediator to DV, path b) |
d = 0.23* | [0.07, 0.40] | |
| Obesity → dysregulation (total effect, path c) |
d = 0.09* | [0.02, 0.16] | |
| Obesity → dysregulation (direct effect, path c′) |
d = 0.07 | [0.00, 0.13] | |
| Obesity → dysregulation (indirect effect) |
d = 0.02* | [0.01, 0.04] | 27% |
Note: Statistical-significance levels and 95% confidence intervals (CIs) are based on models using robust standard errors. Models used latent change in physiological dysregulation z scores as the outcome variable. Models were adjusted for baseline physiological dysregulation, age, sex, ethnicity (White vs. other), degree qualification, household wealth, medication usage, chronic conditions, and depressive symptoms. DV = dependent variable; IV = independent variable; OR = odds ratio.
p < .01.
Robustness tests
Consistent with the main study results, robustness tests showed that weight discrimination explained 30% of the longitudinal association between a continuous BMI measure and increased physiological dysregulation over time (b = 0.010, 95% CI = [0.004, 0.016], p < .01, reduced to b = 0.007, 95% CI = [0.00, 0.014], p > .01). Similarly, we found that weight discrimination explained one quarter of the longitudinal association between waist circumference and increased physiological dysregulation (b = 0.004, 95% CI = [0.002, 0.007], p < .01, reduced to b = 0.003, 95% CI = [0.00, 0.006], p > .01). Thus, it appears that weight discrimination explains a similar portion of the link between initial adiposity and subsequent physiological dysregulation regardless of whether obesity status, continuous BMI, or waist circumference is used to measure adiposity in this sample.
Next, we examined the role of gender. A similar percentage of men (12.3%) and women (13.6%) with obesity reported experiencing weight-based discrimination. The association between initial obesity and longitudinal change in physiological dysregulation was not moderated by gender (b = −0.02, 95% CI = [–0.16, 0.12], p > .01), nor was the association between perceived weight discrimination and changes in physiological dysregulation (b = 0.07, 95% CI = [–0.25, 0.38], p > .01). These analyses suggest that there is little evidence that the key mediation paths examined differed markedly between men and women.
In additional analyses, we showed that the relationship between weight discrimination and subsequent dysregulation was not substantially attenuated when more stringent controls for initial adiposity were introduced (model adjusted for obesity and covariates: d = 0.23, 95% CI = [0.07, 0.40], p < .01; model adjusted for initial BMI, waist circumference, and covariates: d = 0.22, 95% CI = [0.04, 0.40], p > .01).
Finally, we assessed the association between perceived weight discrimination and longitudinal change in each biomarker variable. The point estimates for the linkages between weight discrimination and changes in biomarkers were positive in all cases, as shown in Table S5 in the Supplemental Material. Associations between weight discrimination and individual biomarkers were not, however, statistically significant. It therefore appears that a robust association between weight discrimination and changes in biological functioning is primarily evident when biomarker variables are examined in concert (d = 0.23, 95% CI = [0.07, 0.40], p < .01), demonstrating the advantage of drawing on an integrated physiological-dysregulation index to capture the physiological changes following weight discrimination.
Discussion
To date, it has been largely assumed that the adverse effects that obesity has on health are driven by the biological strain that excess body fat has on physiological systems that regulate cardiovascular and metabolic functioning. In this study, we tested whether the widespread discrimination experienced by individuals living with obesity may play an important, but underappreciated, role in explaining why obesity is associated with the deterioration of healthy physiological functioning. Our study was designed to move beyond initial studies indicating that weight discrimination may in part explain cross-sectional obesity-related decrements in self-reported health (Jackson et al., 2015; Hunger & Major, 2015).
Consistent with prior research (Kahn et al., 2006; Van Gaal et al., 2006), our findings revealed that obesity was associated with increases in physiological dysregulation over 4 years in a large sample of older English adults. Critically, this longitudinal association was accounted for in part by perceived weight discrimination; participants with obesity were more likely to report experiencing weight-based discrimination, and these stigmatizing experiences predicted adverse changes in physiological dysregulation over time. Specifically, perceived weight discrimination was associated with an increase of 0.23 standard deviations in physiological dysregulation, as gauged by cardiovascular, inflammatory, and lipid and metabolic dysregulation, and this accounted for 27% of the relation between obesity and subsequent increases in dysregulation. Importantly, these associations were observed after adjustment for initial sociodemographic characteristics, dysregulation levels, medication usage, and health conditions.
These findings are in line with prior cross-sectional research that has documented linkages between perceived weight-based discrimination and high levels of clinical indicators of ill health, such as C-reactive protein and glycated hemoglobin (Potter et al., 2015; Sutin et al., 2014). Our longitudinal findings show that these relations may reflect the direction of influence from weight discrimination to inflammation and glucose metabolism. Previously, a single longitudinal study has linked initial weight discrimination to raised levels of physiological dysregulation measured 10 years later (Vadiveloo & Mattei, 2017). This study was hampered by the absence of objective measures of obesity and physiological dysregulation at baseline. The current study provides the first prospective evidence of an association between perceived weight discrimination and longitudinal changes in physiological dysregulation, as determined using objectively measured clinical indicators of health status. The cumulative toll of these alterations in biological processes could account for part of the recently documented association between weight discrimination and premature mortality (Seeman et al., 2001; Sutin et al., 2015). Our mediation findings also provide the first longitudinal evidence that directly implicates weight-based discrimination as a channel through which obesity generates long-run detrimental biological effects across a range of physiological parameters.
We suggested that these consequential health effects may occur because weight-based social stigmatization acts as an interpersonal stressor that can set in motion a cycle of daily stress, negative affect, and maladaptive coping responses (Schvey et al., 2014; Sutin, Robinson, et al., 2016; Sutin, Stephan, et al., 2016; Vartanian & Porter, 2016). Such stress-related responses could influence physiological dysregulation directly through biological mechanisms (Epel et al., 2001; Jackson et al., 2016) or act indirectly by impairing weight management (Tomiyama, 2014). For example, individuals experiencing weight stigma fear negative evaluation from other people and report overeating and reduced motivation to engage in exercise, which could hamper weight control (Vartanian & Porter, 2016; Vartanian & Shaprow, 2008). Perceived weight discrimination could also contribute to adverse health effects by impeding chronic illness management. For example, community-dwelling adults with diabetes who report experiencing weight-based discrimination have shown poor diabetes self-care in key areas (i.e., diet, physical activity, blood glucose monitoring) and also impaired glycemic control (Potter et al., 2015).
Further research is now needed to articulate the behavioral and biological pathways through which unfair treatment based on body weight may contribute to physiological dysregulation and help explain the obesity-related disease burden. This work could help inform the development of interventions that aim to promote adaptive coping responses and inoculate people with obesity against the harmful effects of weight-based stigma and discrimination. Regardless of the precise pathways linking weight discrimination to health, the current findings highlight the importance of developing stigma-prevention policies, including informing the public that the denigration of people with obesity may have damaging health consequences (Tomiyama et al., 2018). Our findings suggest that intervention strategies that are successful in reducing the stigmatization of obesity (Pearl, 2018) may also act to reduce the physiological consequences and disease burden of obesity.
Whereas we identified evidence that the social experience of body weight may partially explain the biomedical consequences of obesity, our investigation was limited in several respects. First, we focused on middle-age and older adults, and further work is now needed to test whether this process may be observed in younger adults. Obesity may be stigmatized most among younger individuals (Hebl, Ruggs, Singletary, & Beal, 2008), and weight-based discrimination has been linked to distress, maladaptive health behaviors, and poor health in young adults (Pearl, 2018; Puhl & Heuer, 2009). Thus, it is feasible that weight discrimination may play a similar role in explaining the health effects of obesity in younger samples. Second, our sample identified predominantly as White, so research drawing on more racially diverse samples is needed to decipher whether the study findings extend to other ethnic groups. Third, our measure of weight discrimination assessed whether participants reported everyday experiences of discrimination at a single time point. Administering longer form measures at multiple time points would increase the reliability of the assessment and help ensure that estimates of the indirect effect of weight discrimination were not attenuated by measurement error. Finally, perceived weight discrimination is part of a broader constellation of related psychosocial constructs (e.g., perceived weight, weight stigma concerns, weight bias internalization) that may combine to mediate the pathway from obesity to physiological dysregulation (Daly et al., 2017; Hunger & Major, 2015). For instance, a recent study suggested that the tendency of individuals with obesity to self-stigmatize by internalizing weight bias may also contribute to greater cardiometabolic risk among people with obesity (Pearl et al., 2017). Researchers may seek to investigate this pathway more extensively.
Conclusion
In a large sample of English adults, weight-based discrimination predicted increases in physiological dysregulation over time and partly explained the longitudinal association between obesity and cardiovascular, metabolic, and immune function. These findings provide initial evidence that the psychosocial strain of weight stigma may account for a notable proportion of the obesity-related disease burden.
Supplemental Material
Supplemental material, DalyTables_S1-S4_rev for Perceived Weight Discrimination Mediates the Prospective Association Between Obesity and Physiological Dysregulation: Evidence From a Population-Based Cohort by Michael Daly, Angelina R. Sutin and Eric Robinson in Psychological Science
Supplemental Material
Supplemental material, Daly_OpenPracticesDisclosure_rev for Perceived Weight Discrimination Mediates the Prospective Association Between Obesity and Physiological Dysregulation: Evidence From a Population-Based Cohort by Michael Daly, Angelina R. Sutin and Eric Robinson in Psychological Science
Footnotes
Action Editor: Brent W. Roberts served as action editor for this article.
Author Contributions: All the authors developed the study concept and were responsible for the study design. M. Daly analyzed the data and drafted the manuscript, and E. Robinson and A. R. Sutin provided critical revisions. All the authors approved the final manuscript for submission.
ORCID iD: Michael Daly  https://orcid.org/0000-0003-1557-8326
https://orcid.org/0000-0003-1557-8326
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: This research received no external funding. E. Robinson’s salary is in part funded by the Economic and Social Research Council and Medical Research Council, and he has previously received research funding from Unilever and the American Beverage Association. A. R. Sutin was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (1R15HD083947).
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/0956797619849440
Open Practices: All data and materials have been made available to academic researchers via the UK Data Service and can be accessed at https://discover.ukdataservice.ac.uk/series/?sn=200011 (Study Number, or SN, 5050). The design and analysis plans for the present study were not preregistered.
References
- Andreyeva T., Puhl R. M., Brownell K. D. (2008). Changes in perceived weight discrimination among Americans, 1995–1996 through 2004–2006. Obesity, 16, 1129–1134. [DOI] [PubMed] [Google Scholar]
- Craig R., Deverill C., Pickering K. (2006). Quality control of blood, saliva and urine analytes. In Sproston K., Mindell J. (Eds.), Health survey for England 2004: Methodology and documentation (pp. 34–41). Leeds, England: The Information Centre. [Google Scholar]
- Daly M., Robinson E., Sutin A. R. (2017). Does knowing hurt? Perceiving oneself as overweight predicts future physical health and well-being. Psychological Science, 28, 872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E., Lapidus R., McEwen B., Brownell K. (2001). Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology, 26, 37–49. [DOI] [PubMed] [Google Scholar]
- GBD 2015 Obesity Collaborators. (2017). Health effects of overweight and obesity in 195 countries over 25 years. The New England Journal of Medicine, 377, 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guh D. P., Zhang W., Bansback N., Amarsi Z., Birmingham C. L., Anis A. H. (2009). The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health, 9, Article 88. doi: 10.1186/1471-2458-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson S. E., Goldberg L. R., Vogt T. M., Hillier T. A., Dubanoski J. P. (2009). Using physiological dysregulation to assess global health status: Associations with self-rated health and health behaviors. Journal of Health Psychology, 14, 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenbuehler M. L., Keyes K. M., Hasin D. S. (2009). Associations between perceived weight discrimination and the prevalence of psychiatric disorders in the general population. Obesity, 17, 2033–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebl M. R., Ruggs E. N., Singletary S. L., Beal D. J. (2008). Perceptions of obesity across the lifespan. Obesity, 16(Suppl. 2), S46–S52. [DOI] [PubMed] [Google Scholar]
- Himmelstein M. S., Incollingo Belsky A. C., Tomiyama A. J. (2015). The weight of stigma: Cortisol reactivity to manipulated weight stigma. Obesity, 23, 368–374. [DOI] [PubMed] [Google Scholar]
- Hunger J. M., Major B. (2015). Weight stigma mediates the association between BMI and self-reported health. Health Psychology, 34, 172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S. E., Beeken R. J., Wardle J. (2015). Obesity, perceived weight discrimination, and psychological well-being in older adults in England. Obesity, 23, 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S. E., Kirschbaum C., Steptoe A. (2016). Perceived weight discrimination and chronic biochemical stress: A population-based study using cortisol in scalp hair. Obesity, 24, 2515–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster R. P., McEwen B. S., Lupien S. J. (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews, 35, 2–16. [DOI] [PubMed] [Google Scholar]
- Kahn S. E., Hull R. L., Utzschneider K. M. (2006). Mechanisms linking obesity to insulin resistance and Type 2 diabetes. Nature, 444, 840–846. [DOI] [PubMed] [Google Scholar]
- Major B., Eliezer D., Rieck H. (2012). The psychological weight of weight stigma. Social Psychological and Personality Science, 3, 651–658. [Google Scholar]
- Pascoe E. A., Smart Richman L. (2009). Perceived discrimination and health: A meta-analytic review. Psychological Bulletin, 135, 531–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl R. L. (2018). Weight bias and stigma: Public health implications and structural solutions. Social Issues and Policy Review, 12, 146–182. [Google Scholar]
- Pearl R. L., Wadden T. A., Hopkins C. M., Shaw J. A., Hayes M. R., Bakizada Z. M., . . . Alamuddin N. (2017). Association between weight bias internalization and metabolic syndrome among treatment-seeking individuals with obesity. Obesity, 25, 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter L., Wallston K., Trief P., Ulbrecht J., Juth V., Smyth J. (2015). Attributing discrimination to weight: Associations with well-being, self-care, and disease status in patients with Type 2 diabetes mellitus. Journal of Behavioral Medicine, 38, 863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl R. M., Heuer C. A. (2009). The stigma of obesity: A review and update. Obesity, 17, 941–964. [DOI] [PubMed] [Google Scholar]
- Robinson E., Sutin A., Daly M. (2017). Perceived weight discrimination mediates the prospective relation between obesity and depressive symptoms in U.S. and U.K. adults. Health Psychology, 36, 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M. H., Ferraro K. F. (2011). The stigma of obesity: Does perceived weight discrimination affect identity and physical health? Social Psychology Quarterly, 74, 76–97. [Google Scholar]
- Schvey N. A., Puhl R. M., Brownell K. D. (2014). The stress of stigma: Exploring the effect of weight stigma on cortisol reactivity. Psychosomatic Medicine, 76, 156–162. [DOI] [PubMed] [Google Scholar]
- Seeman T. E., McEwen B. S., Rowe J. W., Singer B. H. (2001). Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences, USA, 98, 4770–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seplaki C. L., Goldman N., Glei D., Weinstein M. (2005). A comparative analysis of measurement approaches for physiological dysregulation in an older population. Experimental Gerontology, 40, 439–439. [DOI] [PubMed] [Google Scholar]
- Steptoe A., Breeze E., Banks J., Nazroo J. (2012). Cohort profile: The English Longitudinal Study of Ageing. International Journal of Epidemiology, 42, 1640–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streiner D. L. (2003). Being inconsistent about consistency: When coefficient alpha does and doesn’t matter. Journal of Personality Assessment, 80, 217–222. [DOI] [PubMed] [Google Scholar]
- Sutin A., Robinson E., Daly M., Terracciano A. (2016). Weight discrimination and unhealthy eating-related behaviors. Appetite, 102, 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin A. R., Stephan Y., Grzywacz J. G., Robinson E., Daly M., Terracciano A. (2016). Perceived weight discrimination, changes in health, and daily stressors. Obesity, 24, 2202–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin A. R., Stephan Y., Luchetti M., Terracciano A. (2014). Perceived weight discrimination and C-reactive protein. Obesity, 22, 1959–1961. [DOI] [PubMed] [Google Scholar]
- Sutin A. R., Stephan Y., Terracciano A. (2015). Weight discrimination and risk of mortality. Psychological Science, 26, 1803–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama A. J. (2014). Weight stigma is stressful. A review of evidence for the cyclic obesity/weight-based stigma model. Appetite, 8, 8–15. [DOI] [PubMed] [Google Scholar]
- Tomiyama A. J., Carr D., Granberg E. M., Major B., Robinson E., Sutin A. R., Brewis A. (2018). How and why weight stigma drives the obesity ‘epidemic’ and harms health. BMC Medicine, 16, Article 123. doi: 10.1186/s12916-018-1116-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turvey C. L., Wallace R. B., Herzog R. (1999). A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. International Psychogeriatrics, 11, 139–148. [DOI] [PubMed] [Google Scholar]
- Vadiveloo M., Mattei J. (2017). Perceived weight discrimination and 10-year risk of allostatic load among US adults. Annals of Behavioral Medicine, 51, 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gaal L. F., Mertens I. L., De Block C. E. (2006). Mechanisms linking obesity with cardiovascular disease. Nature, 444, 875–880. [DOI] [PubMed] [Google Scholar]
- Vartanian L. R., Porter A. M. (2016). Weight stigma and eating behavior: A review of the literature. Appetite, 102, 3–14. [DOI] [PubMed] [Google Scholar]
- Vartanian L. R., Shaprow J. G. (2008). Effects of weight stigma on exercise motivation and behavior: A preliminary investigation among college-aged females. Journal of Health Psychology, 13, 131–138. [DOI] [PubMed] [Google Scholar]
- Williams D. R., Yu Y., Jackson J. S., Anderson N. B. (1997). Racial differences in physical and mental health: Socio-economic status, stress and discrimination. Journal of Health Psychology, 2, 335–351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DalyTables_S1-S4_rev for Perceived Weight Discrimination Mediates the Prospective Association Between Obesity and Physiological Dysregulation: Evidence From a Population-Based Cohort by Michael Daly, Angelina R. Sutin and Eric Robinson in Psychological Science
Supplemental material, Daly_OpenPracticesDisclosure_rev for Perceived Weight Discrimination Mediates the Prospective Association Between Obesity and Physiological Dysregulation: Evidence From a Population-Based Cohort by Michael Daly, Angelina R. Sutin and Eric Robinson in Psychological Science