Abstract
BACKGROUND
Patients with stage II-III colorectal cancer (CRC) treated with adjuvant chemotherapy, gain a 25% survival benefit. In the context of personalized medicine, there is a need to identify patients with CRC who may benefit from adjuvant chemotherapy. Molecular profiling could guide treatment decisions in these patients. Thymidylate synthase (TYMS) gene polymorphisms, KRAS and BRAF could be included in the molecular profile under consideration.
AIM
To investigate the association of TYMS gene polymorphisms, KRAS and BRAF mutations with survival of CRC patients treated with chemotherapy.
METHODS
A retrospective study studied formalin-fixed paraffin-embedded tissues (FFPEs) of consecutive patients treated with adjuvant chemotherapy during January/2005-January/2007. FFPEs were analysed with PCR for the detection of TYMS polymorphisms, mutated KRAS (mKRAS) and BRAF (mBRAF). Patients were classified into three groups (high, medium and low risk) according to 5’UTR TYMS polymorphisms Similarly, based on 3’UTR polymorphism ins/loss of heterozygosity (LOH) patients were allocated into two groups (high and low risk of relapse, respectively). Cox regression models examined the associated 5-year survival outcomes.
RESULTS
One hundred and thirty patients with early stage CRC (stage I-II: 55 patients; stage III 75 patients; colon: 70 patients; rectal: 60 patients) were treated with surgery and chemotherapy. The 5-year disease free survival and overall survival rate was 61.6% and 73.9% respectively. 5’UTR polymorphisms of intermediate TYMS polymorphisms (2RG/3RG, 2RG/LOH, 3RC/LOH) were associated with lower risk for relapse [hazard ratio (HR) 0.320, P = 0.02 and HR 0.343, P = 0.013 respectively] and death (HR 0.368, P = 0.031 and HR 0.394, P = 0.029 respectively). The 3’UTR polymorphism ins/LOH was independently associated with increased risk for disease recurrence (P = 0.001) and death (P = 0.005). mBRAF (3.8% of patients) was associated with increased risk of death (HR 4.500, P = 0.022) whereas mKRAS (39% of patients) not.
CONCLUSION
Prospective validating studies are required to confirm whether 2RG/3RG, 2RG/LOH, 3RC/LOH, absence of ins/LOH and wild type BRAF may indicate patients at lower risk of relapse following adjuvant chemotherapy.
Keywords: Colorectal neoplasms, Thymidylate synthase, Untranslated regions, Fluorouracil, KRAS, BRAF, Prognosis
Core tip: There is a need to identify patients with colorectal cancer (CRC) who may benefit from adjuvant chemotherapy. We investigated the survival in 130 patients with stage II-III CRC treated with adjuvant chemotherapy based on thymidylate synthase (TYMS) gene polymorphisms, KRAS and BRAF status. We found that TYMS polymorphisms and BRAF status associate independently with the survival outcomes. Prospective validating studies are required.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer in the United States of America while worldwide it is expected to increase by 60% to more than 2.2 million new cases and 1.1 million deaths by 2030[1,2]. In 2014, almost 153000 patients died from CRC in the European Union, where it is the second leading cause of cancer death (Eurostat. Cancer statistics – specific cancers)[3]. At diagnosis, 74%-76% of patients have a localized or regional CRC. Fluoropyrimidines remain the backbone of adjuvant chemotherapy for early stage CRC patients after curative surgery[4,5]. Fluoro-pyrimidines exert their action by different ways mainly by inhibiting the de novo formation of thymidylate (dTMP) from uridylate (dUMP)[6]. Other mechanisms of action are more complex than simply inhibition of TS expression, as they involve inhibition of DNA synthesis and function through misincorporation of FdUTP into cellular DNA and inhibition of RNA processing and mRNA translation through the incorporation of FUTP into cellular RNA[7]. The use of fluoropyrimidines is associated with reduction of recurrence in only 25% of patients with stage III CRC[8,9]. Only 3%-7% of patients with stage II CRC will benefit from adjuvant chemotherapy[10]. The variability of observed survival outcomes has been largely attributed to molecular heterogeneity and KRAS, BRAF and thymidylate synthase (TYMS) are being investigated to this end[11]. KRAS belongs to the RAS subfamily of genes that encodes a 21-kDa small-GTPase[12]. Activating mutations in RAS result in activation of major signaling pathways downstream of epidermal growth factor receptor (EGFR) stimulating cell proliferation and inhibiting apoptosis[13]. In the metastatic disease setting, KRAS mutations (mKRAS) is a predictor of resistance to EGFR inhibitors and is directly linked to poor patient survival, while its role in the adjuvant setting is under investigation[14-16].
BRAF is an essential part of the RAS/RAF/MAP2K (MEK)-MAPK signaling cascade and its mutations have been likewise associated with inferior survival in CRC patients after curative resection and adjuvant chemotherapy[17,18].
The TYMS gene (GeneID 7298) is located on the short arm of chromosome 18 (18p11.32). There is conflicting evidence on the role of TYMS polymorphisms in predicting response to 5FU–based chemotherapy[19-25]. The loss of heterozygosity (LOH) at the TYMS locus on chromosome 18 has been implicated as a factor affecting the TYMS-related resistance to fluoropyrimidine-based therapy[26].
A TYMS polymorphism of the 5’ untranslated region (5’UTR) results by the insertion of a 28 base-pair (bp) sequence (rs34743033)[19]. From the resulting alleles that may include two or three 28bp tandem repeats (2R or 3R respectively), the 3R allele was associated with increased TYMS protein expression and TYMS enzyme activity[27,28]. G->C single nucleotide polymorphism (SNP) in the tandem repeat sequence [rs2853542] was found to reduce the translational efficiency of a 3R to a 2R[19,29]. Based on the presence of SNP polymorphisms (G or C) 3R are characterized as 3RG and 3RC. In addition, the 3’UTR may contain a 6 bp polymorphism (rs34489327) affecting the TYMS mRNA stability, and resulting in increased intratumoral TYMS mRNA[19,30]. Depending on the presence of this 6 bp polymorphism, the three resulting genotypes are ins/ins (homozygous for insertion of 6bp), del/del (homozygous for deletion) and ins/del (heterozygous).
Based on all the above, the identification of potential markers that could elucidate which patients’ subgroups could benefit most from fluoropyrimidine-based therapy remains an unmet clinical need.
The present study aims to investigate the associations of TYMS polymorphisms, LOH, mKRAS and BRAF mutations (mBRAF) with clinicopathologic characteristics and survival outcomes of patients with CRC treated with fluoropyrimidine-based adjuvant chemotherapy.
MATERIALS AND METHODS
Patients and clinical data
This was a retrospective study carried out by a single institution (University General Hospital “ATTIKON”). Formalin-fixed paraffin-embedded tissues (FFPE) and clinical data of consecutive patients with CRC referred for adjuvant chemotherapy from January 2005 to January 2007 were retrieved. Of these, only patients with histologies reporting R0 surgical margins and treated with fluoropyrimidine-based adjuvant chemotherapy (and therefore with no redisual disease) were included in the analysis. In these cases, the integrity of mesocolon/mesorectum was preserved.
DNA extraction protocol
DNA was extracted from 5 μm thick FFPE sections, containing at least 30% malignant cells, using a commercially available kit (Purelink Genomic DNA kit, Thermo Fisher Scientific, Germany). DNA was quantified by qPCR (Quant-iT™ PicoGreen® dsDNA Assay Kit, Thermo Fisher Scientific, Germany) and was diluted accordingly to achieve a concentration of 10 ng/μL for TYMS polymorphisms and 4 ng/μL for mKRAS detection.
TYMS polymorphisms
Analysis was carried-out as previously described[31,32]. PCR was performed using 1U of Platinum® Taq DNA Polymerase (Thermo Fisher Scientific, Germany), 1.5 mmol/L of Mg and 200 nmol/L of dNTPs and primers. Althoug the same primers were used, 5’-UTR amplification was performed using a GC rich amplification kit (PCRX Enhancer System, Thermo Fisher Scientific, Germany) adding 1× of PRCx Enhancer. Genotyping for the 2R/3R polymorphism was performed by running 10 μL of the PCR product on a 1.5% agarose gel and staining with Ethidium Bromide as previously described (Ntavatzikos et al[31]). Similarly, for the 12G>C substitution, 10 μL of PCR product was digested with 1U of HaeIII restriction enzyme (Takara, Japan) at 37 oC for 1 h and run on an 8% 19:1 polyacrylamide gel. Polyacrylamide gels were used for the analysis of the 3’UTR. LOH analysis was achieved by analyzing the intensity of the 5’UTR and 3’UTR bands of the pictures acquired using the GeneTools software (Syngene, United Kingdom). The sample was categorized as having LOH if one of the bands had an intensity score of < 50% of the other. Samples showing LOH were defined as 2R/3RGLOH, 2RLOH/3RG, 2R/3RCLOH and 2RLOH/3RC indicating the allele that was partially lost. For quality control, selected products were sequenced to verify the sequence amplified. The amplified product was 242 bp for 3R and 214 bp for 2R polymorphisms, as revealed by the blast of the sequenced products and the alignment with the latest human assemblies.
Mutational analysis
Detection of mKRAS in codons 12 and 13 and BRAF activating mutation V600E were performed as previously described with a commercially available Real-Time PCR kit (Therascreen KRAS, DxS Diagnostics, United Kingdom) detecting 6 mutations of codon 12 (G12D, G12A, G12V, G12S, G12R, G12C) and 1 mutation of codon 13 (G13D)[31,33]. A positive reaction mix for all mutations was included. To avoid false negative results caused by PCR inhibitors, a second exogenous reaction was simultaneously taking place. If the sample’s ΔCt (Ct of control reaction-Ct mutation reaction) was lower than the value set by the manufacturer, then it was characterized as bearing a mutation. BRAF activating mutation V600E was identified using molecular beacons as previously described[33]. One beacon for the wild type and one for the mutant allele were added at a final concentration of 100 nmol/L in a 25 μL PCR reaction containing 1× PCR Buffer, 6 mmol/L MgCl2, 200 nmol/L dNTPs, 300 nmol/L of each primer and 1U of Platinum® Taq. PCR profile applied was 95 oC 2 min, followed by 40 cycles of 95 oC for 10 sec, 62 oC for 60 sec and 72 oC for 20 sec. DNA extracts from the series of melanoma cell lines SKMEL2 and SKMEL20 were used as positive controls for both the wild type and mutant allele (CLS, Germany). The ABI 7500 Fast (Thermo Fisher Scientific, Germany) was used to perform all Real-Time PCR experiments.
TYMS-gene polymorphisms stratification model
Based on the predicted TYMS protein expression, 5’UTR polymorphisms were assigned into low (2RG/2RG, 2RG/3RC, 3RC/3RC), medium (2RG/3RG, 2RG/3RCLOH, 2RG/3RGLOH, 2RGLOH/3RC) and high TYMS protein expression group (3RG/3RG, 3RG/3RC, 2RGLOH/3RG)[31]. The effect of each 3’UTR polymorphism was examined against all the others by applying univariate analysis and it was found that only the ins/LOH polymorphism had a statistically significant effect. Based on this finding, 3’UTR polymorphisms were allocated into two groups depending on the presence or not of ins/LOH. This classification is depicted in Table 1.
Table 1.
TYMS polymorphisms’ groups according to risk group and level of expression respectively
| Groups | Polymorphisms |
| 3’UTR | |
| A (low risk) | del/del |
| del/LOH | |
| ins/del | |
| ins/ins | |
| B (high risk) | ins/LOH |
| 5’UTR | |
| A (low expression) | 2RG |
| 2RG/3RC | |
| 3RC | |
| B (medium expression) | 2RG/3RG |
| 2RG/3RCLOH | |
| 2RG/3RGLOH | |
| 2RGLOH/3RC | |
| C (high expression) | 3RG |
| 3RG/3RC | |
| 2RGLOH/3RG |
UTR: Untranslated region; LOH: Loss of heterozygosity.
Statistical analysis
Association of TYMS polymorphisms with selected clinicopathological characteristics was performed using the χ2 test with a 2-sided significance of 0.05. Time-to-event distributions were estimated using the Kaplan-Meier method. For all associations, the level of statistical significance was set at a = 0.05. Overall survival (OS) was defined as the interval between initiation of adjuvant chemotherapy and death of any cause. Disease-free survival (DFS) was defined as the time from adjuvant chemotherapy initiation to the first recurrence or death by any cause.
Surviving patients were censored at the date of last contact. Cox proportional hazards model was used to estimate the relationship of clinicopathological parameters and TYMS polymorphisms with OS and DFS. The relationship of TYMS polymorphisms and the groups to which classified with OS and DFS was assessed by univariate Cox regression analysis. The final multivariate model was selected using a backward selection procedure, starting from an initial model that included all potential risk factors and TYMS polymorphisms. Model selection was based on likelihood ratio test, while the removal criterion was set at 0.10. All statistical analyses were performed using the SPSS software version 24.0 (SPSS Inc, Chicago, IL, United States). The statistical methods of this study were reviewed by Georgia Vourli from the Department of Hygiene, Epidemiology and Medical Statistics, Medical School University of Athens.
RESULTS
Patient characteristics
Medical records of 130 consecutive patients and their FFPE were retrieved for analysis. Patients’ clinicopathologic data including age, gender, primary tumor site, histological grade, treatment and survival are shown in Table 2. With a median follow-up of 71.2 mo (range 0.5-157), 51 patients (39.2%) experienced disease recurrence while 45 patients (34.6%) died. The 5-year OS and DFS rate was 73.9% and 61.6% respectively.
Table 2.
Clinicopathologic data for colorectal cancer patients treated with adjuvant chemotherapy
| Clinicopathologic data | Total (n = 130) |
| Median age (range) | 67 (37-88) |
| Male | 79 (60.8) |
| Primary site | |
| Rectum | 60 (46.2) |
| Positive lymph nodes | 76 (58.5) |
| Stage according to AJCC | |
| I | 1 (0.8) |
| II | 54 (41.5) |
| III | 75 (57.7) |
| Histological grade | |
| I + II | 83 (63.8) |
| III + IV | 47 (36.2) |
| KRAS mutation | 48 (36.9) |
| BRAF V600E mutation | 5 (3.8) |
| TYMS LOH | 34 (26.2) |
| Overall survival | |
| Deaths n (%) | 45 (34.6) |
| Mean time month (95%CI) | 110.0 (99.5-120.5) |
| Disease-free survival | |
| Events n (%) | 51 (39.2) |
| Mean time month (95%CI) | 100.1 (88.3-112.0) |
| Median follow up in months (range) | 71.2 (0.5-156.8) |
AJCC: American Joint Committee on Cancer 7th edition; TYMS: Thymidylate synthase gene; LOH: Loss of heterozygosity; CI: Confidence interval.
The frequency of TYMS polymorphisms involving G>C SNP and LOH are presented in Table 3. Significant associations were found among patients’ tumor characteristics and polymorphisms as shown in Table 4.
Table 3.
Frequency of TYMS 5’UTR, 3’UTR genotypes
| Genotype | Total n (%) |
| TYMS 5’UTR | 130 (100) |
| 2R | 13 (10.0) |
| 2R/3R | 78 (60.0) |
| 2R/3RG | 34 (26.1) |
| 2R/3RG | 20 (15.4) |
| 2R/3RGLOH | 8 (6.2) |
| 2RLOH/3RG | 6 (4.6) |
| 2R/3RC | 44 (33.8) |
| 2R/3RC | 24 (18.5) |
| 2R/3RCLOH | 13 (10.0) |
| 2RLOH/3RC | 7 (5.4) |
| 3R | 39 (30.0) |
| 3RG | 10 (7.7) |
| 3RG/3RC | 20 (15.4) |
| 3RC | 9 (6.9) |
| TYMS 3’UTR | 130 (100) |
| ins/ins | 28 (21.5) |
| ins/LOH | 27 (20.8) |
| ins/del | 52 (40.0) |
| del/LOH | 7 (5.4) |
| del/del | 16 (12.3) |
TYMS: Thymidylate synthase gene; UTR: Untranslated region; SNP: Single nucleotide polymorphism; LOH: Loss of heterozygosity.
Table 4.
Associations between patient characteristics and TYMS polymorphisms
| Patient characteristics | Polymorphisms | RR (95%CI) | P value |
| Birth after 1942 | 3RG/3RG | 5.128 (1.131-23.26) | 0.025 |
| 3RC/3RC and 3RC/LOH | 0.296 (0.088-0.988) | 0.035 | |
| Male | 3RG/3RG and 3RG/LOH | 4.519 (1.072-19.06) | 0.030 |
| Grade III-IV | 3RG/3RC | 2.646 (1.167-6.024) | 0.022 |
| Stage III | 3RG/3RG and 3RG/3RC and 3RG/LOH | 2.198 (1.126-4.292) | 0.020 |
| 3RG/3RC | 4.149 (1.280-13.51) | 0.008 | |
| Without any 3RG allele | 0.733 (0.546-0.984) | 0.050 | |
| 3RC/3RC and 3RC/LOH | 0.333 (1.229-0.904) | 0.030 | |
| 3RC/LOH | 0.122 (0.015-0.986) | 0.045 | |
| KRAS mutation | 3RG/3RC | 3.135 (1.344-7.299) | 0.010 |
| 3RC/3RC and 3RC/LOH | 0.241 (0.057-1.015) | 0.030 |
TYMS: Thymidylate synthase gene; RR: Relative risk; CI: Confidence interval; LOH: Loss of heterozygosity.
Univariate survival analysis
Univariate Cox regression analysis of TYMS polymorphisms, mKRAS and mBRAF, LOH and selected clinicopathological patients’ characteristics are shown in Table 5. Univariate analysis indicated a trend for a better DFS and OS in the group of 5’UTR polymorphisms with medium expression profile (group B), while ins/LOH polymorphism of the 3’UTR were associated with a trend for worse DFS and OS. The analysis of mKRAS showed no significant effect on survival whereas BRAF V600E mutation was associated with increased risk of death. Clinical variables, close to statistical significance, were age (< 65years old vs ≥ 65years old), primary site (rectal vs colon), histological grade (III-IV vs I-II) and stage (III vs Ι and II).
Table 5.
Univariate Cox regression analysis for clinicopathological features and genotypes
| Variable | HR | DFS 95%CI | P value | HR | OS 95%CI | P value |
| Age < 65 yr | 1.513 | 0.873-2.621 | 0.140 | 1.229 | 0.682-2.213 | 0.492 |
| Rectal Ca | 1.550 | 0.890-2.703 | 0.121 | 1.282 | 0.713-2.306 | 0.406 |
| Stage III vs I%II | 2.532 | 1.368-4.695 | 0.003 | 1.877 | 1.009-3.494 | 0.047 |
| Grade III and IV vs I and II | 1.984 | 1.143-3.436 | 0.015 | 2.097 | 1.166-3.770 | 0.013 |
| KRAS mutation | 1.330 | 0.761-2.326 | 0.321 | 1.283 | 0.702-2.346 | 0.418 |
| BRAF V600E mutation | 1.276 | 0.310-5.255 | 0.736 | 2.743 | 0.845-8.902 | 0.093 |
| TYMS 5’UTR | 0.397 | 0.766 | ||||
| 2R | 1 | 1 | ||||
| 2R/3R | 1.213 | 0.498-2.958 | 0.671 | 0.745 | 0.332-1.672 | 0.475 |
| 3R | 1.690 | 0.678-4.213 | 0.260 | 0.846 | 0.355-2.020 | 0.707 |
| TYMS 5’UTR | 0.596 | 0.615 | ||||
| 2RG/3RG | 1 | 1 | ||||
| 2RG/2RG | 1.038 | 0.377-2.858 | 0.942 | 1.750 | 0.672-4.559 | 0.252 |
| 2RG/3RC | 1.523 | 0.684-3.394 | 0.303 | 1.625 | 0.702-3.760 | 0.257 |
| 3RC/3RC | 1.414 | 0.482-4.148 | 0.528 | 0.680 | 0.146-3.162 | 0.623 |
| 3RG/3RC | 2.128 | 0.902-5.018 | 0.085 | 1.782 | 0.686-4.625 | 0.235 |
| 3RG/3RG | 1.489 | 0.466-4.672 | 0.502 | 1.996 | 0.610-6.532 | 0.253 |
| TYMS 5’UTR | 0.204 | 0.589 | ||||
| 2RG/3RG | 1 | 1 | ||||
| 2RG/2RG | 1.702 | 0.343-8.441 | 0.515 | 3.322 | 0.787-14.03 | 0.102 |
| 2RG/3RC | 2.935 | 0.778-11.08 | 0.112 | 3.034 | 0.803-11.46 | 0.102 |
| 2RG/3RCLOH | 5.387 | 1.427-20.34 | 0.013 | 3.879 | 0.967-15.56 | 0.056 |
| 2RG/3RGLOH | 2.138 | 0.431-10.60 | 0.352 | 2.026 | 0.408-10.06 | 0.388 |
| 2RGLOH/3RC | 3.178 | 0.640-15.78 | 0.157 | 3.109 | 0.626-15.44 | 0.165 |
| 2RGLOH/3RG | 7.402 | 1.648-33.24 | 0.009 | 6.127 | 1.358-27.64 | 0.018 |
| 3RC/3RC | 4.326 | 1.031-18.15 | 0.045 | 1.733 | 0.288-10.42 | 0.548 |
| 3RG/3RC | 4.865 | 1.336-17.72 | 0.016 | 3.438 | 0.888-13.32 | 0.074 |
| 3RG/3RG | 3.413 | 0.761-15.30 | 0.109 | 3.994 | 0.887-17.99 | 0.071 |
| TYMS 5’UTR groups | 0.130 | 0.223 | ||||
| A1 | 1.136 | 0.574-2.251 | 0.714 | 1.882 | 0.917-3.861 | 0.085 |
| B2 | 1 | 1 | ||||
| C3 | 1.908 | 0.980-3.713 | 0.057 | 1.309 | 0.637-2.692 | 0.464 |
| TYMS 3’UTR | 0.791 | 0.846 | ||||
| del/del | 1.170 | 0.496-2.760 | 0.721 | 1.145 | 0.456-2.873 | 0.773 |
| ins/del | 1.244 | 0.664-2.329 | 0.495 | 1.219 | 0.622-2.387 | 0.564 |
| ins/ins | 1 | 1 | ||||
| TYMS 3’UTR | 0.299 | 0.391 | ||||
| del/del | 0.624 | 0.244-1.595 | 0.324 | 0.634 | 0.228-1.761 | 0.382 |
| del/LOH | 0.374 | 0.086-1.630 | 0.190 | 0.408 | 0.093-1.797 | 0.236 |
| ins/del | 0.634 | 0.329-1.224 | 0.175 | 0.743 | 0.372-1.482 | 0.399 |
| ins/LOH | 1 | 1 | ||||
| ins/ins | 0.417 | 0.172-1.016 | 0.054 | 0.391 | 0.140-1.087 | 0.072 |
| ins/ins vs ELSE | 0.593 | 0.267-1.318 | 0.200 | 0.511 | 0.201-1.297 | 0.158 |
| ins/LOH vs ELSE | 1.807 | 1.000-3.266 | 0.050 | 1.650 | 0.877-3.104 | 0.120 |
| ins/del vs ELSE | 0.976 | 0.556-1.713 | 0.933 | 1.131 | 0.626-2.044 | 0.684 |
| del/del vs ELSE | 0.964 | 0.411-2.262 | 0.934 | 0.907 | 0.358-2.299 | 0.837 |
| del/LOH vs ELSE | 0.565 | 0.137-2.327 | 0.430 | 0.570 | 0.138-2.359 | 0.438 |
| LOH | 1.480 | 0.833-2.629 | 0.181 | 1.350 | 0.732-2.487 | 0.336 |
| SNP G->C | 1.542 | 0.878-2.707 | 0.132 | 1.108 | 0.617-1.992 | 0.731 |
Low expression profile;
Medium expression profile;
High expression profile. DFS: Disease-free survival; OS: Overall survival; HR: Hazard ratios; CI: Confidence interval; Ca: Cancer; TYMS: Thymidylate synthase gene; UTR: Untranslated region; LOH: Loss of heterozygosity; 5FU: 5-fluorouracil; SNP: Single nucleotide polymorphism.
Multivariate survival analysis
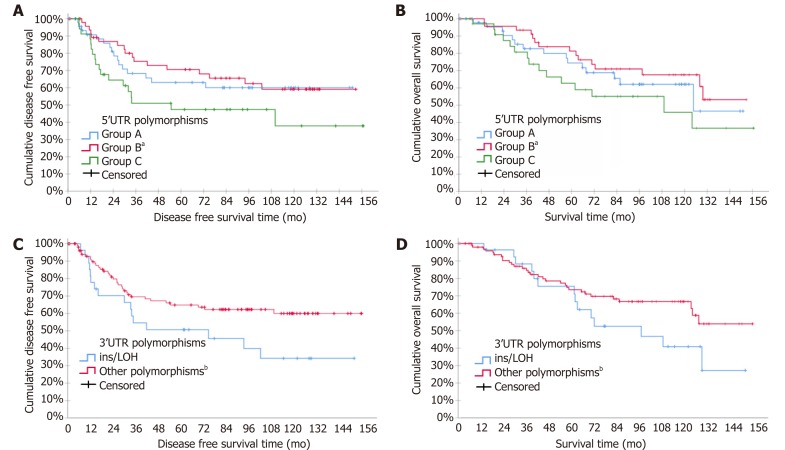
Results of the multivariate analysis including TYMS polymorphisms, mBRAF and selected clinicopathological characteristics are shown in Table 6. From the 5’UTR polymorphisms, the group A (2RG/2RG, 2RG/3RC, 3RC/3RC) and group C (3RG/3RG, 3RG/LOH, 3RG/3RC) were associated with higher risk for disease recurrence and death as compared to group B (2RG/3RG, 2RG/LOH and 3RC/LOH). Similarly, group B of 3’UTR polymorphism (ins/LOH) was associated with increased risk of relapse and death as compared to group A.
Table 6.
Multivariate Cox regression analysis for clinicopathological features and selected genotypes
| Variable | HR | DFS 95%CI | P value | HR | OS 95%CI | P value |
| Stage III vs I and II | 2.432 | 1.279-4.625 | 0.007 | |||
| Grade III and IV vs I and II | 1.715 | 0.951-3.091 | 0.073 | 1.860 | 0.982-3.525 | 0.057 |
| TYMS 5’UTR groups | 0.031 | 0.052 | ||||
| A | 3.122 | 1.193-8.169 | 0.020 | 2.715 | 1.093-6.739 | 0.031 |
| B | 1 | 1 | ||||
| C | 2.919 | 1.258-6.772 | 0.013 | 2.540 | 1.098-5.876 | 0.029 |
| TYMS 3’UTR groups | ||||||
| A (without ins/LOH) | 1 | |||||
| B (ins/LOH) | 4.124 | 1.744-9.753 | 0.001 | 3.335 | 1.474-7.548 | 0.004 |
| BRAF V600E mutation | 4.500 | 1.241-16.32 | 0.022 |
DFS: Disease-free survival; OS: Overall survival; HR: Hazard ratio; CI: Confidence interval; TYMS: Thymidylate synthase gene; UTR: Untranslated region; LOH: Loss of heterozygosity.
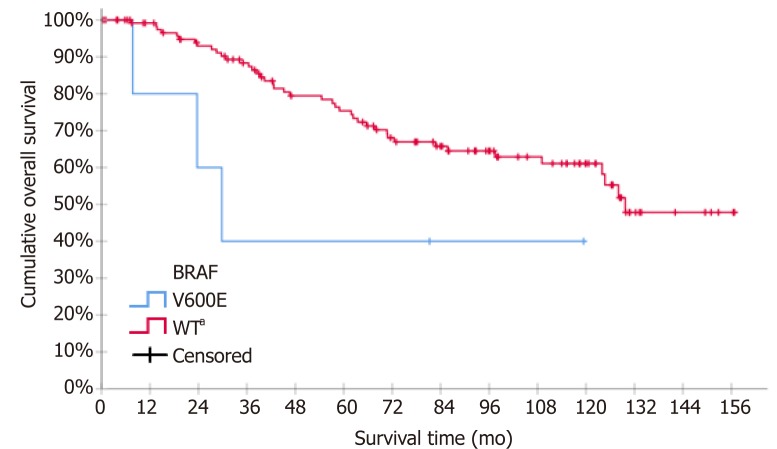
Kaplan-Meier curves for DFS and OS according to TYMS 3’UTR and 5’UTR polymorphisms groups are shown in Figure 1. Stage III increased independently the risk for relapse while the BRAF mutation increased independently the risk for death. Kaplan-Meier curves for OS according to mBRAF are shown in Figure 2.
Figure 1.
Kaplan-Meier curves for disease free survival and overall survival according to thymidylate synthase polymorphisms: A: Disease free survival (DFS) according to 5’ untranslated region (UTR); B: Overall survival (OS) according to 5’UTR; C: DFS according to 3’UTR; D: OS according to 3’UTR. aP < 0.05 vs Group A and C; bP < 0.005. LOH: Loss of heterozygosity.
Figure 2.
Kaplan-Meier survival curve for overall survival according to BRAF mutation status (V600E vs WT - wild type). aP < 0.05.
DISCUSSION
This is a retrospective study of 130 patients with CRC treated with surgery and adjuvant chemotherapy, studying for the first time the correlation of TYMS polymorphisms, LOH, mKRAS and mBRAF with survival outcomes. We report that the 3’UTR and 5’UTR TYMS polymorphisms were independent factors associated with risk of disease relapse and death. In particular, ins/LOH increased risk of disease relapse and death, while the group of 5’UTR polymorphisms containing 2RG/3RG, 2RG/LOH and 3RC/LOH decreased the risk of disease relapse and death. The study of mKRAS pointed out that it did not associate with disease relapse or related death, while the mBRAF increased independently the risk of death.
Since the early studies of adjuvant chemotherapy treatment with 5FU, 23 years ago, there have been two landmark advances in the field[34]. The first one involved the incorporation of oral capecitabine as an alternative to intravenously administered 5FU[35]. The second was the addition of oxaliplatin to 5FU that lead to a 4.2% absolute improvement in OS of patients with T4 and N1 disease (stage III disease; MOSAIC trial) whereas stage II patients did not benefit[36,37]. As clinicopathologic parameters are important but not sufficiently useful in deciding which patients with stage II-III will benefit from adjuvant chemotherapy, molecular markers are essential[38]. Several studies reported the association of TYMS polymorphisms, TYMS mRNA and TYMS protein expression with survival in patients with CRC but with inconsistent findings[20-22,24,39-43]. A meta-analysis indicated that patients with advanced CRC tumors expressing high levels of TYMS had a poorer OS compared to tumors expressing low levels[44]. On the contrary, a subsequent prospective, blinded analysis of TYMS expression in the adjuvant treatment of CRC concluded that TYMS expression did not show a significant prognostic value[45]. None of the studies included in their multivariate analysis the mBRAF status nor the different TYMS polymorphisms.
5’UTR polymorphisms
In this study TYMS polymorphisms emerged as prognostic factors for survival outcomes in patients treated with surgery and adjuvant chemotherapy. More specifically, the group B (2RG/3RG, 2RG/3RCLOH, 2RG/3RGLOH, 2RGLOH/3RC) was shown to have the lowest risk of recurrence and a trend for lower risk of death when compared to the other two groups A (2RG/2RG, 2RG/3RC, 3RC/3RC) and C (3RG/3RG, 3RG/3RC, 2RGLOH/3RG). Similarly, a previous study showed that 5’UTR polymorphisms associated with survival. In particular, they reported that ‘low risk’ polymorphisms (2RG/2RG, 2RG/3RC, 3RC/3RC) were associated with improved DFS regardless chemotherapy treatment[40]. On the contrary, a previous study indicated that TYMS 5’UTR polymorphisms do not predict clinical outcome of CRC patients treated with 5-FU based chemotherapy[39]. Nevertheless, neither of these two studies took into consideration a combined analysis of 3’UTR polymorphisms, LOH or mBRAF status. In addition, the categorization of the TYMS 5’UTR polymorphisms into only two groups (high expression group: 2RG/3RG, 3RC/3RG, 3RG/3RG and low expression group: 2RG/2RG, 2RG/3RC, 3RC/3RC), albeit it facilitates statistical processing it also entails the risk of classification error. Indeed, in this way both studies placed the 2RG/3RG with the high expression 3RG/3RG, although 2RG/3RG is a member of the group of heterozygous 5’UTR polymorphisms group that are generally considered to have an intermediate expression profile[27,46]. Our study identified heterozygotes such as 2RG/3RG, 2RG/LOH and 3RC/LOH, as independent good prognostic factors for recurrence and death in CRC patients treated with surgery and adjuvant chemotherapy.
3’UTR polymorphisms
In our study, 3’UTR polymorphism ins/LOH was found to independently increase the risk for both relapse and death. Comparably, two other studies outlined the negative effect of the ins allele in the therapeutic outcome of CRC patients treated with adjuvant chemotherapy and neoadjuvant setting in rectal cancer patients[41,47]. On the contrary, another study found that ins/ins with 2R/3R and any 3’UTR polymorphism with 3R/3R predict longer DFS and OS in CRC patients treated with adjuvant 5FU-based chemotherapy[22]. However, in the later study the SNP G>C and LOH status were not taken into consideration.
KRAS and BRAF
The present study showed that the rate of mBRAF identified in our population (3.8%) was lower than expected, as previously reported rates in the adjuvant setting ranged from 7.9% to 17%[17,36,48]. Albeit mBRAF was not associated with the risk for relapse, mBRAF independently increased the risk of death. In agreement with our study, three previous studies linked mBRAF to poor survival in relation with MSI status[17,48,49]. A fourth study reported that mBRAF was an adverse prognostic factor for both DFS and OS, independently of MSI status[50]. Contrary to these studies, another study indicated that BRAF mutations did not confer a worse prognosis[36]. Differently to our study, none of the above studies took into consideration TYMS polymorphisms.
In this study mutated KRAS did not emerge as a predictive factor for survival in the univariate analysis. Similar to ours, two previous studies indicated that mKRAS was not associated with survival in stage II/III CRC patients[48,51]. On the contrary, a more recent study reported that the risk of recurrence was higher for mKRAS compared to wild type KRAS tumors[52]. More recently, another study reported that mKRAS had prognostic impact on DFS and OS independently of microsatellite instability status[50]. None of the above studies took into consideration TYMS polymorphisms.
Other findings of the analysis
We found that patients born from 1943 onwards had more frequently the polymorphism 3RG/3RG and high-grade malignancy tumors (RR 1.730, 95%CI: 1.088-2.747; P = 0.030). Two previous studies have also linked age to TYMS polymorphisms and protein expression in CRC[53,54]. As more data gather, the differences in the frequency of polymorphisms among generations are of great interest. These differences could derive from epigenetic modifications induced by environmental changes during the course of human life[55]. Another important open question is whether in younger generations TYMS polymorphisms associate with higher risk of developing aggressive cancer due to changes in the genetic substrate.
We report for the first time that mKRAS had a strong correlation with the polymorphism 3RG/3RC and with polymorphisms that contain only 3RC allele (3RC/3RC, 3RC/LOH). Contrary to our findings, a previous study reported no significant relationship between any of the TYMS polymorphisms with tumor characteristics[56]. However, in the understudy grouping of TYMS polymorphisms, LOH was not considered.
Limitations
Although the size of this study’s patient cohort is one of the largest reported, still it makes it difficult to analyze the large sum of polymorphisms resulting from the combination of 3’UTR and 5’UTR polymorphisms, SNP G>C and LOH. Another limitation is that subsequent chemotherapy lines following disease relapse were not included in the survival analysis. An important limitation is that classification of TYMS polymorphisms into groups was based on our statistical analysis and previously published data but requires further validation in prospective trials.
Another important limitation is that the levels of TYMS protein expression and activity were not examined. Although immunohistochemical analysis of TYMS protein expression is considered important, several studies have shown that TYMS protein expression is affected by several factors, like p53 mutation and other genes which proved to affect the final level of TYMS expression, like astrocyte elevated gene-1 (AEG-1) and enolase superfamily member 1 (ENOSF1) during the course of the disease[57-60]. It has been reported that there is discordance in TYMS mRNA expression and TYMS protein levels between primary tumors and their metastasis[61-63]. Furthermore, the binding of TYMS protein to its own mRNA, as well as the binding of TYMS to p53 mRNA causes translational repression, in an autoregulatory translational manner[64-66]. Other significant prognostic and predictive markers such as NRAS, PIK3CA exon 20 and MMR/MSI were not included in this analysis[64-66].
In conclusion, the group of TYMS polymorphisms 2RG/3RG, 2RG/LOH and 3RC/LOH and the absence of ins/LOH was associated with better prognosis in CRC patients treated with adjuvant chemotherapy while mBRAF was associated with increased risk of death. Proof of concept, prospective studies are required to validate our findings.
ARTICLE HIGHLIGHTS
Research background
A large proportion of patients with colorectal cancer (CRC) do not benefit from fluoro-pyrimidine-based adjuvant chemotherapy (FBAC). Fluoropyrimidines are thymidylate synthase (TYMS) inhibitors. Single nucleotide polymorphism (SNP) and various polymorphisms have been discovered in the 5’ untranslated region (UTR) and in the 3’UTR of the TYMS gene and their association with the survival of CRC patients is under consideration but with conflicting results. Molecular profiling could help clinicians to identify patients with CRC who may benefit from adjuvant chemotherapy, as shown by the associations of BRAF mutations with inferior survival in CRC patients after adjuvant chemotherapy. Also, although KRAS mutations have been found to be associated with poor patient survival, their role in the adjuvant setting is under investigation
Research motivation
There is a need to study the association of the numerous combinations of TYMS polymorphisms (3’UTR, 5’UTR and SNP) with CRC patient survival in a multivariate model including clinicopathological patients’ features and KRAS/BRAF mutations. The loss of heterozygosity (LOH) affects polymorphisms and should be included in such a study.
Research objectives
This study aimed to investigate the association of all known TYMS gene polymorphisms, LOH, KRAS and BRAF mutations with the survival of CRC patients treated with adjuvant chemotherapy.
Research methods
Formalin-fixed paraffin-embedded tissues of 130 consecutive patients treated with FBAC were analysed for the detection of TYMS polymorphisms, mKRAS and mBRAF. Patients were classified according to 5’UTR TYMS polymorphisms and the predicted expression profile, into three groups (high, medium and low expression), utilizing the current literature. This categorization could reduce classification errors. Based on the presence or absence of the 3’UTR polymorphism ins/LOH patients were allocated into two groups (high and low risk of relapse), utilizing the results from univariate analysis of the 3’UTR TYMS polymorphisms. Cox regression models examined the associated 5-year survival outcomes
Research results
In this study, where BRAF, TYMS polymorphisms including SNP G>C and LOH were taken into consideration, both 3’UTR and 5’UTR polymorphisms emerged as independent prognostic factors of survival outcome after adjuvant chemotherapy for CRC. More specifically, the group of patients with tumors bearing 5’UTR polymorphisms 2RG/3RG, 2RG/LOH and 3RC/LOH was associated with better survival. On the contrary, patients with ins/LOH polymorphism in the 3’UTR had worse survival outcome. Also, mBRAF was found to correlate independently with worse prognosis.
Research conclusions
Knowledge of TYMS gene polymorphisms and BRAF status indicates prognosis and could aid clinicians to distinguish the group of patients in need for adjuvant chemotherapy.
Research perspectives
The study of the effect on the survival of CRC patients of the numerous genotypes resulting from the combinations of the 3’UTR and 5’UTR polymorphisms, the SNP and LOH requires larger prospective studies. These studies could validate our findings. Also, they could facilitate the grouping of the TYMS polymorphisms in more than just two groups and thus reduce the classification errors.
ACKNOWLEDGEMENTS
We dedicate this manuscript to the late Petros Karakitsos, our mentor and colleague who founded the lab of cellular and molecular biology where this work was carried out. He will always be remembered with love.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Institutional Review Board and Ethical Committee of University General Hospital Attikon, Athens, Greece.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: All authors declare no conflicts-of-interest related to this article.
Peer-review started: March 15, 2019
First decision: April 16, 2019
Article in press: June 13, 2019
Specialty type: Oncology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arslan NC, Kasi PM, Watanapokasin R S-Editor: Ji FF L-Editor: A E-Editor: Xing YX
Contributor Information
Anastasios Ntavatzikos, Hematology-Oncology Unit, 4th Department of Internal Medicine, Medical School, National and Kapodistrian University of Athens, “ATTIKON” University Hospital, Athens 12462, Greece. dmaal2@yahoo.gr.
Aris Spathis, Department of Cytopathology, National and Kapodistrian University of Athens, Medical School, “ATTIKON” University Hospital, Athens 12462, Greece.
Paul Patapis, 3rd Department of Surgery, Medical School, National and Kapodistrian University of Athens, “ATTIKON” University Hospital, Athens 12462, Greece.
Nikolaos Machairas, 3rd Department of Surgery, Medical School, National and Kapodistrian University of Athens, “ATTIKON” University Hospital, Athens 12462, Greece.
Georgia Vourli, Department of Hygiene, Epidemiology and Medical Statistics, Medical School, National and Kapodistrian University of Athens, Athens 11527, Greece.
George Peros, Department of Surgery, Medical School, National and Kapodistrian University of Athens, Evgenideio Therapeutirio S.A., “I AGIA TRIAS”, Athens 11528, Greece.
Iordanis Papadopoulos, Department of Surgery, Medical School, National and Kapodistrian University of Athens, Evgenideio Therapeutirio S.A., “I AGIA TRIAS”, Athens 11528, Greece.
Ioannis Panayiotides, 2nd Department of Pathology, University of Athens, Medical School, “ATTIKON” University Hospital, Athens 12462, Greece.
Anna Koumarianou, Hematology-Oncology Unit, 4th Department of Internal Medicine, Medical School, National and Kapodistrian University of Athens, “ATTIKON” University Hospital, Athens 12462, Greece.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 3.Eurostat. Cancer statistics - specific cancers 2017. Available from: URL: http://ec.europa.eu/eurostat/statistics-explained/index.php/Cancer_statistics_-_specific_cancers. [Google Scholar]
- 4.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010;375:1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 6.Bijnsdorp IV, Comijn EM, Padron JM, Gmeiner WH, Peters GJ. Mechanisms of action of FdUMP[10]: metabolite activation and thymidylate synthase inhibition. Oncol Rep. 2007;18:287–291. doi: 10.3892/or.18.1.287. [DOI] [PubMed] [Google Scholar]
- 7.Humeniuk R, Menon LG, Mishra PJ, Gorlick R, Sowers R, Rode W, Pizzorno G, Cheng YC, Kemeny N, Bertino JR, Banerjee D. Decreased levels of UMP kinase as a mechanism of fluoropyrimidine resistance. Mol Cancer Ther. 2009;8:1037–1044. doi: 10.1158/1535-7163.MCT-08-0716. [DOI] [PubMed] [Google Scholar]
- 8.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 9.de Gramont A, Larsen AK, Tournigand C, Louvet C, André T GERCOR (French Oncology Research Group) Update on targeted agents for adjuvant treatment of colon cancer in 2006. Gastrointest Cancer Res. 2007;1:S47–S49. [PMC free article] [PubMed] [Google Scholar]
- 10.Quasar Collaborative Group. Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 11.Sinicrope FA, Okamoto K, Kasi PM, Kawakami H. Molecular Biomarkers in the Personalized Treatment of Colorectal Cancer. Clin Gastroenterol Hepatol. 2016;14:651–658. doi: 10.1016/j.cgh.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–531. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adjei AA. Blocking oncogenic Ras signaling for cancer therapy. J Natl Cancer Inst. 2001;93:1062–1074. doi: 10.1093/jnci/93.14.1062. [DOI] [PubMed] [Google Scholar]
- 14.De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V, Papamichael D, Laurent-Puig P, Penault-Llorca F, Rougier P, Vincenzi B, Santini D, Tonini G, Cappuzzo F, Frattini M, Molinari F, Saletti P, De Dosso S, Martini M, Bardelli A, Siena S, Sartore-Bianchi A, Tabernero J, Macarulla T, Di Fiore F, Gangloff AO, Ciardiello F, Pfeiffer P, Qvortrup C, Hansen TP, Van Cutsem E, Piessevaux H, Lambrechts D, Delorenzi M, Tejpar S. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 15.Deng Y, Wang L, Tan S, Kim GP, Dou R, Chen D, Cai Y, Fu X, Wang L, Zhu J, Wang J. KRAS as a predictor of poor prognosis and benefit from postoperative FOLFOX chemotherapy in patients with stage II and III colorectal cancer. Mol Oncol. 2015;9:1341–1347. doi: 10.1016/j.molonc.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taieb J, Zaanan A, Le Malicot K, Julié C, Blons H, Mineur L, Bennouna J, Tabernero J, Mini E, Folprecht G, Van Laethem JL, Lepage C, Emile JF, Laurent-Puig P. Prognostic Effect of BRAF and KRAS Mutations in Patients With Stage III Colon Cancer Treated With Leucovorin, Fluorouracil, and Oxaliplatin With or Without Cetuximab: A Post Hoc Analysis of the PETACC-8 Trial. JAMA Oncol. 2016;2:643–653. doi: 10.1001/jamaoncol.2015.5225. [DOI] [PubMed] [Google Scholar]
- 17.Ogino S, Shima K, Meyerhardt JA, McCleary NJ, Ng K, Hollis D, Saltz LB, Mayer RJ, Schaefer P, Whittom R, Hantel A, Benson AB, 3rd, Spiegelman D, Goldberg RM, Bertagnolli MM, Fuchs CS. Predictive and prognostic roles of BRAF mutation in stage III colon cancer: results from intergroup trial CALGB 89803. Clin Cancer Res. 2012;18:890–900. doi: 10.1158/1078-0432.CCR-11-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu L, Dong C, Cao Y, Fang X, Zhong C, Li D, Yuan Y. Prognostic Role of BRAF Mutation in Stage II/III Colorectal Cancer Receiving Curative Resection and Adjuvant Chemotherapy: A Meta-Analysis Based on Randomized Clinical Trials. PLoS One. 2016;11:e0154795. doi: 10.1371/journal.pone.0154795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Library of Medicine. USNLoM. In: Information NCfB, editor; 2017. Available from: https://www.nlm.nih.gov/ [Google Scholar]
- 20.Ciaparrone M, Quirino M, Schinzari G, Zannoni G, Corsi DC, Vecchio FM, Cassano A, La Torre G, Barone C. Predictive role of thymidylate synthase, dihydropyrimidine dehydrogenase and thymidine phosphorylase expression in colorectal cancer patients receiving adjuvant 5-fluorouracil. Oncology. 2006;70:366–377. doi: 10.1159/000098110. [DOI] [PubMed] [Google Scholar]
- 21.Edler D, Glimelius B, Hallström M, Jakobsen A, Johnston PG, Magnusson I, Ragnhammar P, Blomgren H. Thymidylate synthase expression in colorectal cancer: a prognostic and predictive marker of benefit from adjuvant fluorouracil-based chemotherapy. J Clin Oncol. 2002;20:1721–1728. doi: 10.1200/JCO.2002.07.039. [DOI] [PubMed] [Google Scholar]
- 22.Hitre E, Budai B, Adleff V, Czeglédi F, Horváth Z, Gyergyay F, Lövey J, Kovács T, Orosz Z, Láng I, Kásler M, Kralovánszky J. Influence of thymidylate synthase gene polymorphisms on the survival of colorectal cancer patients receiving adjuvant 5-fluorouracil. Pharmacogenet Genomics. 2005;15:723–730. doi: 10.1097/01.fpc.0000175598.42141.59. [DOI] [PubMed] [Google Scholar]
- 23.Kostopoulos I, Karavasilis V, Karina M, Bobos M, Xiros N, Pentheroudakis G, Kafiri G, Papakostas P, Vrettou E, Fountzilas G. Topoisomerase I but not thymidylate synthase is associated with improved outcome in patients with resected colorectal cancer treated with irinotecan containing adjuvant chemotherapy. BMC Cancer. 2009;9:339. doi: 10.1186/1471-2407-9-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koumarianou A, Tzeveleki I, Mekras D, Eleftheraki AG, Bobos M, Wirtz R, Fountzilas E, Valavanis C, Xanthakis I, Kalogeras KT, Basdanis G, Pentheroudakis G, Kotoula V, Fountzilas G. Prognostic markers in early-stage colorectal cancer: significance of TYMS mRNA expression. Anticancer Res. 2014;34:4949–4962. [PubMed] [Google Scholar]
- 25.Tsuji T, Hidaka S, Sawai T, Nakagoe T, Yano H, Haseba M, Komatsu H, Shindou H, Fukuoka H, Yoshinaga M, Shibasaki S, Nanashima A, Yamaguchi H, Yasutake T, Tagawa Y. Polymorphism in the thymidylate synthase promoter enhancer region is not an efficacious marker for tumor sensitivity to 5-fluorouracil-based oral adjuvant chemotherapy in colorectal cancer. Clin Cancer Res. 2003;9:3700–3704. [PubMed] [Google Scholar]
- 26.Uchida K, Hayashi K, Kawakami K, Schneider S, Yochim JM, Kuramochi H, Takasaki K, Danenberg KD, Danenberg PV. Loss of heterozygosity at the thymidylate synthase (TS) locus on chromosome 18 affects tumor response and survival in individuals heterozygous for a 28-bp polymorphism in the TS gene. Clin Cancer Res. 2004;10:433–439. doi: 10.1158/1078-0432.ccr-0200-03. [DOI] [PubMed] [Google Scholar]
- 27.Kawakami K, Salonga D, Park JM, Danenberg KD, Uetake H, Brabender J, Omura K, Watanabe G, Danenberg PV. Different lengths of a polymorphic repeat sequence in the thymidylate synthase gene affect translational efficiency but not its gene expression. Clin Cancer Res. 2001;7:4096–4101. [PubMed] [Google Scholar]
- 28.Marsh S. Thymidylate synthase pharmacogenetics. Invest New Drugs. 2005;23:533–537. doi: 10.1007/s10637-005-4021-7. [DOI] [PubMed] [Google Scholar]
- 29.Mandola MV, Stoehlmacher J, Muller-Weeks S, Cesarone G, Yu MC, Lenz HJ, Ladner RD. A novel single nucleotide polymorphism within the 5' tandem repeat polymorphism of the thymidylate synthase gene abolishes USF-1 binding and alters transcriptional activity. Cancer Res. 2003;63:2898–2904. [PubMed] [Google Scholar]
- 30.Mandola MV, Stoehlmacher J, Zhang W, Groshen S, Yu MC, Iqbal S, Lenz HJ, Ladner RD. A 6 bp polymorphism in the thymidylate synthase gene causes message instability and is associated with decreased intratumoral TS mRNA levels. Pharmacogenetics. 2004;14:319–327. doi: 10.1097/00008571-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Ntavatzikos A, Spathis A, Patapis P, Machairas N, Peros G, Konstantoudakis S, Leventakou D, Panayiotides IG, Karakitsos P, Koumarianou A. Integrating <i>TYMS</i>, <i>KRAS</i> and <i>BRAF</i> testing in patients with metastatic colorectal cancer. World J Gastroenterol. 2017;23:5913–5924. doi: 10.3748/wjg.v23.i32.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lecomte T, Ferraz JM, Zinzindohoué F, Loriot MA, Tregouet DA, Landi B, Berger A, Cugnenc PH, Jian R, Beaune P, Laurent-Puig P. Thymidylate synthase gene polymorphism predicts toxicity in colorectal cancer patients receiving 5-fluorouracil-based chemotherapy. Clin Cancer Res. 2004;10:5880–5888. doi: 10.1158/1078-0432.CCR-04-0169. [DOI] [PubMed] [Google Scholar]
- 33.Spathis A, Georgoulakis J, Foukas P, Kefala M, Leventakos K, Machairas A, Panayiotides I, Karakitsos P. KRAS and BRAF mutation analysis from liquid-based cytology brushings of colorectal carcinoma in comparison with formalin-fixed, paraffin-embedded tissue. Anticancer Res. 2010;30:1969–1975. [PubMed] [Google Scholar]
- 34.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, Ungerleider JS, Emerson WA, Tormey DC, Glick JH, Veeder MH, Mailliard JA. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med. 1995;122:321–326. doi: 10.7326/0003-4819-122-5-199503010-00001. [DOI] [PubMed] [Google Scholar]
- 35.Twelves C, Wong A, Nowacki MP, Abt M, Burris H, 3rd, Carrato A, Cassidy J, Cervantes A, Fagerberg J, Georgoulias V, Husseini F, Jodrell D, Koralewski P, Kröning H, Maroun J, Marschner N, McKendrick J, Pawlicki M, Rosso R, Schüller J, Seitz JF, Stabuc B, Tujakowski J, Van Hazel G, Zaluski J, Scheithauer W. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696–2704. doi: 10.1056/NEJMoa043116. [DOI] [PubMed] [Google Scholar]
- 36.André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 37.Sanoff HK, Carpenter WR, Stürmer T, Goldberg RM, Martin CF, Fine JP, McCleary NJ, Meyerhardt JA, Niland J, Kahn KL, Schymura MJ, Schrag D. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol. 2012;30:2624–2634. doi: 10.1200/JCO.2011.41.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Auclin E, Zaanan A, Vernerey D, Douard R, Gallois C, Laurent-Puig P, Bonnetain F, Taieb J. Subgroups and prognostication in stage III colon cancer: future perspectives for adjuvant therapy. Ann Oncol. 2017;28:958–968. doi: 10.1093/annonc/mdx030. [DOI] [PubMed] [Google Scholar]
- 39.Park CM, Lee WY, Chun HK, Cho YB, Yun HR, Heo JS, Yun SH, Kim HC. Relationship of polymorphism of the tandem repeat sequence in the thymidylate synthase gene and the survival of stage III colorectal cancer patients receiving adjuvant 5-flurouracil-based chemotherapy. J Surg Oncol. 2010;101:22–27. doi: 10.1002/jso.21412. [DOI] [PubMed] [Google Scholar]
- 40.Sulzyc-Bielicka V, Bielicki D, Binczak-Kuleta A, Kaczmarczyk M, Pioch W, Machoy-Mokrzynska A, Ciechanowicz A, Gołębiewska M, Drozdzik M. Thymidylate synthase gene polymorphism and survival of colorectal cancer patients receiving adjuvant 5-fluorouracil. Genet Test Mol Biomarkers. 2013;17:799–806. doi: 10.1089/gtmb.2013.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dotor E, Cuatrecases M, Martínez-Iniesta M, Navarro M, Vilardell F, Guinó E, Pareja L, Figueras A, Molleví DG, Serrano T, de Oca J, Peinado MA, Moreno V, Germà JR, Capellá G, Villanueva A. Tumor thymidylate synthase 1494del6 genotype as a prognostic factor in colorectal cancer patients receiving fluorouracil-based adjuvant treatment. J Clin Oncol. 2006;24:1603–1611. doi: 10.1200/JCO.2005.03.5253. [DOI] [PubMed] [Google Scholar]
- 42.Matsui T, Omura K, Kawakami K, Morita S, Sakamoto J. Genotype of thymidylate synthase likely to affect efficacy of adjuvant 5-FU based chemotherapy in colon cancer. Oncol Rep. 2006;16:1111–1115. [PubMed] [Google Scholar]
- 43.Wang J, Shi D, Guo X, Zhang J, Yu S, Song J, Cao Z, Wang J, Ji M, Dong W. Thymidylate synthase genetic polymorphisms and colorectal cancer risk: a meta-analysis. Clin Res Hepatol Gastroenterol. 2014;38:481–490. doi: 10.1016/j.clinre.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Popat S, Matakidou A, Houlston RS. Thymidylate synthase expression and prognosis in colorectal cancer: a systematic review and meta-analysis. J Clin Oncol. 2004;22:529–536. doi: 10.1200/JCO.2004.05.064. [DOI] [PubMed] [Google Scholar]
- 45.Popat S, Chen Z, Zhao D, Pan H, Hearle N, Chandler I, Shao Y, Aherne W, Houlston R. A prospective, blinded analysis of thymidylate synthase and p53 expression as prognostic markers in the adjuvant treatment of colorectal cancer. Ann Oncol. 2006;17:1810–1817. doi: 10.1093/annonc/mdl301. [DOI] [PubMed] [Google Scholar]
- 46.Horie N, Aiba H, Oguro K, Hojo H, Takeishi K. Functional analysis and DNA polymorphism of the tandemly repeated sequences in the 5'-terminal regulatory region of the human gene for thymidylate synthase. Cell Struct Funct. 1995;20:191–197. doi: 10.1247/csf.20.191. [DOI] [PubMed] [Google Scholar]
- 47.Stoehlmacher J, Goekkurt E, Mogck U, Aust DE, Kramer M, Baretton GB, Liersch T, Ehninger G, Jakob C. Thymidylate synthase genotypes and tumour regression in stage II/III rectal cancer patients after neoadjuvant fluorouracil-based chemoradiation. Cancer Lett. 2008;272:221–225. doi: 10.1016/j.canlet.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 48.Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, Dietrich D, Biesmans B, Bodoky G, Barone C, Aranda E, Nordlinger B, Cisar L, Labianca R, Cunningham D, Van Cutsem E, Bosman F. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–474. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 49.Seppälä TT, Böhm JP, Friman M, Lahtinen L, Väyrynen VM, Liipo TK, Ristimäki AP, Kairaluoma MV, Kellokumpu IH, Kuopio TH, Mecklin JP. Combination of microsatellite instability and BRAF mutation status for subtyping colorectal cancer. Br J Cancer. 2015;112:1966–1975. doi: 10.1038/bjc.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kadowaki S, Kakuta M, Takahashi S, Takahashi A, Arai Y, Nishimura Y, Yatsuoka T, Ooki A, Yamaguchi K, Matsuo K, Muro K, Akagi K. Prognostic value of KRAS and BRAF mutations in curatively resected colorectal cancer. World J Gastroenterol. 2015;21:1275–1283. doi: 10.3748/wjg.v21.i4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogino S, Meyerhardt JA, Irahara N, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Schaefer P, Whittom R, Hantel A, Benson AB 3rd, Goldberg RM, Bertagnolli MM, Fuchs CS. Cancer and Leukemia Group B, North Central Cancer Treatment Group, Canadian Cancer Society Research Institute, Southwest Oncology Group. KRAS mutation in stage III colon cancer and clinical outcome following intergroup trial CALGB 89803. Clin Cancer Res. 2009;15:7322–7329. doi: 10.1158/1078-0432.CCR-09-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hutchins G, Southward K, Handley K, Magill L, Beaumont C, Stahlschmidt J, Richman S, Chambers P, Seymour M, Kerr D, Gray R, Quirke P. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 2011;29:1261–1270. doi: 10.1200/JCO.2010.30.1366. [DOI] [PubMed] [Google Scholar]
- 53.Odin E, Wettergren Y, Nilsson S, Carlsson G, Gustavsson B. Colorectal carcinomas with microsatellite instability display increased thymidylate synthase gene expression levels. Clin Colorectal Cancer. 2007;6:720–727. doi: 10.3816/CCC.2007.n.042. [DOI] [PubMed] [Google Scholar]
- 54.Fariña-Sarasqueta A, Gosens MJ, Moerland E, van Lijnschoten I, Lemmens VE, Slooter GD, Rutten HJ, van den Brule AJ. TS gene polymorphisms are not good markers of response to 5-FU therapy in stage III colon cancer patients. Cell Oncol (Dordr) 2011;34:327–335. doi: 10.1007/s13402-011-0030-z. [DOI] [PubMed] [Google Scholar]
- 55.Marsh S, Collie-Duguid ES, Li T, Liu X, McLeod HL. Ethnic variation in the thymidylate synthase enhancer region polymorphism among Caucasian and Asian populations. Genomics. 1999;58:310–312. doi: 10.1006/geno.1999.5833. [DOI] [PubMed] [Google Scholar]
- 56.Etienne-Grimaldi MC, Mahamat A, Chazal M, Laurent-Puig P, Olschwang S, Gaub MP, Formento JL, Formento P, Sudaka A, Boige V, Abderrahim-Ferkoune A, Benchimol D, André T, Houry S, Faucheron JL, Letoublon C, Gilly FN, Delpero JR, Lasser P, Pradere B, Pezet D, Penault-Llorca F, Milano G. Molecular patterns in deficient mismatch repair colorectal tumours: results from a French prospective multicentric biological and genetic study. Br J Cancer. 2014;110:2728–2737. doi: 10.1038/bjc.2014.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nief N, Le Morvan V, Robert J. Involvement of gene polymorphisms of thymidylate synthase in gene expression, protein activity and anticancer drug cytotoxicity using the NCI-60 panel. Eur J Cancer. 2007;43:955–962. doi: 10.1016/j.ejca.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 58.Yoo BK, Gredler R, Vozhilla N, Su ZZ, Chen D, Forcier T, Shah K, Saxena U, Hansen U, Fisher PB, Sarkar D. Identification of genes conferring resistance to 5-fluorouracil. Proc Natl Acad Sci U S A. 2009;106:12938–12943. doi: 10.1073/pnas.0901451106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosmarin D, Palles C, Church D, Domingo E, Jones A, Johnstone E, Wang H, Love S, Julier P, Scudder C, Nicholson G, Gonzalez-Neira A, Martin M, Sargent D, Green E, McLeod H, Zanger UM, Schwab M, Braun M, Seymour M, Thompson L, Lacas B, Boige V, Ribelles N, Afzal S, Enghusen H, Jensen SA, Etienne-Grimaldi MC, Milano G, Wadelius M, Glimelius B, Garmo H, Gusella M, Lecomte T, Laurent-Puig P, Martinez-Balibrea E, Sharma R, Garcia-Foncillas J, Kleibl Z, Morel A, Pignon JP, Midgley R, Kerr D, Tomlinson I. Genetic markers of toxicity from capecitabine and other fluorouracil-based regimens: investigation in the QUASAR2 study, systematic review, and meta-analysis. J Clin Oncol. 2014;32:1031–1039. doi: 10.1200/JCO.2013.51.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vignoli M, Nobili S, Napoli C, Putignano AL, Morganti M, Papi L, Valanzano R, Cianchi F, Tonelli F, Mazzei T, Mini E, Genuardi M. Thymidylate synthase expression and genotype have no major impact on the clinical outcome of colorectal cancer patients treated with 5-fluorouracil. Pharmacol Res. 2011;64:242–248. doi: 10.1016/j.phrs.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 61.Aschele C, Debernardis D, Tunesi G, Maley F, Sobrero A. Thymidylate synthase protein expression in primary colorectal cancer compared with the corresponding distant metastases and relationship with the clinical response to 5-fluorouracil. Clin Cancer Res. 2000;6:4797–4802. [PubMed] [Google Scholar]
- 62.Kumamoto K, Kuwabara K, Tajima Y, Amano K, Hatano S, Ohsawa T, Okada N, Ishibashi K, Haga N, Ishida H. Thymidylate synthase and thymidine phosphorylase mRNA expression in primary lesions using laser capture microdissection is useful for prediction of the efficacy of FOLFOX treatment in colorectal cancer patients with liver metastasis. Oncol Lett. 2012;3:983–989. doi: 10.3892/ol.2012.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marsh S, McKay JA, Curran S, Murray GI, Cassidy J, McLeod HL. Primary colorectal tumour is not an accurate predictor of thymidylate synthase in lymph node metastasis. Oncol Rep. 2002;9:231–234. [PubMed] [Google Scholar]
- 64.Chu E, Koeller DM, Casey JL, Drake JC, Chabner BA, Elwood PC, Zinn S, Allegra CJ. Autoregulation of human thymidylate synthase messenger RNA translation by thymidylate synthase. Proc Natl Acad Sci U S A. 1991;88:8977–8981. doi: 10.1073/pnas.88.20.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chu E, Voeller DM, Jones KL, Takechi T, Maley GF, Maley F, Segal S, Allegra CJ. Identification of a thymidylate synthase ribonucleoprotein complex in human colon cancer cells. Mol Cell Biol. 1994;14:207–213. doi: 10.1128/mcb.14.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Q, Yu Z, Xiang Y, Wu N, Wu L, Xu B, Wang L, Yang P, Li Y, Bai L. Prognostic and predictive significance of thymidylate synthase protein expression in non-small cell lung cancer: a systematic review and meta-analysis. Cancer Biomark. 2015;15:65–78. doi: 10.3233/CBM-140432. [DOI] [PubMed] [Google Scholar]